Abstract
Cytomegalovirus (CMV) is the most common cause of congenital infection worldwide and occurs as a result of transplacental transmission of the virus. The human neonate is highly susceptible to infection due to a combination of immaturity of the immune system and antigenic inexperience. This study uses the in vivo model of congenital CMV to examine both the humoral and cell-mediated immune responses in vertically infected neonates and their mothers. Ten pairs of matched neonates and their mothers were evaluated for specific IgM responses to three immunodominant CMV antigens: pp38 (pUL80a), pp52 (pUL44) and pp150 (pUL32). In contrast to conventional enzyme immunoassay (EIA) testing for CMV-specific IgM, which found five of the mothers and four of the neonates to be positive, Western immunoblotting showed all 10 adults and nine newborns to be positive. Eight mothers and nine newborns had serological evidence of primary infection. All neonates showed a response to pp38, an assembly protein, nine responded to the pp52 immediate early antigen but only four had reactivity to the pp150 tegument associated protein. Of the mothers, eight had pp38 reactivity, 10 showed a response to the pp52 antigen and seven to the pp150 antigen. T cell-mediated immunity was assessed by measuring cytokines using a multiplex microarray assay. Levels of interferon (IFN)-γ were high in both groups [mean ± standard error of the mean (s.e.m.): neonates = 657 ± 238 pg/ml, mothers = 1072 ± 677 pg/ml, pNS]; however, neonates had significantly higher levels of interleukin (IL)-8 (316 ± 136 pg/ml versus 48 ± 28 pg/ml, P < 0·005). Similar levels of IL-2, IL-7, IL-10 and IL-12 were measured in both groups, but levels of IL-1α, IL-1β, IL-4, IL-6 and tumour necrosis factor (TNF)-α were either absent or low. In response to CMV, neonates and adults mount a predominant T helper 1 (Th1) response, as evidenced by the presence of IL-2, IL-8, IL-12 and IFN-γ with concomitant lack of IL-4. These findings suggest that the neonate, when presented with infection in utero, is capable of mounting an individual response; however, the lower IFN-γ and higher IL-8 levels suggest reduced immune responsiveness when compared to their adult counterparts.
Keywords: congenital, cytomegalovirus, IFN-γ, IgM, IL-8
Introduction
Cytomegalovirus (CMV) is a double-stranded DNA virus and is one of the most ubiquitous human pathogens being transmitted vertically and horizontally. Primary CMV infection is always followed by an acute, often asymptomatic infection followed by lifelong latency without clinical illness. Severe symptomatic CMV infection has been shown to occur in immunosuppressed individuals such as in transplant recipients, patients with AIDS and following in utero infection. CMV is the most commonly acquired congenital viral infection occurring in about 0·2% to 3% of all live births [1]. It is an infectious cause of prenatal neurological damage, which is particularly severe when primary maternal infection occurs during the first 16 weeks of gestation, at the time of organ development and neuronal migration [2]. Currently, more than four CMV vaccines are in clinical trials [3]. A better understanding of the immune response in early life, factors which might predict clinical sequelae and the impact of reactivation would help in the design of suitable vaccines.
IgM antibodies generally do not cross the placenta during normal pregnancy presumably because of their large pentameric structure and the lack of a specific transporter mechanism, as for IgG [4]. Therefore, in a normal non-infected neonate, IgM, if present, is predominantly naturally occurring and not as a response to stimulation by foreign antigen. Several strong lines of evidence have supported the possibility that the naturally occurring antibody repertoire in human neonates contains certain stereotyped specificities [5,6]. The presence of IgM in the neonatal circulation at birth is indicative of intrauterine infection. This IgM is thought to be derived locally, although the potential for the presence of maternal IgM in the neonatal circulation in such cases of infection cannot be eliminated totally. Studies of neonatal T cells in vitro have revealed reduced capacity to respond to primary antigen, stimulatory monoclonal antibodies and toxic shock toxin resulting in deficient interleukin (IL)-2 production [7–9]. In contrast to functional studies showing reduced cytolytic activity [10], a recent study has shown mature CD8+ T cell responses to CMV in congenitally infected newborns and in a fetus of 28 weeks' gestation [11].
