Abstract
Both genetic and environmental factors contribute to autoimmune disease development. Previously, we evaluated genetic factors in a humanized mouse model of Hashimoto's thyroiditis (HT) by immunizing human leucocyte antigen DR3 (HLA-DR3) and HLA-DQ8 transgenic class II-knock-out non-obese diabetic (NOD) mice. DR3+ mice were susceptible to experimental autoimmune thyroiditis (EAT) induction by both mouse thyroglobulin (mTg) and human (h) Tg, while DQ8+ mice were weakly susceptible only to hTg. As one environmental factor associated with HT and tested in non-transgenic models is increased sodium iodide (NaI) intake, we examined the susceptibility of DR3+ and/or DQ8+ mice to NaI-induced disease. Mice were treated for 8 weeks with NaI in the drinking water. At 0·05% NaI, 23% of DR3+, 0% of DQ8+ and 20% of DR3+DQ8+ mice had thyroid destruction. No spleen cell proliferation to mTg was observed. Most mice had undetectable anti-mTg antibodies, but those with low antibody levels usually had thyroiditis. At 0·3% NaI, a higher percentage of DR3+ and DR3+DQ8+ mice developed destructive thyroiditis, but it was not statistically significant. However, when DR3+ mice had been depleted of CD4+CD25+ regulatory T cells prior to NaI treatment, destructive thyroiditis (68%) and serum anti-mTg antibodies were exacerbated further. The presence of DQ8 molecules does not alter the susceptibility of DR3+DQ8+ mice to NaI-induced thyroiditis, similar to earlier findings with mTg-induced EAT. Susceptibility of DR3+ mice to NaI-induced EAT, in both the presence and absence of regulatory T cells, demonstrates the usefulness of HLA class II transgenic mice in evaluating the roles of environmental factors and immune dysregulation in autoimmune thyroid disease.
Keywords: CD4+CD25+ regulatory T cells, DR3 transgene, experimental autoimmune thyroiditis, HLA-DR3, sodium iodide
Introduction
Murine experimental autoimmune thyroiditis (EAT) is an organ-specific disease which serves as a model for Hashimoto's thyroiditis (HT), a hypothyroid syndrome. EAT susceptibility is based on H2 class II genes [1]. Recently, we have used HLA class II transgenic mice as a HT model to examine DR and DQ polymorphisms in susceptibility to mouse thyroglobulin (mTg)-induced EAT in an endogenous class II-knock-out strain (Ab0), and compared their responses to EAT induction with mTg and human (h) Tg. This strategy demonstrated the important role of human leucocyte antigen DR3 (HLA-DR3) (DRB1*0301) allele in susceptibility [2], in support of certain patient studies [3]. We further showed that DQ8 (HLA-DQA1*0301/DQB1*0302)-transgenic mice were resistant to mTg induction of EAT, but were weakly susceptible to hTg-induced EAT [4]. When these studies were conducted in the class II-knock-out mice on the autoimmune disease-prone non-obese diabetic (NOD) background (Ab0/NOD), the difference in class II gene permissiveness for both hTg- and mTg-induced EAT was again observed [5,6]. However, the influence of background genes was also notable; on the NOD background, the milder hTg-induced EAT became more severe, whereas mTg-induced EAT, already severe, was unaltered [5]. Moreover, the DQ8 resistance transgene reduced only hTg-induced thyroiditis severity in double transgenic DR3+DQ8+ mice without influencing mTg-induced EAT, suggesting that DQ8 had no down-regulating effect when mice were immunized with mTg [6].
It is now accepted that the DR3 class II allele plays a prominent role in autoimmune thyroid disease [7], but environmental factors also contribute. One factor is iodine intake (reviewed in [8]). The evidence is clear in the NOD mouse with EAT-susceptible alleles; treatment of NOD (IAg7) and NOD.H2h4 (IAk) mice with sodium iodide (NaI, 0·05%) in the drinking water for 4–8 weeks resulted in an increase in thyroiditis incidence [9–11]. Epidemiological surveys in humans, however, show that the extent of influence of iodine intake is less clear and is highly dependent on genetic/ethnic differences and less on dietary customs [12–14].
Another factor in susceptibility to autoimmune thyroid disease is the presence of naturally occurring regulatory T (Treg) cells and their control of peripheral T cells specific for thyroid autoantigens. These Treg cells, which constitute approximately 5–10% of peripheral CD4+ T cells, suppress autoreactive T cell responses, which can either be beneficial (i.e. autoimmunity) or detrimental (i.e. anti-tumour immunity) [15,16]. Most Treg cells express constitutively the interleukin-2 receptor α chain, CD25 [17], which provides a convenient marker for monoclonal antibody (MoAb)-mediated Treg cell modulation. We have used an anti-CD25 MoAb to show the role of Treg cells in mediating tolerance to mTg-induced EAT. In EAT-susceptible CBA/J mice, depletion of CD4+CD25+ T cells interfered with tolerance induction with mTg and abrogated already established tolerance [18]. Moreover, in the absence of tolerance induction, depletion of naturally occurring CD25+ Treg cells exacerbated EAT [19,20]. In traditionally EAT-resistant C57BL/10 [20] and BALB/c mice [21], Treg cell depletion enabled the induction of moderate EAT.
Using our single DR3+ and double DR3+DQ8+ class II transgenic Ab0/NOD mouse models, we have examined the effect of NaI consumption and whether depletion of CD4+CD25+ Treg cells exacerbated EAT in these mice.
