Abstract
The flexion reflex in human spinal cord injury (SCI) is believed to incorporate interneuronal circuits that consist elements of the stepping generator while ample evidence suggest that hip proprioceptive input is a controlling signal of locomotor output. In this study, we examined the expression of the non-nociceptive flexion reflex in response to imposed sinusoidal passive movements of the ipsilateral hip in human SCI. The flexion reflex was elicited by low-intensity stimulation (300 Hz, 30 ms pulse train) of the right sural nerve at the lateral malleolus, and recorded from the tibialis anterior (TA) muscle. Sinusoidal hip movements were imposed to the right hip joint at 0.2 Hz by a Biodex system while subjects were supine. The effects of leg movement on five leg muscles along with hip, knee, and ankle joint torques were established simultaneously with the modulation pattern of the flexion reflex during hip oscillations. Phase-dependent modulation of the flexion reflex was present during hip movement, with the reflex to be significantly facilitated during hip extension and suppressed during hip flexion. The phase-dependent flexion reflex modulation coincided with no changes in TA pre- and post-stimulus background ongoing activity during hip extension and flexion. Reflexive muscle and joint torque responses, induced by the hip movement and substantiated by excitation of flexion reflex afferents, were entrained to specific phases of hip movement. Joint torque responses were consistent with multi-joint spasmodic muscle activity, which was present mostly during the transition phase of the hip from flexion to extension and from mid- to peak extension. Our findings provide further evidence on the interaction of hip proprioceptors with spinal interneuronal circuits engaged in locomotor pathways, and such interaction should be considered in rehabilitation protocols employed to restore sensorimotor function in people with SCI.
Keywords: EMG, Interneurons, Locomotion, Paraplegia, Reflex circuits, Rehabilitation, Spasms
Introduction
The flexion reflex can be elicited via stimulation of skin, muscle, and high-threshold afferents inducing a generalized flexion of the limb. Because of the similar action of these afferents (excitation of flexors and inhibition of extensors) they were grouped under the collective term of flexion reflex afferents (FRA) (see review of Lundberg, 1979). Excitation of FRA induces two distinct responses, a short and a long reflex loop response (SRL and LRL, respectively) (Spaich et al., 2004), while their latency and amplitude depend on the stimulus intensity strength and site of stimulation (Andersen et al., 2001; Spaich et al., 2004). In spinal-intact subjects, the nociceptive flexion withdrawal reflex, which combines flight and defense movements (Sherrington, 1910), has a short central conduction time observed at latencies of 40–60 ms, while the long-latency response occurs at latencies of 85–120 ms or beyond (Shahani and Young, 1971; Hagbarth and Finer, 1963). After a spinal cord injury (SCI), the SRL response is still observable, but when non-nociceptive stimulus is employed it is rarely observed (Roby-Brami and Bussel, 1987; Knikou and Conway, 2005; Knikou et al., 2006b).
Beyond the nociceptive function of the flexion reflex, evidence derived from experiments conducted in low spinalized and decerebrated animals suggest that the spinal interneurons that belong to reflex pathways in which actions of FRA are integrated participate in the generation of spinal locomotor activity. Specifically, FRA volleys in l-DOPA (a precursor for dopamine and noradrenaline) anesthetized spinal cats induces marked changes in the neuronal activity of the spinal cord (Anden et al., 1966a) by depressing short-latency effects and inducing long lasting depolarization of Ia afferent terminals (in both flexors and extensors) (Anden et al., 1966b; Grillner et al., 1967). This reflex reorganization is even more pronounced when Nialamide is given before l-DOPA, and be present as alternating bursts of flexor and extensor activity (Jankowska et al., 1967a,b). Stimulation of FRA during walking enhances the ongoing flexion or resets the step cycle to flexion (Schomburg et al., 1998) signifying the important role of flexion reflex pathways to stepping.
A similar half-center organization to the l-DOPA induced long-latency flexion reflexes in acute spinal animals has been reported in SCI subjects, during which long-latency flexion reflexes are released without drug administration (Roby-Brami and Bussel, 1987). More specifically, contralateral FRA volleys induce inhibition of the ipsilateral TA H-reflex mediated by presynaptic inhibition of Ia transmission to alpha motoneurons, while evidence suggest that long-latency flexion reflexes in human SCI are under a reciprocal innervation (Roby-Brami and Bussel, 1990, 1992). Spontaneous alternating stepping-like movements in the lower extremities have been reported in SCI patients lying supine with extended hips (Bussel et al., 1988; Calancie et al., 1994), while imposed sinusoidal hip movements induce muscle and joint torque responses in a manner consistent with locomotion (Steldt and Schmit, 2004).
Extensive series of experiments in our laboratory have provided substantial evidence to propose that hip-mediated sensory signals interact with key spinal interneuronal circuits involved in the neural control of movement in health and disease. In people with chronic SCI, spinal reflex inhibitory mechanisms acting either on Ia afferent terminals or on alpha motoneurons are modulated in a hip-phase-dependent manner consistent to that observed during human walking (Knikou et al., 2006a). Further, imposed static hip angle changes depress the soleus H-reflex and the long-latency flexion reflex with the hip flexed and facilitate both spinal reflexes in spinal-intact subjects and in people with a motor complete or incomplete SCI (Knikou and Rymer, 2002a,b; Knikou et al., 2006b). Lastly, a graded flexion reflex modulation is observed when hip proprioceptors interact with plantar cutaneous afferents (Knikou, 2006; Knikou et al., 2006b). It is thus apparent that input from the hip region is integrated by interneuronal circuits associated with motor control. In this respect, hip position entrains the activity of flexors and extensors during fictive locomotion in spinal animals (Hiebert et al., 1996; Kriellaars et al., 1994) while similar effects have been reported in adults with SCI during partial body-weight-assisted treadmill walking (Dobkin et al., 1995; Dietz et al., 1998, 2002).
The objective of the present study was to establish the modulation pattern of the flexion reflex during imposed sinusoidal movements of the hip in a group of SCI subjects. The underlying spastic reflex effects associated with the imposed hip movement were monitored during reflex testing using measurements of ongoing EMG and joint torque responses at the hip, knee, and ankle.
