Abstract
Sulfatases are involved in several biological functions such as degradation of macromolecules in the lysosomes. In patients with multiple sulfatase deficiency, mutations in the SUMF1 gene cause a reduction of sulfatase activities because of a posttranslational modification defect. We have generated a mouse line carrying a null mutation in the Sumf1 gene. Sulfatase activities are completely absent in Sumf1−/− mice, indicating that Sumf1 is indispensable for sulfatase activation and that mammals, differently from bacteria, have a single sulfatase modification system. Similarly to multiple sulfatase deficiency patients, Sumf1−/− mice display frequent early mortality, congenital growth retardation, skeletal abnormalities, and neurological defects. All examined tissues showed progressive cell vacuolization and significant lysosomal storage of glycosaminoglycans. Sumf1−/− mice showed a generalized inflammatory process characterized by a massive presence of highly vacuolated macrophages, which are the main site of lysosomal storage. Activated microglia were detected in the cerebellum and brain cortex associated with remarkable astroglyosis and neuronal cell loss. Between 4 and 6 months of age, we detected a strong increase in the expression levels of inflammatory cytokines and of apoptotic markers in both the CNS and liver, demonstrating that inflammation and apoptosis occur at the late stage of disease and suggesting that they play an important role in both the systemic and CNS phenotypes observed in lysosomal disorders. This mouse model, in which the function of an entire protein family has been silenced, offers a unique opportunity to study sulfatase function and the mechanisms underlying lysosomal storage diseases.
Keywords: apoptosis, macrophages, sulfatase modifying factor 1
Sulfatases catalyze the hydrolysis of sulfate ester bonds from a wide variety of substrates, ranging from complex molecules, such as glycosaminoglycans (GAGs), to sulfolipids and steroid sulfates (1). Seventeen sulfatase genes are present in the human genome (2). Based on current knowledge, two main functional categories can be identified. The first category includes six sulfatases that are localized into the lysosomes and exert their enzymatic activity at an acidic pH. These are primarily involved in the catabolism of GAGs and sulfatides. The second category includes sulfatases acting at neutral pH and localized in other subcellular compartments such as the ER and the Golgi apparatus. These sulfatases are more likely to be involved in biosynthetic, rather than catabolic, pathways (2–4).
Eight known metabolic disorders are caused by the deficiency of individual sulfatase activities. These disorders are all inherited as monogenic traits and are associated with impaired desulfation of specific substrate metabolites. Six of them are lysosomal storage diseases (LSDs), including five different types of mucopolysaccharidoses (MPSs) and metachromatic leukodystrophy (5, 6), whereas the remaining two disorders are due to deficiencies of nonlysosomal sulfatases (4, 7). Although the natural substrates and metabolic pathways of sulfatases involved in human diseases have been elucidated in detail, the function of orphan sulfatases remains largely elusive (2).
In a rare autosomal recessive disorder, known as multiple sulfatase deficiency (MSD), the activity of all sulfatases is profoundly reduced (1). The phenotype of MSD patients combines, with some phenotypic variability, all of the clinical symptoms observed in each individual sulfatase deficiency (8). We and others discovered that MSD is caused by mutations in the SUMF1 gene which is involved in the posttranslational modification of sulfatases resulting in the conversion of a cysteine located in the catalytic site of sulfatases into α-formylglycine (9, 10). In mammals, SUMF1 is the only factor known to be involved in the sulfatase modification machinery and belongs to a gene family that has been highly conserved from prokaryotes to eukaryotes. Interestingly, sulfatases appear to be the only proteins undergoing this unique biochemical modification (2).
We generated Sumf1 KO mice in which, differently from MSD patients, the activities of all sulfatases are completely absent. The phenotype of these mice is severe and progressive and resembles the clinical features of patients with MSD. Massive GAG accumulation and cell vacuolization were observed in all tissues and were associated with systemic inflammation, apoptosis, and neurodegeneration.
Results
Generation of a Sumf1−/− Mouse Strain.