Materials and methods
Mice
All strains were raised in the pathogen-free Immunogenetics Mouse Core facility at the Mayo Clinic. NOD HLA-DR3 or HLA-DQ8 transgenic mice in the absence of endogenous H2 class II molecules were created as described previously [6]. Briefly, HLA-DRA/DRB1*0301 (DR3) or HLA-DQA1*0301/DQB1*0302 (DQ8) transgenes were introduced into class II-negative Ab0 mice [22] and back-crossed to C57BL/10 mice [2,23]. Mice were back-crossed repeatedly to NOD mice, generating class II-negative, DR3 transgenic NOD (DR3+ Ab0/NOD) [5] or class II-negative, DQ8 transgenic NOD (DQ8+ Ab0/NOD) mice [24], which were then intercrossed to obtain double transgenic DR3+DQ8+ Ab0/NOD mice. Mice of both sexes were used at 8–16 weeks of age. Flow cytometric analysis of peripheral blood leucocytes was used to determine the HLA-DR3 and/or HLA-DQ8 expression. MoAbs used were L227 (anti-DRB1) [25] and IVD12 (anti-DQ) [26]. The polymerase chain reaction technique was used to determine the homozygous or heterozygous presence of H2Ag7, based on the absence or presence of the neo marker for Ab°, respectively [22].
Sodium iodide (NaI) treatment
Mice were supplied with either normal drinking water or water supplemented with NaI (Sigma Chemical Co., St Louis, MO, USA) at 0·05% or 0·3% for 8 weeks.
Regulatory T cell depletion
The MoAb to CD25, PC61 [rat IgG, λ light chain; American Type Culture Collection (ATCC, Rockville, MD, USA)], was produced by infusing 5 × 107 B cell hybridoma cells into a FiberCell hollow fibre tissue culture module (FiberCell Systems, Inc., Frederick, MD, USA); secreted antibodies were harvested as per the manufacturer's protocol and purified by two 50% ammonium sulphate precipitations. The rat λ light chain content was determined by enzyme-linked immunosorbent assay (ELISA), using mouse anti-rat IgG (Fc fragment-specific, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) as capture antibody and biotinylated mouse anti-rat Ig λ chain B46-5 (PharMingen, San Diego, CA, USA) as detection antibody. To deplete CD25+ cells, mice were injected twice intravenously (i.v.) with 1 mg PC61, 4 days apart; cell depletion was confirmed by fluorescence activated cell sorter (FACS) analysis of peripheral blood leucocytes 6–8 days after the second PC61 injection. Control mice were injected with either phosphate-buffered saline (PBS) or rat IgG (Jackson ImmunoResearch Laboratories); no differences in CD4+CD25+ cell percentages were observed between the two control groups.
Antigen
mTg used for proliferative assays and ELISA was prepared from frozen mouse thyroids as described previously [27]. The thyroid extract was fractionated on a Sephadex G-200 column. The mTg fraction contained < 0·1 ng/ml lipopolysaccharide (LPS) at 40 µg mTg/ml (cell culture dose), as determined by Limulus amebocyte assay (Associates of Cape Cod, Woods Hole, MA, USA) [28].
Serum antibody titres
Titres for mTg antibodies were determined by ELISA generally as described previously [29]. Briefly, Immulon I plates (Dynex Technologies, Chantilly, VA, USA) were coated with 1 µg mTg/well overnight (4°C), followed by dilutions of sera overnight (4°C). Then, alkaline phosphatase-conjugated goat anti-mouse IgG (1 : 4000, Southern Biotechnology Associates, Birmingham, AL, USA) was added for 60 min (37°C), followed by substrate (p-nitrophenyl phosphate, Sigma). The reaction was stopped after 30 min at 25°C with 3 N NaOH, and optical densities (OD) were determined (405 nm). Anti-mTg standard serum and normal mouse serum were used as positive and negative controls. Data from individual mice are presented after subtraction of background values.
Evaluation of thyroiditis
Mononuclear cell infiltration was determined at the end of the 8-week treatment by histological examination of the thyroid. The pathology score for each mouse was determined by examining 60–70 vertical sections throughout both thyroid lobes (totalling 10–15 step levels). The score, graded on a scale of 0–4, is expressed as a percentage of thyroid gland infiltration: 0, no infiltration; 0·5, > 0–10% infiltration of the thyroid, with multiple foci of infiltration but no follicular destruction; 1·0, > 10–20% thyroid infiltration with follicular destruction; 2·0, > 20–40% thyroid infiltration; 3·0, > 40–80% thyroid infiltration; and 4·0, > 80–100% thyroid infiltration [30,31]. Statistical differences between groups were determined by the non-parametric Mann–Whitney U-test; P < 0·05 was considered significant.
Results
Oral NaI treatment induces EAT in HLA-DR3 transgenic Ab0/NOD mice
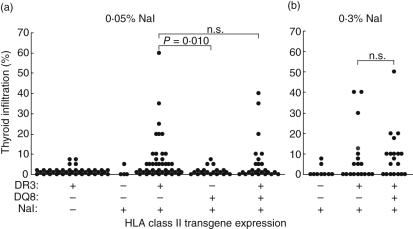
The susceptibility of DR3+ and/or DQ8+ Ab0/NOD mice to NaI-induced EAT was examined by adding 0·05% NaI to their drinking water for 8 weeks. As seen in Fig. 1a, almost 50% of DR3+ mice demonstrated some level of thyroiditis. However, because of the NOD background, we also observed a low incidence of ∼10% (four of 39) of non-destructive thyroiditis in 6–9-month-old, untreated DR3+ Ab0/NOD mice. Therefore, we compared the thyroiditis-inducing effects of NaI by only comparing mice with thyroid destruction (i.e. = 10% thyroid involvement). In this context, 23% (10 of 43) of DR3+ mice developed thyroiditis versus 0% (0/20) of DQ8+ mice. Comparable to DR3+ mice, 20% (five of 25) of DR3+DQ8+ mice had thyroiditis, indicating a lack of down-regulating effect of the non-permissive DQ8 molecule.
