Abstract
Previously we reported that proteasome inhibitors were able to overcome Bcl-2-mediated protection from apoptosis. Here we show that inhibition of the proteasome activity in Bcl-2-overexpressing cells accumulates the proapoptotic Bax protein to mitochondria/cytoplasm, where it interacts to Bcl-2 protein. This event was followed by release of mitochondrial cytochrome c into the cytosol and activation of caspase-mediated apoptosis. In contrast, proteasome inhibition did not induce any apparent changes in Bcl-2 protein levels. In addition, treatment with a proteasome inhibitor increased levels of ubiquitinated forms of Bax protein, without any effects on Bax mRNA expression. We also established a cell-free Bax degradation assay in which an in vitro-translated, 35S-labeled Bax protein can be degraded by a tumor cell protein extract, inhibitable by addition of a proteasome inhibitor or depletion of the proteasome or ATP. The Bax degradation activity can be reconstituted in the proteasome-depleted supernatant by addition of a purified 20S proteasome or proteasome-enriched fraction. Finally, by using tissue samples of human prostate adenocarcinoma, we demonstrated that increased levels of Bax degradation correlated well with decreased levels of Bax protein and increased Gleason scores of prostate cancer. Our studies strongly suggest that ubiquitin/proteasome-mediated Bax degradation is a novel survival mechanism in human cancer cells and that selective targeting of this pathway should provide a unique approach for treatment of human cancers, especially those overexpressing Bcl-2.
Apoptosis, an evolutionarily conserved form of cell suicide, occurs in two physiological stages: commitment and execution (1). Most recent experiments have demonstrated that mitochondria play an essential role in the process of apoptotic commitment (2, 3). It has been found that several Bcl-2 family proteins are located in the outer mitochondrial membrane, where they control release of some caspase-activating proteins (such as cytochrome c) into the cytosol (2, 3). The released cytochrome c then activates effector caspases, a hallmark of apoptotic execution (4, 5), that in turn cleave a number of important cellular target proteins, including poly(ADP-ribose) polymerase (PARP) (6) and retinoblastoma protein (7), resulting in disassembly of the cell.
Release of cytochrome c can be induced by proapoptotic members of Bcl-2 family (such as Bax, Bad, and Bid), but inhibited by antiapoptotic Bcl-2 family members (such as Bcl-2 and Bcl-XL) (2, 3). The ratio of proapoptotic to antiapoptotic proteins, therefore, is involved in determination of cellular fate. In addition, posttranslational modifications on Bcl-2 family proteins also determine their active or inactive conformations (2, 3). For example, activation of Bax involves its subcellular translocation and dimerization. Phosphorylation of Bad by the serine/threonine kinase Akt or cAMP-dependent protein kinase results in its sequestration in the cytosol, whereas dephosphorylated Bad interacts with Bcl-XL or Bcl-2 in mitochondria, triggering apoptosis. Bcl-2 protein also is phosphorylated in vivo, which is associated with inactivation of Bcl-2 function in several systems. Tumor necrosis factor α or Fas treatment triggers cleavage of Bid by caspase-8, and the cleaved Bid inserts into the mitochondrial membrane, resulting in release of cytochrome c (2, 3).
It becomes more and more clear that the ubiquitin/proteasome system plays an important role in the degradation of cellular proteins that are involved in regulating different cellular processes including apoptosis (8, 9). Recently, several groups, including ours, found that proteasome inhibitors were potent apoptosis inducers when used in multiple cancer and transformed cell lines (10–13). In addition, we also reported that inhibition of the proteasome activity was sufficient to overcome Bcl-2- or Bcr-Abl-mediated protective function from apoptosis (13, 14). In the present study, we report that Bax is a direct target protein of the ubiquitin/proteasome pathway. Inhibition of this pathway by a proteasome inhibitor in Bcl-2-overexpressing Jurkat T cells resulted in accumulation of Bax and its ubiquitinated forms, but had no effect on Bax mRNA level. The increased Bax-immunofluorescent signals were localized primarily to mitochondria/cytoplasm, associated with increased levels of Bax-Bcl-2 interaction. This event was followed by the mitochondrial cytochrome c release and the caspase activation. Furthermore, correlated to decreased Bax expression, levels of Bax degradation were significantly increased in aggressive prostate cancer tissue samples.
Materials and Methods
Materials.
Tripeptidyl protease inhibitors, phosphocreatinine, creatine phosphokinase, ATP, ATP-γ-S, ubiquitin, and other chemicals were purchased from Sigma. Purified 20S proteasome, lactacystin, and clasto-lactacyctin β-lactone (β-lactone) were from Calbiochem. Stocks of proteasome and protease inhibitors were prepared in DMSO as described (14). Purified mouse mAbs to human Bax (clone 6A7) and proliferating cell nuclear antigen (clone PC-10) were purchased from Santa Cruz Biotechnology, to human Bcl-2 (clone 2–124) from Dako, to human cytochrome c (clone 7H8.2C12) from PharMingen, to cytochrome oxidase subunit II (clone 12c4-f12) from Molecular Probes, and to 20S proteasome subunit α6 (clone HC2) from Affiniti Research Products (Exeter, U.K.). Rabbit polyclonal antibody to human PARP was from Boehringer Manheim, to human Bax (clone N-20) and actin (clone C11) from Santa Cruz Biotechnology, and to human ubiquitin from Sigma.