Using congenital CMV as an in vivo model of viral infection to analyse the primary repertoire accurately, this study shows that neonates are capable of mounting an independent primary humoral response with a preferential specificity to the pp38 CMV assembly protein antigen. Although T helper 1 (Th1) responses predominated in response to viral infection, the lower levels of interferon (IFN)-γ and higher IL-8 levels observed in newborns suggests reduced immune responsiveness when compared to adults. Our data reveal the development of specific humoral and cellular immune responses from a naive state in humans, and insight into this process is essential in understanding immunity and development of effective vaccination strategies.
Materials and methods
Patients' sera
Sera were obtained retrospectively from the National Virus Reference Laboratory archive sera bank. A cohort of 10 newborns and their mothers' sera were included in the study and were compiled at random from laboratory records. Direct early antigen fluorescent foci (DEAFF) tests were positive in all newborns for urine collected within 4 days of birth [12]. This test is used routinely to allow rapid detection of CMV in urine specimens. The corresponding mothers' archived booking bloods were retrieved. Ethical approval for the study was obtained from the Human Research Ethics Committee, University College Dublin.
CMV IgM enzyme immunoassay (EIA)
CMV IgM detection was performed according to the manufacturer's instructions (Trinity Biotech, Wicklow, Ireland). Briefly, 100 µl of sera were added to the wells at a dilution of 1/100 and incubated for 60 min at 37°C. After washing, 100 µl of conjugate (1/100) was added to all wells and plates were incubated for a further 60 min at 37°C. After a final wash, 100 µl of substrate was added for 30 min at room temperature; 100 µl of 2 N sulphuric acid was added to stop the reaction. Optical density was measured using an enzyme-linked immunosorbent assay (ELISA) reader (Biotek Instrument, ELX 808, Launch Diagnostics, Dublin, Ireland). Index values of < 0·9 were considered negative, 0·9–1·1 were considered equivocal and > 1·1 were considered positive.
CMV IgG avidity assay
Avidity assays were performed according to the manufacturer's recommendations (BioMerieux, Basingstoke, UK). Briefly, 100 µl of sera were added to two strips coated with CMV antigens. To the second strip, a dissociating buffer containing 6 M urea was added. Strips were then slotted into the Vidas instrument (Vitek Systems, Basingstoke, UK) for the assay to proceed. Appropriate positive control and control with urea buffer were included to standardize the assay. For a given serum sample, the avidity index (AI) was calculated using the following formula (OD for the well washed with dissociating urea buffer/OD for the well washed with control buffer). An AI of < 0·3 indicates primary infection, 0·3–0·8 cannot distinguish between recent or former infection and ≥ 0·8 is a strong indication of primary infection ≥ 3 months previously.
Immunoblotting for the detection of IgM to specific CMV antigens
CMV IgM Western blot kits supplied by Genelabs Diagnostics (Geneva, Switzerland) were used. Nitrocellulose strips incorporated with three CMV recombinant antigenic proteins (pp38, pp52 and pp150) and an anti-human IgM control was blocked with blotting buffer and then incubated with patient sera (1/100 dilution) at room temperature on a rocking platform. After 60 min, strips were washed three times and then incubated with 2 ml of alkaline phosphatase goat anti-human IgM conjugate (1/1000) for a further 60 min. After washing, substrate (5-bromo-4-chloro-3 indolyl-phosphate and nitroblue tetrazolium) was added for 15 min. Strips were rinsed thoroughly with distilled water to stop the reaction. Images were captured on an UVP BioImaging System (Cambridge, UK) using Labworks software and an Autochemi camera system. For CMV IgM positivity, reactivity to the pp52 and one other antigen was required. Band intensity of pp52 > pp150 was indicative of primary infection. Reactivity to p38 only indicates negativity for CMV IgM. Intensity of bands were graded from 0 to 4, where 0 indicated absence of staining and 4 indicated intense staining.
Polymerase chain reaction (PCR) for CMV
DNA extraction was carried out using the QIAmp DNA mini kit (Roche Diagnostics Systems Inc., NJ, USA). Briefly, 20 µl of Qiagen protease and 200 µl of lysis buffer were added to 200 µl of patient's plasma. After pulse vortexing the sample was incubated at 56°C for 10 min; 200 µl of 96–100% ethanol was added to the sample, which was further vortexed for 15 s. The lysed solution was placed in a QIAmp spin column and centrifuged at 6000 g for 1 min. The filtrate was discarded and the spin column washed twice with 500 µl of wash buffer 1 and then once with wash buffer 2. The sample was eluted from the column matrix with 200 µl of buffer and then subjected to PCR using the following conditions: 3 min at 50°C, 10 min at 95°C and 38 cycles of 15 s at 95°C and 1 min at 60°C. The primers used were: forward primer 5′-GCG TGC TTT TTA GCC TCT GCA-3′; reverse primer 5′-AAA AGT TTG TGC CCC AAC GGT A-3′. This assay is a qualitative method and results are interpreted as being positive or negative depending on the result of the positive and negative controls used.