Materials and methods
Subjects
The experimental protocol was approved by the Office for the Protection of Human Subjects of the Northwestern University (IRB #1236-001) and was conducted in accordance with the 1964 Declaration of Helsinki. Signed informed consent was obtained from each participant before testing. Eight subjects with lesion ranging from cervical 5 to thoracic 11 spinal segments with clinically motor incomplete and complete spinal lesions participated in this study (Table 1). These same participants also volunteered in previous studies (Knikou et al., 2006a,b; Knikou, 2005, 2006), and were identified with the same numbers. At the time of the study, half (4 out of 8) of the subjects reported taking antispastic medications.
Table 1.
SCI subjects' characteristics1
| S# | Gender | Age (years) | Post-injury (months) | Ashworth score | ASIA | Lesion level | Sensation | Spasms/Clonus | Medication per day | Range of movement (ROM) at the hip |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | M | 35 | 96 | 1 | C | C5 | Intact | Extensor crossed | – | 43° |
| S3 | M | 29 | 36 | 0 | C | C5 | Intact | Frequent | – | 45° |
| S4 | M | 47 | 78 | 2 | C | C6 | Intact | Ankle clonus | Baclofen 60 mg | 55° |
| S5 | M | 22 | 45 | 2 | C | T11 | Intact | Ankle clonus | Baclofen 20 mg | 65° |
| S7 | F | 61 | 50 | 0 | C | T9 | Intact | Spasms present | – | 57° |
| S10 | M | 52 | 210 | 1 | C | C5 | No sensation of cold/warm | Ankle clonus | Baclofen 30 mg | 55° |
| S11 | M | 52 | 186 | 3 | C | C5 | No sensation of cold/warm | Extensor spasms Ankle clonus | – | 60° |
| S12 | M | 36 | 36 | 2 | B | T8 | Intact | Severe flexor spasms | Baclofen pump | 58° |
Lesion completeness was classified according to the ASIA impairment scale (Maynard et al., 1997) with ASIA C representing a sensory and motor incomplete lesion where more than half of muscles below the injury level have strength of less than 3 out of 5, and ASIA B representing sensory incomplete but motor complete spinal cord lesion. Spasticity at the ankle was scaled according to the Ashworth scale (Ashworth, 1964). S#: subject number; F, Female; M, Male.
Experimental apparatus
The experimental apparatus used in the current study was identical to that previously employed in the same SCI subjects (Knikou et al., 2006a). With subjects supine, the right leg was secured to a leg brace with built-in single-axis torque transducers (Himmelstein Inc., Hoffman Estates, IL, USA) that were aligned with the axes of rotation for the knee and ankle joints. The leg brace was affixed to the motor head of the Biodex (System 3, Biodex Medical Systems Inc., Shirley, NY). The hip torque was measured directly from the Biodex system. The hip–knee and knee–ankle links were adjustable to accommodate each subject's leg length. The footplate of the leg brace included a clamp to hold the fore-foot and a strap to secure the heel. The pelvis was secured with a strap across the lower trunk and the contralateral leg was supported with the hip and knee semi-flexed.
Surface electromyograms (EMGs) were recorded from the tibialis anterior (TA), medialis gastrocnemius (MG), vastus medialis (VM), rectus femoris (RF), and medial hamstrings (MH) muscles through differential bipolar electrodes (DE-2.1; DelSys, MA, USA). All electrodes were placed following light mechanical abrasion of the skin and were secured via self-adhesive tape. EMG signals were band pass filtered (20–250 Hz) before being sampled at 5 kHz using a data acquisition card (National Instruments, Austin TX) and customized data acquisition programs (LabVIEW software, National Instruments, Texas, USA).
TA non-nociceptive flexion reflex elicitation and recording protocol
The flexion reflex was evoked by electrical stimulation of the sural nerve, a pure sensory nerve, according to procedures previously described in detail (Knikou et al., 2006b; Knikou and Conway, 2005). The sural nerve was stimulated at the right lateral submalleolar region through two disposable pre-gelled Ag-AgCl electrodes (Ambu Inc., Denmark). At the beginning of each test, the stimulus delivered to the sural nerve was set at 0 mA and was increased slowly so to observe the evoked response on the digital oscilloscope screen. The stimulus intensity during which the initial EMG TA activity was induced was identified as the reflex threshold (RT). This reflex response was categorized as SRL if its latency was less than 100 ms, and as LRL when its latency was beyond 120 ms (Spaich et al., 2004). Further increments in stimulus strengths were delivered so to observe if the expression of the flexion reflex changed with increments in the stimulation intensity. During testing, the sural nerve was stimulated at 1.5× RT. At this intensity level, SCI subjects with intact sensation reported no pain indicating that the test afferent volley included large muscle and cutaneous afferents.
Conditioning of the TA flexion reflex by passive sinusoidal hip movements
Sinusoidal hip movements in the sagittal plane were imposed by the Biodex system using sinusoidal pattern files from the Researcher's Toolkit (Biodex). During the sinusoidal oscillations, the hip was passively moved through 10 full cycles of the subject's unresisted flexion and extension at 0.2 Hz. At this frequency, each hip movement cycle lasted 5 s. In Table 1, the total range of movement (ROM) at the hip is indicated for each subject. Across subjects, we tried to match the ROM at the hip, but in few cases this was not possible because of an increased hip adductor tone limiting hip flexion. The right ankle and knee joints were set at 20° of plantar flexion and 30° of flexion, respectively.
With the brace secured to the right leg, the ROM at the hip was determined (Table 1), and was used to set the sinusoidal hip movement produced by the Biodex. Pattern files based on the hip ROM for each subject were used to drive the hip throughout the experiment. As the leg moved from flexion to extension, a customized LabVIEW program collected the torque, EMG, and position data. Depending on the test condition requirement, the software also delivered a trigger signal (pulse train) to a constant current stimulator (Digitimer, Hertfordshire, UK) timed with the hip flexion or extension movement. The stimulator trigger was sent either mid-extension or mid-flexion.
TA flexion reflexes elicited while the hip was extending or flexing were considered the conditioned reflexes, and were evoked only once in every hip movement cycle. These reflexes were compared to the reflex recorded in the same subject when lying supine (control reflex). The control reflexes were evoked by a 30-ms pulse train of 1-ms pulses delivered at 300 Hz once every 10 s (Knikou and Conway, 2005; Knikou et al., 2006b). Conditioned and control reflexes were recorded randomly. In every experiment, at least two control flexion reflexes were recorded. A total of three conditioned trials (10 reflexes elicited every 5 s in each trial) were recorded for each hip movement (flexion and extension) randomly across subjects.
