Abstract
The macrolide antibiotics are now well known to have anti-inflammatory effects. Because dendritic cells (DCs) orchestrate immune responses, we examined the in vitro effects of clarithromycin (CAM), azithromycin (AZM) and midecamycin (MDM) on the expression of co-stimulatory molecules and production of cytokines [interleukin (IL)-10, IL-6, interferon (IFN)-γ, IL-12p40, tumour necrosis factor (TNF)-α] of murine bone marrow-derived DCs by lipopolysaccharide (LPS) stimulation. A 15-membered macrolide, AZM, and a 14-membered macrolide, CAM, significantly enhanced the intensity of a co-stimulatory molecule, CD80, on DCs but not CD86 and CD40. AZM significantly increased the production of IL-10 and CAM significantly inhibited the production of IL-6 by DCs. However, a 16-membered macrolide, MDM, did not have any significant effect on these surface markers and cytokine productions. Moreover, AZM increased IL-10 and CAM decreased IL-2 productions significantly, when naive T cells derived from spleen were co-cultured with DCs treated in advance with LPS and these macrolides. These findings suggest that 14-membered and 15-membered, but not 16-membered macrolides play as anti-inflammatory agents, at least in part, through modulating the functions of DCs. However, each macrolide affects them in different ways.
Keywords: bone marrow-derived dendritic cells, co-stimulatory molecules, cytokines, macrolides
Introduction
The anti-inflammatory and immunomodulatory activities of macrolide antibiotics have been established since the effectiveness of low-dose and long-term treatment with erythromycin in diffuse panbronchiolitis (DPB) was reported by Kudoh and colleagues [1]. Efficacy of other 14- and 15-membered macrolides such as clarithromycin (CAM) and azithromycin (AZM) in DPB has also been clarified [2,3]. Recent studies have demonstrated similar benefits of macrolide therapy in patients with cystic fibrosis (CF), having similar clinical features to DPB [4,5]. Macrolides have no anti-microbial activities against Pseudomonas aeruginosa, as the maximum achievable serum or sputum concentrations of macrolides are below the MICs of P. aeruginosa [6]. Therefore, it has been suggested that their therapeutic effects might be derived from anti-inflammatory and immunomodulatory activities, rather than anti-bacterial properties [7]. Several therapeutic benefits of macrolides have been reported in immunocellular functions such as in neutrophils [8], lymphocytes [9,10] and monocytes [11,12], or cytokine production of interleukin (IL)-8 [13,14] and IL-6 [15], or bacterial activities such as the biofilm formation [16] and the quorum-sensing of P. aeruginosa [17]. However, the exact mechanisms of macrolides in patients with DPB or CF are still unknown, and their anti-inflammatory and immunomodulatory activities are of particular concern.
Dendritic cells (DCs) are the most potent, professional antigen-presenting cells with the unique ability to prime naive T cells and play a central role in the initiation and regulation of immune responses. DCs, distributed widely in virtually all organs except the brain, are situated preferentially in the mucosal surface [18]. In DPB it has been reported that the number of mature DCs was increased in the bronchial tissues of patients [19], suggesting that DCs may play an important role in the mucosal immune modulation in DPB. Macrolides therefore might modulate immune responses in patients with DPB or CF by altering DC functions. However, to our knowledge, there is only one report demonstrating the association between erythromycin and DCs (human monocyte-derived DCs) [20]; thus, we examined the effects of macrolides on the expression of co-stimulatory molecules and the production of several cytokines induced by lipopolysaccharide (LPS) in murine bone marrow-derived DCs. Here we show CAM (14-membered macrolide) and AZM (15-membered), but not midecamycin (MDM, 16-membered) function as anti-inflammatory agents, at least in part, through modulating the functions of DCs, and that each macrolide acts by different effects on cytokine production. Our present data might solve the question why there are many DPB cases showing treatment with one macrolide is effective, while others are ineffective [3,21].
Materials and methods
Reagents
Three kinds of macrolides, CAM (Taishotoyama, Tokyo, Japan), AZM (Pfizer, Groton, CT, USA) and MDM (Meiji Seika, Tokyo, Japan), were donated generously from the respective companies. Macrolides were dissolved in ethanol. The final concentration of ethanol in culture was 0·02%, which did not affect the functions of DCs in our preliminary experiment. LPS derived from P. aeruginosa (lot 87F4009) was also obtained from Sigma (St Louis, MO, USA). Recombinant mouse granulocyte–macrophage colony-stimulating factor (rmGM-CSF) was purchased from R&D Systems (Oxford, UK). The concentrations of tumour necrosis factor (TNF)-α, interferon (IFN)-γ, IL-2, IL-4, IL-6, IL-10 or IL-12p40 in the culture supernatants were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (Duo Set; R&D Systems) according to the manufacturer's instructions.