Multiplex T cell cytokine microarray
Using the proteoplex microarray assay supplied by Novagen (MP Biomedicals, Geneva, Switzerland), 12 cytokines [IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12, granulocyte–macrophage colony-stimulating factor (GM-CSF), IFN-γ and tumour necrosis factor (TNF)-α] were measured simultaneously. Patients' sera and control sera were diluted according to the manufacturer's protocol and then incubated for 60 min on an orbital shaker at room temperature. After washing, incubation with human detection antibody cocktail was performed for a further 60 min. After rinsing, the detection system (sensilight PBLX fluorophore) was added for 90 min at room temperature in the dark. Slides were rinsed, dried and disassembled and returned to the manufacturer for scanning (633 nm Ex, 660 nm Em at 10 µm resolution) and analysis. Standard curves for all 12 cytokines analysed had R2 values of between 0·94 and 1·00.
Statistical analysis
The Mann–Whitney U-test was used to compare maternal and neonatal cytokine levels.
Results
Demographic, clinical and diagnostic evaluation of the study cohort
Ten pairs of matched newborns (1–4 days old) and mothers (age range 16–34 years) were included in the study. Laboratory diagnosis of congenital CMV is established by isolation of the virus and detection using the urine DEAFF test. All neonates, of whom five were female, were DEAFF-test positive. Table 1 outlines several clinical characteristics of all the neonates at birth. Six of the 10 neonates were asymptomatic but were exposed when their mothers had CMV infection during pregnancy. Two newborns were premature and had low birth weight. Table 2 shows the profile for neonatal CMV IgM serology and PCR to detect CMV DNA. Using EIA, only four neonates were positive for primary infection; however, Western blotting showed nine positive with one neonate (N7) interpreted as negative, as only reactivity to the p38 band was observed. PCR for CMV showed detectable virus levels in two of the five newborns studied. PCR was not as informative in this study, as appropriate blood samples were not received. Table 3 shows the laboratory details of the matched maternal group in the study. At the time of sampling, five mothers were reported with primary infection using conventional EIA assays. In contrast, Western blotting to detect CMV-specific IgM to three immunodominant CMV antigens revealed that eight mothers had primary infection and two showed reactivation (M2 and M9). Unlike M2, who had an AI of 0·895, M9 had a low AI of 0·220 and positive serology suggesting possible primary infection. Avidity index studies showed that six samples had values < 0·3 indicative of recent infection.
Table 1.
Clinical characteristics of neonates in the study cohort.
| Neonate | Gestation age* | Birth weight** | Clinical symptoms |
|---|---|---|---|
| N1 | 36+ 5 | 4·20 | Hepatosplenamegaly |
| N2 | 35 | 1·44 | Pre-mature baby, TORCH screen*** |
| N3 | 39 + 3 | 3·40 | Asymptomatic, mother had CMV infection during pregnancy |
| N4 | 33 + 2 | 1·15 | Pre-mature weight/hepatosplenamegaly |
| N5 | 37 + 5 | 3·35 | Asymptomatic, mother had CMV infection during pregnancy |
| N6 | 39 + 2 | 3·15 | Asymptomatic, mother had CMV infection during pregnancy |
| N7 | 40 + 1 | 3·21 | Hepatosplenamegaly |
| N8 | 41 | 3·24 | Asymptomatic, mother had CMV infection during pregnancy |
| N9 | 38 + 5 | 3·70 | Asymptomatic, mother had CMV infection during pregnancy |
| N10 | 38 + 4 | 3·10 | Asymptomatic, mother had CMV infection during pregnancy |
CMV: cytomegalovirus.
Weeks and days;
kilograms;
toxoplasma, rubella, CMV and herpes screen.
Table 2.
