Abstract
Patients with coeliac disease (CD) on a gluten-free diet may still have gastrointestinal symptoms. On clinical grounds cow's milk (CM) protein sensitivity may be suspected. Here, using rectal protein challenge, we investigated the local inflammatory reaction to gluten and CM protein in adult patients with CD in remission. Rectal challenges with wheat gluten and dried CM powder were performed in 20 patients with CD and 15 healthy controls. Fifteen hours after challenge the mucosal reaction was recorded by the mucosal patch technique with measurements of local release of neutrophil and eosinophil granule constituents; myeloperoxidase (MPO) and eosinophil cationic protein (ECP). We measured the mucosal production of nitric oxide (NO) simultaneously. Six of the patients who reacted to CM were also challenged with α-lactalbumin and casein. In 18 of 20 patients gluten challenge induced neutrophil activation defined as increased MPO release and increased NO synthesis. Ten of these 20 patients showed a similarly strong inflammatory reaction to CM challenge. Six of the CM sensitive patients were challenged with specific CM proteins: casein and α-lactalbumin. Casein, in contrast to α-lactalbumin, induced an inflammatory response similar to that produced by CM. A mucosal inflammatory response similar to that elicited by gluten was produced by CM protein in about 50% of the patients with coeliac disease. Casein, in particular, seems to be involved in this reaction.
Keywords: coeliac disease, milk hypersensitivity, rectal challenge
Introduction
A diagnosis of coeliac disease (CD) in adults relies on the presence of a structurally abnormal intestinal mucosa, followed by a clear clinical remission on a gluten-free diet [1]. An initial finding of circulating antibodies and their disappearance on a gluten-free diet further supports the diagnosis [1]. Many patients, however, do not have a total histological or clinical recovery in spite of a total gluten-free diet [2]. The two principal reasons that may be suspected when symptoms persist in spite of a gluten-free diet are (1) that there are still trace amounts of gluten in the diet and (2) that besides gluten sensitivity the patients also have a non-gluten food intolerance [3,4]. Only a few studies have focused on non-gluten dietary intolerance in coeliac patients and much of what is written and said is based on clinical experience and case reports. The only non-gluten food intolerance that is well described in CD is secondary lactose intolerance [5]. If symptoms do persist in spite of a lactose-free diet, physicians may recommend elimination of cow's milk (CM) protein or soy protein [6,7].
Food hypersensitivities/allergies might be due to IgE- or non-IgE-mediated immune mechanisms. Skin prick tests and radioallergosorbent test (RAST) analysis are rapid methods that are useful for excluding IgE-mediated food allergies. A double-blinded placebo-controlled food challenge has been considered the gold standard for diagnosing food allergy, but is not widely available and false negatives do occur [8]. The objective test procedures available for identifying a non-IgE sensitivity are excellent for gluten but have several limitations for other food antigens [9]. We have recently described the mucosal patch technique as a sensitive method for evaluating inflammatory reactions in the rectal mucosa [10]. We have used this technique for studying the mucosal reaction to gluten and we have found that neutrophil activation as defined by the luminal release of myeloperoxidase (MPO) is pronounced in CD [11]. The mucosal patch technique allows simultaneous measurement of nitric oxide (NO), another indicator of mucosal inflammation [12]. By using this technique we have made an attempt to evaluate the possible inflammatory mucosal response to CM protein as an indication of CM sensitivity in adult patients with CD.
Materials and methods
Study subjects
Twenty adult patients (six men) with CD and 15 adult healthy control subjects (10 men) were included. The mean age of the coeliac patients was 49 years (range 25–68) and that of the controls was 34 years (19–58). Criteria for a diagnosis of CD were: (a) a small bowel biopsy specimen showing total/subtotal or partial villous atrophy at the time of the diagnosis and (b) improvement of the histopathological abnormalities after a gluten-free diet. Prior to dietary treatment 17 of the CD patients had total/subtotal villous atrophy and three had partial villous atrophy. At the time of the present investigation all patients had been on a gluten-free diet for more than 2 years (range 2–22). On a gluten-free diet, small bowel biopsy results became normal in 11 of 20 patients and the other nine had partial remission. At the time of the present study, all patients had serum IgA tissue transglutaminase (tTG) and IgG/IgA gliadin antibodies within the normal range and no detectable serum IgA endomysial antibodies. The healthy controls had no gastrointestinal symptoms, no subjective CM intolerance and none had a rise in IgA antibodies to gliadin or endomysium. None of the controls had reported symptoms that could be related to CM–milk protein/lactose intolerance. One control subject had a borderline value for IgA antibodies to tTG but no other signs of CD and a normal duodenal biopsy. Serum IgE-antibodies to CM or wheat proteins and IgA and IgG antibodies to casein and α-lactalbumin were measured in accordance with the manufacturer's instructions (Pharmacia Diagnostics AB, Uppsala, Sweden).