Fig. 1.
Human leucocyte antigen DR3 (HLA-DR3) molecules are permissive for NaI-induced thyroiditis, even in the presence of non-permissive HLA-DQ8 molecules. On week 0, DR3+, DQ8+ or DR3+DQ8+ Ab0/non-obese diabetic (NOD) mice were given 0·05% (a) or 0·3% NaI (b) in the drinking water. DR3+ Ab0/NOD mice given regular drinking water (a) were also evaluated for background infiltration. Thyroiditis in individual mice was determined on week 8; n.s., not significant.
When the NaI concentration in the drinking water was raised to 0·3%, an increase in the incidence of destructive thyroiditis was observed in both DR3+ (five of 18 = 28%) and DR3+DQ8+ mice (nine of 19 = 47%), but these increases were not statistically significant (Fig. 1bversus1a). Despite the destructive thyroiditis seen in some mice, no T cell proliferation to mTg was detected in vitro (data not shown). Most mice also had undetectable titres of mTg antibody, but those with positive antibody titres usually had some thyroid destruction (data not shown). The higher NaI percentage also did not raise the background infiltration in control mice lacking both DR3 and DQ8 expression (Fig. 1aversus1b). Therefore, mice shown previously to be susceptible to mTg-induced EAT [5,6] also exhibit a similar susceptibility to NaI-induced EAT.
Depletion of CD4+CD25+ Treg cells exacerbates NaI-induced EAT
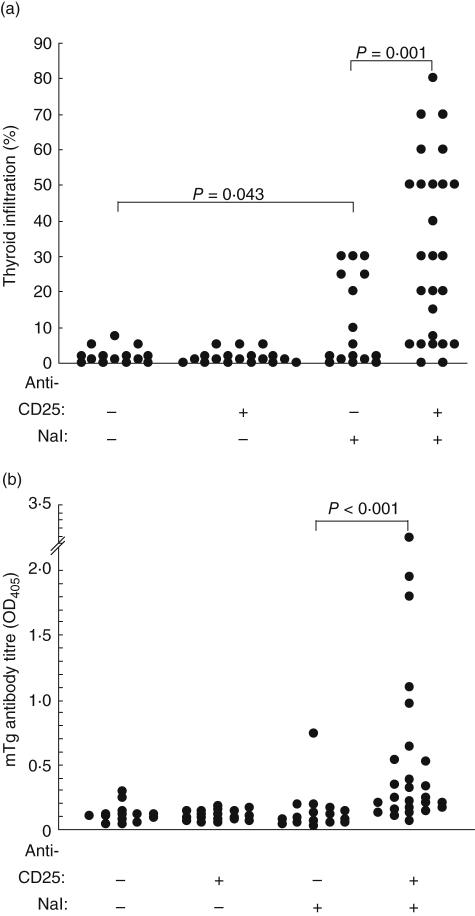
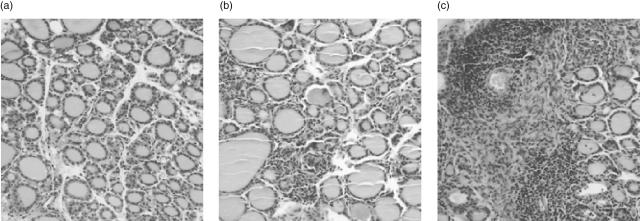
CD4+CD25+ Treg cells have been shown to play an inhibitory role in several autoimmune diseases, including EAT [18–21]. To determine if the reduction in CD25+ Treg cells would result in an increase in the incidence of NaI-induced EAT in DR3+ mice, mice were injected with rat IgG or CD25 MoAb PC61 prior to oral intake of 0·3% NaI for 8 weeks. In the absence of Treg cell depletion and NaI treatment, some DR3+ control mice developed non-destructive thyroiditis (Fig. 2a) at a low incidence similar to transgene-negative mice (Fig. 1). When only Treg cells were depleted without NaI administration, no increase in this low background incidence was observed. As in Fig. 1, oral intake of 0·3% NaI raised the incidence of destructive thyroiditis. However, a much higher thyroiditis incidence and severity were seen when Treg cells had been depleted prior to NaI exposure; nearly 70% of mice displayed some thyroid destruction and over half of those involved 40–80% of the thyroid (Fig. 2a). Again, no proliferation to mTg was detected in vitro after prior depletion of Treg cells (data not shown). However, most of these mice also showed higher levels of mTg antibodies (Fig. 2b). In the more severe cases of thyroiditis, fibrosis was present along with follicular destruction (Fig. 3c) compared to focal infiltration seen in the group treated with only NaI (Fig. 3b). Thus, while depletion of Treg cells alone was not sufficient to exacerbate nondestructive thyroiditis, Treg cell depletion combined with NaI significantly increased the severity and incidence of thyroiditis and anti-mTg levels.