Mice
Female BALB/c mice (5–12 weeks) were purchased from Charles River (Yokohama, Japan) and housed in a specific pathogen-free facility. The experimental protocol was approved by the Nagasaki University School of Medicine committee on animal research.
Generation of bone marrow-derived DCs
DCs were prepared from bone marrow (BM) progenitor cells as described previously by Lutz et al. [22]. Briefly, femurs and tibias of BALB/c mice were removed and dissected from the surrounding muscle tissues after BALB/c mice were killed. The ends were then cut and the marrow flushed with phosphate-buffered saline (PBS). After washing with PBS, a density of 2 × 106 cells/ml of leucocytes were suspended in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) containing 10% heat-inactivated fetal calf serum (FCS) (Gibco brl Products, Grand Island, NY, USA), 1% penicillin-streptomycin (Invitrogen) and 50 µM 2-mercaptoethanol (Sigma), referred to as cRPMI. The cells were cultured with 10 ml of 20 ng/ml rmGM-CSF in 100-mm dishes. On day 3 another 10 ml of cRPMI containing 20 ng/ml rmGM-CSF was added to the dishes. On days 6 and 8, half the culture supernatant was collected and centrifuged, and then the cells were resuspended in the fresh cRPMI containing 20 ng/ml rmGM-CSF, to be given back into the original dishes. On day 10 non-adherent cells were collected, centrifuged, resuspended in fresh cRPMI containing 20 ng/ml rmGM-CSF and then incubated with/without 1 µg/ml LPS for 24 h. On days 8 and 10, each macrolide was added at the concentration 10 µg/ml. In the preliminary experiments we used concentrations of 1, 10, 30, 50 and 100 µg/ml of macrolides. However, at concentrations > 50 µg/ml DCs were dead because of ethanol solvent used to dissolve these macrolides. Thus, we used 10 µg/ml of macrolides, which is the physiological concentration found in lung tissue [23,24]. On day 11 the supernatants were collected, and then the DCs (about 1–2 × 105 cells/ml) were washed twice with PBS and used for fluorescence activated cell sorter (FACS) analysis or co-culture with naive T cells. The percentage of CD11c+ DCs was > 90% in all DC groups without regard to macrolides. In all experiments incubation was conducted in humidified 5% atmosphere at 37°C. The supernatants were collected and stored at −80°C until analysis using ELISA.
Surface markers expression by flow cytometry
DCs were examined by direct immunofluorescence staining using fluorescein isothiocyanate-labelled antibodies to I-A/I-E or CD11c and phycoerythrin-labelled antibody to CD11c, CD40, CD80 or CD86 (all from BD PharMingen, San Diego, CA, USA). The stained cells were analysed on a flow cytometer (FACScan; Becton Dickinson). Data are presented as mean fluorescence intensity (MFI).
DC and T cell co-cultures
Pan T cells were isolated from spleen cells of BALB/c mice by depletion of non-T cells (negative selection) using the Pan T cell isolation kit (Miltenyi Biotec, Auburn, CA, USA) and naive T cells were isolated from pan T cells by positive selection using CD62L (l-selectin) MicroBeads (Miltenyi Biotec), according to the manufacturer's instructions. These naive T cells were consistently > 90% CD3+ CD62L+ (both from BD PharMingen). DCs treated with/without AZM or CAM on day 11 as described above were washed twice with PBS and cultured with naive T cells (ratio of DCs : T cells = 1 : 10; 1·25 × 105 : 1·25 × 106 cells/ml) in 48-well plates in cRPMI for 5 days. Concurrently DCs or naive T cells alone (1·25 × 105 or 1·25 × 106 cells/ml, respectively) were cultured in the same plate. On day 16 the supernatants were collected, respectively, and stored at −80°C until analysis.
Statistical analysis
Data were expressed as mean ± standard error (s.e.) of five experiments. Differences between multiple groups were compared by one-way analysis of variance (anova). The post-hoc test for multiple comparisons was Fisher's partial least squares difference (PLSD) test. Significance was assumed at P < 0·05.