All subjects underwent rectal challenge with gluten and CM, and mucosal measurements were performed before and based on our previous kinetic studies after gluten challenge [11] 15 h after challenge. All participants were asked about CM allergy or intolerance before the measurements.
The ethics committee of the Medical Faculty, Uppsala University, approved the study. All subjects gave informed consent to participation.
Rectal challenge
The CD patients and control subjects were challenged with wheat gluten 6·2–6·5 g (crude wheat gluten, Sigma Chemical Co., St Louis, MO, USA) and dried milk powder 6·2–6·5 g (Semper AB, Stockholm, Sweden) suspended in 25 ml of 0·9% NaCl solution. The suspension was instilled into the rectum with a syringe with the participant lying in the left lateral position. The subjects were then allowed to move about as they wished and were instructed to retain the enema for at least 60 min. Rectal challenge was performed between 4 and 6 p.m. and measurements were made 15 h later, between 7 a.m. and 9 a.m. The subjects were told to fast for 1 h before and 1 h after the challenge and also from midnight before the measurements.
Six of the 10 patients who had a mucosal inflammatory reaction after to CM challenge were challenged with specific milk proteins in amounts proportional to their concentrations in 6·5 g dried CM powder. Thus, six patients were challenged with 1·9 g casein from CM with the normal milk proportions of α- and α-casein milk protein (Sigma Chemical Co.). Five of these patients were also challenged with 0·2 g of α-lactalbumin (Sigma Chemical Co.).
Mucosal evaluation
To elucidate the occurrence of mucosal inflammation after the rectal challenge with CM, casein and α-lactalbumin and gluten we measured MPO, eosinophil cationic protein (ECP) and NO, using the mucosal patch technique (Fig. 1) and in accordance with the procedure described previously [10,12]. The samples obtained were frozen at −70°C until analysed in duplicates using radioimmunoassay (RIA) and later an enzyme-linked immunosorbent assay (ELISA) to measure the concentrations of MPO and ECP according to the manufacturer's instructions (Pharmacia Diagnostics AB). The mean value of the duplicate measurements was used for calculations and presentations. ELISA and RIA results correlated well (r = 0·997 for MPO and r = 0·989 for ECP). NO was measured with a chemiluminescence NO analyser (model Sievers NOA 280; Ionics Instrument Business Group, Boulder, CO, USA). The calibration of the system, collection and analysis of samples were performed as described previously [12].
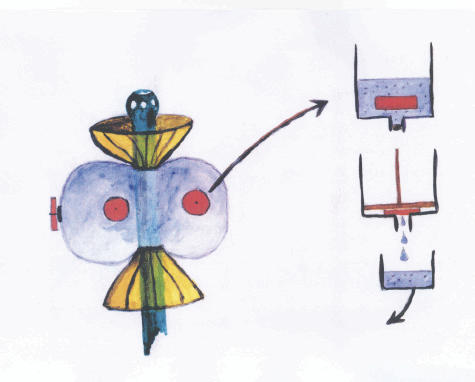
Fig. 1.
The mucosal patch technique for measurement of the inflammatory condition of the rectal mucosa is illustrated. The instrument used is a plastic catheter with a silicon balloon at the end of the catheter, with three patches of highly absorptive cellulose material attached to the balloon. The figure illustrates when the instrument is positioned in the rectal ampulla and the balloon is inflated with air (60–80 ml), allowing the patches to be in contact with the mucosa. After 20 min the balloon is deflated and the air is collected in glass syringe for analysis of nitric oxide (NO). After removal of the catheter the patches are cut off and immediately placed in 2 ml of 0·3% N-cetyl-N,N,N-trimethyl ammonium bromide (CTAB) to extract the contents.