By searching the Baygenomics gene-trapping database, we identified an ES cell clone that was supposed to contain an insertion of the trapping vector within the Sumf1 gene. We carefully characterized both the insertion site and the Sumf1 fusion transcript in this ES cell clone. Genomic PCR [see supporting information (SI) Fig. 8 a and b] and Southern blotting (data not shown) experiments demonstrated that the trapping vector was inserted in intron 3 of the Sumf1 gene, and RT-PCR revealed that the fusion transcript was composed by the first three exons of Sumf1 fused by β-Geo mRNA (see SI Fig. 8c). This ES clone was injected into mouse blastocysts, and several chimeric animals were obtained. Germ-line transmission was obtained, and three heterozygous animals were born. Mating of these heterozygous mice generated homozygous Sumf1−/− individuals, as demonstrated by genomic PCR (SI Fig. 8b). Nineteen litters have been generated so far by crossing Sumf1+/− heterozygous mice, yielding a total of 138 mice born. Among them, 38 were Sumf1+/+ (27.5%), 62 Sumf1+/− (45%), and 38 Sumf1−/− (27.5%), as expected by Mendelian laws, indicating that the absence of all sulfatase activities is not embryonic-lethal in mice, at least in the mixed C57B6/S129J background. RT-PCR from tail mRNA demonstrated the presence of the fusion transcript in both the homozygous −/− mice and in the heterozygotes. Homozygous −/− mice totally lacked the WT Sumf1 transcript, demonstrating that they carry a null mutation (SI Fig. 8c). Identical results were obtained by using liver and brain mRNA (data not shown).
Sumf1−/− Mice Completely Lack All Sulfatase Activities.
We tested the activities of eight different sulfatases, arylsulfatase A, B, C, and E (ARSA, ARSB, ARSC, and ARSE), sulfamidase (SGSH), iduronate-2-sulfatase (IDS), N-acetylglucosamine-6-sulfatase (G6S), and N-acetylgalactosamine-6-sulfatase (GAL6S), each involved in a human disease (4–7), and of a control enzyme (β-galactosidase), in total homogenates of mouse embryonic fibroblasts (MEFs) as well as in liver, kidney, and brain samples (data not shown) from Sumf1−/− mice and found them to be completely absent (Fig. 1).
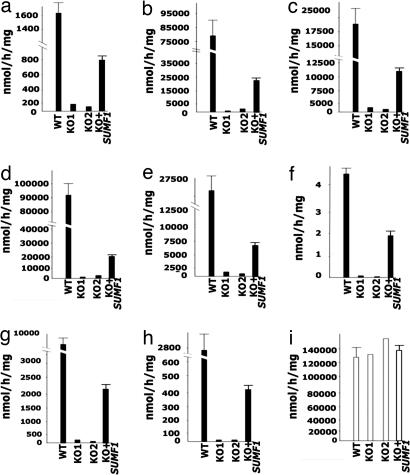
Fig. 1.
Sulfatase activities in Sumf1−/− MEFs. ARSA (a), ARSB (b), ARSC (c), ARSE (d), SGSH (e), iduronate-2-sulfatase (IDS) (f), G6S (g), GAL6S (h), and B-Gal (i) activities were measured in MEFs derived from two separate Sumf1−/− mice (KO1 and KO2) and WT littermates. Sulfatase activities are undetectable in the Sumf1−/− MEFs and are partially rescued when the cells are transduced by a lentiviral vector containing the human SUMF1 cDNA (KO+SUMF1). WT indicates average values and standard deviations of four WT mice. KO+SUMF1 indicate average values and standard deviations of two Sumf1−/− MEF lines from two different mice, each transduced in duplicate. All enzymatic activities are indicated as nmol/h/mg.
Transduction of Sumf1−/− MEFs by using a lentiviral vector containing the human SUMF1 cDNA resulted in significant rescue of the activities of all sulfatases (Fig. 1). The rescue was not complete because of the presence of several (≈70%) nontransduced cells. Sulfatase activities were also tested in Sumf1+/− heterozygous mice and found within WT ranges (data not shown).
A Severe and Progressive Phenotype in Sumf1−/− Mice.
In principle, the phenotype of Sumf1−/− mice should combine those observed in each individual sulfatase deficiency. Sumf1−/− mice display congenital growth retardation and frequent mortality in the first weeks of life, with only 10% reaching 3 months of age (Fig. 2a). We observed that smaller Sumf1−/− mice live significantly less compared with those with higher weights (P < 0.0002, see Methods) (Fig. 2b). Growth and survival rates of Sumf1+/− heterozygous mice were indistinguishable from those of WT littermates. Fig. 2 c and d shows pictures of a 40-day-old Sumf1−/− mouse and a WT littermate. Growth retardation and a flat facial profile are evident in the Sumf1−/− mouse. Starting at approximately 2 weeks of life, Sumf1−/− mice show a severe kyphosis, short limbs (Fig. 3a) and skull (Fig. 3b), loss of the spinal process of dorsal vertebrae, and joint deformities (Fig. 3 c and d). Hind limb clasping, head tremor, and seizures were also detected starting at 1 month of age, indicating neurological involvement (data not shown). All these features are important components of the phenotype observed in patients with MSD (8) and in human patients and murine models of MPSs (5–7, 11–16).