Cell Culture and Drug Treatment.
Human breast cancer MCF-7 cells, Jurkat T cells, and Jurkat T cells stably transfected with pRcCMV vector containing a complete human bcl-2 cDNA (obtained from Hong-gang Wang, Moffitt Cancer Center & Research Institute) were grown in RPMI 1640 growth medium (13). Treatment of cell with a proteasome inhibitor was performed as described (13, 14).
Western Blot Analysis and Immunoprecipitation.
Whole-cell extract (7), whole tissue extracts (15), and cytosol and mitochondria fractions (16) were prepared as described. The enhanced chemiluminescence Western blot assay was performed as described (7). To perform a coupled immunoprecipitation-Western blot assay, a whole-cell or tissue extract (200 μg protein) first was precleared by incubating with protein A plus protein G agarose beads (Calbiochem) at 4°C for 2 h. The collected supernatant then was incubated for at least 3 h with either 10 μl of agarose beads conjugated with the N20 Bax antibody (Santa Cruz Biotechnology) or the 6A7 Bax antibody, followed by incubation with protein A/protein G beads at 4°C overnight. The washed Bax immunoprecipitates were boiled in SDS sample buffer and used for Western blotting with antibodies to Bax, Bcl-2, or ubiquitin.
In Vitro Bax Degradation Assay.
Human bax-α cDNA subcloned into pcDNA3 was a gift from Hong-gang Wang. Human full-length bcl-2-α cDNA was cloned from Jurkat T cells and subcloned into pcDNA3.1(−) (G. Gao and Q.P.D., unpublished work). Both bax and bcl-2 plasmids were used for coupled in vitro transcription/translation (Promega) in the presence of [35S]methionine (Amersham Pharmacia). Protein extracts were prepared from either MCF-7 cells or prostate tumor tissues in buffer Y (50 mM Tris⋅HCl, pH 7.4/250 mM NaCl/1% Triton X-100/0.1% SDS/1 mM EDTA), and used for Bax (or Bcl-2) degradation assay. Briefly, 1 μl of 35S-labeled Bax (or Bcl-2) protein was incubated at 37°C for 2–4 h with 100–200 μg protein extract in buffer Z (50 mM Tris⋅HCl, pH 7.4/5 mM MgCl2/3 mM DTT/10 mM ATP/10 mM phosphocreatine/10 μg/ml creatine phosphokinase/10 μg/ml aprotinin/10 μg/ml leupeptin/10% glycerol/2 μg/ml ubiquitin). After incubation, the samples were subjected to gel electrophoresis and autoradiography. Under the cell-free assay conditions, the calpain-mediated Bax cleavage activity was blocked by omission of calcium and addition of the protease inhibitor leupeptin (17). To deplete the proteasome, MCF-7 cell lysates were either immunoprecipitated with the 20S proteasome subunit α6 antibody or ultracentrifugated at 100,000 × g for 6 h. The proteasome-enriched pellet fraction was resuspended in buffer Y.
Immunocytochemistry and Reverse Transcriptase-PCR (RT-PCR).
Immunocytochemistry was performed with the rabbit polyclonal Bax antibody (N20) and an FITC-labeled goat anti-rabbit antibody (Southern Biotechnology), followed by counterstaining nuclei with propidium iodide (Sigma) (18). To perform RT-PCR, total RNA was isolated from Jurkat T cells by an Advantage RT-for-PCR kit (CLONTECH). The primer pairs used for amplification of Bax mRNA (538 bp) were: forward, 5′-CAGCTCTGAGCAGATCATGAAGACA-3′ and reverse, 5′-GCCCATCTTCTTCCAGATGGTGAGC-3′ (19). PCR was conducted by using a MasterTaq DNA polymerase kit (Eppendorf), followed by agarose gel analysis. All results were normalized to glyceraldehyde-3-phosphate dehydrogenase mRNA (983 bp; CLONTECH).
Results
Proteasome Inhibitors Are Able to Accumulate Bax Protein and Subsequently Induce Cytochrome c-Dependent Apoptosis in Jurkat T Cells Overexpressing Bcl-2 Protein.
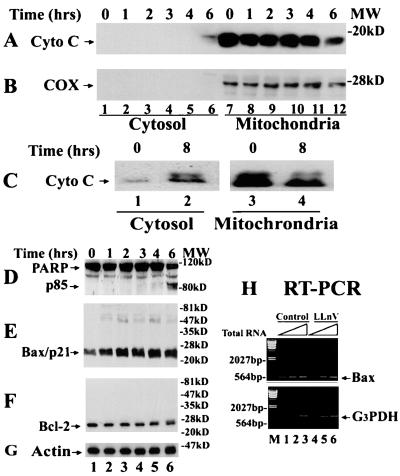
We first determined whether cytochrome c release is associated with proteasome inhibition-induced apoptosis in Bcl-2-expressing cells. Treatment of Bcl-2-overexpressing Jurkat cells with the tripeptidyl proteasome inhibitor N-carbobenzoxy-l-leucyl-l-leucyl-norvalinal (LLnV) for 6–8 h increased the level of cytosolic cytochrome c, accompanied by a decrease in the level of the mitochondrial cytochrome c (Fig. 1 A and C). The increased cytosolic cytochrome c was not the result of contamination from the mitochondria preparation because expression of cytochrome oxidase, an enzyme that is localized in mitochondria (20), was detected only in the membrane-bound, but not the cytosolic, fraction (Fig. 1B). Release of cytochrome c in LLnV-treated Bcl-2 cells was associated with induction of apoptosis, as demonstrated by cleavage of PARP (Fig. 1D). These data suggest that proteasome inhibition-induced apoptosis in Bcl-2-overexpressing cells is associated with cytochrome c release.
