Abstract
Circulating immune complexes (IC) and levels of IC-induced cytokines have been correlated with complement activation and autoantibody profiles in systemic lupus erythematosus (SLE). SLE sera were analysed concerning levels of immune complexes (IC), classical complement function and different antinuclear and anti-C-reactive protein (CRP) autoantibodies. Blood mononuclear cells from healthy donors were stimulated with isolated IC and production of interleukin (IL)-10, IL-6 and IL-12p40 was measured. Functional experiments revealed that increased levels of IC-induced cytokines were associated with both increased classical complement activation and the occurrence of anti-Sjögren's syndrome A (SSA) and anti-SSB but not other autoantibodies. Biochemical measurement of circulating IC showed that the degree of complement activation and the occurrence of anti-SSA were synergistically associated with levels of circulating IC in SLE sera, as complement activation was a prerequisite for the enhancing effect of anti-SSA. Anti-CRP was associated with complement activation, but not with other autoantibodies. Our results indicate that anti-SSA and possibly anti-SSB antibodies influence IC formation and subsequent IC-induced cytokine induction, and that they thereby participate in the inflammatory process in active SLE.
Keywords: complement, cytokines, immune complex, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a prototype immune complex (IC)-mediated disease [1] associated with IC-induced classical complement activation [2]. Antibodies bound to autoantigens released from apoptotic cells contribute to IC formation [3]. A number of autoantibodies against nuclear constituents are associated with SLE, e.g. antibodies against double-stranded (ds) DNA, Sjögren's syndrome A [SSA(Ro)], SSB(La), U1-snRNP and Sm antigens. Whereas levels of antibodies against dsDNA covary with SLE disease activity [3] this is not obvious for anti-SSA/anti-Ro and anti-SSB/anti-La [4]. Anti-SSA has therefore not been considered as a disease activity marker but as a marker of primary Sjögren's syndrome and SLE associated with photosensitivity, interstitial lung disease and homozygous deficiency of C2 or C4, as well as a marker of subacute cutaneous lupus erythematosus [5]. Interleukin (IL)-6 and IL-10 are both considered important in SLE pathogenesis [6,7]. Elevated levels of IL-6 [8] and/or IL-10 [9,10] mediate enhanced IgG production, including the formation of autoantibodies and IC [9], and IL-10 levels correlate with SLE disease activity [11]. IL-12, a monokine promoting Th1-like responses, has been reported to be decreased in SLE as a consequence of IL-10-mediated suppression [12]. In addition, serum levels of IL-12 have been reported to correlate inversely with IL-10 in SLE [13], although positive correlations between serum IL-10 and IL-12 have also been reported in SLE [14].
In contrast to other systemic inflammatory conditions, circulating C-reactive protein (CRP) levels often remain low in SLE despite high inflammatory activity [15]. Supplementation of CRP reduces autoantibody formation, delays onset of nephritis and prolongs the survival of lupus-prone mice [16]. Many of the biological properties of CRP may explain the salutogenic implications in relation to SLE, e.g. its opsonizing properties due to affinity for certain microbes, apoptotic material (including nuclear autoantigens) and Fc-gamma receptors (FcγR), its activating and modulating effects on complement activation and its interaction with IgG-containing IC [17,18]. Raised levels of autoantibodies against the monomeric form of CRP occur frequently in parallel with disease activity in SLE [19].
We have reported previously that polyethylene glycol (PEG)-precipitated IC from SLE patients induce IL-6 and IL-10 production from peripheral blood mononuclear cells (PBMC) [20]. We have also described how IC-induced production of IL-10 and IL-12p40 were regulated in association with activation of the classical complement pathway in vitro [21,22]. In the present study we aimed to investigate how complement activation in vivo and levels of autoantibodies correlate with the amount of circulating IC in vivo and IC-induced cytokine production in vitro. A second aim was to investigate whether the earlier-described reciprocal findings of increased circulating IL-10 and decreased IL-12 in SLE could be attributed to divergent IC-mediated regulation.
We determined an association between autoantibodies, classical complement activation and levels of circulating IC in vivo and induction of the cytokines IL-10, IL-6 and IL-12 by PEG-precipitated SLE IC in vitro. Notably, classical complement activation and anti-SSA/anti-SSB but not other autoantibodies were synergistically associated with circulating IC levels in vivo and with levels of SLE IC-induced cytokines in vitro. Thus anti-SSA and anti-SSB antibodies may participate directly in the inflammatory process in SLE by enhancing IC formation and subsequent production of cytokines.
Materials and methods
Serum samples
Serum samples were obtained from patients with a clinical diagnosis of SLE and which had been investigated previously concerning classical complement function, and levels of C3 and C3d as a measure of disease activity. A total of 147 serum samples from 63 SLE patients (51 women and 12 men, median age 37 years, range 8–81) were studied. All samples had been separated and frozen at −70°C within 4 h of sampling. Prior to the present study the serum samples had been quickly thawed, aliquoted, anonymized and frozen again. The ethics committee of Uppsala University approved the study protocol.