In each experimental session, surface EMG, and joint torques were also collected during imposed hip movements (10 cycles) when the sural nerve was not stimulated. The data from the hip movement cycle during which muscles were quiescent were used as a measurement of the combined effects of inertia, gravity, and passive resistance of the limb and instrumented leg brace.
Data analysis
The flexion reflex was measured as the area under the full-wave rectified waveform, and was defined as SRL or as LRL based on their latency (SRL < 100 ms, LRL > 120 ms). The conditioned reflexes were expressed as a percentage of the mean size of the associated control reflex recorded with subjects in a relaxed supine position. For each subject, a one-way analysis of variance (ANOVA) was applied to the experimental data sets, with the hip movement condition as the independent variable and α set at 0.05. The average flexion reflex of each subject was then grouped based on the hip movement and ANOVA for repeated measures was conducted to establish differences between reflexes recorded during hip flexion and extension.
For each subject, the TA background ongoing activity was measured from the rectified EMG recordings at an interval of 50 ms before sural nerve stimulation, and 200–250 ms after sural nerve stimulation (to avoid the LRL). For both cases the mean rectified EMG was computed for a period of 50 ms. This calculation was conducted separately for reflexes elicited with the hip in mid-flexion and mid-extension. Then, the pre- and post-stimulus TA EMG activity during hip flexion was compared to the associated ones during hip extension using a paired t-test. The average of pre- and post-stimulus TA EMG activity from each subject was grouped based on the phase of the movement that the stimulus was delivered (flexion or extension) and ANOVA for repeated measures was applied to the data.
The pattern of muscle responses produced by the sinusoidal oscillation at the hip was characterized for each subject in order to establish the effects of hip movement on the timing and duration of the muscles responses when the sural nerve was either stimulated or not. For each subject and trial, the EMG data for each muscle were band-pass filtered (20–500 Hz, Butterworth 2nd order filters with no phase lag), full-wave rectified, smoothed and averaged over the 10 consecutive hip movement cycles. Then, the average EMG signal for each muscle was normalized by its average integrated area under the rectified curve, and the resultant normalized EMG was plotted as a function of time.
The joint torque data produced by the imposed sinusoidal hip oscillations during which the sural nerve was not stimulated and muscles were quiescent, were used to determine the underlying reflexive effects produced at the hip, knee, and ankle when FRA were excited during hip oscillations. For each subject, the hip, knee, and ankle joint torques from the hip movement cycle during which the sural nerve was not stimulated and muscles were quiescent, were aligned with the respective joint torques produced when flexion reflexes during hip movement were recorded. The difference between them was calculated, representing the reflexive torque response initiated by the hip movement. These signals were low pass filtered at 2 Hz and the average of the filtered data from 10 hip movement cycles was identified as the torque response for each specific joint and subject. Results are presented as mean values along with the standard error of the mean (SEM).
Results
Effects of imposed sinusoidal hip movements on the TA flexion reflex
The TA SRL response was observed only in two out of eight subjects. In the first subject (S7), this reflex was depressed during hip extension and facilitated during hip flexion reaching overall amplitudes of 55 ± 2.5% and 230 ± 8.7% of the control SRL, respectively. These effects can be clearly seen in Figs. 1Ai and Aii where the average reflex recorded during hip movement and under control conditions is indicated. In the second subject (S5), the SRL response was present only during control conditions and was not present during imposed hip movements, thus the effects of imposed passive hip movement on the SLR in this subject could not be established (data not shown graphically).
Fig. 1.
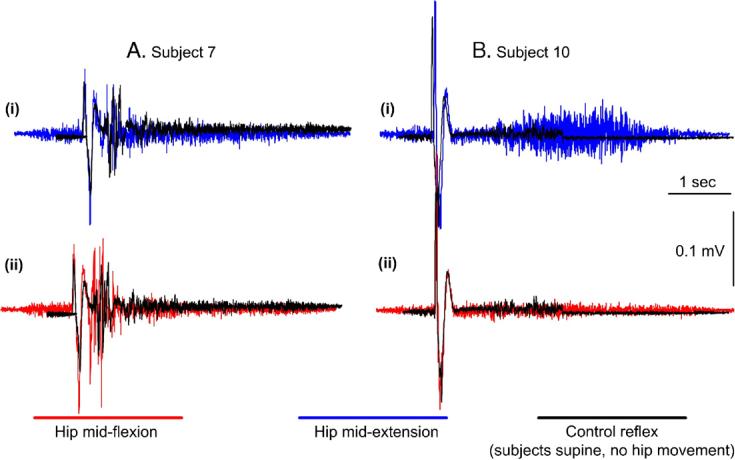
TA flexion reflex modulation during sinusoidal hip movements. For two subjects (S7 and S10) the average control (n = 10) TA flexion reflex recorded with subjects supine (no hip movement) is indicated superimposed to the reflexes recorded with the hip at mid-extension and mid-flexion. In subject 7, the short reflex loop (SRL) response was depressed with the hip at mid-extension (Ai) and facilitated at mid-flexion (Aii), while in subject 10 the long reflex loop (LRL) response was facilitated with the hip at mid-extension (Bi) and depressed at mid-flexion (Bii). In this subject (S10) the SRL was absent.
The LRL response (>120 ms) was consistently observed under all conditions in all subjects, and was present independent of whether or not a SRL response was present. Representative examples of the LRL during hip movement are illustrated in Fig. 1B. It is clear that the TA flexion reflex was remarkably facilitated (Fig. 1Bi) during hip extension and suppressed (Fig. 1Bii) (in some cases it was even completely abolished) during hip flexion.
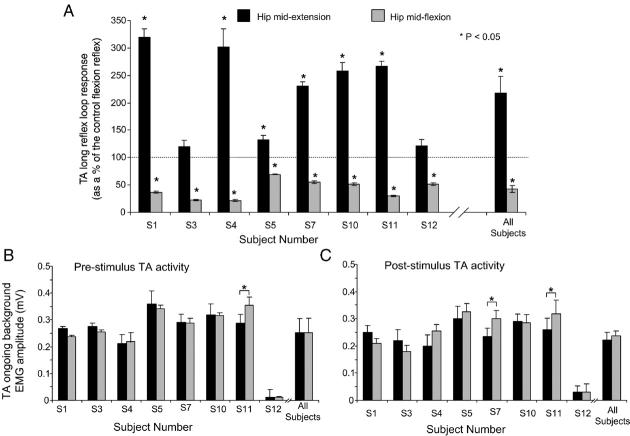
For each subject, the average (n = 30) size of the LRL response while the hip moved in flexion and extension is illustrated in Fig. 2A. Only in two subjects (S3 and S12) the LRL was not statistically significant different from control reflex values (P > 0.05). Across subjects, the LRL response was significantly different during hip flexion and hip extension. The LRL response reached an overall amplitude of 220 ± 29% and 43 ± 6% of the control long-latency flexion reflex when the hip moved in hip extension and flexion, respectively.