Serological cytomegalovirus (CMV)-specific IgM analysis in neonatal sera.
| Western blotting CMV-IgM | |||||
|---|---|---|---|---|---|
| Neonate | EIA CMV-IgM | p38 | p52 | p150 | PCR |
| N1 | 0·407 | 3* | 2 | 2 | Negative |
| N2 | 1·376 | 1 | 4 | 0 | Positive |
| N3 | 0·634 | 1 | 2 | 2 | NT** |
| N4 | 2·398 | 2 | 1 | 0 | Negative |
| N5 | 4·186 | 4 | 4 | 4 | NT |
| N6 | 0·542 | 1 | 3 | 0 | NT |
| N7 | 0·235 | 1 | 0 | 0 | Positive |
| N8 | 1·229 | 2 | 1 | 0 | Negative |
| N9 | 0·584 | 3 | 2 | 2 | NT |
| N10 | 0·357 | 2 | 1 | 0 | NT |
Scoring for intensity, see Materials and methods;
not tested. EIA: enzyme immunoassay; PCR: polymerase chain reaction.
Table 3.
Serological evaluation for cytomeagalovirus (CMV)-specific IgM and IgG avidity index (AI) in maternal sera.
| Western blotting CMV IgM | |||||
|---|---|---|---|---|---|
| Mother | EIA CMV-IgM | p38 | p52 | p150 | AI |
| M1 | 2·243 | 1 | 3 | 2 | 0·252 |
| M2 | 0·234 | 0 | 1 | 3 | 0·895 |
| M3 | 3·975 | 2 | 4 | 4 | 0·170 |
| M4 | 0·509 | 2 | 3 | 2 | 0·285 |
| M5 | 1·196 | 0 | 2 | 1 | 0·129 |
| M6 | 1·609 | 4 | 4 | 4 | 0·260 |
| M7 | 0·466 | 1 | 2 | 2 | 0·540 |
| M8 | 0·197 | 1 | 1 | 0 | 0·844 |
| M9 | 4·240 | 2 | 1 | 2 | 0·220 |
| M10 | 0·736 | 1 | 2 | 0 | 0·856 |
Detection of IgM specific for three CMV antigens by Western blotting
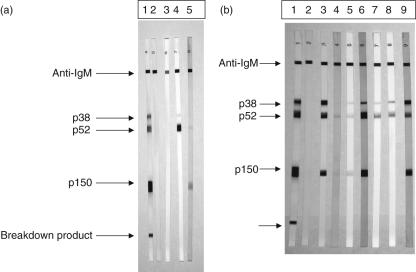
A higher rate of CMV infection was detected in both groups using the immunoblot assay compared to EIA testing. Figure 1a shows the sensitivity of the blotting assay used and its correlation with laboratory serology results. Lanes 1 and 2 represent positive and negative controls, respectively. Samples from a neonate (N2) received at 1 day of birth (lane 3) and at 22 days (lane 4) showed appearance of the pp38 antigen and dominant pp52 early antigen, indicating primary infection. The matched mother's serum (M2, lane 5) had IgM reactivity to the pp52 and pp150 antigens only. Figure 1b shows IgM responses in three matched pairs of neonates and mothers. Lanes 3 and 4 shows a paired sample of newborn (N5) and mother (M5) who were both EIA IgM positive. The newborn shows responses to all three antigens, whereas the maternal response is restricted to the pp52 and pp150 antigens only. Interestingly, this maternal serum (M5) had an avidity index of 0·129 indicating primary infection. Lanes 5 and 6 show another pair where the newborn (N3) was EIA IgM negative (lane 5) and the mother (M3) positive and with an AI of 0·17. Both had reactivity to all three antigens. Lanes 7 and 8 show the same newborn (N6) studied 10 days apart. Response to the pp38 antigen has increased over this time period and no pp150 reactivity was observed. Lane 9 shows the matched maternal response (M6) to all three antigens. These findings show that the newborn is capable of mounting an individual and detectable immune response to CMV infection in utero.
Fig. 1.
(a) Western blot analysis of IgM to three cytomegalovirus (CMV) recombinant proteins, p38, p52 and p150. Lanes 1 and 2 show positive and negative controls. Lanes 3 and 4 show the same newborn (N2) studied at 1 day old and 22 days old. Lane 5 shows the matched maternal IgM response (M2). (b) Analysis of three matched pairs of neonates and their mothers with regard to CMV-specific IgM responses assessed by Western blotting. Positive and negative controls are shown in lanes 1 and 2, respectively. Two newborn and mother pairs are represented in lanes 3 and 4 (N5, M5) and 5 and 6 (N3, M3). Lanes 7 and 8 are the same newborn (N6) studied 10 days apart while lane 9 is the matched maternal serum response (M6).