Experimental set-up
In a first experiment aimed primarily at examining the effect of complement activation, pairs of sera with markedly discrepant classical complement function from 19 SLE patients (38 samples) were investigated concerning the activity of purified IC to induce cytokine production. The purpose of the second experiment was to investigate the effect of various autoantibodies on cytokine production. Seventy-eight SLE samples with normal classical complement function (complement function or C3 not depressed) and divergent autoantibody profiles were investigated in the same way as in experiment 1. The 78 sera represented 40 patients. The objective of the third experiment was to study the combined effect of complement activation and autoantibodies on the formation of circulating IC. In this setting C1q-binding IC were measured in the 115 samples with complete data of anti-nuclear antibody (ANA)-associated autoantibodies and IC levels compared both to complement activation and to the occurrence of specific autoantibodies in a two-way analysis of variance (anova).
PEG precipitation of immune complexes
The precipitates were purified and washed in a single-step centrifugation procedure as described previously [23,24]. Briefly, 1 ml of phosphate-buffered saline (PBS) containing 5% human serum albumin (HSA) and 2·5% PEG 6000 (PBS–HSA–PEG) was added to 1·5 ml autoclaved Eppendorf tubes. Plastic cylinders made from 5 ml autoclaved pipette tips (by cutting off about 1·5 cm of the tips) were introduced into the Eppendorf tubes containing PBS–HSA–PEG. The sera precipitated overnight were diluted 1 : 3 in RPMI-1640 containing 2·5% PEG 6000 and then placed on top of the PBS–HSA–PEG in the pipette tips. An interface was formed with the less dense, red RMPI-1640 solution on top. The tubes were then centrifuged at 2100 g, 4°C for 20 min, whereby the precipitates in the upper 2·5% PEG–RPMI solution were centrifuged down to the bottom of the Eppendorf tube. The remaining PBS–HSA–PEG solution was removed and the precipitated pellet was immediately resolubilized in ice-cold sterile PBS to the original serum volume. The precipitates were totally resolved in PBS leaving no insoluble aggregates. The dissolved PEG precipitates were then placed on ice until used in cell culture experiments.
Preparation of peripheral blood mononuclear cells (PBMC) and cell cultures
Buffy coats from healthy blood donors were density gradient separated and cultured according to [24]. The cell culture media used was RPMI-1640 (Flow Laboratories, Irvine, Scotland, UK) supplemented with 1% glutamine, 1% penicillin streptomycin, 1% HEPES and 1% Ultroser G® (Flow Laboratories). In previous studies we have found that Ultroser G® sustain IC-induced cytokine production in otherwise serum-free systems. Freshly prepared PEG-precipitated IC were added to the cells (10% v/v) within 2 h of preparation and cultured for 20 h in standardized 300 µl cultures in flat-bottomed 96-microwell plates. PBMC donors might differ considerably concerning their IC-induced cytokine responses. As in our earlier studies [20,24,25], PBMC from two donors were therefore investigated in parallel and data from the PBMC donor exhibiting the strongest net IC reactivity (highest intra-assay CV between investigated samples, always exceeding 60%) was used in the calculations.
Cytokine enzyme-linked immunosorbent assays (ELISAs)
Levels of IL-10, IL-6 and IL-12p40 in cell culture supernatants were measured by ELISA following a recently described protocol [21,22]. Alkaline phosphatase was replaced by horseradish peroxidase (R&D Systems, Abingdon, UK) employing 3,3′-5,5′-tetramethylbenzidine (Dako, Glostrup, Denmark) as substrate. Monoclonal antibodies from Mabtech (Stockholm, Sweden) against IL-6 (13A5) and IL-12p40 (IL-12I) were used as primary antibodies, and 39C3 (IL-6) and IL-12II (IL-12p40) were used as secondary antibodies. For IL-10 measurement we used F(ab′)2-fragmented antibodies (Hu IL-10 FlexiaTM; Biosource, Nivelles, Belgium).
Measurement of circulating IC, ANA and specific ANA-associated autoantibodies
Levels of circulating IC were measured by a solid-phase C1q-assay (Bindazyme C1q binding kit; Binding Site, Birmingham, UK). ANA and anti-dsDNA antibodies were analysed by indirect immunofluorescence (IF) microscopy using HEp-2 cells and Crithidia luciliae, respectively (both from Immunoconcepts, Sacramento, CA, USA). Antibodies against SSA, SSB, U1-snRNP and Sm were analysed by double radial immunodiffusion (DID) for 48 h (Immunoconcepts). Of the 115 samples investigated in experiment 3, 95/115 (82·6%) were IF ANA positive (titre ≥ 200), 34 positive (titre ≥ 10) for anti-dsDNA, 35 were positive for anti-SSA, 18 were positive for both anti-SSA and anti-SSB, six were positive for anti-U1-snRNP and three were positive for anti-Sm. Five of 100 blood donor controls had IF ANA above the cut-off titre, all without any ANA-specific autoantibodies.