Fig. 2.
Effects of sinusoidal hip movements on the TA flexion reflex. (A) Average (n = 30) size of the TA long reflex loop (LRL) response as a percentage of the associated control flexion reflex during hip extension and flexion for each subject along with the population data is illustrated. Only in two subjects (S3 and S12), the reflex was not statistically significantly different from control reflex values (P > 0.05) with the hip at mid-extension. (B, C) For each subject as well as for the average from all subjects tested, the pre-stimulus TA background EMG activity measured from the rectified EMG recordings 50 ms before sural nerve stimulation for a duration of 50 ms, and the post-stimulus TA background EMG activity measured 200–250 ms after sural nerve stimulation for a duration of 50 ms is illustrated for cases where the flexion reflex was elicited while the hip was moving in flexion and in extension. For all graphs, the subject number is identified on the abscissa, and asterisks indicate cases of statistically significant differences. Error bars designate the standard error of the mean.
The hip-phase-dependent flexion reflex modulation coincided with no changes on TA ongoing background activity. The pre-stimulus TA activity was not significant different during hip extension and flexion in all but one subject (S11) reaching an overall amplitude of 0.25 ± 0.07 and 0.253 ± 0.04, respectively (P > 0.05) (Fig. 2B). In a similar way, the post-stimulus TA ongoing background did not vary significantly with changes in the direction of the hip movement, while no statistically significant differences were encountered between pre- and post-stimulus TA activity with the hip in either mid-extension or mid-flexion (P = 0.26 and P = 0.38, respectively).
Muscle and joint torque responses during sinusoidal hip movements
Imposed sinusoidal hip movements induced a reflex activation of many muscles throughout the leg, resulting in a phase-dependent modulation of EMG and joint torques responses that was qualitatively similar to those previously described in the same SCI subjects upon excitation of low-threshold flexor and extensor group I afferents (Knikou et al., 2006a).
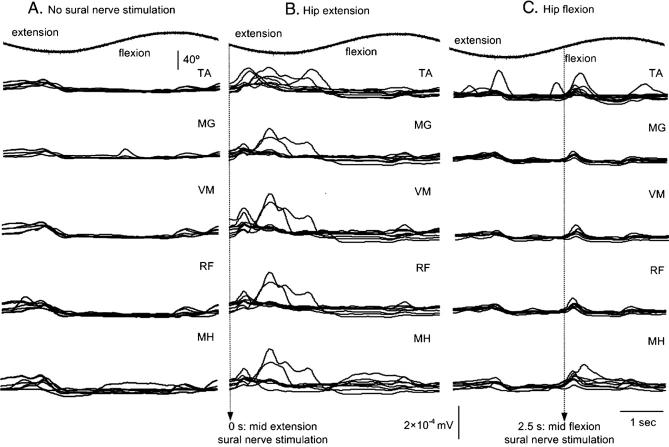
The underlying reflexly induced muscle activation patterns due to hip movement were established on the basis that they might be directly related to the modulation of the flexion reflex. The sinusoidal hip motion, as recorded by the Biodex for a single movement cycle, is shown at the top traces of Fig. 3. The time that the TA flexion reflex was elicited with respect to the phase of the hip movement is identified by vertical dotted lines in Figs. 3B and C. The underlying pattern of muscle responses across subjects when the sural nerve was not stimulated was similar to cases when FRAs were excited at either mid-extension or mid-flexion. In the former case, responses in flexors and extensors were present during the transition of the hip from flexion to extension and from mid- to peak extension (Fig. 3A). The same pattern of muscle responses, but larger in amplitude, was also observed when sural nerve was excited while the hip was moving in extension, as illustrated in Fig. 3B, suggesting that FRA excitation with the hip in mid-extension prolongs significantly the muscle responses. The increased activity in all five muscles during peak extension is consistent with the presence of spasms in human SCI. The normalized activity of all muscles when the TA flexion reflex was elicited with the hip at mid-flexion is shown in Fig. 3C. Although, muscle responses coincided mostly with the timing of the stimulus, responses were also present just before peak extension, supporting further that the EMG responses, initiated by the hip oscillation, followed a stereotyped pattern regardless the presence and/or the timing of the stimulus relative to the phase of the hip movement.
Fig. 3.
Normalized average muscle responses during sinusoidal hip movements while the sural nerve was not stimulated (A), or stimulated with the hip in extension (B) and in flexion (C). The top trace indicates the sinusoidal motion at the hip as recorded by the Biodex system. The vertical dotted lines in panels B and C indicate the position of the hip (0 s for mid-extension and 2.5 s for mid-flexion) during which the sural nerve was stimulated. It is clear that regardless the timing or the presence of the sural nerve stimulation EMG responses were modulated in a hip-phase-dependent manner. See text for further details.
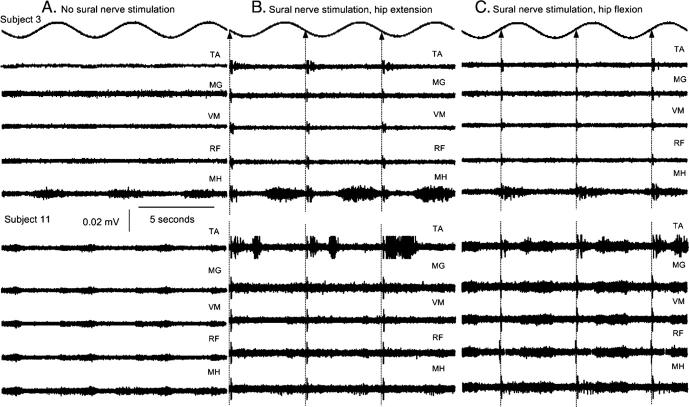
The stimulus to the sural nerve appeared to have an effect on the underlying reflex responses to passive hip oscillation as seen in Fig. 3C. However, muscle responses exhibited an oscillatory activation pattern regardless of the stimulus timing. A representative example of this activity is illustrated in Fig. 4, which represents raw muscle activity for three consecutive hip movement cycles (each cycle lasts 5 s) for two subjects (S3 and S11) when the sural nerve was not stimulated (Fig. 4A), when the TA flexion reflex was elicited with the hip at mid-extension (Fig. 4B) and at mid-flexion (Fig. 4C). Muscle responses when FRAs were not excited were smaller in amplitude but appeared in similar phases of hip movement compared to those observed when the sural nerve was stimulated. This suggests that the spasmodic muscle activity seen in Figs. 4B and C was a combined result of hip oscillation and excitation of FRA. Muscle and torque (see below) responses due to oscillation at the hip suggest an activation pattern that is consistent with involuntary spasms throughout the leg.