Results
Effect of macrolides on the DC surface markers
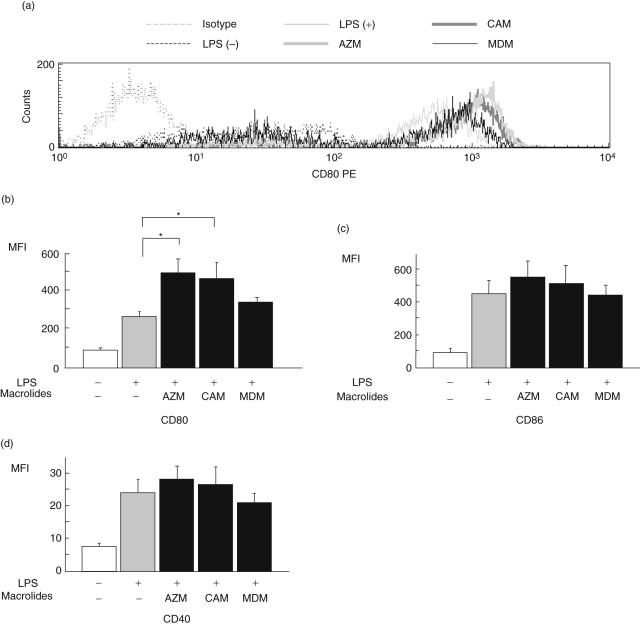
Figure 1 shows the MFI of co-stimulatory molecules of CD80, CD86 or CD40 on DCs pretreated with/without macrolides. LPS stimulation enhanced the intensity of CD80, CD86 and CD40 on DCs (control). AZM and CAM significantly increased the intensity of CD80 on DCs compared with control, but MDM did not. However, all macrolides examined did not affect the intensity of CD86, CD40, I-A/I-d or CD11c compared to controls (I-A/I-d and CD11c: data not shown).
Fig. 1.
The effect of macrolides on the surface markers of co-stimulatory molecules of dendritic cells (DCs). DCs were treated without lipopolysaccharide (LPS), with LPS alone (control) or LPS with azithromycin (AZM), clarithromycin (CAM) or midecamycin (MDM). The expression of CD80 (a,b), CD86 (c) or CD40 (d) on DCs was analysed by flow cytometry after treatment. Values of mean fluorescence intensity (MFI) are expressed as the mean ± standard error of five experiments. *P < 0·05 compared with control.
Effect of macrolides on the cytokine production by DCs
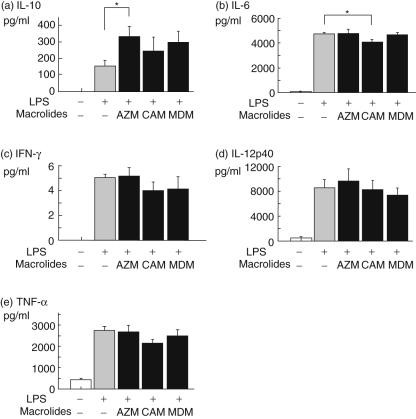
As shown in Fig. 2, the production of IL-10, IL-6, IFN-γ, IL-12p40 or TNF-α by DCs was increased by LPS stimulation (control). Moreover, AZM significantly augmented the production of IL-10 compared with control, and CAM significantly inhibited the production of IL-6 compared with control. However, MDM did not show any significant effects on these cytokines. There were no statistical significances in the production of IFN-γ, IL-12p40 and TNF-α among DCs treated with/without each macrolide.
Fig. 2.
The effect of macrolides on dendritic cells (DCs) cytokine production. DCs were treated without lipopolysaccharide (LPS), with LPS alone or with LPS and azithromycin (AZM), clarithromycin (CAM) or midecamycin (MDM). The production of interleukin (IL)-10 (a), IL-6 (b), interferon (IFN)-γ (c), IL-12p40 (d) and tumour necrosis factor (TNF)-α (e) by DCs were measured by enzyme-linked immunosorbent assay. Values are expressed as the mean ± standard error of five experiments. *P < 0·05 compared with LPS alone.