Statistics and calculations
The results are presented as mean ± standard error of the mean (s.e.m.) and range within brackets unless otherwise stated. The Mann–Whitney U-test (between groups), sign test (within groups) and Spearman's rank correlation were used for statistical calculations.
Results
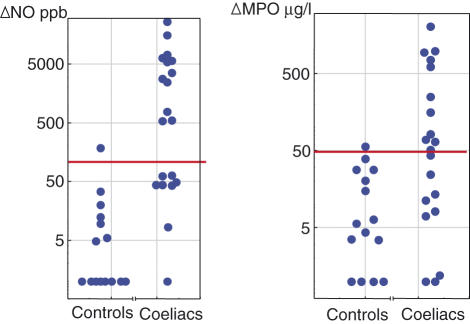
Compared to healthy controls, patients with CD showed significant increases in rectal NO and MPO concentrations measured 15 h after challenge with both CM and gluten (P < 0·001), while ECP was increased to a similar extent in the two groups (Table 1). Figure 2 illustrates the individual increases in NO and MPO after CM challenge. Twelve of the coeliac patients had an increase in NO (ΔNO) of more than 500 parts per billion (ppb) after rectal milk challenge and these increases were clearly above the mean ΔNO ± 2 standard deviations (s.d.) level (123 ppb) in the controls. Eleven patients had ΔMPO values above the mean ΔMPO ± 2 s.d. level (49 µg/l) in the controls. Ten of the patients had an increase in both ΔMPO and ΔNO. After gluten challenge 19 of the patients showed significant increases in rectal ΔNO and 18 patients had significant increases in ΔMPO. In the CD group the rectal NO and MPO values correlated both after CM (r = 0·73, P < 0·001) and gluten challenges (r = 0·54, P < 0·05). No correlation was found between ECP and the other variables after challenge.
Table 1.
Mean changes ± standard error of the mean (s.e.m.) in rectal luminal nitric oxide [ΔNO, parts per billion (ppb)] and granulocyte granule constituents [rectal mucosal concentration of myeloperoxidase (MPO) (ΔMPO) and eosinophil cationic protein (ECP) ΔECP, µg/l] in patients with coeliac disease (n = 20) and controls (n = 15) 15 h after rectal challenges with gluten and cow's milk.
| Gluten challenge | Cow's milk challenge | |||||
|---|---|---|---|---|---|---|
| ΔMPO | ΔECP | ΔNO | ΔMPO | ΔECP | ΔNO | |
| Control subjects | 8 ± 2 | 12 ± 16 | −10 ± 6 | 13 ± 5 | 8 ± 7 | 5 ± 15 |
| Coeliac patients | 211 ± 47*** | 7 ± 5 | 6474 ± 1808*** | 299 ± 117*** | 19 ± 7 | 3893 ± 1474*** |
P < 0·001, Mann–Whitney U-test, for comparison between groups.
Fig. 2.
Increase in rectal luminal nitric oxide (ΔNO) and rectal mucosal concentration of myeloperoxidase (MPO) (ΔMPO) in patients with coeliac disease (n = 20) 15 h after rectal milk challenge. The level of two standard deviations (s.d.) above mean of the control subjects (n = 15) is marked by a line.
Subjective milk intolerance in relation to rectal challenge results
Gastrointestinal symptoms, subjective intolerance to CM and positive reaction after rectal CM challenge are compared to the histological findings of the small bowel (Table 2) in our coeliac patients. Before the challenge studies, seven of the 20 coeliac patients reported symptoms which they related to CM intake. Two of these patients had excluded only lactose from the diet and one of them had a confirmed lactose intolerance. Five patients claimed that they excluded all food containing CM protein. Four of these seven patients who attributed their symptoms to CM or CM protein had significant increases in both MPO and NO after CM challenge. The other 13 patients had not suspected that CM could contribute to their symptoms and had not excluded lactose or CM protein from the diet. Five of them showed a significant increase in both NO and MPO. Patients with incomplete histological remission had significantly higher NO increase than those with total remission, 4933 ± 1527 versus 3038 ± 2413 ppb (P < 0·05). All controls tolerated CM.
Table 2.