Measurement of classical complement function, C3 and C3d levels
Functional activity of the classical complement pathway was measured according to Nilsson et al. [26]. Briefly, 20 µl from each SLE serum and from a reference serum pool was diluted 1 : 5 before mixing with 100 µl of a 40% solution of rabbit IgM-coated sheep erythrocytes and incubated for 20 min on a shaker at 37°C. The reaction was stopped by adding 3 ml of 0·01 M ethylenediamine tetraacetic acid (EDTA), whereupon the samples were centrifuged at 650 g for 10 min at 4°C; 250 µl from each supernatant were added to ELISA plates and the absorbance was measured at 541 nm. Haemolytic activity of the classical complement pathway was defined as the absorbance in the samples divided by the absorbance of the reference serum pool. Levels of C3 were measured using rate nephelometry (Immage; Beckman Coulter, Stockholm, Sweden) according to the manufacturer's instructions. The reference range for C3 was based on investigations of 180 individuals from Danderyd Hospital, Sweden, and related to the international calibrator CRM 470. C3d levels were also measured using rate nephelometry and an unconjugated polyclonal rabbit anti-C3d antibody (A0063; Dako Sweden AB, Stockholm, Sweden). Prior to C3d measurements the sera were mixed with 20% PEG 6000 and incubated for 90 min at 4°C before centrifugation at 1920 g for 30 min at 4°C. The reference range for C3d was based on a mean value from 100 healthy blood donors ± 3 standard deviations (s.d.).
Measurement of anti-CRP antibodies and CRP
IgG anti-CRP antibodies were measured exactly as described previously [19]. Anti-CRP was investigated in 125 patients, 100 of whom were included in experiment 3.
Possible interference by IC with anti-CRP measurement was assessed. IC from eight SLE sera were thus PEG-precipitated as described above and rediluted in PBS to the original serum volumes. The samples were again diluted 1 : 20 in PBS-Tween and applied to a CRP-coated microtitre plate in quadruplicates. The subsequent procedure was identical to that used for anti-CRP antibody analysis. High sensitivity CRP determinations were made using nephelometry (Bayer HealthCare, Advia 1650, NY, USA).
Statistics
The Mann–Whitney U-test was used for comparisons between groups in the unpaired design, whereas the Wilcoxon signed-rank test was employed for paired comparisons and the Spearman's rank correlation test was used to determine correlations. Differences between proportions were analysed using the χ2 test. Two-way analysis of variance (anova) was used to investigate the combined effects of complement activation and various autoantibodies on levels of circulating IC. P-values < 0·05 were considered significant.
Results
IC-induced production of IL-10 and IL-12 versus complement activation
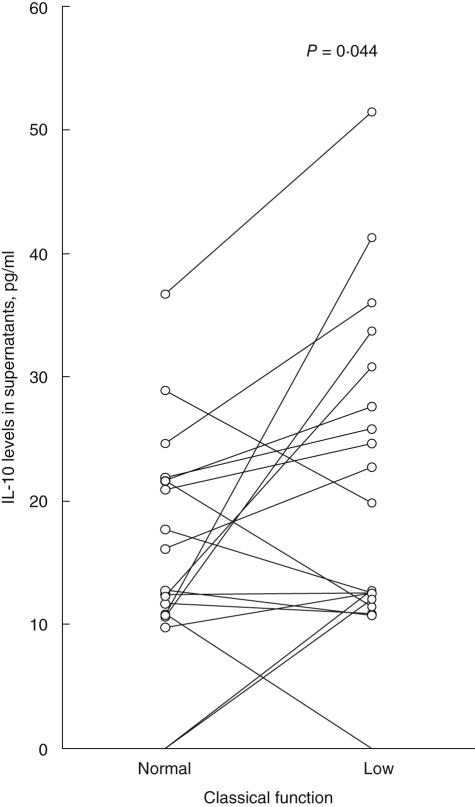
Increased complement activation, measured either as decreased function of the classical complement pathway (Fig. 1) or as raised C3d/C3 ratios, was associated with increased IC-induced IL-10 production (P = 0·044 for both comparisons). For IL-6 there was no significant difference. Decreased levels of C3 were associated with increased IL-12p40 production (P = 0·033; data not included).
Fig. 1.
Activation of the classical complement pathway is associated with increased immune complex (IC)-induced interleukin (IL)-10 production. Paired sera from 19 systemic lupus erythematosus (SLE) patients taken on two separate occasions with either normal (left) or depressed (right) function of the classical complement pathway were polyethylene glycol (PEG)-precipitated. The paired samples were drawn a median of 5 months apart (range 1–10). PEG-precipitates were added to healthy peripheral blood mononuclear cell cultures and levels of IL-10 were measured in supernatants after 20 h of culture.