Fig. 2.
Depletion of CD4+CD25+ regulatory T cells exacerbates NaI-induced EAT in DR3+ Ab0/non-obese diabetic (NOD) mice. Mice were injected with 1 mg anti-CD25 monoclonal antibody PC61 (weeks −2 and −1·5) before being given NaI (0·3% in drinking water) for 8 weeks. Mice were killed, and the extent of thyroid infiltration (a) and serum (1 : 50) antibody titres to mTg of individual mice (b) were determined.
Fig. 3.
Depletion of CD4+CD25+ regulatory T cells with CD25 monoclonal antibody (MoAb) exacerbates thyroiditis induced by 0·3% NaI in the drinking water for 8 weeks. Thyroid sections showing mononuclear cell infiltration in DR3+ Ab0/non-obese diabetic (NOD) mice on regular water after MoAb treatment alone (a, 0% thyroid involvement), on 0·3% NaI after phosphate-buffered saline treatment (b, 20% thyroid involvement with destruction) and on 0·3% NaI after anti-CD25 treatment (c, 50% thyroid involvement with destruction and fibrosis) (original magnification, 200×).
Discussion
Our previous work has examined the susceptibility of HLA class II transgenic DR3+ and/or DQ8+ Ab0 mice and the extent of background gene influence on both mTg- and hTg-induced EAT [2,4,6,32]. In brief, we noted differences not only in susceptibility (DR3 > DQ8), but also differences in severity of thyroiditis (DR3 > DQ8). Furthermore, class II gene permissiveness for the Tg species used for EAT induction also varies both for DR3 (mTg > hTg) and DQ8 (hTg > mTg) and background genes for the class II-knock-out mice (NOD > C57BL/10). Background genes were also important in demonstrating the down-regulation by DQ8 molecules on DR3-mediated, mTg-induced EAT (C57BL/10 > NOD). It is this combination of background genes from the autoimmune disease-prone NOD mouse and the differences in permissiveness of DR3 and DQ8 molecules for EAT that was examined in this study, using NaI consumption as an environmental factor to examine the effects of increased dietary iodine on disease.
Regardless of the level of NaI in the drinking water, both DR3+ and DR3+DQ8+ mice developed thyroiditis at a higher incidence and with greater severity than either DQ8+ or the DR3–DQ8– control mice. Also, in DR3+DQ8+ mice, the DQ8 molecules did not appear to down-regulate thyroiditis. These findings are similar to our previous results with mTg-induced EAT [5,6] and, in that context, are probably not surprising. These results are also consistent with other NaI studies on the NOD background with mouse class II genes present; NaI induces thyroiditis only when the mice express an EAT-susceptible class II allele, such as IAg7[9] or IAk[10,11]. Similarly, the combination of the autoimmune disease-prone background genes of the NOD mouse and the DR3 class II allele permissive for EAT is important for susceptibility to NaI-induced EAT. NaI does not induce thyroiditis in EAT-susceptible CBA/J (IAk) mice [10]. Moreover, having only background genes from the NOD mouse is also insufficient; DQ8+ Ab0/NOD mice remained resistant to NaI-induced thyroiditis (Fig. 1a).
The use of 0·05% NaI to induce EAT in NOD.H2h4 mice has led to both positive [10,11] and negative [33] results, despite the estimate that it represented ∼550 times the normal intake of iodine [34]. This led others to use 0·15% without reported toxic effects [35]. We selected 0·05% and 0·3% to ensure an effective NaI concentration for EAT induction in this human class II transgenic model. Agreeing with others [34], the iodine appeared to mediate its effects through immunological, not toxicological, mechanisms. We did not observe any signs of follicular damage or unusual appearance of cells other than mononuclear in all single DR3+ or double DR3+DQ8+ mice. Moreover, the incidence and severity of thyroiditis after 0·05% or 0·3% treatment were not significantly different. Also, in the absence of functional lymphocytes, DR3–DQ8– mice treated with 0·3% did not show signs of thyroid damage or associated inflammation (Fig. 1b). It should be noted that past studies showing toxic effects of NaI treatment were conducted in iodine-depleted spontaneous thyroiditis-prone obese strain chicken [36] and NOD mouse [37]. In contrast, our studies did not include prior iodine depletion. More importantly, the finding that depletion of CD4+CD25+ Treg cells resulted in a higher incidence and greater severity of thyroiditis (Fig. 2) is consistent with an autoimmune mechanism. Whether the NaI intake alters the pathogenicity of mTg epitopes other than those on the primary hormonogenic sites, wherein the role of iodination is secondary [29], by increasing iodotyrosyl groups [38] is currently unknown.
Despite the thyroiditis induced by increased NaI intake and Treg cell depletion, other parameters of the immune response, i.e. mTg antibody titres and cellular proliferation to mTg, were more difficult to detect. In other models of NaI-induced thyroiditis, increased iodine consumption by NOD.H2h4 mice (IAk) correlated with increased thyroiditis and higher mTg antibody levels [9–11]. However, NOD mice (IAg7), which also developed thyroiditis, did not have detectable antibody levels [9]. In our study, the DR3+ mice with positive antibody titres typically had some thyroid destruction, while some mice with thyroid destruction had little to no antibody titre. Even with PC61 treatment resulting in a greater incidence of thyroiditis and mTg antibody titres for DR3+ mice, some mice were again negative for antibodies (Fig. 2). Clearly, the varied responses are strain-dependent. Furthermore, the weak antibody titres in some mice may be due to the use of normally iodinated mTg in the ELISA. Because iodination of hTg altered its immunoreactivity for antibodies, resulting in the loss of natural epitopes and the generation of new epitopes [39], it is conceivable that the use of iodinated mTg in the ELISA may have yielded higher antibody titres.