Histology of the small bowel, gastrointestinal symptoms at the investigation and subjective tolerance to cow's milk (CM) in coeliac patients on gluten-free diet. Those patients who before the present investigation had excluded CM/CM protein from the diet are indicated. Those patients who had a positive reaction to rectal CM challenge are also presented.
| Patient no. | Histology after gluten-free diet | GI symptoms at the investigation | Subjective tolerance to CM before tests | CM/CM protein excluded before tests | Positive reaction to CM rectal challenge |
|---|---|---|---|---|---|
| 1 | Increased number of IEL | Yes | T | No | Yes |
| 2 | Increased number of IEL | Yes | T | No | Yes |
| 3 | Increased number of IEL | No | T | No | No |
| 4 | Normal | No | T | No | No |
| 5 | Normal | No | T | No | No |
| 6 | Partial villous atrophy | No | T | No | Yes |
| 7 | Normal | No | T | No | No |
| 8 | Normal | Yes | IT | Yes | No |
| 9 | Normal | No | T | No | Yes |
| 10 | Increased number of IEL | No | IT | Yes | Yes |
| 11 | Partial villous atrophy | Yes | IT | Yes | Yes |
| 12 | Partial villous atrophy | Yes | IT | Yes | Yes |
| 13 | Partial villous atrophy | Yes | IT | Yes | Yes |
| 14 | Normal | Yes | IT | Yes | No |
| 15 | Normal | Yes | IT | Yes | No |
| 16 | Normal | Yes | T | No | No |
| 17 | Normal | Yes | T | No | No |
| 18 | Normal | Yes | T | No | No |
| 19 | Normal | Yes | T | No | Yes |
| 20 | Partial villous atrophy | No | T | No | No |
IEL = intraepithelial lymphocytes, GI = gastrointestinal, T = tolerant, IT = intolerant.
IgA and IgG antibodies to CM proteins
The mean serum levels of IgA and IgG antibodies to gliadin, casein, and α-lactalbumin were not higher in the patients with CD than in the controls. Neither did those patients who reacted with mucosal inflammation (defined as a combined increase in MPO and NO) after challenge have different antibody levels compared to those with no reaction (Table 3). RAST-test for IgE antibodies (Pharmacia Diagnostics AB) for CM or wheat proteins were negative in patients and controls.
Table 3.
Mean serum levels ± standard error of the mean (s.e.m.) of IgA and IgG antibodies to gliadin, casein and α-lactalbumin in control subjects and patients with coeliac disease, with and without cow's milk sensitivity defined by the inflammatory response to rectal challenge with cow's milk.
| Antibodies | Coeliac disease (n = 20) | Milk sensitive (n = 10) | Non- milk sensitive (n = 10) | Controls (n = 15) |
|---|---|---|---|---|
| IgG anti-gliadin kU/l | 4·5 ± 0·7 | 3·5 ± 0·6 | 5·9 ± 1·5 | 5·1 ± 1·4 |
| IgA anti-gliadin kU/l | 25·0 ± 1·9 | 26·4 ± 2·9 | 23·1 ± 2·3 | 20·4 ± 1·8 |
| IgG anti-casein mg/l | 12·0 ± 1·9 | 10·2 ± 2·0 | 12·6 ± 4·1 | 7·1 ± 1·8 |
| IgA anti-casein mg/l | 2·3 ± 0·3 | 2·0 ± 0·3 | 2·7 ± 0·8 | 2·8 ± 0·8 |
| IgG anti-α- lactalbumin mg/l | 3·8 ± 0·7 | 3·5 ± 0·6 | 4·3 ± 1·6 | 2·4 ± 0·2 |
| IgA anti-α-lactalbumin mg/l | 1·2 ± 0·1 | 1·2 ± 0·1 | 1·1 ± 0·1 | 1·2 ± 0·1 |
Challenge with casein and α-lactalbumin
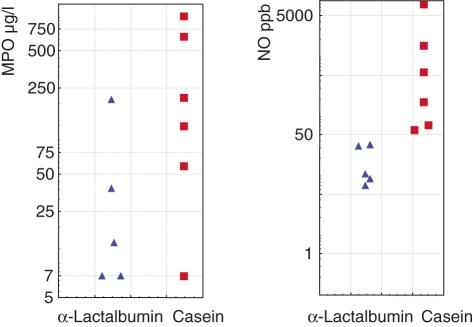
Six patients reactive to CM accepted further investigation and were challenged with casein and five of them also accepted challenge with α-lactalbumin (Fig. 3). All showed an increase in MPO and/or NO in responses to casein and one patient had a significant increase in ECP. Mean rectal ΔMPO was 303 ± 27 µg/l after casein challenge and 16 ± 27 µg/l after challenge with α-lactalbumin. Mean ΔNO was 1697 ± 1242 ppb after casein challenge and 1 ± 9 ppb after α-lactalbumin challenge. Five of six patients reported subjective clinical improvement of symptoms (diarrhoea, abdominal distension/pain or tiredness) after being informed about the results and excluding CM protein from their diet. The subjective improvement was reported to have begun after about 6 weeks.