Serum cytokine levels in response to CMV
Due to limited sample volume and the retrospective nature of the study, T cell-mediated immunity was assessed by measuring serum cytokine levels using a multiplex microarray assay. Table 4 tabulates the cytokine levels in the neonatal and maternal groups as well as showing the reference ranges for these cytokines published previously in healthy neonates and adults[13–25]. Although maternal sera had higher levels of IFN-γ than paired neonatal sera, statistical significance was not reached. In contrast, neonatal sera contained significantly higher levels of IL-8 (P < 0·005) when compared to their mothers. IL-1α, IL-1β and GM-CSF were barely detectable, while IL-4, IL-6 and TNF-α levels were low in both groups, ranging from 0 to 20 pg/ml. Although not statistically significant, neonatal levels of IL-2 and IL-12 were slightly higher than their mothers (IL-2: 59·5 ± 14·1 pg/ml versus 43·7 ± 8·5 pg/ml, IL-12: 106·8 ± 45·9 pg/ml versus 83·6 ± 44·9 pg/ml), whereas levels of IL-7 was similar in both study groups (45·8 ± 9·4 pg/ml versus 44·1 ± 12·3 pg/ml). Compared to the previously published values of cytokines in healthy neonates and adults, IL-2, IL-7, IL-8, IL-12 and IFN-γ were raised in both our study groups indicating a predominant Th1 response to viral infection.
Table 4.
Serum levels of cytokines in congenitally infected newborns and their mothers.
| Cytokine | Newborn | Mothers | Normal range* | References |
|---|---|---|---|---|
| IL-1α | 0·3 ± 0·2** | 0·9 ± 0·6 | Undetectable | Ziegler et al. [13] |
| IL-1β | 0·5 ± 0·2 | 0·3 ± 0·2 | < 0·52 Median 27·9 | Ziegler et al. [13] Atici et al. [14] |
| IL-2 | 59·5 ± 14·1 | 43·7 ± 8·5 | 30·3 ± 2·4 | Bagci Ceyhan et al. [15] |
| IL-4 | 3·1 ± 0·6 | 8·1 ± 2·5 | 19·9–27·7 0·18–0·25 | Pagani et al. [16] Kanakoudi-Tsakalidon et al. [17] |
| IL-6 | 8·4 ± 1·6 | 4·4 ± 0·9 | 0·2–60·1 3·12–8·6 | Pagani et al. [16] Santana et al. [18] |
| IL-7 | 45·8 ± 9·4 | 44·1 ± 12·3 | 9·59 ± 5·94 | Wasilewska et al. [19] |
| IL-8 | 316·1 ± 136·9 | 48·5 ± 28·6 | 8·6–33·9 0·4–38·0 | Santana et al. [18] Nupponen et al. [20] |
| IL-10 | 10·2 ± 3·1 | 14·9 ± 4·1 | 2·3 ± 2·5 19–50 | Bagci Ceyhan et al. [15] Romagnoli et al. [21] |
| IL-12 | 106·8 ± 45·9 | 83·6 ± 44·9 | 3–31·2 | Pagani et al. [16] |
| GM-CSF | 2·9 ± 0·9 | 0·2 ± 0·1 | 0·0–6·5 | Rizk et al. [22] |
| IFN-γ | 657·4 ± 238·1 | 1072·2 ± 677·2 | 12–76 Not detected | Skogstrand et al. [23] Dumler et al. [24] |
| TNF-α | 11·5 ± 4·3 | 13·1 ± 2·5 | 2·8–1063·7 < 15, not detected | Pagani et al. [16] Sweed et al. [25], Dumler et al. [24] |
IL: interleukin; IFN: interferon; GM-CSF: granulocyte–macrophage colony-stimulating factor; TNF: tumour necrosis factor.
The normal range of cytokine levels quoted in the literature for healthy neonates and adults are shown with the corresponding references;
cytokine levels are shown as mean pg/ml ± s.e.m.