Similarly, the use of highly iodinated mTg might facilitate the detection of spleen cell proliferation, which usually correlates with thyroid infiltration [40]. Others have also reported only weak [8,10] or no [34] cellular proliferation in NOD.H2h4 mice to mTg. Given the weak stimulus of a self-antigen and the absence of an adjuvant to boost the immune response, the lack of proliferation may have been due to the majority of Tg-specific cells being present in the thyroid and/or the draining lymph nodes, in addition to our use of normally iodinated mTg isolated from the mouse. A combined use of T cells isolated from the thyroid or draining lymph nodes and Tg-containing thyroid extracts from NaI-treated mice could increase the sensitivity of the assay either by [3H]-thymidine uptake or interferon-γ release.
Our results showing differences with NaI-induced thyroiditis in DR3+ and/or DQ8+ transgenic mice are consistent with epidemiological studies which have suggested a greater role for genetic versus environmental factors. Analysis of autopsy material from British and Japanese subjects showed a higher incidence of lymphocytic thyroiditis in British cases, and the lower incidence in Japanese cases was similar between normal and iodine-rich diets [12]. White Americans were twice as likely to have thyroiditis as black Americans, but the incidence is similar to their British counterparts despite higher iodine consumption in the United States [13]. The importance of genetic/racial factors, while evident in iodine-sufficient diets, is also important when iodine is deficient. Most studies have shown an increase in the incidence of symptoms of autoimmune thyroid disease after prophylactic iodine administration to iodine-deficient areas [41–43], again reflecting a probable genetic predisposition exacerbated by over-compensation with a more immunogenic Tg [39,44,45]. These studies, along with others in both humans and rodents (reviewed in [8,46]), suggest that those who are genetically prone to autoimmune thyroid disease may develop symptoms with a high iodine diet.
While genetics and an environmental factor such as NaI play different roles in this autoimmune disease model, alterations in Treg cell function also are important as CD4+ CD25+ Treg cells play a major role in inhibiting the autoimmune responses to mTg. We have shown that these Treg cells function to maintain self-tolerance; their prior depletion enables EAT induction in resistant strains and exacerbates thyroiditis severity in susceptible mice [19,20]. Because of the differences in class II gene permissiveness, the severity in EAT-resistant strains does not reach the level seen in EAT-susceptible strains [20,21]. Treg cells appear to suppress autoreactive T cells directly in a contact-dependent manner [47]. In EAT, this contact-dependent mechanism can be demonstrated by in vitro co-culture of purified Treg cells with mTg-primed effector cells [18]. Treg cells can be purified from normal [19,20] or mTg-tolerized mice and express forkhead box P3 (Foxp3) [18,20]. EAT-susceptible CBA/J mice are resistant to EAT induction if self-tolerance is first strengthened by elevating circulatory mTg level for 2–3 days, ostensibly by expanding naturally existing Treg cells [48,49]. However, depletion of CD4+CD25+ Treg cells abrogates this mTg-induced tolerance, thus restoring susceptibility [18]. Clearly, in our current study the loss of Treg cells alone, in the absence of an environmental trigger such as NaI consumption, was insufficient to exacerbate the low-level thyroiditis that develops on this autoimmunity-prone background with time (Fig. 2a). However, when Treg cell depletion was combined with an environmental trigger, represented by prolonged increase in NaI consumption, a greater incidence and severity of thyroiditis occurred.
After Treg cell depletion and during the 8-week NaI treatment, Treg cells would be expected to emerge gradually from the thymus to replenish the periphery. From our previous studies on the role of CD4+ and CD8+ T cell subsets in EAT pathogenesis, we observed that CD4+ T cells required almost 2 months to recover to 70% of pretreatment levels [50]. Thus, in our current study, the return of CD4+ Treg cells in anti-CD25-treated mice would be gradual and incomplete, well after NaI intake had initiated thyroid autoimmunity. We have also shown that, once thyroiditis development was under way, inhibition by Treg cells was ineffective, as mTg-activated thyroiditogenic T cells can transfer thyroiditis to mTg-tolerized mice [51]. In PC61-treated DR3+ Ab0/NOD mice, the variation in the thyroiditis incidence and severity is due more probably to the varied DR3 transgene expression.
Although these results are consistent with our previous studies on the role of Treg cells in EAT-susceptible strains, they differ from a study examining the resistance of B cell-deficient NOD.H2h4 (IAk) mice to NaI-induced EAT [52]. In that study, wild-type NOD.H2h4 mice depleted of CD25+ Treg cells beginning at 10 days of age and prior to NaI treatment (0·05% in drinking water) actually developed less thyroiditis than Treg cell-intact NOD.H2h4 mice. Anti-mTg levels were not increased. The authors suggested that the effector T cells may also have been depleted by the anti-CD25 MoAb treatment. While possible, it would not explain why we saw an increase in incidence and severity when Treg cells were depleted at 8–16 weeks of age. Another explanation for the differences between the two studies could be the NaI-induced EAT susceptibility of the mice. For example, over 90% of NOD.H2h4 mice develop thyroiditis (with destruction of ∼25% of the gland in the majority of mice) [52], compared to ∼50% of DR3+ Ab0/NOD mice (with < 20% thyroid infiltration) (see Figs 1 and 2). Because almost 100% of the NOD.H2h4 mice already have significant thyroid destruction in the absence of Treg cell depletion, it may be more difficult to demonstrate the exacerbating effects of Treg cell depletion. Differences in susceptibility could also be due to the different class II molecules (Akversus DR3), which would affect the antigenic specificity of both effector and Treg cells, as well as differences in non-major histocompatibility complex background genes between the two strains.