Fig. 2.
Growth, survival rates in Sumf1−/− mice. (a) Kaplan–Meyer survival curve of Sumf1−/− mice (n = 39) over 180 days. (b) Mean weight values of WT (purple) and individual weight values of Sumf1−/− mice (blue). Crosses indicate the age (days) of death of Sumf1−/− mice. WT are the mean values of four (two females and two males) mice. Significant correlation between growth and survival rates is evident. (c) Sumf1−/− mice (KO) are smaller than their WT littermates at P40. (d) Sumf1−/− mice (on the left) display the typical flat facial profile of patients affected by MPSs.
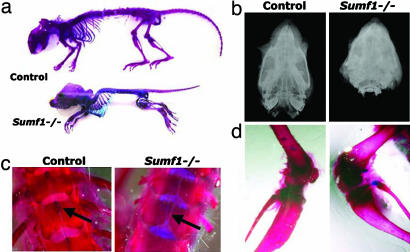
Fig. 3.
Skeletal abnormalities in Sumf1−/− mice. (a) Alizarin red- and Alcian blue-stained skeletons from a P30 WT and a Sumf1−/− mouse. Growth retardation, spinal kyphosis, reduced length of long bones, and skull deformities are evident in the Sumf1−/−mouse. (b) Facitron radiography of a P30 control mouse (Left) and a Sumf1−/− littermate (Right) shows that the Sumf1−/− skull is significantly shorter. (c) Loss of spinous process of dorsal vertebrae in P30 Sumf1−/− mice (Right) compared with control (Left). (d) Analysis of distal tibia shows enlarged epiphysis and metaphysis in Sumf1−/− mice (Right) compared with control (Left).
GAG Storage in Macrophages.
Extraordinarily high levels of GAG staining, increasing with age, were detected in liver, kidney, and heart (Fig. 4a) as well as in brain, lung, synovium, heart valves, aorta, and trachea (data not shown) from Sumf1−/− mice, indicating significant storage that is likely due to the simultaneous deficiency of several lysosomal sulfatases. Immunohistochemical analysis using MOMA-2 [monoclonal antibody against macrophages (17)] antibody revealed a massive presence of macrophages in all tissues examined from Sumf1−/− mice as early as at postnatal day (P)5, indicating the presence of a systemic inflammatory process (Fig. 4b). Interestingly, the sites of GAG storage appeared to have a similar distribution to the presence of macrophages. Immunofluorescence analysis performed in liver by using antibodies against macrophages (MOMA-2) and heparan sulfate (HS), demonstrated that macrophages are the primary site of GAG storage, thus establishing a link between storage and inflammation (Fig. 4c). Analysis of semithin sections of Sumf1−/− liver showed that, whereas vacuoles of macrophages are clear and very large in size, often filling the entire cytoplasm, hepatocytes display smaller and more closely packed vacuoles (Fig. 4 d and e). Fig. 5 shows that a significant number of macrophages was also detected in heart valves, synovium, and larynx.
Fig. 4.
GAG staining and immunohistochemical detection of activated macrophages in the visceral organs of Sumf1−/− mice. (a) GAG staining. Alcian blue-stained tissue sections of the liver, kidney, and heart from a 3-month-old WT mouse (Upper) and a Sumf1−/− littermate (Lower). Tissues from the Sumf1−/− mice show massive GAG accumulation (blue spots), which is absent in the control. Sections were counterstained with nuclear-fast red reagent. (b) MOMA-2 immunohistochemistry on liver, kidney, and heart from a 3-month-old WT mouse (Upper) and a Sumf1−/− littermate (Lower) shows the presence of macrophages in the Sumf1−/− tissues. (c) Immunofluorescence analyses of liver sections from 3-month-old Sumf1−/− mice by using MOMA-2 and heparan sulfate (HS) antibodies. Merge shows that macrophages are the primary site of GAG storage. (d) Semithin section of liver from 3-month-old Sumf1−/− mouse. Highly vacuolated macrophage (arrow) localize next to hepatocytes. Vacuoles are significantly larger compared with those observed in hepatocytes (arrowhead). (e) Semithin section of liver from 3-month-old Sumf1−/− mouse. Highly vacuolated macrophage (arrowheads) and endothelial cells (arrow) bound liver sinusoids (sin). Vacuoles are significantly larger compared with those observed in hepatocytes (Hep). [Scale bars: 20 μm (a–c); 7 μm (d); 20 μm (e).]
Fig. 5.