Figure 1.
Proteasome inhibitor LLnV induces Bax accumulation, cytochrome c release, and PARP cleavage in Bcl-2-overexpressing Jurkat T cells. (A–C) Jurkat T cells overexpressing Bcl-2 (0 h) were treated with 50 μM LLnV for up to 8 h, followed by preparation of cytosol and mitochondrial fractions. Both fractions were immunoblotted first by an antibody to cytochrome c (Cyto C, 17 kDa; A and C) and then reblotted by anticytochrome oxidase subunit II (COX, 26 kDa; B). Note: 20 μg protein from the cytosol, and 40 μg protein from the mitochondrial, preparation was used in each lane. (D–G) Whole-cell extracts (70 μg per lane) of the above treated cells were immunoblotted with specific antibodies to PARP (D), Bax (clone N-20; E), Bcl-2 (F), or actin (G). Molecular masses of PARP, the PARP cleavage fragment (p85), Bax, Bcl-2, and actin are 113, 85, 21, 26, and 40 kDa, respectively. Positions of protein markers are indicated at right. (H) Bcl-2-expressing Jurkat cells (Control) were treated with 50 μM LLnV for 8 h, followed by reverse transcription–PCR (RT-PCR). For the first-strand cDNA synthesis, 0.2 (lanes 1 and 4), 0.6 (lanes 2 and 5), and 1.8 μg (lanes 3 and 6) of the total RNA were used. The positions of Bax (538 bp) and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) mRNA (983 bp) are indicated. Lane M is DNA molecular weight marker.
To test the hypothesis that Bax is a direct target protein of the ubiquitin/proteasome pathway, Bax protein levels were measured in the same experiment by Western blot assay. The level of Bax protein (Bax/p21) was increased after LLnV treatment for 1 h or longer (Fig. 1E). In contrast, Bax mRNA level remained unchanged during proteasome inhibition (Fig. 1H). The LLnV treatment also increased levels of several Bax-related, high molecular weight polypeptides (Fig. 1E and also see Fig. 2D Upper), suggesting Bax posttranslational modification. Little changes were observed in levels of the overexpressed Bcl-2 protein in these cells (Fig. 1F). Therefore, LLnV treatment of Bcl-2-expressing cells increased the Bax protein level and the Bax/Bcl-2 ratio, which is associated with the ability of this proteasome inhibitor to overcome Bcl-2-mediation protection from apoptosis.
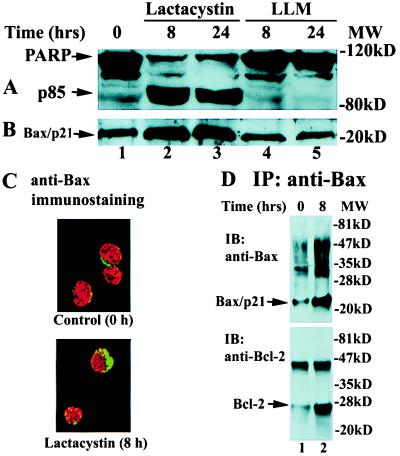
Figure 2.
Proteasome inhibition accumulates Bax to mitochondria/cytoplasm and increases interaction between Bax and Bcl-2 proteins. Jurkat cells (0 h; A and B) or Jurkat cells overexpressing Bcl-2 (0 h; C and D) were treated by either 10 μM lactacystin (A–C), 50 μM LLnV (D), or 50 μM LLM (A and B) for the indicated h. (A and B) Western blotting with antibodies to PARP and Bax were performed as described in Fig. 1. (C) Immunohistochemistry (see Materials and Methods). Localization of Bax protein (green) and nuclei (red) are shown. (D) Bax immunoprecipitates (IP), prepared by an agarose-conjugated N-20 Bax antibody, were immunoblotted (IB) first with the 6A7 Bax antibody (Upper) and then reblotted with a Bcl-2 antibody (Lower).
LLnV inhibits not only the proteasome activity but also some cysteine proteases, such as calpain and cathepsin B (21). To confirm that Bax accumulation and subsequent apoptosis induction are caused by inhibition of the proteasome activity, we used lactacystin, a specific proteasome inhibitor (22), and N-acetyl-l-leucyl-l-leucyl-l-methioninal (LLM), a strong inhibitor of calpain and cathepsin but a very weak inhibitor of the proteasome (21). Treatment of Jurkat T cells with 10 μM lactacystin induced Bax accumulation and PARP cleavage (Fig. 2 A and B, lanes 1–3). In contrast, LLM at 50 μM had no such effects (Fig. 2 A and B, lanes 4 and 5 vs. 1). Therefore, inhibition of the proteasome, but not a cysteine protease, pathway results in Bax accumulation and apoptosis induction (also see Discussion).
We then determined cellular localization of Bax protein accumulated by a proteasome inhibitor by immunofluorescent staining. In untreated Bcl-2-overexpressing Jurkat cells, Bax protein was primarily expressed in the cytoplasm (Fig. 2C Upper). Treatment with LLnV (data not shown) or lactacystin (Fig. 2C Lower) markedly increased the cytoplasmic Bax-immunofluorescent signals, which was consistent with the results obtained from Western blotting (Figs. 1E and 2B). The increased Bax signals remained largely clusters in cytoplasm around nuclei (Fig. 2C), suggesting accumulation of Bax protein in mitochondria.