In summary, we have shown that differences in HLA class II transgenes, which affect mTg-induced EAT susceptibility, play a similar role in NaI-induced EAT. In particular, DR3+ Ab0/NOD mice are susceptible to NaI-induced EAT, which can be exacerbated by depletion of CD4+CD25+ Treg cells. This demonstrates the usefulness of HLA class II transgenic mice in not only evaluating the role of environmental factors, but also the consequences of immunotherapies that may hinder Treg cell function.
Acknowledgments
We are grateful to Julie Hanson and her staff for the breeding and care of mice and to Ann Mazurco for her excellent histological preparation of tissues. This work was supported in part by grant no. DK45960 from the National Institute of Diabetes and Digestive and Kidney Diseases and a grant from the St John Hospital and Medical Center (Y. M. Kong) and grant no. AI14764 from the National Institute of Allergy and Infectious Diseases (C. S. David). This work was presented in part at Immunology 2003, Denver, CO, 6–10 May 2003 (FASEB Journal 2003;17:C38).
References
- 1.Vladutiu AO, Rose NR. Autoimmune murine thyroiditis: relation to histocompatibility (H-2) type. Science. 1971;174:1137–9. doi: 10.1126/science.174.4014.1137. [DOI] [PubMed] [Google Scholar]
- 2.Kong YM, Lomo LC, Motte RW, et al. HLA-DRB1 polymorphism determines susceptibility to autoimmune thyroiditis in transgenic mice: definitive association with HLA-DRB1*0301 (DR3) gene. J Exp Med. 1996;184:1167–72. doi: 10.1084/jem.184.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marga M, Denisova A, Sochnev A, Pirags V, Farid NR. Two HLA DRB 1 alleles confer independent genetic susceptibility to Graves disease: relevance of cross-population studies. Am J Med Genet. 2001;102:188–91. doi: 10.1002/ajmg.1431. [DOI] [PubMed] [Google Scholar]
- 4.Kong YM, David CS, Lomo LC, Fuller BE, Motte RW, Giraldo AA. Role of mouse and human class II transgenes in susceptibility to and protection against mouse autoimmune thyroiditis. Immunogenetics. 1997;46:312–7. doi: 10.1007/s002510050277. [DOI] [PubMed] [Google Scholar]
- 5.Flynn JC, Fuller BE, Giraldo AA, Panos JC, David CS, Kong YM. Flexibility of TCR repertoire and permissiveness of HLA-DR3 molecules in experimental autoimmune thyroiditis in nonobese diabetic mice. J Autoimmun. 2001;17:7–15. doi: 10.1006/jaut.2001.0528. [DOI] [PubMed] [Google Scholar]
- 6.Flynn JC, Wan Q, Panos JC, et al. Coexpression of susceptible and resistant HLA class II transgenes in murine experimental autoimmune thyroiditis: DQ8 molecules downregulate DR3-mediated thyroiditis. J Autoimmun. 2002;18:213–20. doi: 10.1006/jaut.2002.0587. [DOI] [PubMed] [Google Scholar]
- 7.Vaidya B, Kendall-Taylor P, Pearce SHS. Genetics of endocrine disease: the genetics of autoimmune thyroid disease. J Clin Endocrinol Metab. 2002;87:5385–97. doi: 10.1210/jc.2002-020492. [DOI] [PubMed] [Google Scholar]
- 8.Rose NR, Bonita R, Burek CL. Iodine: an environmental trigger of thyroiditis. Autoimmun Rev. 2002;1:97–103. doi: 10.1016/s1568-9972(01)00016-7. [DOI] [PubMed] [Google Scholar]
- 9.Hutchings PR, Verma S, Phillips JM, Harach SZ, Howlett S, Cooke A. Both CD4+ T cells and CD8+ T cells are required for iodine accelerated thyroiditis in NOD mice. Cell Immunol. 1999;192:113–21. doi: 10.1006/cimm.1998.1446. [DOI] [PubMed] [Google Scholar]
- 10.Braley-Mullen H, Sharp GC, Medling B, Tang H. Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Autoimmun. 1999;12:157–65. doi: 10.1006/jaut.1999.0272. [DOI] [PubMed] [Google Scholar]
- 11.Rasooly L, Burek CL, Rose NR. Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clin Immunol Immunopathol. 1996;81:287–92. doi: 10.1006/clin.1996.0191. [DOI] [PubMed] [Google Scholar]
- 12.Okayasu I, Hatakeyama S, Tanaka Y, Sakurai T, Hoshi K, Lewis PD. Is focal chronic autoimmune thyroiditis an age-related disease? Differences in incidence and severity between Japanese and British. J Pathol. 1991;163:257–64. doi: 10.1002/path.1711630312. [DOI] [PubMed] [Google Scholar]
- 13.Okayasu I, Hara Y, Nakamura K, Rose NR. Racial and age-related differences in incidence and severity of focal autoimmune thyroiditis. Am J Clin Pathol. 1994;101:698–702. doi: 10.1093/ajcp/101.6.698. [DOI] [PubMed] [Google Scholar]