Impact of anti-SSA and anti-SSB on IC-induced cytokine production
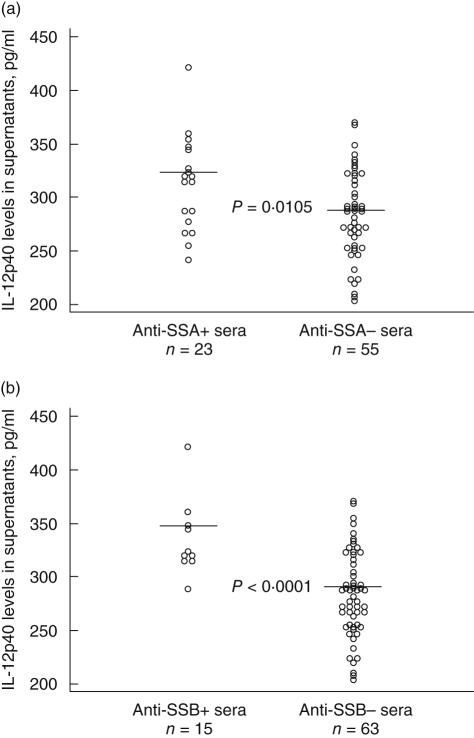
Among the 38 samples with varying complement levels in experiment 1, PEG precipitates from patients with both anti-SSA and anti-SSB antibodies induced higher levels of IL-10 and IL-6 levels compared to sera lacking these antibodies (P = 0·021 and 0·011, respectively; data not included). No such association was determined for other autoantibodies. In the second experiment, encompassing 78 samples with normal complement profile, IL-12p40 levels were increased in cell cultures with PEG-IC from anti-SSA antibody-containing samples (P = 0·0105; Fig. 2a), and this effect was even more pronounced in samples containing both anti-SSA and anti-SSB antibodies (P < 0·0001; Fig. 2b). There was a trend of higher IL-10 production induced by PEG precipitates from sera containing both anti-SSA and anti-SSB antibodies (P = 0·082; data not included). IL-6 was not investigated in the second experiment.
Fig. 2.
Autoantibodies against (a) Sjögren's syndrome A (SSA) and (b) both SSA and SSB are associated with increased interleukin (IL)-12p40 levels induced by systemic lupus erythematosus (SLE) immune complex (IC). Polyethylene glycol (PEG) precipitates from 78 SLE sera with normal complement function and known autoantibody status were used to stimulate cytokine production in vitro. Smaller differences in cytokine production were noted between individual cell cultures in this investigation of serum samples with normal complement function compared to the investigation of samples with both normal and depressed complement activity. Five high outliers for each antibody combination in (a) and (b) are not depicted.
Covariation between SLE IC-induced cytokine levels
In the first experiment with 19 pairs of samples there was a strong correlation between IC-induced levels of IL-10 and IL-6 (r = 0·86, P < 0·0001) and between IC-induced changes in IL-10 and IL-6 (r = 0·75, P = 0·0015). There was a positive correlation between the amount of IC-induced IL-10 and circulating IC measured in serum (r = 0·459, P = 0·0221; data not included). IC-induced levels of IL-12p40 did not correlate with induced levels of IL-6 or IL-10 in experiment 1 (data not included). In the second experiment IC-induced levels of IL-10 and IL-12 were positively correlated (r = 0·58, P < 0·0001; data not included).
Circulating IC versus complement function and anti-SSA antibodies
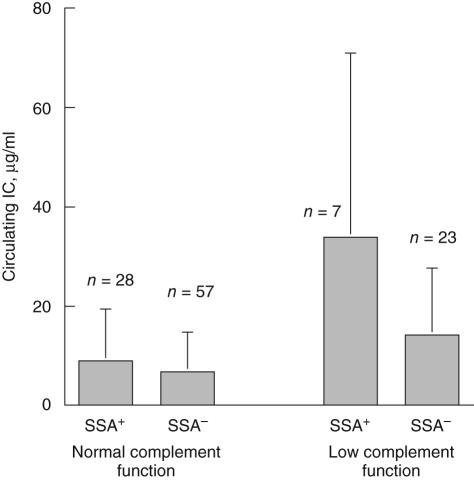
To analyse the impact of antibody status and complement function in a large number of samples in parallel we examined levels of circulating IC instead of IC-induced cytokine production. Both classical complement function and the occurrence of anti-SSA were associated with circulating IC levels, with a strong interaction between decreased complement function and the occurrence of anti-SSA antibodies (complement consumption P < 0·0001, anti-SSA P = 0·0009, interaction between complement consumption and anti-SSA P = 0·0072; Fig. 3). No corresponding association was apparent for anti-dsDNA, the combination of anti-SSA and anti-SSB, anti-U1-snRNP or of anti-CRP antibodies. None of the anti-SSA-positive patients had any known homozygous deficiency for C2 or C4, and complement function was never decreased towards zero as observed in such states.