Fig. 3.
Six coeliac patients reactive to cow's milk protein accepted further investigation and were challenged with casein and five of them also accepted challenge with α-lactalbumin. The figure illustrates rectal luminal nitric oxide (NO) and rectal mucosal concentration of myeloperoxidase (MPO) 15 h after rectal challenge with casein and α-lactalbumin.
Discussion
The major finding in this study is that rectal challenge with CM protein frequently induced a local inflammatory mucosal reaction in patients with CD but not in healthy controls. We have recently described the kinetics of granulocyte activity after rectal gluten challenge in CD [11]. We have also reported that NO production is very pronounced after gluten challenge in coeliac patients and probably as a result of activation of the major inducible isoform of NO synthase, NOS II a [12]. These findings after gluten challenge were confirmed in the present study. Gut mucosal granulocyte activation, defined as MPO release, precedes NO production in coeliac patients challenged with gluten but 15 h after challenge we found both an MPO and NO response [12]. On the basis of this knowledge we designed the timing of post-challenge measurements in the present study. Ten of our 20 patients showed abnormal increases in both MPO and NO as a reaction to CM challenge, but no increase in ECP, indicating the absence of eosinophil activation at least 15 h after challenge.
The jejunal mucosal lesion in untreated CD forms a continuum, with the classical flat lesion at one end of the spectrum and a mucosa with apparently normal architecture but with increased villous lymphocytes at the other end. On a gluten-free diet a return to normal histological features is common in children, while only in about half of adults does this diet lead to complete recovery of the jejunal mucosa [2]. Our 20 patients were already on a gluten-free diet at the time of the present study and nine of them had persistent minor mucosal abnormalities. Failure to normalize the mucosa has been attributed to the fact that complete elimination of gluten is very difficult to achieve and maintain. The trace amounts of gluten allowed according to the Codex Alimentarius Standard for gluten-free foods have also been suggested as a cause of this lack of total recovery [3]. However, others have claimed that persistent mucosal abnormalities in CD are not related to the ingestion of trace amounts of gluten [13]. but have proposed that other food components, especially CM proteins, may induce food reactivity in some coeliac patients [3,4,6,7].
CM has a high nutritional value and is one of the most commonly consumed foods worldwide. Nevertheless, adverse reactions to CM are frequently reported and are attributed mainly to lactase deficiency or allergy to CM proteins [14]. As with food allergy in general, CM allergy may be divided into IgE-mediated and non-IgE-mediated food allergy.
Non-IgE food allergies are more difficult to evaluate, both clinically and in the laboratory, and require procedures with food elimination and food challenges [9,15]. Gluten enteropathy is the best characterized non-IgE food allergy with a food protein-induced enteropathy. CM protein may also induce a non-IgE enteropathy, which is considered to be a transient condition in early childhood but may persist or manifest itself in older children [16]. The histopathological features of the small intestine in CD are often prominent, while the histological inflammatory findings in CM protein-sensitive enteropathy are more discrete, with normal villous architecture [16].
The major food antigens in CD are gliadin and similar prolamines from rye and barley. In active disease increased serum antibodies not only against gliadin but also against CM proteins are seen [17]. However, direct evidence for CM protein allergy in CD is lacking. Most exposed healthy individuals have low levels of antibodies against various food antigens [17,18]. The probably explanation of this physiological phenomenon is that a small fraction of food proteins passes undegraded across the gut barrier [19], and thereby presents to the immune system with subsequent production of antibodies. Certain diseases are characterized by enhanced antibody production against dietary antigens. The elevated levels of IgG and IgA anti-CM protein antibodies observed, for example, in CD [20] and inflammatory bowel disease [21] could be related to the damaged intestinal mucosa, causing increased penetration by undegraded proteins [22]. Our patients with CD had normal serum levels of IgA, IgG and IgE against casein and α-lactalbumin, which might be explained by the fact that they were on a gluten-free diet and therefore had improved the mucosal integrity.