- 14.Kong YM. Animal models of autoimmune thyroiditis: recent advances. In: Weetman AP, editor. Endocrine autoimmunity and associated conditions. Dordrecht: Kluwer Academic Publishers BV; 1998. pp. 1–23. [Google Scholar]
- 15.Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 18.Morris GP, Chen L, Kong YM. CD137 signaling interferes with activation and function of CD4+CD25+ regulatory T cells in induced tolerance to experimental autoimmune thyroiditis. Cell Immunol. 2003;226:20–9. doi: 10.1016/j.cellimm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Morris GP, Kong YM. Naturally-existing CD4+CD25+ regulatory T cells: a peripheral barrier to autoimmune thyroiditis. Turk J Endocrinol Metab. 2004;8(Suppl. 1):28. [Google Scholar]
- 20.Morris GP, Yan Y, David CS, Kong YM. H2A- and H2E-derived CD4+CD25+ regulatory T cells: a potential role in reciprocal inhibition by class II genes in autoimmune thyroiditis. J Immunol. 2005;174:3111–6. doi: 10.4049/jimmunol.174.5.3111. [DOI] [PubMed] [Google Scholar]
- 21.Wei W-Z, Jacob JB, Zielinski JF, et al. Concurrent induction of antitumor immunity and autoimmune thyroiditis in CD4+CD25+ regulatory T cell-depleted mice. Cancer Res. 2005;65:8471–8. doi: 10.1158/0008-5472.CAN-05-0934. [DOI] [PubMed] [Google Scholar]
- 22.Cosgrove D, Gray D, Dierich A, et al. Mice lacking MHC class II molecules. Cell. 1991;66:1051–66. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 23.Nabozny GH, Baisch JM, Cheng S, et al. HLA-DQ8 transgenic mice are highly susceptible to collagen-induced arthritis: a novel model for human polyarthritis. J Exp Med. 1996;183:27–37. doi: 10.1084/jem.183.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham RS, Wilson SB, de Souza NF, Jr, Strominger JL, Munn SR, David CS. NOD background genes influence T cell responses to GAD 65 in HLA-DQ8 transgenic mice. Hum Immunol. 1999;60:583–90. doi: 10.1016/s0198-8859(99)00057-9. [DOI] [PubMed] [Google Scholar]
- 25.Grumet FC, Charron DJ, Fendly BM, Levy R, Ness DB. HLA-DR epitope region definition by use of monoclonal antibody probes. J Immunol. 1980;125:2785–9. [PubMed] [Google Scholar]
- 26.Giles RC, Nunez G, Hurley CK, et al. Structural analysis of a human I-A homologue using a monoclonal antibody that recognizes an MB3-like specificity. J Exp Med. 1983;157:1461–70. doi: 10.1084/jem.157.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong YM, David CS, Giraldo AAEI, Rehewy M, Rose NR. Regulation of autoimmune response to mouse thyroglobulin: influence of H-2D-end genes. J Immunol. 1979;123:15–8. [PubMed] [Google Scholar]
- 28.EIRehewy M, Kong YM, Giraldo AA, Rose NR. Syngeneic thyroglobulin is immunogenic in good responder mice. Eur J Immunol. 1981;11:146–51. doi: 10.1002/eji.1830110216. [DOI] [PubMed] [Google Scholar]
- 29.Kong YM, McCormick DJ, Wan Q, et al. Primary hormonogenic sites as conserved autoepitopes on thyroglobulin in murine autoimmune thyroiditis: secondary role of iodination. J Immunol. 1995;155:5847–54. [PubMed] [Google Scholar]
- 30.Rose NR, Twarog FJ, Crowle AJ. Murine thyroiditis: importance of adjuvant and mouse strain for the induction of thyroid lesions. J Immunol. 1971;106:698–704. [PubMed] [Google Scholar]
- 31.Kong YM. Experimental autoimmune thyroiditis in the mouse. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current protocols in immunology. New York: John Wiley & Sons, Inc.; 1996. pp. 15.7.1–15.7.16. [DOI] [PubMed] [Google Scholar]
- 32.Wan Q, Shah R, Panos JC, Giraldo AA, David CS, Kong YM. HLA-DR and HLA-DQ polymorphism in human thyroglobulin-induced autoimmune thyroiditis: DR3 and DQ8 transgenic mice are susceptible. Hum Immunol. 2002;63:301–10. doi: 10.1016/s0198-8859(02)00360-9. [DOI] [PubMed] [Google Scholar]
- 33.McLachlan SM, Braley-Mullen H, Chen C-R, Aliesky H, Pichurin PN, Rapoport B. Dissociation between iodide-induced thyroiditis and antibody-mediated hyperthyroidism in NOD.H-2h4 mice. Endocrinology. 2005;146:294–300. doi: 10.1210/en.2004-1126. [DOI] [PubMed] [Google Scholar]
- 34.Verma S, Hutchings P, Guo J, McLachlan S, Rapoport B, Cooke A. Role of MHC class I expression and CD8+ T cells in the evolution of iodine-induced thyroiditis in NOD-H2h4 and NOD mice. Eur J Immunol. 2000;30:1191–202. doi: 10.1002/(SICI)1521-4141(200004)30:4<1191::AID-IMMU1191>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Barin JG, Talor MV, Sharma RB, Rose NR, Burek CL. Iodination of murine thyroglobulin enhances autoimmune reactivity in the NOD.H2h4 mouse. Clin Exp Immunol. 2005;142:251–9. doi: 10.1111/j.1365-2249.2005.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagchi N, Brown TR, Sundick RS. Thyroid cell injury is an initial event in the induction of autoimmune thyroiditis by iodine in obese strain chickens. Endocrinology. 1995;136:5054–60. doi: 10.1210/endo.136.11.7588241. [DOI] [PubMed] [Google Scholar]