Fig. 4.
Raw muscle activity during sinusoidal hip movements. EMG data for two subjects (S3 and S11) are illustrated for 3 full cycles of hip movement when the sural nerve was not stimulated (A), and when the flexion reflex was elicited with the hip at mid-extension (B) and at mid-flexion (C). In the first case, muscle responses exhibited a stereotyped pattern during the transition phase of the hip from flexion to extension and from mid- to peak extension. This stereotyped muscle oscillatory activity was pronounced when flexion reflex afferents were excited, suggesting that oscillatory muscle activity was initiated by hip motion and substantiated by low-threshold muscle and cutaneous afferents of the foot.
The reflexive hip, knee, and ankle joint torque data support further that multi-joint spastic reflex activity was present during the hip oscillations, which was initiated by the imposed sinusoidal hip movement and substantiated by FRA excitation. The torque data are summarized for TA flexion reflex recordings during hip extension in Fig. 5A and for flexion in Fig. 5B. The hip and knee joints showed a stereotyped pattern of torque expression, which occurred regardless of the stimulus timing in respect to the hip movement. Hip extension torques were present when the hip had passed mid-extension, while hip flexion torques were present when the hip moved from mid-flexion to mid-extension. The hip extensor and flexor torques coincided with diametrically opposite torques at the knee. The hip and knee joint torques showed an oscillatory behavior that was most prominent when the hip had passed mid-flexion and during the transition of the hip from peak flexion to mid-extension. The behavior of the hip and knee joint torque responses might be related to spastic activity of the leg muscles, which is consistent with the expression of spasms in human SCI.
Fig. 5.
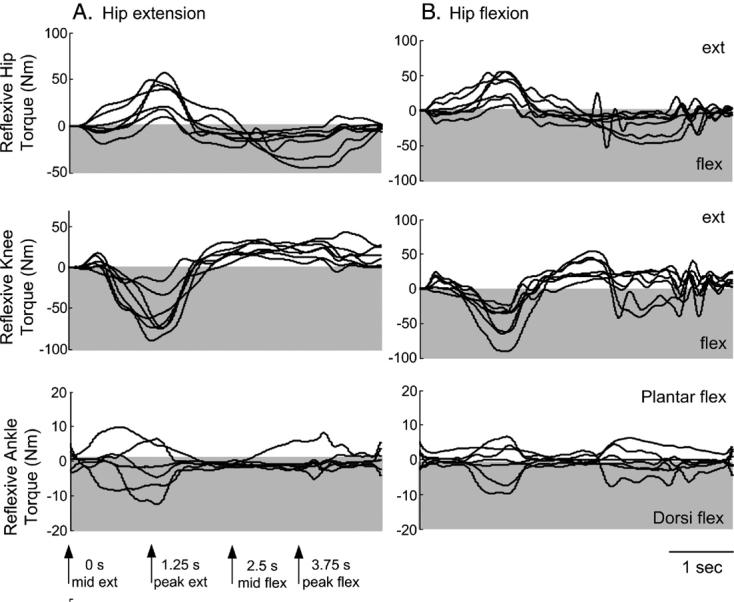
Hip, knee, and ankle reflexive torques (Nm) following excitation of flexion reflex afferents. The reflexive joint torques at the hip, knee, and ankle are indicated for trials where the flexion reflex was elicited with the hip at mid-extension (A) and at mid-flexion (B). Each waveform represents the average of 10 consecutive hip movement cycles (each cycle lasts 5 s). Arrows at the bottom of panel A indicate the position of the hip during a hip sinusoidal movement cycle (mid-extension at 0 s, peak extension at 1.25 s, mid-flexion at 2.5 s, and peak flexion at 3.75 s). For extension trials stimuli were delivered at 0 s and for flexion trials at 2.5 s. Torques above zero designate extension and those below zero designate flexion. Oscillatory torques at the hip, knee, and ankle were mostly observed during the transition phase of the hip from flexion to extension.
In general, the ankle torques were more variable compared to those observed at the hip and knee. Plantar flexor and extensor torques were present when the hip had passed mid-extension and flexion, regardless of the stimulus timing in respect to the phase of the hip movement. Ankle torques coincided with some ankle spastic activity – consistent with the TA and MG responses observed during hip movement – that was most prominent when the leg moved from flexion to extension. The joint torque responses suggest of an increased muscle activity throughout the leg during the hip oscillations.
Discussion
Interaction of FRA pathways with signals registering hip position
The TA flexion reflex was significantly depressed during hip flexion and facilitated during hip extension (Fig. 2A) in line with the reflex modulation pattern previously observed in the same SCI subjects during imposed static hip angle changes (Knikou et al., 2006b). It is thus apparent that FRA pathways are modulated in a similar manner regardless the type of the signal (static vs. dynamic) originated from the hip region. In this study, the imposed sinusoidal hip motion was delivered without a stop in either flexion or extension, providing an ongoing signal concerning the muscle stretch at the hip region. Thus, hip muscle afferents sensitive to stretch and to dynamic changes of muscle length (groups Ia and II), considered also as limb position detectors, might have mediated the observed reflex modulation.
Signals from the hip region contribute to regulation of locomotion (see review of Whelan, 1996). In the acute spinal cat preparation, long-latency flexion reflexes are generated by spinal interneurons that are strongly modulated by signals transmitting hip position (Grillner and Rossignol, 1978). In this respect, imposed flexion movements at the hip during fictive locomotion in l-DOPA/nialamide-treated spinal cats shorten flexor burst duration when applied near the end of the flexor bursts (Andersson and Grillner, 1981, 1983), while assisting hip flexion during the swing phase results in shortening the flexor burst duration (Lam and Pearson, 2001). Possible afferents account for these effects are the hip flexors (Kriellaars et al., 1994; Hiebert et al., 1996; Perreault et al., 1995; Lam and Pearson, 2002). A recent study by McVea et al. (2005) supports further the crucial role that hip-mediated sensory signals have on the walking pattern and account for the initiation of the swing-to-stance transition phase. Although the above studies were conducted in spinalized or decerebrated animals, they signify the impact of hip flexor muscle afferents to locomotor pathways in which the FRA system in SCI humans is involved (Lundberg, 1979).