Fig. 3.
Levels of circulating immune complex (IC) are associated with both classical complement function and the occurrence of anti-Sjögren's syndrome A (SSA) autoantibodies in a synergistic fashion. The graph depicts mean levels ± s.d. of circulating IC in sera with normal and low classical complement function distributed between anti-SSA positive (anti-SSA +) and anti-SSA negative (anti-SSA–) sera. Two-way analysis of variance analysis revealed that levels of circulating IC were associated significantly with classical complement function (P < 0·0001), anti-SSA (P = 0·0009) and the interaction between complement function and anti-SSA (P = 0·0072); 115 sera with data available both concerning circulating IC and autoantibodies were included.
Anti-CRP antibodies
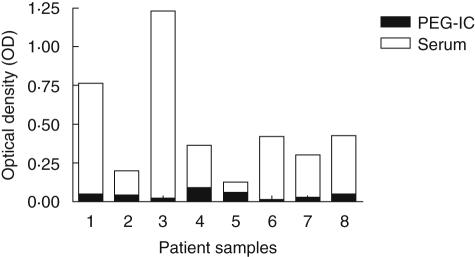
Of the investigated SLE sera, 50 of 125 (40%) exhibited increased anti-CRP levels, highly different from the healthy controls (P < 0·0001). Anti-CRP antibody levels did not differ between SLE sera with and without ANA-associated autoantibodies, elevated levels of CRP or circulating IC levels (data not included). There were no correlations between anti-CRP antibody levels and measures of complement function when all sera were investigated together. When 25 paired samples with markedly different levels of classical complement function were investigated we found that decreased C3 levels (P = 0·0082) and increased C3d/C3 ratios (P = 0·048) were associated with increased anti-CRP antibody levels. IC from SLE patients did not cause false positive results in the anti-CRP analyses (Fig. 4).
Fig. 4.
Sera from systemic lupus erythematosus (SLE) patients (empty bars), but not their corresponding precipitated immune complex (IC) (filled bars) can elicit a positive anti-C-reactive protein (CRP) reaction. Sera and polyethylene glycol (PEG)-precipitated IC from eight patients were introduced into a single CRP-coated enzyme-linked immunosorbent assay plate and analysed in parallel with regard to anti-CRP reactivity.
Discussion
Signs of IC-mediated activation of the classical complement pathway is a hallmark of flare and ongoing organ damage in SLE, and many studies support that anti-dsDNA antibodies can be involved in this process [27]. Levels of other SLE-related autoantibodies, such as anti-SSA/SSB, do not exhibit variations in relation to disease activity; however, in this study we report that SLE-IC induced cytokine levels are associated both with an activated complement system and with the occurrence of antibodies against SSA and SSB. Biochemical measurement of circulating IC including more samples than would be feasible in cell culture experiments revealed a synergistic effect between autoantibodies and complement, as the association of anti-SSA with IC levels was obvious only in serum samples with low classical complement consumption function. This circumstance was restricted to anti-SSA and anti-SSB, and could not be shown for the other SLE-associated autoantibodies, anti-dsDNA, anti-U1-snRNP or anti-CRP. The fact that we did not find any association between anti-dsDNA and IC-levels, IC-induced cytokine production or degree of complement activation is surprising because of the strong associations between anti-dsDNA and SLE shown in other studies. However, our results do not rule out the role of other autoantibodies, e.g. anti-dsDNA in vivo. Anti-dsDNA levels in IC have been shown to be decreased during SLE flares, possibly because of tissue deposition of IC [28]. Also positively charged antibodies [29] and especially anti-dsDNA antibodies [30] bind more strongly to glomerular basement membrane compared to non-cationic antibodies in SLE. Anti-dsDNA can be trapped in the tissue and create IC in situ without any association to circulating IC levels, and this might explain the lack of correlation to complement activation and circulating IC levels for anti-dsDNA and other antibodies besides anti-SSA and possibly also anti-SSB in this study.
Hypothetically, in active SLE, anti-SSA and anti-SSB antibodies form IC with antigens released from apoptotic cells, resulting in complement activation and leucocyte production of cytokines. Conversely, in quiescent disease states these autoantigens are not released, leaving the circulating autoantibodies uncomplexed. This discovery is corroborated by the recent findings of Båve et al. [31], who reported that anti-SSA/SSB-positive sera from patients with Sjögren's syndrome together with either apoptotic or necrotic cells induced IFN-α production from plasmacytoid dendritic cells or PBMC. Although the proportion of ANA-positive SLE sera with an abnormal titre (≥ 200) by IF microscopy was only 82·6% in the present study, this accords with previous recent findings in patients with established SLE according to the 1982 American College of Rheumatology classification criteria [32,33].