Inflammation in heart valves, synovium, and larynx. Immunofluorescence analyses of atrioventricular valve (a and b), synovium (c and d), and larynx (e and f) sections from 1-month-old Sumf1−/− mice and control littermates by using MOMA-2 antibodies revealed massive presence of macrophages in the tissues from Sumf1−/− mice. (Scale bars: 20 μm.)
Neuroinflammation and Neuronal Cell Loss.
Particularly strong signs of inflammation were observed in the entire CNS of Sumf1−/− mice starting at 1 month of age. Activated microglia were detected in the cerebellum, where MOMA-2 labeling revealed that macrophages are located in the Purkinje cell layer (Fig. 6a). Comparison between MOMA-2 and calbindin immunofluorescence analyses showed that macrophages establish engulfing contacts with the few survived Purkinje cells (Fig. 6b). Fig. 6c shows electron microscopy performed in the cerebellum revealing enlarged vacuoles in the cytoplasm of protoplasmic astrocytes, which have a pale nucleus with a narrow rim of heterochromatin, surrounding Purkinje cells (Fig. 6c). Consistently, significant and progressive loss of Purkinje cells was detected in Sumf1−/− mice (Fig. 6d).
Fig. 6.
Inflammation in the CNS of Sumf1−/− mice. (a) Immunofluorescence with MOMA-2 antibody reveals massive presence of activated microglia in the cerebellum of Sumf1−/− mice, particularly along the Purkinje cell layer. (b) Purkinje cells immunostained with calbindin marker (green) are surrounded by microglia. (c) Cerebellum from 3-month-old Sumf1−/− mice. Electron micrograph shows large vacuoles present in the cytoplasm of protoplasmic astrocytes (A) surrounding a Purkinje cell (P). (d) Immunostaining with calbindin shows loss of Purkinje cells in 2- and 6-month-old Sumf1−/− mice. Quantification of progressive loss of Purkinje cells in Sumf1−/− mice. Histogram shows the percentage of Purkinje cells lost in cerebellar sections of Sumf1−/− mice compared with control sections (values represent means of n = 3 mice for each group. ∗, Student's t test P < 0.05). (e) Double staining using MOMA-2 (red) and Neun (green) as macrophage and neuronal markers, respectively, shows that activated microglia are juxtaposed to neurons in the cortex of Sumf1−/− mice. Microglia staining is not detected in control mice. (f) Double staining with GFAP (green) and Neun (red) shows massive astroglyosis (GFAP-positive cells) in the cortex of Sumf1−/− mice. (g) Cortex from 3-month-old Sumf1−/− mouse. Electron micrograph of endothelial cells surrounding a blood capillary vessel (BV) contain cytoplasmic vacuoles (arrowheads). Larger vacuoles are present in the cytoplasm of a neighboring protoplasmic astrocyte (arrows). (h) Measurement of expression levels of TNFα and IL-12 cytokines and MIP1α chemokine at 2 and 6 months of age in RNA from total brain (values represent means value of three independent experiment for each group). ∗, Student's t test P < 0.05. [Scale bars: 20 μm (a, b, d–f); 2.8 μm (c); 1.8 μm (g).]
A similar situation was found in brain cortex, where macrophages completely encircle neurons (Fig. 6e). In addition, a remarkable astroglyosis was evident by the use of GFAP antibody (Fig. 6f). Ultrastructural analysis of brain cortex shows the presence of numerous slightly electron dense granules, often containing membrane remnants, filling the cytoplasm of protoplasmic astrocytes (Fig. 6g), endothelial cells and pericytes surrounding capillaries.
Consistently, an increase in the expression levels of proapoptotic inflammatory cytokines TNFα and IL-12 and of MIP1α chemokine was detected at 6 months of age in RNA from total brain (Fig. 6h). A similar increase in the expression of inflammatory cytokines was also detected in liver RNA (data not shown).
Massive Apoptosis at a Late Stage of Disease.
In situ TUNEL staining of liver sections revealed the presence of apoptotic cells, morphologically identified as hepatocytes, starting at 1 month of age and significantly increasing at 3 months when it becomes massive and generalized (Fig. 7 a–c). Similar results were also observed in the brain cortex (Fig. 7 d–f).
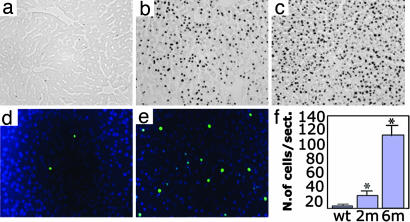
Fig. 7.