In subjects with intact supraspinal drive, suppressive and facilitatory sural nerve induced responses in the TA and MG muscles are reversed depending on the gait cycle (Yang and Stein, 1990; Duysens et al., 1990). The TA responses are facilitatory during the swing phase and suppressive during the transition phase from swing to stance (Yang and Stein, 1990), mediated probably by group II muscle afferents (Van Wezel et al., 2000). A similar modulation pattern has also been demonstrated for the nociceptive flexion reflex (Spaich et al., 2004). Our current findings are in agreement with the reflex modulation pattern postulated during human walking, suggesting that the TA flexion reflex will be modulated by signals mediating hip position before swing phase commences. Reorganization of the flexion reflex during assisted walking in human SCI might be different, but given that imposed hip extension augments the swing phase during body-weight-assisted treadmill walking in human SCI (Dietz et al., 2002), it is likely that similar neuronal mechanisms and afferent systems might be involved in flexion reflex modulation during walking and imposed sinusoidal hip movements. Taken together, a possible mechanism is a hip-initiated switch between suppressive and facilitatory pathways. However, further studies examining the contribution of hip originated sensory signals to locomotion are needed in people with SCI during assisted walking.
The experimental protocol employed in this study was indirect; however, it is worth considering the possible neuronal mechanisms associated with the flexion reflex modulation. The sural nerve was stimulated at non-nociceptive levels suggesting that low-threshold sensory afferents were excited, with their effects to be gated by hip muscle afferents. Ipsilateral and contralateral sural nerve stimulation has been reported to decrease presynaptic inhibition of soleus Ia afferent terminals in spinal-intact subjects (Delwaide et al., 1981; Iles, 1996), a phenomenon that has also been described in people with motor complete SCI (Roby-Brami and Bussel, 1992). Although it is likely that presynaptic mechanisms might be involved in the reflex modulation, postsynaptic reflex actions cannot be ignored.
In this respect, one might consider the possibility that the flexion reflex modulation was mediated by changes in alpha motoneuronal excitability alone and not by actions of hip muscle afferents that influenced directly the excitability of interneurons of the FRA pathway. There is ample evidence to suggest that hip proprioceptors interacted with interneuronal circuits belonging to the FRA system to mediate these effects (Knikou et al., 2006b). While the SRL response was observed only in one subject during sinusoidal hip movements, it was suppressed during hip extension and was facilitated during hip flexion, in accordance to what we have previously observed (Knikou et al., 2006b). Given that the SRL and the LRL responses were modulated differently during imposed hip movements and that the background TA activity was not significant different during hip flexion and extension, it is unlikely that the observed effects were due to a simple increase in motoneuronal excitability.
The human flexion reflex is increased following repetitive stimulation, a phenomenon known as temporal summation. This reflex increment probably reflects integration of excitatory postsynaptic potentials at the spinal level (Price et al., 1977), and depends on the stimulation site relative to the reflex receptive field (Andersen et al., 2005). The temporal summation of the flexion reflex in humans following repeated electrical stimulation is due mainly to a summation of inputs from A-delta and C-fibers (see review of Sandrini et al., 2005). Thus, absence of this phenomenon in this study might be attributed to different stimulation protocols employed in this study compared to others (Serrao et al., 2004; Andersen et al., 2005).
On the spasms initiated by the hip movement and the involved neuronal pathways
Muscle and joint torque responses were generally modulated by hip movement in a phase-dependent manner. The underlying muscle responses were observed during flexion and extension regardless of the stimulus timing in respect to the phase of the hip movement. In a similar manner, the extensor and flexor hip torques coincided with knee flexor and extensor torques, respectively. From the reflexive joint torques produced when FRA pathways were excited with the hip at mid-flexion and extension (Fig. 5) it is clear that spasms were initiated by the hip movement.
Spasms are very common in patients with impaired supraspinal control and can be initiated by a range of stimuli including changes in body posture and excitation of muscle and cutaneous afferents. The intensity and frequency of spasms increase gradually the first 2 years after the SCI, reaching a plateau afterwards (Hiersemenzel et al., 2000). Spasms produced by unilateral extension of the hip in supine SCI subjects are mostly characterized by hip flexion, knee extension, and ankle extension torques (Schmit and Benz, 2002), similar to those observed in the current study when the LRL response was elicited with the hip in mid-extension.
It is highly likely that the spasms observed in the current study might be mediated by the reflexive muscle contractions (Fig. 3) due to hip movement and/or to the stimulus (Nickolls et al., 2004). Nonetheless, spasms have also been attributed to slowly activating voltage-dependent persistent inward currents that enable motoneurons to produce prolonged discharges in response to brief inputs (Gorassini et al., 2004; Bennett et al., 2004), which in turn are under control of descending monoaminergic systems that are impaired in people with a SCI.
Although the neurobehavioral basis of the spasms in humans is unclear, they might constitute a fragmental expression of the spinal stepping generator, and be manifested when body unloading and hip position signals are concomitantly present. Analogously, it can be suggested that the beneficial actions of body-weight-supported treadmill walking in SCI patients (Dietz et al., 1998, 2002) might be associated with a gradual decrement of spasms during this intervention. Nonetheless, further experimentation is needed to support this mechanism.
Application of findings to SCI rehabilitation
The hip-phase-dependent modulation of the TA LRL response coincided with greater joint forces compared to those observed upon excitation of flexor and extensor group I afferents (Knikou et al., 2006a), suggesting that motor output during hip movement when FRAs are excited is increased. Further, the flexion reflex is employed in rehabilitation protocols that use functional electrical stimulation to promote the swing phase during locomotion training in SCI patients (Postans et al., 2004; see review of Barbeau et al., 2002). However, when the flexion reflex is elicited with the leg unloaded, it is possible that spasms may influence motor output. Thus, stimulation protocols should take into consideration this possibility so to maximize the effects of this intervention.