Tissue transglutaminase 2 has been identified as the autoantigen in CD [23] and IgA anti-tTG autoantibodies are a very sensitive marker for this diagnosis [24]. Both the expression of CD and the presence of serum antibodies to tTG are strictly dependent on dietary exposure to gluten [23,24]. Extracellular tTG can form complexes to glutamine-rich proteins, particularly gluten from wheat, in which glutamine constitutes about 40% of the amino acids. Casein is also glutamine-rich and is known to bind to tTG [25]. The observation that the production of an autoantibody is dependent on the intake of a dietary protein such as gluten may seem confusing, but has been proposed to reflect antibodies directed against cross-links between gliadin and tTG [25,26]. Antibodies to gliadin seem to be an epiphenomenon in CD and the pathophysiological role of autoantibodies to tTG remains unclear. Our results in coeliac patients on a gluten-free diet demonstrate that gluten and casein challenges induce a gut mucosal inflammation by pathways until now not identified. It has been demonstrated recently that certain gluten peptides elicit not only an adaptive but also an innate immune response [27–29]. The innate immune system provides an early, so-called pattern-recognition response to various tissue-damaging agents, e.g. viral proteins and bacterial DNA. In individuals with the genetic prerequisites, an innate response to normally harmless dietary proteins might precede and enhance adaptive immunity to such proteins. Activation of the adaptive immune system is one prerequisite for the occurrence of CD and is reflected by the development of gliadin antibodies and auto antibodies. Our finding that, in a fraction of coeliac patients, CM protein challenge may induce an inflammatory reaction of the same magnitude, as did gluten challenge, may also suggest an innate as well as adaptive immune response to CM, and casein in particular. However, lack of increased serum antibodies to casein in our casein-sensitive coeliac patients may suggest that casein is less prone than gliadin to drive adaptive immunity. There are certain similarities between gliadin and casein: both proteins are chemotactic to human leucocytes [30,31] and are stable to digestion because of their content of proline [32]. They also show certain amino acid sequence homologies [33]. CD is associated strongly with certain human leucocyte antigen (HLA) class II alleles, 90% of the patients being HLA DQ2 [25] Additional genes may relate to the recently observed innate immune reactivity to certain gliadin peptides [34]. In the light of the hypothesis that the casein sensitivity found in CD also reflects an innate response, we have to consider the possibility that sensitivity to gluten and casein share common genes related to innate immunity.
In conclusion, our data raise the possibility that sensitivity to CM may be a feature in a proportion of patients with CD and may therefore contribute to persistent symptoms in coeliac patients who are on a gluten-free diet. The finding that casein, but not α-lactalbumin, induced an inflammatory response similar to that produced by CM identifies casein as one candidate behind the observed reaction to CM. Casein has also been suggested as an environmental trigger of other autoimmune disorders [35–37]. However, with the data available we cannot assert that the sensitization to CM protein is a specific pathogenic mechanism operating in a significant proportion of patients with CD. The possibility remains that patients with CD are sensitized to a broad range of dietary proteins. Nevertheless, we have shown a possible way to identify food antigen sensitization, which could be a basis for future proper studies of the possible beneficial effect of food antigen elimination on symptoms and mucosal histology in patients with coeliac disease and persistent symptoms on a gluten-free diet.
Acknowledgments
We acknowledge the technical assistance of Mrs Sneh Ajuha, Mrs Kerstin Lindblad and Mrs Åsa Lidman. This work was supported by the Medical Faculty of the University of Uppsala, Sweden, Pharmacia Diagnostics AB, Uppsala, Sweden, Alimenta Diagnostics AB, Uppsala, Sweden and by the Vardal Foundation − the Swedish Foundation for Health Care Sciences and Allergy Research.