Toward the goal of investigating the functional significance of proteasome inhibition-accumulated Bax protein in Bcl-2-overexpressing cells, we measured the interaction between Bax and Bcl-2 proteins by a coupled immunoprecipitation-Western blot assay. Bax immunoprecipitates were prepared from untreated and LLnV-treated Bcl-2-expressing cells by using a polyclonal Bax antibody, followed by immunoblot with mAbs to Bax and Bcl-2, respectively (Fig. 2D Upper and Lower, respectively). LLnV treatment significantly increased levels of both Bax/p21 and the Bax-bound Bcl-2 protein (Fig. 2D). The nature of a band of ≈46 kDa, detected by the Bcl-2 antibody in Bax immunoprecipitates (Fig. 2D Lower), remains unknown. LLnV also dramatically increased levels of multiple bands in a range of 30 to 60 kDa, most of which were detected by the antibody to Bax, but not to Bcl-2 (Fig. 2D Upper vs. Lower), indicating that most of them contain only Bax protein (also see Fig. 3A). Our data demonstrate that proteasome inhibitor-accumulated Bax protein is able to interact with Bcl-2, which correlates to release of mitochondrial cytochrome c and inhibition of the Bcl-2 antiapoptotic function.
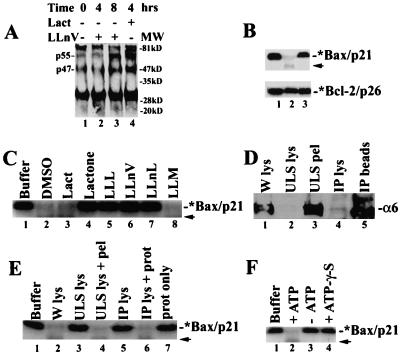
Figure 3.
Bax degradation depends on ubiquitination, proteasome, and ATP. (A) Jurkat cells (0 h) were treated with either 50 μM LLnV or 10 μM lactacystin, followed by preparation of Bax immunoprecipates (with clone 6A7), which were immunoblotted with an ubiquitin antibody. Positions of putative ubiquitinated Bax proteins (p47 and p55) are indicated at left. The nature of the ≈30-kDa and ≈84-kDa bands remains unclear. (B) The 35S-labeled Bax (Upper) or Bcl-2 (Lower) protein (1 μl) were incubated at 37°C for 2 h with either buffer Z only (lane 1) or 100 μg protein extract of MCF-7 cells grown exponentially (lane 2) or pretreated for 8 h with 50 μM LLnV (lane 3). (C) The 35S-labeled Bax protein (1 μl) was incubated with either buffer Z only (lane 1) or 100 μg MCF-7 cell lysate at 37°C for 4 h, in the presence of inhibitors N-carbobenzoxy-L-leucyl-L-leucyl-L-leucinal (LLL), LLnV, N-acetyl-L-leucyl-L-leucyl-norleucinal (LLnL), and LLM (100 μM; lanes 3–8) or an equal volume of DMSO (lane 2) in buffer Z. A weak band of ≈16 kDa (indicated by an arrow) is probably an intermediate product of proteasome-mediated Bax degradation. (D) The proteasome in MCF-7 whole-cell lysate (W lys, lane 1) was precipitated by using either ultraspin (ULS) or a proteasome subunit α6 antibody (IP). Both supernatant (lanes 2 and 4) and pellet (lanes 3 and 5) fractions were examined by Western blot assay using antibody to the proteasome α6 subunit (33 kDa). (E) Bax degradation assay was performed as in B, with addition of buffer Z (lane 1) or 100 μg protein from MCF-7 whole-cell lysate (lane 2), ultraspun supernatant (lane 3) or plus the pellet (lane 4), immunodepleted supernatant (lane 5), or plus a purified 20S proteasome (2 μg; lane 6), or the purified proteasome alone (2 μg; lane 7). (F) Bax degradation assay was performed as in B, in the absence (lane 3) or presence of 10 mM ATP (lane 2) or 10 mM ATP-γ-S (lane 4).
Bax Degradation Depends on Ubiquitin, Proteasome, and ATP.
If Bax is a direct target of the ubiquitin/proteasome pathway, inhibition of the proteasome activity should accumulate ubiquitinated forms of Bax protein. To investigate this possibility, protein extracts of Jurkat T cells treated with lactacystin or LLnV were immunoprecipitated with a Bax mAb, followed by Western blot assay using a polyclonal ubiquitin antibody. Several polypeptide bands including p55 and p47 were detected in the untreated cell lysate (Fig. 3A, lane 1). Treatment with lactacystin for 4 h, or with LLnV for 8 h, significantly increased both p55 and p47 levels (Fig. 3A), suggesting that they are probably polyubiquitinated forms of Bax.