Levels of circulating IC were measured by a commonly used solid-phase C1q-binding assay. In a comparison of the amount of CIC in healthy control sera with sera from patients with RA, SLE and systemic sclerosis the C1q-binding assay was shown to have the highest sensitivity compared to two different C3 binding assays [34]. Autoantibodies to C1q are sometimes found in sera from SLE patients and we can therefore not exclude that these antibodies might in some cases interfere with the C1q binding assay used here.
Treatment of serum samples with PEG might precipitate a number of high molecular weight proteins in parallel to IC [35,36]. In an earlier paper we performed control experiments to investigate the possibility that PEG precipitation itself could form IC and also if rheumatoid factor (RF) could enhance cytokine-inducing properties of these IC formed by PEG precipitation [24]. These experiments showed that neither PEG precipitation nor RF per se could induce IC formation in the absence of preformed IC. The correlation between levels of C1q-binding circulating IC and levels of cytokines induced by PEG precipitates, and the fact that the levels of SLE PEG precipitate-induced IL-10 can be specifically reduced by pretreatment of the responder PBMC with blocking antibodies against FcγRIIa [20], both argue for IC-mediated effects. We have reported previously similar results of FcγRIIa blockade regarding other patient-derived IC : IgG3 cryoglobulin from a myeloma patient [22] and soluble or surface-bound rheumatoid arthritis IC [24,25]. The prevalence of anti-CRP antibodies in this study accords with our previous observations [19,37]. CRP has been identified in SLE IC [38], and a positive anti-CRP antibody test could hypothetically be explained by the presence of circulating CRP-containing IC. The failure of heat-aggregated rabbit IgG to inhibit the reaction [19] and the present demonstration that PEG-precipitated and re-solubilized IC from SLE sera did not induce falsely positive anti-CRP tests contradict this notion. The associations presented in this report between raised anti-CRP levels and complement activation corroborate our previous findings that anti-CRP antibodies are associated with SLE disease activity [19].
In vitro production of IL-10 by PBMC from SLE patients correlates inversely with IL-12 production according to some [12,13,39,40], but not all [14] reports. We determined either positive or no correlation between IC-induced levels of IL-10 and IL-12p40 in our experiments. The present study therefore argues that the earlier described inverse correlation between IL-10 and IL-12 in SLE is not dependent on disease-specific IC. Future investigations of the interplay between production of IL-10 and IL-12 in SLE must take into account not only the degree of complement activation in vivo during creation of IC, but also the availability of an intact complement system during cell culture studies, such as we have employed in other experimental systems [21,22].
Anti-SSA and anti-SSB antibodies are regarded as disease markers, but have not been associated previously with disease activity in SLE. The results of the present study indicate that these antibodies might be of importance in the formation of circulating IC. We suggest that anti-SSA and possibly anti-SSB antibodies may participate directly in the inflammatory process in SLE by enhancing IC formation and subsequent cytokine production.
Acknowledgments
The study was supported financially by grants from the Swedish Rheumatism association, the Swedish Society for Research without Animal Experiments, the Agnes and Mac Rudberg foundation, the Signe and Reinold Sunds foundation for rheumatologic research, the Signe and Olof Wallerius' foundation and the Norrköping Hospital Research Board. We thank Associate Professor R. A. Harris for linguistic advice.
References
- 1.Koffler D, Agnello V, Thoburn R, Kunkel HG. Systemic lupus erythematosus: prototype of immune complex nephritis in man. J Exp Med. 1971;134:169–79. [PMC free article] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement and systemic lupus erythematosus. Arthritis Res. 2002;4(Suppl. 3):S279–93. doi: 10.1186/ar586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481–90. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassan AB, Lundberg IE, Isenberg D, Wahren-Herlenius M. Serial analysis of Ro/SSA and La/SSB antibody levels and correlation with clinical disease activity in patients with systemic lupus erythematosus. Scand J Rheumatol. 2002;31:133–9. [PubMed] [Google Scholar]
- 5.Reichlin M. Systemic lupus erythematosus. Antibodies to ribonuclear proteins. Rheum Dis Clin North Am. 1994;20:29–43. [PubMed] [Google Scholar]