Discussion
In this study, we show that human neonates when exposed to CMV infection in utero are capable of mounting a significant local immune response. Both humoral and cell-mediated immunity as measured by the presence of specific IgM and T cell cytokines indicate that the neonate's immune system is functional at birth but may not achieve a response as effective as adults when presented with viral infection in utero.
In the immunocompetent host, the virus remains efficiently controlled and several components of the immune system are known to play a role. It is known that virus-specific CD4 and CD8 T cell responses are critical components of the immune response in the control of viral replication. The loss of immune control of CMV that is evidenced by the detection of antigenaemia is associated closely with impaired function of CMV-specific CD8 T cells. Furthermore, in CMV-infected cells, the expression of the viral phosphoprotein pp65 inhibits generation of CMV-specific T cell epitopes by preventing antigen processing by the proteosome [26]. It is reduced cytokine production rather than a lower frequency or absolute number of CMV-specific CD4 or CD8 T cells that is thought to be responsible for the loss of immune control [27]. Reduced numbers of cytokines producing CMV-specific CD8 T cells were found in individuals at a higher risk of CMV reactivation [27]. As expected, in response to viral infection, the immunodominant cytokine phenotype observed in this study was Th1. As reported previously, successful pregnancy is associated with a Th2 cytokine phenotype expression at the feto–maternal interface and skewing the immune response to Th1, as in the case of viral infection, can result in deleterious consequences for the fetus [28]. At birth, the neonatal immune system is biased towards a Th2 response generally which is not suited to fighting intracellular pathogens [29]. Cell-mediated immunity is known to be deficient in the human neonate when compared to the same infection in adults [7]. In agreement with previous studies, neonates in our study secreted reduced levels of IFN-γ compared to adults, implying a decreased capacity to contain viral infection [30]. This is supported further by our finding of higher IL-8 levels in neonates. It is known that CMV induces IL-8 production, which in turn can augment CMV replication directly [31]. Previous studies investigating epigenetic regulation have shown that methylation of the IFN-γ promoter at CpG sites is an important mechanism which explains the low IFN-γ production by neonatal T cells [32,33]. In contradiction to the common epigenetic paradigm, in which methylation of the promoter CpG sites silences gene expression, methylation of the IL-8 gene has been shown to enhance IL-8 production in a breast carcinoma cell line [34]. Taken together, the reduced IFN-γ and higher IL-8 in neonates in the present study may be due to common epigenetic modifications and are currently under investigation.
Studies of CMV proteins have suggested that both humoral and cellular immunity are important in preventing disease. Proteins which elicit a long-lasting response such as ppUL32 (pp150), the basic phosphoprotein of the viral tegument, induces a strong immune serological response that can be detected several years after the end of the active viral replication [35]. ppUL44 (pp52), the DNA polymerase accessory protein, induces a strong and broad early response in the majority of individuals following infection, and ppUL80a (pp38), the assembly protein which preferentially induces an IgM response, were examined in this study [35]. Our findings support these observations, as all neonates indicative of primary infection showed a response to pp38 whereas more mothers showed a response to pp150. It would be of interest to follow our neonatal group to determine when the response to pp150 emerges and whether it is sustained with time. Of interest, after a 10-day interval, a neonate (N6) showed a greater intensity of response to pp38 but no reactivity to pp150 had emerged. Although our sample group was small (n = 20), in agreement with previous studies [36] detection of serum IgM to CMV by Western blotting correlated better with virological data than detection by conventional EIA. Comparative analysis showed that Western blotting was more sensitive than EIA in both study groups. Therefore, although throughput is greater with the EIA assay, in some groups of CMV-infected patients it may be more appropriate to use Western blotting analysis. Taken together, our data imply that human neonates are capable of mounting a local and individual primary immune response to CMV infection in utero. These findings may help in elucidating the appropriate design of vaccines suitable for use in early life.
Acknowledgments
The authors wish to thank Fiona Irwin, Martha Neary and Jeff Connell for helpful discussion, Peter Quinn for photography and the National Virus Reference Laboratory for financial support.