Effect of macrolides on the cytokine production by naive T cells co-cultured with DCs
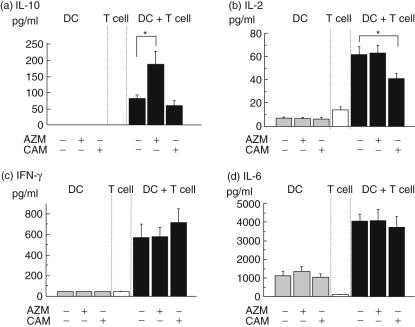
Next, DCs were co-cultured with naive T cells to examine the regulatory effects of DCs on the naive T cells. As shown in Fig. 3, pretreatment with AZM augmented the production of IL-10 by T cells co-cultured with DCs, whereas pretreatment with CAM attenuated the production of IL-2 by T cells co-cultured with DCs. There were no significant differences in the production of IL-6 or IFN-γ among the three groups. The production of IL-10, IL-2, IFN-γ or IL-6 by DCs or naive T cells was low or not detected. The concentration of IL-4 was below the detection limit of our assay in each group.
Fig. 3.
The effect of macrolides on the production of cytokines from naive T cells co-cultured with dendritic cells (DCs). DCs were pretreated with lipopolysaccharide (LPS) alone or LPS with azithromycin (AZM) or clarithromycin (CAM). Subsequently all DCs were co-cultured with naive T cells from mice spleen for 5 days. DCs alone or naive T cells alone were cultured in the same plates. The production of interleukin (IL)-10 (a), IL-2 (b), interferon (IFN)-γ (c) and IL-6 (d) by DCs were measured by enzyme-linked immunosorbent assay. Values are expressed as the mean ± standard error of five experiments. *P < 0·05 compared with control.
Discussion
In the present study, we demonstrated that a 14-membered macrolide such as CAM and a 15-membered macrolide such as AZM had anti-inflammatory effects through modulating the functions of DCs, while a 16-membered macrolide such as MDM had no such effects. In addition, each macrolide affects them in different ways, e.g. CAM inhibits IL-6 and IL-2 production and AZM enhances IL-10 production.
We first examined the effect of macrolides on the co-stimulatory markers of DCs such as CD40, CD80 and CD86. AZM and CAM significantly increased the intensity of CD80 on DCs, but not CD86 and CD40. CD80 is known as one of the B7 family as well as CD86, which is expressed on a variety of antigen-presenting cells including DCs, Langerhans's cells, activated macrophages and B cells [25]. There are several conflicting reports about the functions of CD80 and CD86. The co-stimulatory ligands, CD80 and CD86, appear to differ in their ability to potentiate the differentiation of T helper (Th) cells into Th1 or Th2, e.g. CD80 preferred to Th1 and CD86 preferred to Th2 [26,27]. On the other hand, CD80 and CD86 provide similar co-stimulatory signals for T cell proliferation [28]. Moreover, CD80 and CD86 could engage the same two receptors, the stimulatory CD28 and inhibitory cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) molecules [25]. These molecules are known to play an important role in regulating cytokine production from T cells; thus, we investigated the effects of macrolides on the production of cytokines by DCs and T cells. Interestingly, AZM increased IL-10 and CAM decreased IL-2 production in co-cultured DCs and T cells. These data suggest that AZM and CAM may up-regulate the functions of CTLA-4, because CTLA-4 can reduce IL-2 production and IL-10 inhibits the phosphorylation of CD28 [29,30]. CTLA-4 is thought to be a negative regulator of T cell activation [30]. Therefore, its possible CTLA-4 activation may be an important anti-inflammatory effect of AZM and CAM.
In this study, AZM increased significantly the production of IL-10 from DCs. CAM and MDM also tended to increase IL-10 production from DCs. In addition, AZM increased extremely the production of IL-10 from co-cultured DCs and naive T cells, but CAM did not. These results indicated that AZM affects not only IL-10 production from DCs themselves but also the interaction between DCs and naive T cells. IL-10 potently inhibits the production of IL-1, IL-8 and TNF-α by inflammatory cells. The levels of these proinflammatory cytokines are elevated in the sputum or bronchoalveolar lavage fluid (BALF) of patients with DPB or CF [13,31]. Long-term intermittent administration of AZM twice a week in patients with DPB has proved useful [3]. This efficacy may be derived at least in part from AZM's anti-inflammatory effect through enhancing the production of IL-10 by DCs and T cells. IL-10 is also thought to have an important role in CF patients, because IL-10 is decreased in BALF of patients with CF [31] and is down-regulated even after infection is eradicated [32]. IL-10 deficiency also deteriorates in chronic P. aeruginosa infection [33,34]. Therefore, the increase of IL-10 leads potentially to improvement of these diseases.