Massive apoptosis in Sumf1−/−. (a–c) In situ TUNEL analysis of liver sections of control (a), 1-month-old (b), and 3-month-old (c) Sumf1−/− mice shows a very high number of apoptotic cells in liver. (d and e) In situ TUNEL analysis of brain sections of Sumf1−/− mice shows few apoptotic cells in cerebral cortex in 2-month-old Sumf1−/− brain (d), whereas apoptosis is generalized in cerebral cortex at 6 month of age (e). (f) Apoptotic cell count in the brain cortex from 2- and 6-month-old Sumf1−/− mice and in 6-month-old control mice (values represent mean of n = 3 mice for each group; ∗, Student's t test P < 0.05).
Discussion
Mutations in the SUMF1 gene in humans results in MSD, a recessively inherited Mendelian disorder in which the activity of all sulfatases is profoundly reduced because of a posttranslational modification defect. However, a variable but significant residual sulfatase activity is detected in MSD cases (1), thus raising the possibility that another factor, in addition to SUMF1, participates to the posttranslational modification of sulfatases in mammals, similarly to what was described in bacteria (18). This putative factor may be responsible for the residual activity detected in MSD patients. We have now demonstrated that Sumf1−/− mice have a complete deficiency of sulfatase activities, thus excluding this possibility and indicating that mammals have a single sulfatase modification system. The residual sulfatase activities detected in MSD patients may be due to the hypomorphic nature of the mutations.
Animal models of individual sulfatase deficiencies are found in several species. Some of them represent spontaneous mutants, whereas others were generated as KO mice. The latter are available for five diseases due to individual sulfatase deficiencies, namely metachromatic leukodystrophy, and MPSs (i.e., MPSII, MPSIIIA, MPSIVA, and MPSVI) (11–16). In all instances the biochemical abnormalities in the mouse models replicated those observed in the corresponding human disease. However, in some cases there were differences between the clinical phenotypes of human diseases and those observed in the corresponding animal models. In principle, a Sumf1 KO mouse should recapitulate all of the features found in individual sulfatase deficiencies and, in addition, should display the potential consequences of the deficiency of all of the other sulfatases which have not been associated to a human disease yet.
The majority of Sumf1−/− mice die within the first month of age, indicating that this is a very severe condition, more than any previously described murine model of lysosomal storage diseases. Among the possible causes of death are feeding difficulties due to the severe growth deficiency and skeletal deformities. Heart insufficiency, respiratory problems and neurological impairment may represent either alternative or concomitant causes. Because of the high complexity of MSD at this point we do not know which of the many metabolic disturbances occurring in this disease play a major role in the growth deficiency and mortality observed in Sumf1−/− mice. However, it is likely that several different factors act synergistically. For example, Sumf1−/− mice are smaller than their WT littermates at birth, suggesting a role of factors acting during development. In addition, their postnatal growth curve is clearly abnormal and this maybe related to metabolic dysfunction and feeding difficulties. More detailed studies are needed to clarify these issues.
Predominant aspects of the phenotype observed in Sumf1−/− mice were growth retardation and skeletal abnormalities. Most mice displayed spinal deformities resulting in a severe kyphosis and a short and coarse skull which becomes particularly evident after the first month of life. Joint deformities were also evident in most mice. All these features are important components of the phenotype of LSDs and are observed in patients with MSD (8) and of both human patients and murine models of MPSs (5, 12–16). In addition, bone histological analysis of Sumf1−/− mice showed clear signs of defective osteogenesis (C.S., unpublished data). Growth deficiency and defective osteogenesis may also be related to arylsulfatase E (ARSE) deficiency, causing X-linked recessive chondrodysplasia punctata (CDPX) in humans (7). Further studies of Sumf1−/− mice may help identifying the metabolic pathway involved in this condition and the natural substrate of ARSE which is still unknown.
The hallmark of LSDs is the accumulation of compounds because of a block of a specific catabolic pathway causing significant cell vacuolization (19). A detailed analysis of Sumf1 KO tissue sections showed massive and generalized cell vacuolization, particularly in macrophages. However, although macrophage vacuoles appear clear and very large, often filling the entire cytoplasm, hepatocytes and neurons display smaller and more closely packed vacuoles, suggesting that there may be differences in the origin and nature of vacuoles in macrophages compared with other cell types. The activation of macrophages in LSDs may represent an attempt of the organism to remove undigested material or damaged cells (20). Macrophages were found to encircle other cell types, suggesting the presence of active phagocytosis. A striking example is the Purkinje cell layer, where microglia become the predominant cell type, and are associated with significant neuronal cell loss. Therefore, it is likely that GAG storage in macrophages derives not only from endogenous sources but also from their phagocytic activity. Furthermore, GAG storage in macrophages may be responsible for a more global lysosomal defect affecting intracellular turnover.