- 6.Mongan AE, Ramdahin S, Warrington RJ. Interleukin-10 response abnormalities in systemic lupus erythematosus. Scand J Immunol. 1997;46:406–12. doi: 10.1046/j.1365-3083.1997.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 7.Cross JT, Benton HP. The roles of interleukin-6 and interleukin-10 in B cell hyperactivity in systemic lupus erythematosus. Inflamm Res. 1999;48:255–61. doi: 10.1007/s000110050456. [DOI] [PubMed] [Google Scholar]
- 8.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–23. [PubMed] [Google Scholar]
- 9.Llorente L, Zou W, Levy Y, et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–44. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aljanadi M, Aldalaan A, Alballa S, Alhumaidi M, Raziuddin S. Interleukin-10 (IL−10): secretion in systemic lupus erythematosus and rheumatoid arthritis − Il-10-dependent Cd4+Cd45ro+ T cell B cell antibody synthesis. J Clin Immunol. 1996;16:198–207. doi: 10.1007/BF01541225. [DOI] [PubMed] [Google Scholar]
- 11.Park YB, Lee SK, Kim DS, Lee J, Lee CH, Song CH. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 1998;16:283–8. [PubMed] [Google Scholar]
- 12.Liu TF, Jones BM. Impaired production of IL-12 in systemic lupus erythematosus. I. Excessive production of IL-10 suppresses production of IL-12 by monocytes. Cytokine. 1998;10:140–7. doi: 10.1006/cyto.1997.0268. [DOI] [PubMed] [Google Scholar]
- 13.Liu TF, Jones BM. Impaired production of IL-12 in system lupus erythematosus. II. IL-12 production in vitro is correlated negatively with serum IL-10, positively with serum IFN-gamma and negatively with disease activity in SLE. Cytokine. 1998;10:148–53. doi: 10.1006/cyto.1997.0269. [DOI] [PubMed] [Google Scholar]
- 14.Capper ER, Maskill JK, Gordon C, Blakemore AI. Interleukin (IL)-10, IL-1ra and IL-12 profiles in active and quiescent systemic lupus erythematosus: could longitudinal studies reveal patient subgroups of differing pathology? Clin Exp Immunol. 2004;138:348–56. doi: 10.1111/j.1365-2249.2004.02607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hesselink DA, Aarden LA, Swaak AJ. Profiles of the acute-phase reactants C-reactive protein and ferritin related to the disease course of patients with systemic lupus erythematosus. Scand J Rheumatol. 2003;32:151–5. doi: 10.1080/03009740310002489. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez W, Mold C, Kataranovski M, Hutt J, Marnell LL, Du Clos TW. Reversal of ongoing proteinuria in autoimmune mice by treatment with C-reactive protein. Arthritis Rheum. 2005;52:642–50. doi: 10.1002/art.20846. [DOI] [PubMed] [Google Scholar]
- 17.Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004;30:261–77. doi: 10.1385/IR:30:3:261. [DOI] [PubMed] [Google Scholar]
- 18.Sjöwall C, Bengtsson T, Skogh T. CRP and anti-CRP autoantibodies in systemic lupus erythematosus. Curr Rheumatol Rev. 2005;1:81–9. [Google Scholar]
- 19.Sjöwall C, Bengtsson AA, Sturfelt G, Skogh T. Serum levels of autoantibodies against monomeric C-reactive protein are correlated with disease activity in systemic lupus erythematosus. Arthritis Res Ther. 2004;6:R87–94. doi: 10.1186/ar1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rönnelid J, Tejde A, Mathsson L, Nilsson-Ekdahl K, Nilsson B. Immune complexes from SLE sera induce IL10 production from normal peripheral blood mononuclear cells by an FcgammaRII dependent mechanism: implications for a possible vicious cycle maintaining B cell hyperactivity in SLE. Ann Rheum Dis. 2003;62:37–42. doi: 10.1136/ard.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tejde A, Mathsson L, Ekdahl KN, Nilsson B, Rönnelid J. Immune complex-stimulated production of interleukin-12 in peripheral blood mononuclear cells is regulated by the complement system. Clin Exp Immunol. 2004;137:521–8. doi: 10.1111/j.1365-2249.2004.02569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathsson L, Tejde A, Carlson K, et al. Cryoglobulin-induced cytokine production via FcgammaRIIa: inverse effects of complement blockade on the production of TNF-alpha and IL-10. Implications for the growth of malignant B-cell clones. Br J Haematol. 2005;129:830–8. doi: 10.1111/j.1365-2141.2005.05538.x. [DOI] [PubMed] [Google Scholar]
- 23.Pontese-Carvalho LC, Lannes-Vieira J, Giovanni de-Simone S, Galvao-Castro B. A protein A-binding, polyethylene glycol precipitation-based immunoradiometric assay. Application to the detection of immune complexes and C3 in human sera and of private antigens in cross- reacting parasite extracts. J Immunol Meth. 1986;89:27–35. doi: 10.1016/0022-1759(86)90028-1. [DOI] [PubMed] [Google Scholar]