- 37.Many M-C, Maniratunga S, Varis I, Dardenne M, Drexhage HA, Denef J-F. Two-step development of Hashimoto-like thyroiditisin genetically autoimmune prone non-obese diabetic mice:effects of iodine-induced cell necrosis. J Endocrinol. 1995;147:311–20. doi: 10.1677/joe.0.1470311. [DOI] [PubMed] [Google Scholar]
- 38.Li HS, Carayanniotis G. Iodination of tyrosyls in thyroglobulin generates neoantigenic determinants that cause thyroiditis. J Immunol. 2006;176:4479–83. doi: 10.4049/jimmunol.176.7.4479. [DOI] [PubMed] [Google Scholar]
- 39.Saboori AM, Rose NR, Bresler HS, Vladut-Talor M, Burek CL. Iodination of human thyroglobulin (Tg) alters its immunoreactivity. I. Iodination alters multiple epitopes of human Tg. Clin Exp Immunol. 1998;113:297–302. doi: 10.1046/j.1365-2249.1998.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okayasu I, Kong YM, David CS, Rose NR. In vitro T-lymphocyte proliferative response to mouse thyroglobulin in experimental autoimmune thyroiditis. Cell Immunol. 1981;61:32–9. doi: 10.1016/0008-8749(81)90351-8. [DOI] [PubMed] [Google Scholar]
- 41.Tsatsoulis A, Johnson EO, Andricula M, et al. Thyroid autoimmunity is associated with higher urinary iodine concentrations in an iodine-deficient area of Northwestern Greece. Thyroid. 1999;9:279–83. doi: 10.1089/thy.1999.9.279. [DOI] [PubMed] [Google Scholar]
- 42.Premawardhana LDKE, Parkes AB, Smyth PPA, et al. Increased prevalence of thyroglobulin antibodies in Sri Lankan schoolgirls – is iodine the cause? Eur J Endocrinol. 2000;143:185–8. doi: 10.1530/eje.0.1430185. [DOI] [PubMed] [Google Scholar]
- 43.Zois C, Stavrou I, Kalogera C, et al. High prevalence of autoimmune thyroiditis in schoolchildren after elimination of iodine deficiency in northwestern Greece. Thyroid. 2003;13:485–9. doi: 10.1089/105072503322021151. [DOI] [PubMed] [Google Scholar]
- 44.Saboori AM, Rose NR, Burek CL. Iodination of human thyroglobulin (Tg) alters its immunoreactivity. II. Fine specificity of a monoclonal antibody that recognizes iodinated Tg. Clin Exp Immunol. 1998;113:303–8. doi: 10.1046/j.1365-2249.1998.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai YD, Rao VP, Carayanniotis G. Enhanced iodination of thyroglobulin facilitates processing and presentation of a cryptic pathogenic peptide. J Immunol. 2002;168:5907–11. doi: 10.4049/jimmunol.168.11.5907. [DOI] [PubMed] [Google Scholar]
- 46.Ruwhof C, Drexhage HA. Iodine and thyroid autoimmune disease in animals models. Thyroid. 2001;11:427–36. doi: 10.1089/105072501300176381. [DOI] [PubMed] [Google Scholar]
- 47.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 48.Kong YM, Okayasu I, Giraldo AA, et al. Tolerance to thyroglobulin by activating suppressor mechanisms. Ann NY Acad Sci. 1982;392:191–209. doi: 10.1111/j.1749-6632.1982.tb36108.x. [DOI] [PubMed] [Google Scholar]
- 49.Lewis M, Giraldo AA, Kong YM. Resistance to experimental autoimmune thyroiditis induced by physiologic manipulation of thyroglobulin level. Clin Immunol Immunopathol. 1987;45:92–104. doi: 10.1016/0090-1229(87)90115-2. [DOI] [PubMed] [Google Scholar]
- 50.Fuller BE, Giraldo AA, Waldmann H, Cobbold SP, Kong YM. Depletion of CD4+ and CD8+ cells eliminates immunologic memory of thyroiditogenicity in murine experimental autoimmune thyroiditis. Autoimmunity. 1994;19:161–8. doi: 10.3109/08916939408995691. [DOI] [PubMed] [Google Scholar]
- 51.Fuller BE, Okayasu I, Simon LL, Giraldo AA, Kong YM. Characterization of resistance to murine experimental autoimmune thyroiditis: duration and afferent action of thyroglobulin- and TSH-induced suppression. Clin Immunol Immunopathol. 1993;69:60–8. doi: 10.1006/clin.1993.1150. [DOI] [PubMed] [Google Scholar]
- 52.Yu S, Maiti PK, Dyson M, Jain R, Braley-Mullen HB. Cell-deficient NOD.H-2h4 mice have CD4+CD25+ T regulatory cells that inhibit the development of spontaneous autoimmune thyroiditis. J Exp Med. 2006;203:349–58. doi: 10.1084/jem.20051438. [DOI] [PMC free article] [PubMed] [Google Scholar]