Acknowledgments
Author is in debt to the subjects for their willingness to participate in all series of experiments, and wishes to thank Elizabeth Kay and Debjani Chaudhuri for their help during the experiments. This work was supported by the National Institutes of Health (NIH), National Institute of Child Health and Human Development (NICHD), Grant No. 5R03HD043951-2.
References
- Anden NE, Jukes MG, Lundberg A, Vyklicky L. The effect of Dopa on the spinal cord: 1. Influence on transmission from primary afferents. Acta Physiol. Scand. 1966a;67:373–386. doi: 10.1111/j.1748-1716.1966.tb03324.x. [DOI] [PubMed] [Google Scholar]
- Anden NE, Jukes MG, Lundberg A, Vyklicky L. The effect of Dopa on the spinal cord: 3. Depolarization evoked in the central terminals of ipsi-lateral Ia afferents by volleys in the FRA. Acta Physiol. Scand. 1966b;68:322–336. [Google Scholar]
- Andersen OK, Sonnenborg FA, Arendt-Nielsen L. Reflex receptive fields for human withdrawal reflexes elicited by non-painful and painful electrical stimulation of the foot sole. Clin. Neurophysiol. 2001;112:641–649. doi: 10.1016/s1388-2457(01)00485-0. [DOI] [PubMed] [Google Scholar]
- Andersen OK, Spaich EG, Madeleine P, Arendt-Nielsen L. Gradual enlargement of human withdrawal reflex receptive fields following repetitive painful stimulation. Brain Res. 2005;1042:194–204. doi: 10.1016/j.brainres.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Anderson O, Grillner S. Peripheral control of the cat's step cycle I. Phase dependent effects of ramp-movements of the hip during “fictive locomotion”. Acta Physiol. Scand. 1981;113:89–101. doi: 10.1111/j.1748-1716.1981.tb06867.x. [DOI] [PubMed] [Google Scholar]
- Andersson O, Grillner S. Peripheral control of the cat's step cycle: entrainment of the central pattern generators for locomotion by sinusoidal hip movements during fictive locomotion. Acta Physiol. Scand. 1983;118:229–239. doi: 10.1111/j.1748-1716.1983.tb07267.x. [DOI] [PubMed] [Google Scholar]
- Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practioner. 1964;192:540–542. [PubMed] [Google Scholar]
- Barbeau H, Ladouceur M, Mirbagheri MM, Kearney RE. The effect of locomotor training combined with functional electrical stimulation in chronic spinal cord injured subjects: walking and reflex studies. Brain Res. Rev. 2002;40:274–291. doi: 10.1016/s0165-0173(02)00210-2. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvery PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J. Neurophysiol. 2004;91:2247–2258. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- Bussel B, Roby-Brami A, Azouvi PH, Biraben A, Yakovleff A, Held JP. Myoclonus in a patient with spinal cord transection. Possible involvement of the spinal stepping generator. Brain. 1988;111:1235–1245. doi: 10.1093/brain/111.5.1235. [DOI] [PubMed] [Google Scholar]
- Calancie B, Needham-Shropshire B, Jacobs P, Willer K, Zych G, Green BA. Involuntary stepping after chronic spinal cord injury. Evidence for a central rhythm generator for locomotion in man. Brain. 1994;117:1143–1159. doi: 10.1093/brain/117.5.1143. [DOI] [PubMed] [Google Scholar]
- Delwaide PJ, Crenna P, Fleron MH. Cutaneous nerve stimulation and motoneuronal excitability: I. Soleus and tibialis anterior excitability after ipsilateral and contralateral sural nerve stimulation. J. Neurol., Neurosurg. Psychiatry. 1981;44:699–707. doi: 10.1136/jnnp.44.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Wirtz M, Curt A, Colombo G. Locomotor pattern in paraplegic patients: training effects recovery of spinal cord function. Spinal Cord. 1998;36:380–390. doi: 10.1038/sj.sc.3100590. [DOI] [PubMed] [Google Scholar]
- Dietz V, Müller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626–2634. doi: 10.1093/brain/awf273. [DOI] [PubMed] [Google Scholar]
- Dobkin BH, Harkema SJ, Requejo P, Edgerton VR. Modulation of locomotor-like EMG activity in subjects with complete and incomplete spinal cord injury. J. Neurol. Rehabil. 1995;9:183–190. [PubMed] [Google Scholar]
- Duysens J, Trippel M, Horstmann GA, Dietz V. Gating and reversal of reflexes in ankle muscles during human walking. Exp. Brain Res. 1990;82:351–358. doi: 10.1007/BF00231254. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash M, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–2258. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lundberg A. The effect of Dopa on the spinal cord: 7. Reflex activation of static γ-motoneurones from the FRA. Acta Physiol. Scand. 1967;70:403–411. doi: 10.1111/j.1748-1716.1967.tb03638.x. [DOI] [PubMed] [Google Scholar]
- Grillner S, Rossignol S. Contralateral reflex reversal controlled by limb position in the acute spinal cat injected with clonidine i.v. Brain Res. 1978;144:411–414. doi: 10.1016/0006-8993(78)90169-5. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Finer BL. The plasticity of human withdrawal reflexes to noxious skin stimuli in lower limbs. Prog. Brain Res. 1963;1:65–81. [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J. Neurophysiol. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Hiersemenzel LP, Curt A, Dietz V. From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology. 2000;54:1574–1582. doi: 10.1212/wnl.54.8.1574. [DOI] [PubMed] [Google Scholar]
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J. Physiol. (Lond.) 1996;491:197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Jukes MGM, Lund S, Lundberg A. The effect of DOPA on the spinal cord: 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol. Scand. 1967a;70:369–388. doi: 10.1111/j.1748-1716.1967.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MGM, Lund S, Lundberg A. The effect of DOPA on the spinal cord: 6. Half centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol. Scand. 1967b;70:389–402. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Knikou M. Effects of hip joint angle changes on intersegmental spinal coupling in human spinal cord injury. Exp. Brain Res. 2005;167:381–393. doi: 10.1007/s00221-005-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knikou M. Plantar cutaneous input modulates differently spinal reflexes in subjects with intact and injured spinal cord. Spinal Cord. 2006 doi: 10.1038/sj.sc.3101917. doi 10.1038/sj. sc.3101917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knikou M, Conway B. Effects of electrically induced muscle contraction on flexion reflex in human spinal cord injury. Spinal Cord. 2005;43:640–648. doi: 10.1038/sj.sc.3101772. [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer WZ. Effects of changes in hip joint angle on H-reflex excitability in humans. Exp. Brain Res. 2002a;143:149–159. doi: 10.1007/s00221-001-0978-4. [Erratum in Exp. Brain Res. 144, 558–558] [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer WZ. Hip angle induced modulation of H reflex amplitude, latency and duration in spinal cord injured humans. Clin. Neurophysiol. 2002b;113:1698–1708. doi: 10.1016/s1388-2457(02)00285-7. [DOI] [PubMed] [Google Scholar]