CAM suppressed IL-6 production significantly by DCs in this study and also suppressed the production of IL-2 significantly when co-cultured with naive T cells. IL-6 was identified as a B cell differentiation factor and had various effects on immune response, inflammation and haematopoiesis [35]. Increased levels of IL-6 were observed in the serum of patients with CF, which cause continuous inflammation with hypergammaglobulinaemia [36]. Hypergammaglobulinaemia often represents the severity of CF clinical status and prognosis [37]. The high antibody titres to alginate of P. aeruginosa were observed in the severe clinical conditions as patients with CF [38]. Higher antibody titres to alginate or bacterial permeability increasing anti-neutrophil cytoplasmic autoantibodies (BPI-ANCA) in patients with DPB has also been observed [39]. Our data demonstrate that CAM significantly suppressed the production of IL-6 by DCs, suggesting macrolides may improve disease status, at least in part, through suppressing the activation of B cells by IL-6. CAM also reduced the production of IL-2 when DCs were co-cultured with naive T cells. In addition, IL-2 may participate in the systemic inflammatory response and hypergammaglobulinaemia observed in patients with CF [40]. The proportion of T cells producing large amounts of IL-2 was observed in patients with CF compared with healthy subjects [41]. Thus, CAM may have an anti-inflammatory effect through the reduction of IL-2 and IL-6 produced by DCs and T cells. In this context, the effects of CAM on these cytokine productions were statistically significant, but not a dramatic response. We therefore suggest that long-term treatment with macrolides might be required because of small effects of short-term therapy on cytokine productions.
Recent studies have demonstrated that LPS induces cytokine production via Toll-like receptor (TLR) 4 in several cell types, including DCs [20,42]. In this study, we did not demonstrate the association between these macrolides and TLR, but it has been reported that CAM down-regulate LPS-induced TLR4 expression [43]. By contrast, Yasutomi et al. also demonstrated that erythromycin did not affect mRNA levels of TLR4 [20]. Thus, the different effect of each macrolide in our study may be explained in part by the ability to suppress TLR4.
In this study, we presented the various anti-inflammatory effects of CAM and AZM. We and others have experienced some DPB patients who showed that AZM therapy was more effective, but erythromycin or CAM therapies were ineffective [3,21]. Thus, there are some differences in the effects of each macrolide against DPB. This may be due to the differing macrolide actions in vitro, e.g. productions of cytokines from mononuclear cells [44]. Here we have shown that macrolides have different effects on DCs with regard to cytokine production or inhibition.
Acknowledgments
We thank A. Yokoyama (Nagasaki University School of Medicine) for his excellent technical assistance. This study was supported in part by a research grant from the Ministry of Education, Science, Sports and Culture of Japan.
References
- 1.Kudoh S, Azuma A, Yamamoto M, et al. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med. 1998;157:1829–32. doi: 10.1164/ajrccm.157.6.9710075. [DOI] [PubMed] [Google Scholar]
- 2.Kadota J, Mukae H, Ishii H, et al. Long-term efficacy and safety of clarithromycin treatment in patients with diffuse panbronchiolitis. Respir Med. 2003;97:844–50. doi: 10.1016/s0954-6111(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi H, Takeda H, Sakayori S, et al. [Study on azithromycin in treatment of diffuse panbronchiolitis] Kansenshogaku Zasshi. 1995;69:711–22. doi: 10.11150/kansenshogakuzasshi1970.69.711. [DOI] [PubMed] [Google Scholar]
- 4.Wolter J, Seeney S, Bell S, et al. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax. 2002;57:212–6. doi: 10.1136/thorax.57.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Equi A, Balfour-Lynn IM, Bush A, et al. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet. 2002;360:978–84. doi: 10.1016/s0140-6736(02)11081-6. [DOI] [PubMed] [Google Scholar]