It is conceivable that, in LSDs, damaged cells stimulate the infiltration of macrophages. In our mice, we cannot distinguish between infiltrating and resident macrophages. However, a study performed in a murine model of Sandhoff disease demonstrated that macrophages in the brain derive from the recruitment of circulating blood monocytes, which is mediated by the MIP1α chemokine (21). Consistent with this observation we detected a significant increase of MIP1α Sumf1−/− mice. Overall, these data also demonstrate that the inflammatory response in LSDs is not linked to the presence of a specific type of lysosomal storage, because the storage material in Sandhoff disease (i.e., gangliosides) is very different from that accumulating in Sumf1−/− mice.
The precise role of inflammation in the phenotype of LSDs is still a matter of debate. MPS patients suffer from degenerative joint disease, breathing abnormalities, and heart valves insufficiency, which is one of the main causes of death in MPS VI (4). Interestingly, in Sumf1−/− mice, we found massive macrophage infiltration in all of these tissues, as observed in inflammatory diseases. For example, it is known that expansion of monocytes/macrophages in synovium is a key pathological feature of rheumatoid arthritis (22). This suggests that some features of LSDs result from inflammatory conditions similar to those observed in autoimmune diseases.
Many LSDs are associated with neurodegeneration (23), even though the pathways leading from lysosomal storage to neuronal cell damage or loss are not well understood. Previous studies described an inflammatory response in the CNS of patients and murine models of MPSs and other LSDs (24–27). Crossing a mouse model of LSD, i.e., Sandhoff disease, with a KO mouse lacking the MIP1α leukocyte chemokine, caused a reduction of macrophage infiltration, thus decreasing apoptosis and improving neurological phenotype. In addition, the treatment of this mouse model with antiinflammatory drugs resulted in phenotypic improvement (28). In Sumf1−/− mice, the elevation of proapoptotic inflammatory cytokines, as well as the presence of massive apoptosis, were observed only at late stages suggesting that inflammation-mediated apoptosis represents a final step in LSD pathogenesis.
In conclusion, silencing of the activity of the entire sulfatase protein family in Sumf1−/− mice offers the opportunity to study sulfatase function and the cascade of events leading from lysosomal dysfunction to cell damage and death in LSDs.
Methods
Generation of MEFs.
MEFs were isolated by trypsinization of littermate embryos dissected at 14 days of gestation from a cross of heterozygous SUMF1 mutant mice. Homogeneous cell suspensions were plated in six-well plates in DMEM supplemented with 20% FBS and penicillin/streptomycin.
Sulfatase Enzymatic Assays.
Experiments with MEFs were performed in early passages (p < 6). For ARSA, ARSB, ARSC, and ARSE, the enzymatic activity was tested as described (10). Iduronate 2-sulfatase, sulfamidase, N-acetylglucosamine 6-sulfatase and N-acetylgalactosamine-6-sulfatase were assayed with fluorogenic substrates. α-d-galactosidase enzymatic activity was measured by using 4-MU-α-d-galactose as a substrate. The activity was determined by incubating cell homogenates with 2 mM 4 MU-α-d-galactose in 0.5 M sodium acetate buffer (pH 5.0) in 300 μl of incubation mixture.
Infections with Lentiviral Vectors.
The human SUMF1 cDNA was cloned into the plasmid pHRcPPT.CMV. MEFs were infected by incubation for 16 h with 50 ng of p24 SUMF1 virus in 0.5 ml of fresh culture medium for 2 days before harvesting for sulfatase tests.
Genotyping of Sumf1 KO Mice.
We genotyped the mice by PCR on tail DNAs using β-gal-specific primers coupled with Sumf1-specific primers. RT-PCR was performed by using the RNeasy kit (Qiagen, Valencia, CA), followed by cDNA synthesis performed with SuperScript First Strand kit (Invitrogen, Carlsbad, CA). Animal use and analyses were conducted in accordance with the guidelines of the Animal Care and Use Committee of Cardarelli Hospital in Naples and authorized by the Italian Ministry of Health. All mice to be killed were deeply anesthetized with 100 mg/kg phenobarbital and subsequently perfused through the left ventricle with PBS.
Skeletal Staining.
Carcasses were fixed overnight in ethanol l00% and stained with 30 mg of Alcian blue (Sigma, St. Louis, MO) in 1:5 ethanol/acetic acid (final volume 100 ml) for 3 days. Bones were stained by using 1% KOH and 75 μg/ml Alizarin red S (Sigma).
Antibodies.