References
- 1.Stagno S, Reynolds DW, Huang ES, et al. Congenital cytomegalovirus infection: occurrence in an immune population. N Engl J Med. 1977;296:1254–8. doi: 10.1056/NEJM197706022962203. [DOI] [PubMed] [Google Scholar]
- 2.Hicks T, Fowler K, Richardson M, et al. Congenital cytomegalovirus infection and neonatal auditory screening. J Pediatr. 1993;123:779–82. doi: 10.1016/s0022-3476(05)80859-5. [DOI] [PubMed] [Google Scholar]
- 3.Pass RF, Burke RL. Development of cytomegalovirus vaccines: prospects for prevention of congenital cytomegalovirus infection. Semin Ped Infect Dis. 2002;13:196–204. doi: 10.1053/spid.2002.125863. [DOI] [PubMed] [Google Scholar]
- 4.Goudeau A, Yvonnet G, Lesage F, et al. Lack of anti-HBc IgM in neonates with HbsAg carrier mothers argues against transplacental transmission of hepatitis B virus infection. Lancet. 1983;2:1103–4. doi: 10.1016/s0140-6736(83)90625-6. [DOI] [PubMed] [Google Scholar]
- 5.Feeney AJ, Lugo G, Escuro G. Human cord blood κ repertoire. J Immunol. 1997;158:3761–8. [PubMed] [Google Scholar]
- 6.Messmer BT, Sullivan JJ, Chiorazzi M, et al. Two human neonatal IgM antibodies encoded by different variable-region genes bind the same linear peptide: evidence for a stereotyped repertoire of epitope recognition. J Immunol. 1999;162:2182–92. [PubMed] [Google Scholar]
- 7.Hassan J, Reen DJ. Reduced primary antigen specific T cell precursor frequencies in neonates is associated with deficient IL-2 production. Immunology. 1996;87:604–8. doi: 10.1046/j.1365-2567.1996.476587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan J, Reen DJ. Cord blood CD4+ CD45RA+ T cells achieve a lower magnitude of activation when compared to their adult counterparts. Immunology. 1997;90:397–401. doi: 10.1111/j.1365-2567.1997.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imanishi K, Seo K, Kato H, et al. Post-thymic maturation of migrating human thymic single positive T cells: thymic CD1a-CD4+ T cells are more susceptible to anergy induction by toxic shock syndrome toxin-1 than cord blood CD4+ T cells. J Immunol. 1998;160:2845–55. [PubMed] [Google Scholar]
- 10.Lewis DB, Wilson CB. Developmental immunology and role of host defenses in fetal and neonatal susceptibility to infection. In: Remington JS, Klein JO, editors. Infectious diseases of the fetus and newborn infant. Philadelphia: W. B. Saunders Company; 2001. pp. 25–138. [Google Scholar]
- 11.Marchant A, Appay V, van der Sande M, et al. Mature CD8+ T lymphocyte response to viral infection during fetal life. J Clin Invest. 2003;111:1747–55. doi: 10.1172/JCI17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gleaves CA, Smith TF, Shuster EA, Pearson GR. Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J Clin Microbiol. 1984;19:917–19. doi: 10.1128/jcm.19.6.917-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziegler H, Tepfer C, Joura EA, et al. Serum interleukin 1 in ovarian cancer patients. Eur J Cancer. 1998;34:931–3. doi: 10.1016/s0959-8049(97)10107-1. [DOI] [PubMed] [Google Scholar]
- 14.Atici A, Satar M, Alsparslan N. Serum interleukin 1 in neonatal sepsis. Acta Paediatr. 1996;85:371–4. doi: 10.1111/j.1651-2227.1996.tb14036.x. [DOI] [PubMed] [Google Scholar]
- 15.Bagci Ceyhan B, Yilmaz Enc F, Sahin S. IL-2 and IL-10 levels in induced sputum and serum samples of asthmatics. J Invest Allergol Clin Immunol. 2004;14:80–5. [PubMed] [Google Scholar]
- 16.Pagani S, Meazza C, Travaglino P, et al. Serum cytokine levels in GH-deficient children during substitutive GH therapy. Eur J Endocrinol. 2005;152:207–10. doi: 10.1530/eje.1.01827. [DOI] [PubMed] [Google Scholar]
- 17.Kanakoudi-Tsakalidou F, Drossou-Agakidou V, Noutsia C, et al. Intracellular and plasma cytokine profile in neonates born to non-atopic parents: the impact of breast feeding. Eur J Pediatr. 2004;163:395–401. doi: 10.1007/s00431-004-1463-4. [DOI] [PubMed] [Google Scholar]
- 18.Santana C, Guideo MC, Gonzalez G, et al. Cord blood levels of cytokines as predictors of early neonatal sepsis. Acta Paediatr. 2001;90:1176–81. doi: 10.1080/080352501317061602. [DOI] [PubMed] [Google Scholar]