- 6.Sakata K, Yajima H, Tanaka K, et al. Erythromycin inhibits the production of elastase by Pseudomonas aeruginosa without affecting its proliferation in vitro. Am Rev Respir Dis. 1993;148:1061–5. doi: 10.1164/ajrccm/148.4_Pt_1.1061. [DOI] [PubMed] [Google Scholar]
- 7.Yanagihara K, Kadoto J, Kohno S. Diffuse panbronchiolitis – pathophysiology and treatment mechanisms. Int J Antimicrob Agents. 2001;18(Suppl. 1):S83–7. doi: 10.1016/s0924-8579(01)00403-4. [DOI] [PubMed] [Google Scholar]
- 8.Kadota J, Sakito O, Kohno S, et al. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am Rev Respir Dis. 1993;147:153–9. doi: 10.1164/ajrccm/147.1.153. [DOI] [PubMed] [Google Scholar]
- 9.Mukae H, Kadota J, Kohno S, et al. Increase in activated CD8+ cells in bronchoalveolar lavage fluid in patients with diffuse panbronchiolitis. Am J Respir Crit Care Med. 1995;152:613–18. doi: 10.1164/ajrccm.152.2.7633715. [DOI] [PubMed] [Google Scholar]
- 10.Morikawa K, Zhang J, Nonaka M, et al. Modulatory effect of macrolide antibiotics on the Th1- and Th2-type cytokine production. Int J Antimicrob Agents. 2002;19:53–9. doi: 10.1016/s0924-8579(01)00457-5. [DOI] [PubMed] [Google Scholar]
- 11.Khan AA, Slifer TR, Araujo FG, et al. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int J Antimicrob Agents. 1999;11:121–32. doi: 10.1016/s0924-8579(98)00091-0. [DOI] [PubMed] [Google Scholar]
- 12.Morikawa K, Watabe H, Araake M, et al. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother. 1996;40:1366–70. doi: 10.1128/aac.40.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakito O, Kadota J, Kohno S, et al. Interleukin 1 beta, tumor necrosis factor alpha, and interleukin 8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: a potential mechanism of macrolide therapy. Respiration. 1996;63:42–8. doi: 10.1159/000196514. [DOI] [PubMed] [Google Scholar]
- 14.Mukae H, Kadota J, Ashitani J, et al. Elevated levels of soluble adhesion molecules in serum of patients with diffuse panbronchiolitis. Chest. 1997;112:1615–21. doi: 10.1378/chest.112.6.1615. [DOI] [PubMed] [Google Scholar]
- 15.Takizawa H, Desaki M, Ohtoshi T, et al. Erythromycin suppresses interleukin 6 expression by human bronchial epithelial cells: a potential mechanism of its anti-inflammatory action. Biochem Biophys Res Commun. 1995;210:781–6. doi: 10.1006/bbrc.1995.1727. [DOI] [PubMed] [Google Scholar]
- 16.Nagata T, Mukae H, Kadota J, et al. Effect of erythromycin on chronic respiratory infection caused by Pseudomonas aeruginosa with biofilm formation in an experimental murine model. Antimicrob Agents Chemother. 2004;48:2251–9. doi: 10.1128/AAC.48.6.2251-2259.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tateda K, Comte R, Pechere JC, et al. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2001;45:1930–3. doi: 10.1128/AAC.45.6.1930-1933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambrecht BN, Prins JB, Hoogsteden HC. Lung dendritic cells and host immunity to infection. Eur Respir J. 2001;18:692–704. [PubMed] [Google Scholar]
- 19.Todate A, Chida K, Suda T, et al. Increased numbers of dendritic cells in the bronchiolar tissues of diffuse panbronchiolitis. Am J Respir Crit Care Med. 2000;162:148–53. doi: 10.1164/ajrccm.162.1.9907015. [DOI] [PubMed] [Google Scholar]
- 20.Yasutomi M, Ohshima Y, Omata N, et al. Erythromycin differentially inhibits lipopolysaccharide- or poly(I:C)-induced but not peptidoglycan-induced activation of human monocyte-derived dendritic cells. J Immunol. 2005;175:8069–76. doi: 10.4049/jimmunol.175.12.8069. [DOI] [PubMed] [Google Scholar]
- 21.Nagata T, Mukai H, Kadokawa T, et al. [Two cases successfully treated with long-term administration of low-dosage azithromycin] Jpn J Antibiot. 2001;54(Suppl. C):5–8. [PubMed] [Google Scholar]
- 22.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Meth. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 23.Morris DL, De Souza A, Jones JA, et al. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur J Clin Microbiol Infect Dis. 1991;10:859–61. doi: 10.1007/BF01975842. [DOI] [PubMed] [Google Scholar]