References
- 1.Walker-Smith JAGS, Schmitz J, Schmerling DH, Visakopri JK. Revised criteria for diagnosis of coeliac disease. Arch Dis Child. 1990;65:909–11. doi: 10.1136/adc.65.8.909. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grefte JM, Bouman JG, Grond J, Jansen W, Kleibeuker JH. Slow and incomplete histological and functional recovery in adult gluten sensitive enteropathy. J Clin Pathol. 1988;41:886–91. doi: 10.1136/jcp.41.8.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faulkner-Hogg KB, Selby WS, Loblay RH. Dietary analysis in symptomatic patients with coeliac disease on a gluten-free diet: the role of trace amounts of gluten and non-gluten food intolerances. Scand J Gastroenterol. 1999;34:784–9. doi: 10.1080/003655299750025714. [DOI] [PubMed] [Google Scholar]
- 4.Baker AL, Rosenberg IH. Refractory sprue: recovery after removal of nongluten dietary proteins. Ann Intern Med. 1978;89:505–8. doi: 10.7326/0003-4819-89-4-505. [DOI] [PubMed] [Google Scholar]
- 5.Plotkin GR, Isselbacher KJ. Secondary disaccharidase deficiency in adult celiac disease (nontropical sprue) and other malabsorption states. N Engl J Med. 1964;271:1033–7. doi: 10.1056/NEJM196411122712003. [DOI] [PubMed] [Google Scholar]
- 6.Ament ME, Rubin CE. Soy protein − another cause of the flat intestinal lesion. Gastroenterology. 1972;62:227–34. [PubMed] [Google Scholar]
- 7.Walker-Smith J, Harrison M, Kilby A, Phillips A, France N. Cows' milk-sensitive enteropathy. Arch Dis Child. 1978;53:375–80. doi: 10.1136/adc.53.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freed DL. False-negative food challenges. Lancet. 2002;359:980–1. doi: 10.1016/S0140-6736(02)08006-6. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff S, Crowe SE. Gastrointestinal food allergy: new insights into pathophysiology and clinical perspectives. Gastroenterology. 2005;128:1089–113. doi: 10.1053/j.gastro.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Kristjansson G, Venge P, Wanders A, Lööf L, Hällgren R. Clinical and subclinical intestinal inflammation assessed by the mucosal patch technique: studies of mucosal neutrophil and eosinophil activation in inflammatory bowel diseases and irritable bowel syndrome. Gut. 2004;53:1806–12. doi: 10.1136/gut.2003.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristjánsson GSJ, Lööf L, Venge P, Hällgren R. The kinetics of mucosal granulocyte activation after gluten challenge in coeliac disease. Scand J Gastroenterol. 2005;40:662–9. doi: 10.1080/00365520510015566. [DOI] [PubMed] [Google Scholar]
- 12.Kristjansson G, Högman M, Venge P, Hällgren R. Gut mucosal granulocyte activation precedes nitric oxide production: studies in coeliac patients challenged with gluten and corn. Gut. 2005;54:769–74. doi: 10.1136/gut.2004.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selby WS, Painter D, Collins A, Faulkner-Hogg KB, Loblay RH. Persistent mucosal abnormalities in coeliac disease are not related to the ingestion of trace amounts of gluten. Scand J Gastroenterol. 1999;34:909–14. doi: 10.1080/003655299750025390. [DOI] [PubMed] [Google Scholar]
- 14.Bahna SL. Cow's milk allergy versus cow milk intolerance. Ann Allergy Asthma Immunol. 2002;89:56–60. doi: 10.1016/s1081-1206(10)62124-2. [DOI] [PubMed] [Google Scholar]
- 15.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113:805–19. doi: 10.1016/j.jaci.2004.03.014. quiz 820. [DOI] [PubMed] [Google Scholar]
- 16.Kokkonen J, Haapalahti M, Laurila K, Karttunen TJ, Maki M. Cow's milk protein-sensitive enteropathy at school age. J Pediatr. 2001;139:797–803. doi: 10.1067/mpd.2001.118882. [DOI] [PubMed] [Google Scholar]