The antibodies used were MOMA-2 (rat anti mouse 1:250; Serotech, Ontario, Canada), GFAP (G-A-5 1:200; Sigma), NeuN (MAB377 1:100; Chemicon, Temecula, CA), anti-Calbindin-d-28K (EG-20 1:200; Sigma) anti-heparan sulfate (F58–10E4 1:200; Seigagaku, Tokyo, Japan). The secondary antibodies were from Molecular Probes (Eugene, OR) (Invitrogen).
Immunofluorescence.
Organs were fixed in 4% paraformaldehyde in PBS, pH 7.4, overnight and embedded in optimal cutting temperature compound (Tissue Tek). Cryostat sections were cut at 10 μm. The immunofluorescence was performed by using a standard protocol. Photographs were taken by using a fluorescence microscope Zeiss (Thornwood, NY) Axioplan 2 integrated with the AxioCam MR camera.
Histology and Immunohistochemistry.
Histological analyses were performed by using standard operating procedures generated by the EMPReSS platform (European Mouse Phenotyping Resource of Standardised Screens). Immunohistochemistry was performed by using Vectastain ABC kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions.
In Situ Detection of Apoptotic Cells (TUNEL).
TUNEL staining was performed by using the ApopTag Peroxidase In Situ Apoptosis Detection kit (Oncor, Gaithersburg, MD) according to the manufacturer's instructions.
GAG Staining.
GAG staining was performed on organs fixed in methacarn (methanol 60%, chloroform 30%, and acetic acid 10%) and stained with Alcian blue.
Real-Time PCR.
Real-time PCR was performed by using the iQ SYBR green supermix kit (Bio-Rad, Hercules, CA) following the manufacturer instructions and by using an iCycler iQ system (Bio-Rad).
Electron Microscopy and Semithin Sections.
For semithin sections, 0.5- to 0.7-μm sections were stained with toluidine blue. For electron microscopy, gray-silver sections were observed with a FEI CM10 or Tecnai 12G2.
Statistical Analysis.
The experiment was carried out on 16 Sumf1−/− mice. We divided the observed samples into two groups based on the survival time (Ts). Group 1 (n1 = 9) Ts was <40 days, and group 2 (n2 = 7) Ts was >40 days. For the statistical analysis, we measured the weight of each mouse at postnatal day (P)25, and then we computed an unpaired t test. The corresponding P value was 0.0002.
Student's t tests were used to compare the mean levels of three independent experiments in Figs. 6 and 7. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Consuelo Venturi for electron microscopy and Pietro De Camilli, Graciana Diez-Roux, Alessandro Fraldi, Gerard Karsenty, Alberto Mantovani, Giancarlo Parenti, Marco Sardiello, Antonio Sica, and Michèle Studer for helpful suggestions. C. Settembre is the recipient of a predoctoral fellowship of the European School of Molecular Medicine (SEMM). This work was supported by the Italian Telethon Foundation and by the Italian Ministry of Agriculture (MiPAF).
Abbreviations
- Sumf1
sulfatase-modifying factor 1
- MPS
mucopolysaccharidoses
- LSD
lysosomal storage disease
- GAG
glycosaminoglycan
- MEF
mouse embryonic fibroblast.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700382104/DC1.
References
- 1.Hopwood JJ, Ballabio A. The Metabolic and Molecular Basis of the Inherited Disease. New York: McGraw–Hill; 1997. pp. 3725–3732. [Google Scholar]
- 2.Sardiello M, Annunziata I, Roma G, Ballabio A. Hum Mol Gen. 2005;14:3203–3217. doi: 10.1093/hmg/ddi351. [DOI] [PubMed] [Google Scholar]
- 3.Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. J Biol Chem. 2002;277:49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballabio A, Shapiro LJ. The Metabolic and Molecular Basis of the Inherited Disease. New York: McGraw–Hill; 2001. pp. 4241–4262. [Google Scholar]
- 5.Neufeld EF, Muenzer J. The Metabolic and Molecular Basis of the Inherited Disease. New York: McGraw–Hill; 2001. pp. 3421–3452. [Google Scholar]