To further study the proteasome activity that degrades Bax protein, we developed a cell-free Bax degradation assay by using an in vitro-translated, 35S-labeled Bax protein as substrate (Fig. 3B Upper, lane 1). The Bax degradation activity is present in protein extracts prepared from exponentially growing MCF-7 (Fig. 3), K562, VA-13, WI-38, Jurkat T, or HL-60 cells (data not shown). The labeled Bax was almost completely degraded by a MCF-7 cell extract after a 2- to 4-h incubation at 37°C (Fig. 3 B Upper and C, lanes 2 vs. 1). In contrast, no or little decrease in the level of a labeled Bcl-2 was detected after in vitro incubation (Fig. 3B Lower, lanes 2 vs. 1). When MCF-7 cells were pretreated with the proteasome inhibitor LLnV, the cell-free Bax degradation activity was inhibited (Fig. 3B, lanes 3 vs. 2). The Bax degradation process also was blocked by a 10-min preincubation of the cell extract with the tripeptide proteasome inhibitor N-carbobenzoxy-l-leucyl-l-leucyl-l-leucinal (LLL), LLnV, or N-acetyl-l-leucyl-l-leucyl-norleucinal (LLnL), but not with the tripeptide cysteine protease inhibitor LLM (Fig. 3C, lanes 5–8 vs. 2). In addition, Bax degradation activity was blocked by β-lactone, the active product of lactacystin (23), but not by lactacystin itself (Fig. 3C, lanes 3 and 4 vs. 2), suggesting failure of lactacystin to convert to β-lactone under the cell-free conditions. However, Bax degradation activity was not inhibited by several other protease inhibitors, including leupeptin, aprotinin, N-ethylmaleimide, PMSF, benzamidine, tosyl-l-lysine chloromethyl ketone, acetyl-YVAD-chloromethyl ketone, and acetyl-DEVD-fluoromethyl ketone (data not shown).
We then determined the effects of proteasome depletion on the cell-free Bax degradation (Fig. 3 D and E). An ultracentrifugation of the MCF-7 whole-cell lysate resulted in precipitation of the proteasome, as judged by Western blot assay using a specific antibody to the proteasome subunit α6 (Fig. 3D, lanes 1–3). The proteasome-depleted supernatant also had lost its Bax degradation activity, which was reconstituted by addition of the pellet fraction (Fig. 3E, lanes 2–4). The proteasome complex in the MCF-7 cell lysate was also successfully immunodepleted by using the proteasome α6 antibody (Fig. 3D, lanes 4 and 5), associated with loss of Bax degradation activity, which could be recovered by addition of a purified 20S proteasome (Fig. 3E, lanes 5 and 6). Addition of the purified proteasome alone was not sufficient to degrade Bax protein (Fig. 3E, lane 7), which is consistent with the idea that Bax ubiquitination is required for its degradation.
The cell-free Bax degradation assay was performed in the presence of ATP (see Materials and Methods), suggesting requirement for ATP. Indeed, Bax was not degraded if ATP was omitted or replaced by ATP-γ-S, a nonhydrolyzable analog of ATP (Fig. 3F, lanes 3 and 4 vs. 2). Taken together, both in vivo and in vitro studies have demonstrated that Bax is regulated by an ATP- and ubiquitin-dependent, proteasome-mediated degradation pathway.
Decreased Levels of Bax Protein Correlate with Increased Levels of Bax Degradation in Advanced Human Prostate Cancer.
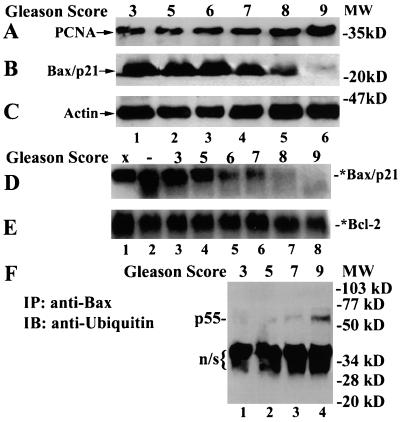
If constant degradation of the apoptosis inducer Bax by the proteasome is a cancer cell survival mechanism, the levels of Bax degradation activity should be increased in aggressive cancers. To test this hypothesis, we analyzed Bax protein expression and Bax degradation activity in frozen specimens of prostate adenocarcinomas. We obtained 38 cases of prostate tumor samples, which included 22 cases with Gleason scores 3–6 (low grade), 10 cases with Gleason score 7 (moderate), and six cases with Gleason scores 8–10 (high grade). In a selected subset of tumor samples (16 cases; Table 1 and Fig. 4), progression of prostate cancer (marked by increased Gleason scores) was confirmed by increased levels of proliferating cell nuclear antigen expression (Fig. 4A), an indicator of cell proliferation (24). We observed that levels of Bax/p21 protein were high in low-grade tumors, decreased in mid-grade tumors, and further decreased in high-grade cancers (Table 1 and Fig. 4B). Furthermore, the low-grade prostate tumors containing high levels of Bax protein displayed low levels of Bax degradation activity whereas the high-grade tumors with reduced Bax expression had enhanced proteolytic activity for Bax (Table 1 and Fig. 4D). These data suggest a tight correlation among decreased Bax protein expression, increased Bax degradation activity, and increased Gleason scores in this subset of prostate cancer samples.
Table 1.