- 24.McCarty JM. Clarithromycin in the management of community-acquired pneumonia. Clin Ther. 2000;22:281–94. doi: 10.1016/S0149-2918(00)80033-8. discussion 65. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Chen L. Co-signaling molecules of the B7-CD28 family in positive and negative regulation of T lymphocyte responses. Microbes Infect. 2004;6:759–66. doi: 10.1016/j.micinf.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Harris NL, Ronchese F. The role of B7 co-stimulation in T-cell immunity. Immunol Cell Biol. 1999;77:304–11. doi: 10.1046/j.1440-1711.1999.00835.x. [DOI] [PubMed] [Google Scholar]
- 27.Kuchroo VK, Das MP, Brown JA, et al. B7–1 and B7–2 co-stimulatory molecules activate differentially the Th1/Th2developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 28.Schweitzer AN, Borriello F, Wong RC, et al. Role of co-stimulators in T cell differentiation: studies using antigen-presenting cells lacking expression of CD80 or CD86. J Immunol. 1997;158:2713–22. [PubMed] [Google Scholar]
- 29.Joss A, Akdis M, Faith A, et al. IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur J Immunol. 2000;30:1683–90. doi: 10.1002/1521-4141(200006)30:6<1683::AID-IMMU1683>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 30.Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- 31.Bonfield TL, Panuska JR, Konstan MW, et al. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–8. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 32.Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol. 1997;24:137–42. doi: 10.1002/(sici)1099-0496(199708)24:2<137::aid-ppul13>3.0.co;2-3. discussion 59–61. [DOI] [PubMed] [Google Scholar]
- 33.Saadane A, Soltys J, Berger M. Role of IL-10 deficiency in excessive nuclear factor-kappaB activation and lung inflammation in cystic fibrosis transmembrane conductance regulator knockout mice. J Allergy Clin Immunol. 2005;115:405–11. doi: 10.1016/j.jaci.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 34.Chmiel JF, Konstan MW, Saadane A, et al. Prolonged inflammatory response to acute Pseudomonas challenge in interleukin-10 knockout mice. Am J Respir Crit Care Med. 2002;165:1176–81. doi: 10.1164/ajrccm.165.8.2107051. [DOI] [PubMed] [Google Scholar]
- 35.Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–68. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 36.Nixon LS, Yung B, Bell SC, et al. Circulating immunoreactive interleukin-6 in cystic fibrosis. Am J Respir Crit Care Med. 1998;157:1764–9. doi: 10.1164/ajrccm.157.6.9704086. [DOI] [PubMed] [Google Scholar]
- 37.Jones MM, Seilheimer DK, Pollack MS, et al. Relationship of hypergammaglobulinemia, circulating immune complexes, and histocompatibility antigen profiles in patients with cystic fibrosis. Am Rev Respir Dis. 1989;140:1636–9. doi: 10.1164/ajrccm/140.6.1636. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen SS, Espersen F, Hoiby N, et al. Immunoglobulin A and immunoglobulin G antibody responses to alginates from Pseudomonas aeruginosa in patients with cystic fibrosis. J Clin Microbiol. 1990;28:747–55. doi: 10.1128/jcm.28.4.747-755.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi H. Airway biofilms: implications for pathogenesis and therapy of respiratory tract infections. Treat Respir Med. 2005;4:241–53. doi: 10.2165/00151829-200504040-00003. [DOI] [PubMed] [Google Scholar]
- 40.Greally P, Hussain MJ, Vergani D, et al. Serum interleukin-1 alpha and soluble interleukin-2 receptor concentrations in cystic fibrosis. Arch Dis Child. 1993;68:785–7. doi: 10.1136/adc.68.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hubeau C, Le Naour R, Abely M, et al. Dysregulation of IL-2 and IL-8 production in circulating T lymphocytes from young cystic fibrosis patients. Clin Exp Immunol. 2004;135:528–34. doi: 10.1111/j.1365-2249.2003.02385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaisho T, Akira S. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 2001;22:78–83. doi: 10.1016/s1471-4906(00)01811-1. [DOI] [PubMed] [Google Scholar]
- 43.Park JY, Kim HY, Lee JY, et al. Macrolide-affected Toll-like receptor 4 expression from Helicobacter pylori-infected monocytes does not modify interleukin-8 production. FEMS Immunol Med Microbiol. 2005;44:171–6. doi: 10.1016/j.femsim.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Takizawa H. Airway inflammation and macrolides. Jpn J Antibiotics. 1998;52:46–9. [Google Scholar]