- 19.Wasilewska A, Zoch-Zwierz WM, Tomaszewska B, et al. Relationship of serum interleukin-7 concentration and the coagulation state in children with nephritic syndrome. Pediatr Int. 2005;47:424–9. doi: 10.1111/j.1442-200x.2005.02078.x. [DOI] [PubMed] [Google Scholar]
- 20.Nupponen I, Andersson S, Jarvenpaa A-L, et al. Neutrophil CD11b expression and circulating interleukin-8 as diagnostic markers for early-onset neonatal sepsis. Pediatrics. 2001;108:12–7. doi: 10.1542/peds.108.1.e12. [DOI] [PubMed] [Google Scholar]
- 21.Romagnoli C, Frezza S, Cingolani A, et al. Plasma levels of interleukin-6 and interleukin-10 in preterm neonates evaluated for sepsis. Eur J Pediatr. 2001;160:345–50. doi: 10.1007/pl00008445. [DOI] [PubMed] [Google Scholar]
- 22.Rizk EA, El-Gendy WM, Hassab H, et al. Granulocyte–macrophage colony-stimulating factor (GM-CSF) in children with acute immune thrombocytopenic purpura. Med Sci Monit. 2004;10:CR330–35. [PubMed] [Google Scholar]
- 23.Skogstrand K, Thorsen P, Norgaard-Pedersen B, et al. Simultaneous measurement of 25 inflammatory markers and neutrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem. 2005;51:1854–66. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- 24.Dumler JS, Trigiani ER, Bakken JS, et al. Serum cytokine responses during acute human granulocytic ehrlichiosis. Clin Diag Lab Immunol. 2000;7:6–8. doi: 10.1128/cdli.7.1.6-8.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweed Y, Reen D, Puri P. Association of tumour necrosis factor and haemodynamic shock in a newborn undergoing surgery for sacrococcygeal teratoma. Pediatr Surg Int. 1994;9:216–8. [Google Scholar]
- 26.Ploegh HL. Viral strategies of immune evasion. Science. 1998;280:248–59. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 27.Reusser P, Riddell SR, Meyers JD, et al. Cytotoxic T-lymphocytes response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373. [PubMed] [Google Scholar]
- 28.Wegmann TG, Lin H, Guilbert L, et al. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 29.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–46. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 30.Wilson CB, Westall J, Johnson L, et al. Decreased production of interferon-γ by human neonatal cells: intrinsic and regulatory deficiencies. J Clin Invest. 1986;77:860–7. doi: 10.1172/JCI112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murayama T, Kuno K, Jisaki F, et al. Enhancement of human cytomegalovirus replication in a human fibroblast cell line by interleukin-8. J Virol. 1994;68:7582–5. doi: 10.1128/jvi.68.11.7582-7585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penix LA, Sweetser MT, Weaver WM, et al. The proximal regulatory element of the interferon-γ promoter mediates selective expression in T cells. J Biol Chem. 1996;271:31964–72. doi: 10.1074/jbc.271.50.31964. [DOI] [PubMed] [Google Scholar]
- 33.White GP, Watt PM, Holt BJ, et al. Differential patterns of methylation of the IFNγ promoter at CpG and non-CpG sites underlie differences in IFNγ gene expression between neonatal and adult CD45RO- T-cells. J Immunol. 2002;168:2828–7. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 34.De Larco JE, Wuertz BRK, Yee D, et al. Atypical methylation of the interleukin-8 gene correlates strongly with the metastatic potential of breast carcinoma cells. Proc Natl Acad Sci. 2003;100:13988–93. doi: 10.1073/pnas.2335921100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landini MP, Lazzarotto T, Ertl PF. Humoral immune response to human cytomegalovirus DNA polymerase. J Clin Microbiol. 1993;31:724–6. doi: 10.1128/jcm.31.3.724-726.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazzarotto T, Maine GT, Dal Monte P, et al. Detection of serum immunoglobulin M to human cytomegalovirus by Western blotting correlates better with virological data than detection by conventional enzyme immunoassay. Clin Diag Lab Immunol. 1996;3:597–600. doi: 10.1128/cdli.3.5.597-600.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]