- 6.Von Figura K, Gieselmann V, Jaeken J. The Metabolic and Molecular Basis of the Inherited Disease. New York: McGraw–Hill; 2001. pp. 3695–3724. [Google Scholar]
- 7.Franco B, Meroni G, Parenti G, Levilliers J, Bernard L, Gebbia M, Cox L, Maroteaux P, Sheffield L, Rappold GA, et al. Cell. 1995;81:15–25. doi: 10.1016/0092-8674(95)90367-4. [DOI] [PubMed] [Google Scholar]
- 8.Bischel M, Austin J, Kemeny M. Arch Neurol. 1966;15:13–28. doi: 10.1001/archneur.1966.00470130017002. [DOI] [PubMed] [Google Scholar]
- 9.Dierks T, Schmidt B, Borissenko LV, Peng J, Preusser A, Mariappan M, von Figura K. Cell. 2003;113:435–444. doi: 10.1016/s0092-8674(03)00347-7. [DOI] [PubMed] [Google Scholar]
- 10.Cosma MP, Pepe S, Annunziata I, Newbold RF, Grompe M, Parenti G, Ballabio A. Cell. 2003;113:445–456. doi: 10.1016/s0092-8674(03)00348-9. [DOI] [PubMed] [Google Scholar]
- 11.Hess B, Saftig P, Hartmann D, Coenen R, Lullmann-Rauch R, Goebel HH, Evers M, von Figura K, D'Hooge R, Nagels G, et al. Proc Natl Acad Sci USA. 1996;93:14821–14826. doi: 10.1073/pnas.93.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evers M, Saftig P, Schmidt P, Hafner A, McLoghlin DB, Schmahl W, Hess B, von Figura K, Peters C. Proc Natl Acad Sci USA. 1996;93:8214–8219. doi: 10.1073/pnas.93.16.8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muenzer J, Lamsa JC, Garcia A, Dacosta J, Garcia J, Treco DA. Acta Paediatr Suppl. 2002;91:98–99. doi: 10.1111/j.1651-2227.2002.tb03115.x. [DOI] [PubMed] [Google Scholar]
- 14.Bhaumik M, Muller VJ, Rozaklis T, Johnson L, Dobrenis K, Bhattacharyya R, Wurzelmann S, Finamore P, Hopwood JJ, Walkley SU, et al. Glycobiology. 1999;9:1389–1396. doi: 10.1093/glycob/9.12.1389. [DOI] [PubMed] [Google Scholar]
- 15.Tomatsu S, Orii KO, Vogler C, Nakayama J, Levy B, Grubb JH, Gutierrez MA, Shim S, Yamaguchi S, Nishioka T, et al. Hum Mol Genet. 2003;12:3349–3358. doi: 10.1093/hmg/ddg366. [DOI] [PubMed] [Google Scholar]
- 16.Platt FM, Walkley SU. Lysosomal Disorders of the Brain. Oxford: Oxford Univ Press; 2004. pp. 257–269. [Google Scholar]
- 17.Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Proc Natl Acad Sci USA. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marquordt C, Fang Q, Will E, Peng J, von Figura K, Dierks T. J Biol Chem. 2003;24:2212–2218. doi: 10.1074/jbc.M209435200. [DOI] [PubMed] [Google Scholar]
- 19.Futerman AH, van Meer G. Nat Rev Mol Cell Biol. 2004;5:554–565. doi: 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- 20.Streit WJ, Walter SA, Pennell NA. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 21.Wu YP, Proia RL. Proc Natl Acad Sci USA. 2004;101:8425–8430. doi: 10.1073/pnas.0400625101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler DM, Malfait AM, Mason LJ, Warden PJ, Kollias G, Maini RN, Feldmann M, Brennan FM. J Immunol. 1997;159:2867–2876. [PubMed] [Google Scholar]
- 23.Platt FM, Walkley SU. Lysosomal Disorders of the Brain. Oxford: Oxford Univ Press; 2004. pp. 50–75. [Google Scholar]
- 24.Wada R, Tifft CJ, Proia RL. Proc Natl Acad Sci USA. 2000;26:10954–10959. doi: 10.1073/pnas.97.20.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myerowitz1 R, Lawson D, Mizukami H, Mi Y, Tifft CJ, Proia RL. Hum Mol Genet. 2002;15:1343–1350. doi: 10.1093/hmg/11.11.1343. [DOI] [PubMed] [Google Scholar]
- 26.Jeyakumar M, Thomas R, Elliot-Smith E, Smith DA, van der Spoel AC, d'Azzo A, Hugh Perry V, Butters TD, Dwek RA, Platt FM. Brain. 2003;126:974–987. doi: 10.1093/brain/awg089. [DOI] [PubMed] [Google Scholar]
- 27.Mizukami H, Mi Y, Wada R, Kono M, Yamashita T, Liu Y, Werth N, Sandhoff R, Sandhoff K, Proia RL. J Clin Invest. 2002;109:1215–1221. doi: 10.1172/JCI14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeyakumar M, Smith DA, Williams IM, Cortina Borja M, Neville DCA, Butters TD, Raymond A, Dwek FRS, Platt FM. Ann Neurol. 2004;56:642–649. doi: 10.1002/ana.20242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.