- 17.Falth-Magnusson K, Jansson G, Stenhammar L, Magnusson KE. Serum food antibodies analyzed by enzyme-linked immunosorbent assay (ELISA) and diffusion-in-gel (DIG) − ELISA methods in children with and without celiac disease. J Pediatr Gastroenterol Nutr. 1994;18:56–62. doi: 10.1097/00005176-199401000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Labrooy JT, Hohmann AW, Davidson GP, Hetzel PA, Johnson RB, Shearman DJ. Intestinal and serum antibody in coeliac disease: a comparison using ELISA. Clin Exp Immunol. 1986;66:661–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Husby S, Foged N, Host A, Svehag SE. Passage of dietary antigens into the blood of children with coeliac disease. Quantification and size distribution of absorbed antigens. Gut. 1987;28:1062–72. doi: 10.1136/gut.28.9.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hvatum M, Scott H, Brandtzaeg P. Serum IgG subclass antibodies to a variety of food antigens in patients with coeliac disease. Gut. 1992;33:632–8. doi: 10.1136/gut.33.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerner A, Rossi TM, Park B, Albini B, Lebenthal E. Serum antibodies to cow's milk proteins in pediatric inflammatory bowel disease. Crohn's disease versus ulcerative colitis. Acta Paediatr Scand. 1989;78:384–9. doi: 10.1111/j.1651-2227.1989.tb11097.x. [DOI] [PubMed] [Google Scholar]
- 22.Scott H, Ek J, Havnen J, et al. Serum antibodies to dietary antigens: a prospective study of the diagnostic usefulness in celiac disease of children. J Pediatr Gastroenterol Nutr. 1990;11:215–20. [PubMed] [Google Scholar]
- 23.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 24.Sulkanen S, Halttunen T, Laurila K, et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology. 1998;115:1322–8. doi: 10.1016/s0016-5085(98)70008-3. [DOI] [PubMed] [Google Scholar]
- 25.Sollid LM. Molecular basis of celiac disease. Annu Rev Immunol. 2000;18:53–81. doi: 10.1146/annurev.immunol.18.1.53. [DOI] [PubMed] [Google Scholar]
- 26.Freitag T, Schulze-Koops H, Niedobitek G, Melino G, Schuppan D. The role of the immune response against tissue transglutaminase in the pathogenesis of coeliac disease. Autoimmun Rev. 2004;3:13–20. doi: 10.1016/S1568-9972(03)00054-5. [DOI] [PubMed] [Google Scholar]
- 27.Maiuri L, Ciacci C, Ricciardelli I, et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–7. doi: 10.1016/S0140-6736(03)13803-2. [DOI] [PubMed] [Google Scholar]
- 28.Tuckova L, Novotna J, Novak P, et al. Activation of macrophages by gliadin fragments: isolation and characterization of active peptide. J Leukoc Biol. 2002;71:625–31. [PubMed] [Google Scholar]
- 29.Hue S, Mention JJ, Monteiro RC, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–77. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Langner A, Christophers E. Human leukocyte chemotaxis induced by gluten. Br J Dermatol. 1977;97:460–1. doi: 10.1111/j.1365-2133.1977.tb14261.x. [DOI] [PubMed] [Google Scholar]
- 31.Lewis SL, Van Epps DE. Demonstration of specific receptors for fluoresceinated casein on human neutrophils and monocytes using flow cytometry. Inflammation. 1983;7:363–75. doi: 10.1007/BF00916301. [DOI] [PubMed] [Google Scholar]
- 32.Hausch F, Shan L, Santiago NA, Gray GM, Khosla C. Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol. 2002;283:G996–1003. doi: 10.1152/ajpgi.00136.2002. [DOI] [PubMed] [Google Scholar]
- 33.Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuppan D, Esslinger B, Dieterich W. Innate immunity and coeliac disease. Lancet. 2003;362:3–4. doi: 10.1016/S0140-6736(03)13843-3. [DOI] [PubMed] [Google Scholar]
- 35.Triolo G, Accardo-Palumbo A, Dieli F, et al. Humoral and cell mediated immune response to cow's milk proteins in Behcet's disease. Ann Rheum Dis. 2002;61:459–62. doi: 10.1136/ard.61.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monetini L, Barone F, Stefanini L, et al. Establishment of T cell lines to bovine beta-casein and beta-casein-derived epitopes in patients with type 1 diabetes. J Endocrinol. 2003;176:143–50. doi: 10.1677/joe.0.1760143. [DOI] [PubMed] [Google Scholar]
- 37.Riemekasten G, Marell J, Hentschel C, et al. Casein is an essential cofactor in autoantibody reactivity directed against the C-terminal SmD1 peptide AA 83–119 in systemic lupus erythematosus. Immunobiology. 2002;206:537–45. doi: 10.1078/0171-2985-00202. [DOI] [PubMed] [Google Scholar]