- 24.Mathsson L, Lampa J, Mullazehi M, Rönnelid J. Immune complexes from rheumatoid arthritis synovial fluid induce FcgammaRIIa dependent and rheumatoid factor correlated production of tumour necrosis factor-alpha by peripheral blood mononuclear cells. Arthritis Res Ther. 2006;8:R64. doi: 10.1186/ar1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullazehi M, Mathsson L, Lampa J, Rönnelid J. Surface-bound anti-type II collagen-containing immune complexes induce production of tumor necrosis factor alpha, interleukin-1beta, and interleukin-8 from peripheral blood monocytes via Fc gamma receptor IIA: a potential pathophysiologic mechanism for humoral anti-type II collagen immunity in arthritis. Arthritis Rheum. 2006;54:1759–71. doi: 10.1002/art.21892. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson UR, Nilsson B. Simplified assays of hemolytic activity of the classical and alternative pathways. J Immunol Meth. 1984;72:49–59. doi: 10.1016/0022-1759(84)90432-0. [DOI] [PubMed] [Google Scholar]
- 27.Tang Lui S, Lai KN SL. Pathogenesis of lupus nephritis; an update. Nephrology (Carlton) 2005;10:783–9. doi: 10.1111/j.1440-1797.2005.00392.x. [DOI] [PubMed] [Google Scholar]
- 28.Bengtsson A, Nezlin R, Shoenfeld Y, Sturfelt G. DNA levels in circulating immune complexes decrease at severe SLE flares-correlation with complement component C1q. J Autoimmun. 1999;13:111–19. doi: 10.1006/jaut.1999.0300. [DOI] [PubMed] [Google Scholar]
- 29.Gauthier VJ, Mannik M. A small proportion of cationic antibodies in immune complexes is sufficient to mediate their deposition in glomeruli. J Immunol. 1990;145:3348–52. [PubMed] [Google Scholar]
- 30.Suenaga R, Abdou NI. Cationic and high affinity serum IgG anti-dsDNA antibodies in active lupus nephritis. Clin Exp Immunol. 1993;94:418–22. doi: 10.1111/j.1365-2249.1993.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Båve U, Nordmark G, Lövgren T, Rönnelid J, Cajander S, Eloranta ML, Alm GV, Rönnblom L. Activation of the type I interferon system in primary Sjogren's syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005;52:1185–95. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- 32.Nordmark G, Rorsman F, Rönnblom L, et al. Autoantibodies to alpha-fodrin in primary Sjogren's syndrome and SLE detected by an in vitro transcription and translation assay. Clin Exp Rheumatol. 2003;21:49–56. [PubMed] [Google Scholar]
- 33.Salonen EM, Miettinen A, Walle TK, Koskenmies S, Kere J, Julkunen H. Anti-telomere antibodies in systemic lupus erythematosus (SLE): a comparison with five antinuclear antibody assays in 430 patients with SLE and other rheumatic diseases. Ann Rheum Dis. 2004;63:1250–4. doi: 10.1136/ard.2003.011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanilova SA, Slavov ES. Comparative study of circulating immune complexes quantity detection by three assays − CIF-ELISA, C1q-ELISA and anti-C3 ELISA. J Immunol Meth. 2001;253:13–21. doi: 10.1016/s0022-1759(01)00370-2. [DOI] [PubMed] [Google Scholar]
- 35.Robinson MW, Scott DG, Bacon PA, Walton KW, Coppock JS, Scott DL. What proteins are present in polyethylene glycol precipitates from rheumatic sera? Ann Rheum Dis. 1989;48:496–501. doi: 10.1136/ard.48.6.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crowley-Nowick PA, Campbell E, Schrohenloher RE, Mestecky J, Jackson S. Polyethylene glycol precipitates of serum contain a large proportion of uncomplexed immunoglobulins and C3. Immunol Invest. 1996;25:91–101. doi: 10.3109/08820139609059293. [DOI] [PubMed] [Google Scholar]
- 37.Sjöwall C, Eriksson P, Almer S, Skogh T. Autoantibodies to C-reactive protein is a common finding in SLE, but not in primary Sjogren's syndrome, rheumatoid arthritis or inflammatory bowel disease. J Autoimmun. 2002;19:155–60. doi: 10.1006/jaut.2002.0608. [DOI] [PubMed] [Google Scholar]
- 38.Maire MA, Barnet M, Carpentier N, Miescher PA, Lambert PH. Identification of components of IC purified from human sera. I. Immune complexes purified from sera of patients with SLE. Clin Exp Immunol. 1983;51:215–24. [PMC free article] [PubMed] [Google Scholar]
- 39.Horwitz DA, Gray JD, Behrendsen SC, et al. Decreased production of interleukin-12 and other Th1-type cytokines in patients with recent-onset systemic lupus erythematosus. Arthritis Rheum. 1998;41:838–44. doi: 10.1002/1529-0131(199805)41:5<838::AID-ART10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 40.Liu TF, Jones BM, Wong RW, Srivastava G. Impaired production of IL-12 in systemic lupus erythematosus. III. deficient IL-12 p40 gene expression and cross-regulation of IL-12, IL-10 and IFN-gamma gene expression. Cytokine. 1999;11:805–11. doi: 10.1006/cyto.1999.0512. [DOI] [PubMed] [Google Scholar]