Correlation between Bax protein level, Bax degradation activity (BDA), and tumor grade (Gleason score) in human prostate adenocarcinomas
| Tumor # | Gleason score | Bax protein | BDA | Bcl-2 protein |
|---|---|---|---|---|
| 1 | 3 | +++ | + | +++ |
| 2 | 5 | +++ | + | N/A |
| 3 | 5 | +++ | + | +++ |
| 4 | 6 | +++ | + | N/A |
| 5 | 6 | +++ | + | ++ |
| 6 | 6 | +++ | + | + |
| 7 | 6 | +++ | ++ | ++ |
| 8 | 7 | +++ | ++ | ++ |
| 9 | 7 | ++ | ++ | +++ |
| 10 | 7 | + | ++ | +++ |
| 11 | 7 | + | ++ | + |
| 12 | 7 | + | +++ | + |
| 13 | 8 | + | +++ | + |
| 14 | 9 | + | +++ | + |
| 15 | 9 | + | +++ | ++ |
| 16 | 10 | + | +++ | ++ |
Bax or Bcl-2 protein levels were assessed by immunoblot normalized to actin levels: +++, a strong signal; ++, a moderate signal; +, a weak signal; N/A, not available. BDA was assessed by comparison to control (buffer only) after 4-h incubation: +, <30% degradation; ++, 30–60% degradation; >60% degradation.
Figure 4.
Correlation of decreased Bax protein levels and increased Bax degradation in advanced human prostate cancer specimens (marked by increased Gleason scores). (A–C) Whole-tissue extracts (100 μg/lane) were immunoblotted with specific antibodies to proliferating cell nuclear antigen (PCNA, 36 kDa; A), Bax (N-20; B) or actin (C). (D and E) Bax or Bcl-2 degradation activity was assayed by incubating an 35S-labeled Bax or Bcl-2 protein (1 μl) with either buffer Z only (lane 1) or 200 μg protein extract prepared from either prostate adenocarcinomas with different grades (lanes 3–8) or a benign prostate hyperplasia (as a control; lane 2) at 37°C for 4 h in buffer Z. (F) Whole tissue extracts (200 μg) were immunoprecipated (IP) with 6A7 Bax antibody, followed by immunoblot (IB) with an ubiquitin antibody. Position of the putative ubiquitinated Bax, p55, is indicated. n/s indicates a possible nonspecific band.
We also searched for a correlation between Bax levels and Bax degradation activity, or tumor grade and Bax levels, or tumor grade and Bax degradation activity in all of the samples. We found that all eight low-Bax-containing cases expressed high (5/8) or moderate (3/8) levels of Bax degradation activity, whereas most of 17 high-Bax-containing cases had low (6/17) or moderate (8/17) levels of Bax degradation activity (Fisher's exact test, P < 0.05). Furthermore, all six high-grade tumors expressed low (4/6) or moderate (2/6) levels of Bax protein, whereas 17 of 32 low- and mid-grade tumors contained high levels of Bax protein and only few of these cases (4/32) expressed low levels of Bax protein (P < 0.05). Finally, all six cases of high-grade tumors contained high levels of Bax degradation activity, and most low-grade tumors contained low (8/32) or moderate (17/32) levels of Bax degradation activity (P < 0.05). In contrast to Bax, no correlation was observed between levels of Bcl-2 protein and Gleason scores of prostate cancer (Table 1). Furthermore, the levels of Bcl-2 degradation activity were only slightly increased in high-grade tumors (Fig. 4E).
To try to examine whether levels of Bax ubiquitination also are increased in advanced prostate cancers, Bax immunoprecipitates were prepared from different prostate tumor samples, followed by Western blotting using an ubiquitin antibody. Levels of a p55 band were undetected in low-grade prostate tumor samples (Gleason scores 3 and 5), slightly increased in a grade-7 sample, and significantly increased in a grade-9 tumor specimen (Fig. 4F). The increased p55 levels were detected in several different high-grade prostate tumor samples. Our data are consistent with increased levels of Bax ubiquitination and degradation during progression of prostate adenocarcinoma.
Discussion
In the current study, we report that (i) proteasome inhibition results in Bax accumulation before release of cytochrome c and induction of apoptosis, which is associated with the ability of proteasome inhibitors to overcome Bcl-2-mediated antiapoptotic function; (ii) Bax is regulated by an ATP/ubiquitin/proteasome-dependent degradation pathway; and (iii) decreased levels of Bax protein correlate with increased levels of Bax degradation in aggressive human prostate cancer.
Previously, we reported that proteasome inhibitors were able to induce apoptosis in human Jurkat cells overexpressing Bcl-2 protein (13). Another group also reported a similar finding by using Bcl-2-overexpressing prostate cancer cells (25). In the current study, we investigated the molecular basis for the ability of proteasome inhibitors to overcome Bcl-2 antiapoptotic function. We have demonstrated that Bax, an inhibitor of Bcl-2, is a direct target of the proteasome (Figs. 1–3). The following arguments suggest that Bax accumulation by proteasome inhibition is associated with the proteasome inhibitor's ability to overcome the Bcl-2 protective function. First, Bax protein levels were increased before release of cytochrome c from mitochondria to the cytosol (Fig. 1 E vs. A and C). Second, Bax was primarily accumulated in cytoplasm during proteasome inhibition; the observation that the increased Bax signals clustered around nuclei suggests accumulation in mitochondria (Fig. 2C). Third, proteasome inhibition-accumulated Bax protein was able to interact with Bcl-2 (Fig. 2D). Finally, Bcl-2 protein levels remained relatively unchanged during proteasome inhibition (Fig. 1F). Our studies are consistent with the reported functional role of Bax and Bcl-2 proteins in forming ion channels in mitochondria membrane where they regulate cytochrome c leakage into cytosol during apoptosis (ref. 26; reviewed in refs. 2 and 3). It has been found that dephosphorylated Bad and cleaved Bid are able to interact with Bcl-XL or Bcl-2 in mitochondria and overcome their antiapoptotic function (2, 3). Whether proteasome inhibitors also induce dephosphorylation of Bad and cleavage of Bid in our systems remains to be investigated.