- Knikou M, Chaudhuri D, Kay E, Schmit BD. Pre- and post-alpha motoneuronal control of the soleus H-reflex during sinusoidal hip movements in human spinal cord injury. Brain Res. 2006a;1103:123–139. doi: 10.1016/j.brainres.2006.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knikou M, Kay E, Rymer WZ. Modulation of flexion reflex induced by hip angle changes in human spinal cord injury. Exp. Brain Res. 2006b;168:577–586. doi: 10.1007/s00221-005-0112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriellaars DJ, Brownstone RM, Noga BR, Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. J. Neurophysiol. 1994;71:2074–2086. doi: 10.1152/jn.1994.71.6.2074. [DOI] [PubMed] [Google Scholar]
- Lam T, Pearson KG. Proprioceptive modulation of hip flexor activity during swing phase of locomotion in decerebrate cats. J. Neurophysiol. 2001;86:1321–1332. doi: 10.1152/jn.2001.86.3.1321. [DOI] [PubMed] [Google Scholar]
- Lam T, Pearson KG. Sartorius muscle afferents influence the amplitude and timing of flexor activity in walking decerebrate cats. Exp. Brain Res. 2002;147:175–185. doi: 10.1007/s00221-002-1236-0. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Multisensory control of spinal reflex pathways. In: Granit R, Pomeiano O, editors. Reflex Control of Posture and Movement. Elsevier; Amsterdam: 1979. pp. 11–28. [DOI] [PubMed] [Google Scholar]
- Maynard FM, Bracken MB, Creasey G, Ditunno JF, Donovan WH, Ducker TB, Garber SL, Marini RJ, Stover SL, Tator CH, Waters RL, Wilberger JP, Young W. International standards for neurological and functional classification of spinal cord injury. Spinal Cord. 1997;5:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- McVea DA, Donelan JM, Tachibana A, Pearson KG. A role for hip position in initiating the swing-to-stance transition in walking cats. J. Neurophysiol. 2005;94:3497–3508. doi: 10.1152/jn.00511.2005. [DOI] [PubMed] [Google Scholar]
- Nickolls P, Collins DF, Gorman RB, Burke D, Gandevia SC. Forces consistent with plateau-like behaviour of spinal neurons evoked in patients with spinal cord injuries. Brain. 2004;127:660–670. doi: 10.1093/brain/awh073. [DOI] [PubMed] [Google Scholar]
- Perreault MC, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. J. Physiol. (London) 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postans NJ, Hasler JP, Granat MH, Maxwell DJ. Functional electric stimulation to augment partial weight-bearing supported treadmill training for patients with acute incomplete spinal cord injury: a pilot study. Arch. Phys. Med. Rehabil. 2004;85:604–610. doi: 10.1016/j.apmr.2003.08.083. [DOI] [PubMed] [Google Scholar]
- Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B. Long latency spinal reflex in man after flexor reflex afferent stimulation. Brain. 1987;110:707–725. doi: 10.1093/brain/110.3.707. [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B. Effects of FRA stimulation on the soleus H-reflex in patients with a complete spinal cord lesion: evidence for presynaptic inhibition of Ia transmission. Exp. Brain Res. 1990;81:593–601. doi: 10.1007/BF02423509. [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B. Inhibitory effects on flexor reflexes in patients with a complete spinal cord lesion. Exp. Brain Res. 1992;90:201–208. doi: 10.1007/BF00229272. [DOI] [PubMed] [Google Scholar]
- Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC. The lower limb flexion reflex in humans. Prog. Neurobiol. 2005;77:353–395. doi: 10.1016/j.pneurobio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Schmit BD, Benz EN. Extensor reflexes in human spinal cord injury: activation by hip proprioceptors. Exp. Brain Res. 2002;145:520–527. doi: 10.1007/s00221-002-1134-5. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Petersen N, Barajon I, Hultborn H. Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Exp. Brain Res. 1998;122:339–350. doi: 10.1007/s002210050522. [DOI] [PubMed] [Google Scholar]
- Serrao M, Rossi P, Sandrini G, Parisi L, Amabile GA, Nappi G, Pierelli F. Effects of diffuse noxious inhibitory controls on temporal summation of the RIII reflex in humans. Pain. 2004;112:353–360. doi: 10.1016/j.pain.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Shahani BT, Young RR. Human flexor reflexes. J. Neurol., Neurosurg. Psychiatry. 1971;34:616–627. doi: 10.1136/jnnp.34.5.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. Flexion-reflex of the limb, crossed extension-reflex and reflex stepping and standing. J. Physiol. (Lond.) 1910;40:28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaich EG, Arendt-Nielsen L, Andersen OK. Modulation of lower limb withdrawal reflexes during gait: a topographical study. J. Neurophysiol. 2004;91:258–266. doi: 10.1152/jn.00360.2003. [DOI] [PubMed] [Google Scholar]
- Steldt RE, Schmit BD. Modulation of coordinated muscle activity during imposed sinusoidal hip movements in human spinal cord injury. J. Neurophysiol. 2004;92:673–685. doi: 10.1152/jn.00677.2003. [DOI] [PubMed] [Google Scholar]
- Van Wezel BMH, Van Engelen BGM, Gabreels FJM, Gabreels-Festen AAWM, Duysens J. Aβ fibers mediate cutaneous reflexes during human walking. J. Neurophysiol. 2000;83:2980–2986. doi: 10.1152/jn.2000.83.5.2980. [DOI] [PubMed] [Google Scholar]
- Whelan PJ. Control of locomotion in the decerebrate cat. Prog. Neurobiol. 1996;49:481–515. doi: 10.1016/0301-0082(96)00028-7. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB. Phase-dependent reflex reversal in human leg muscles during walking. J. Neurophysiol. 1990;63:1109–1117. doi: 10.1152/jn.1990.63.5.1109. [DOI] [PubMed] [Google Scholar]