Our in vivo and in vitro studies have demonstrated that Bax is degraded via an ATP-/ubiquitin-dependent proteasome pathway (Figs. 1–3). Treatment of cells with the proteasome inhibitor lactacystin (22) or LLnV (21) accumulated Bax protein (but not Bax mRNA) and the ubiquitinated forms of Bax (Figs. 1E, 2B, and 3A), whereas the cysteine protease inhibitor LLM (21) had no effect (Fig. 2B). In addition, Bax degradation activity was inhibited in vivo and in vitro by a proteasome inhibitor LLnV, N-carbobenzoxy-l-leucyl-l-leucyl-l-leucinal (LLL), N-acetyl-l-leucyl-l-leucyl-norleucinal (LLnL), or β-lactone, but not by the cysteine protease inhibitor LLM (Fig. 3 B and C). Furthermore, cell-free Bax degradation was prevented by removal of the proteasome via ultracentrifugation or immunodepletion, which can be reconstituted by addition of the proteasome-enriched pellet fraction or a purified 20S proteasome (Fig. 3 D and E). Finally, the cell-free Bax degradation process requires ATP (Fig. 3F). All of the above features of Bax degradation are similar to those of previously identified target proteins of ubiquitin/proteasome degradation pathway (8, 9). Most recently, one group reported that Bax/p21 protein levels were increased when HeLa or Saos-2 cells were treated with a proteasome inhibitor (27). However, the authors did not provide direct evidence for Bax as a target protein for the ubiquitin/proteasome pathway in their systems. Such direct evidence has been provided in our current studies.
Most recently, it also has been found that Bcl-2 is specifically degraded after stimulation of human endothelial cells with tumor necrosis factor α (28). Compared with cell-free Bax degradation, no or much less Bcl-2 proteolysis was observed after incubation with a tumor cell or tissue extract (Figs. 3B Lower vs. Upper and 4 E vs. D). In addition, the tumor suppressor p53, another target of the ubiquitin/proteasome pathway (8, 9), was much more resistant than Bax to induction of cell-free degradation (B.L., Y. Peng, J. Chen, and Q.P.D., unpublished data). It seems that our in vitro degradation assay preferably detects degradation of Bax over Bcl-2 and p53.
Under cell-free conditions, in addition to proteasome-mediated degradation, Bax can be cleaved by a calcium-dependent calpain activity (17). However, the following arguments suggest that the calpain-mediated Bax cleavage is not a major mechanism for regulation of Bax in our cell systems. First, the calpain cleavage product of Bax, Bax/p18 fragment, was not observed in exponentially growing Jurkat T cells (Fig. 1E, lane 1), suggesting that under in vivo conditions either Bax is not cleaved by the calpain or Bax/p18 is further cleaved or degraded. Second, treatment of Jurkat cells with the calpain inhibitor LLM, which blocked cell-free Bax cleavage to the p18 fragment (ref. 17 and data not shown), neither increased Bax/p21 levels nor induced apoptosis (Fig. 2 A and B). Third, Bax/p18 was not detected during the process of proteasome inhibitor-induced apoptosis (Fig. 1E), although it was found in cells treated with an anticancer drug (29). This difference probably is because different apoptosis stimuli were used. In any case, our results have demonstrated that inhibition of the proteasome, but not calpain, activity is responsible for the accumulation of Bax protein.
In the current study, we also reported that decreased Bax levels correlated well with increased Bax degradation in aggressive prostate tumor samples, whereas no such a correlation was found between levels of Bcl-2 protein or Bcl-2 degradation activity and Gleason scores of these tumor samples (Table 1 and Fig. 4). Furthermore, all high-grade tumors expressed low/moderate levels of Bax protein and high levels of Bax degradation activity, whereas most of low- and mid-grade tumors contained high levels of Bax protein and low/moderate levels of Bax degradation activity. It should be noted that two previous studies using immunohistochemical assay showed that Bax levels did not correlate with Gleason grade of prostate cancer (30, 31), probably because that immunohistochemistry detected a mixture of Bax/p21 and ubiquitinated Bax whereas Western blotting was able to separate Bax/p21 from its modified forms.
The p55 band, found in both Jurkat T cells treated with a proteasome inhibitor (Fig. 3A) and high-grade prostate cancer tumor samples (Fig. 4F), can be recognized by antibodies to both Bax and ubiquitin proteins, suggesting that it is probably a polyubiquitinated form of Bax. This hypothesis needs to be confirmed by further investigation using cells expressing a tagged ubiquitin.
Human cancer biologic behavior must be controlled by complex molecular mechanisms. In addition, Bax is regulated through multiple signal transduction pathways. Our data suggest that Bax degradation is an important regulatory mechanism for controlling Bax protein levels, which plays an important role in advancing prostate cancer. Discovery of the correlation between proteasome-mediated Bax degradation and prostate cancer progression should have great clinical significance in diagnosis, treatment, and prognosis of human prostate and other cancers.
Acknowledgments
We thank Dr. Hong-gang Wang for providing Bcl-2-overexpressing Jurkat T cells and bax-α pcDNA3 plasmid, Dr. Gui Gao for bcl-2 pcDNA3 plasmid, Ms. Xiaoxia Zhang for technical assistance in reverse transcription–PCR and cell culture, Dr. Nikola Valkov for assistance in confocal microscope, and Dr. Julio Pow-Sang for helpful discussion in prostate cancer data. This work is supported in part by a research fund from H. Lee Miffitt Cancer Center & Research Institute (to Q.P.D.) and by the Tissue Procurement/Pathology, Molecular Biology and Molecular Imaging Core Facilities at H. Lee Miffitt Cancer Center & Research Institute.
Abbreviations
- PARP
poly(ADP-ribose) polymerase
- LLnV
N-carbobenzoxy-l-leucyl-l-leucyl-norvalinal
- LLM
N-acetyl-l-leucyl-l-leucyl-l-methioninal
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070047997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070047997
References
- 1.Steller H. Science. 1995;267:1445–1462. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 2.Green D R, Reed J C. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 3.Gross A, McDonnel J M, Korsmeyer S J. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 4.Martin S J, Green D R. Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- 5.Thornberry N A, Lazebnik Y. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 6.Lazebnik Y A, Kaufmann S H, Desnoyers S, Poirier G G, Earnshaw W C. Nature (London) 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 7.An B, Dou Q P. Cancer Res. 1996;56:438–442. [PubMed] [Google Scholar]
- 8.Hochstrasser M. Curr Opin Cell Biol. 1995;7:215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 9.Dou Q P, Li B. Drug Resist Updates. 1999;2:215–223. doi: 10.1054/drup.1999.0095. [DOI] [PubMed] [Google Scholar]
- 10.Imajoh-Ohmi S, Kawaguchi T, Sugiyama S, Tanaka K, Omura S, Kikuchi H. Biochem Biophys Res Commun. 1995;217:1070–1077. doi: 10.1006/bbrc.1995.2878. [DOI] [PubMed] [Google Scholar]
- 11.Drexler H C. Proc Natl Acad Sci USA. 1997;94:855–860. doi: 10.1073/pnas.94.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopes U G, Erhardt P, Yao R, Cooper G M. J Biol Chem. 1997;272:12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- 13.An B, Goldfarb R H, Siman R, Dou Q P. Cell Death Differ. 1998;5:1062–4075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- 14.Dou Q P, McGuire T F, Peng Y B, An B. J Pharmacol Exp Ther. 1999;289:781–790. [PubMed] [Google Scholar]
- 15.Loda M, Cukor B, Tam S W, Lavin P, Fiorentino M, Draetta G F, Jessup J M, Pagano M. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 16.Fang G, Chang B S, Kim C N, Perkins C, Thompson C B, Bhalla K N. Cancer Res. 1998;58:3202–3208. [PubMed] [Google Scholar]
- 17.Wood D, Thomas A, Devi L A, Berman Y, Beavis R C, Reed J C, Newcomb E W. Oncogene. 1998;17:1069–1078. doi: 10.1038/sj.onc.1202034. [DOI] [PubMed] [Google Scholar]
- 18.Bossy-Wetzel E, Newmeryer D D, Green D R. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T T Y, Phang J M. Cancer Res. 1995;55:2487–2489. [PubMed] [Google Scholar]
- 20.Barrell B G, Bankier A T, Drouin J. Nature (London) 1979;282:189–194. doi: 10.1038/282189a0. [DOI] [PubMed] [Google Scholar]
- 21.Rock K L, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A L. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 22.Fenteany G, Standaert R F, Lane W S, Choi S, Corey E J, Schreiber S L. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 23.Fenteany G, Standaert R F, Reichard G A, Corey E J, Schreiber S L. Proc Natl Acad Sci USA. 1994;91:3358–3362. doi: 10.1073/pnas.91.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harper M E, Glynne-Jones E, Goddard L, Wilson D W, Matenhelia S S, Conn I G, Peeling W B, Griffiths K. Prostate. 1992;20:243–253. doi: 10.1002/pros.2990200309. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann J L, Briones F, Jr, Brisbay S, Logothetis C J, McDonnell T J. Oncogene. 1998;17:2889–2899. doi: 10.1038/sj.onc.1202221. [DOI] [PubMed] [Google Scholar]
- 26.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J J, Mazzei G, et al. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 27.Chang Y C, Lee Y S, Tejima T, Tanaka K, Omura S, Heintz N H, Mitsui Y, Magae J. Cell Growth Differ. 1998;9:79–84. [PubMed] [Google Scholar]
- 28.Dimmeler S, Breitschopf K, Haendeler J, Zeiher A M. J Exp Med. 1999;189:1815–1822. doi: 10.1084/jem.189.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas A, El Rouby S, Reed J C, Krajewski S, Silber R, Potmesil M, Newcomb E W. Oncogene. 1996;12:1055–1062. [PubMed] [Google Scholar]
- 30.Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang H G, Reed J C. Am J Pathol. 1994;145:1323–1336. [PMC free article] [PubMed] [Google Scholar]
- 31.Mackey T J, Borkowski A, Amin P, Jacobs S C, Kyprianou N. Urology. 1998;52:1085–1090. doi: 10.1016/s0090-4295(98)00360-4. [DOI] [PubMed] [Google Scholar]