Abstract
A significant challenge to efforts aimed at inducing effective antitumor immune responses is that CD8+ T cells, which play a prominent role in these responses, may be unable to respond to tumors that lack costimulatory signals and that are protected by an immune suppressive environment such as that mediated by TGF-β produced by tumor cells themselves or by infiltrating Tregs, often resulting in tolerance or anergy of tumor-specific T cells. Here we show that the in vitro activation of Cblb–/– CD8+ T cells does not depend on CD28 costimulation and is resistant to TGF-β suppression. In vivo studies further demonstrated that Cblb–/– mice, but not WT controls, efficiently rejected inoculated E.G7 and EL4 lymphomas that did not express B7 ligands and that introduction of the Cblb–/– mutation into tumor-prone ataxia telangiectasia mutated–deficient mice markedly reduced the incidence of spontaneous thymic lymphomas. Immunohistological study showed that E.G7 tumors from Cblb–/– mice contained massively infiltrating CD8+ T cells. Adoptive transfer of purified Cblb–/– CD8+ T cells into E.G7 tumor-bearing mice led to efficient eradication of established tumors. Thus, our data indicate that ablation of Cbl-b can be an efficient strategy for eliciting immune responses against both inoculated and spontaneous tumors.
Introduction
Immune responses against immunogenic tumors are mediated by CD8+ CTLs (1–3). Interestingly, despite T cell recognition, most tumors are not rejected in the host (4, 5). The mechanisms that may prevent CTL-mediated tumor rejection include inhibition of T cell responsiveness by tumor-derived factors, such as TGF-β and soluble MHC class I–related molecules secreted by tumor cells (6, 7), as well as negative regulation of the host immune system, including suppressive CTLA-4 signaling (8), the effect of CD25+ regulatory T cells (9), and suppression by IL-13 produced by CD4+ NKT cells (10). In addition to these mechanisms of active suppression, lack of effective recognition of tumors by T cells may disable an antitumor immune response, for example in the absence of TCR and/or costimulatory signals (11, 12). It has been recognized that while stimulation of T cells through TCRs and costimulatory receptors such as CD28 leads to T cell activation, triggering of T cells through the TCR alone results in a nonresponsive state (anergy) of these cells (13). The importance of costimulation for antitumor immune response has been demonstrated by experiments in which the enforced expression on tumor cells of B7 or ICOS/B7h, ligands for CD28, results in efficient eradication of inoculated tumors (14–18). However, this approach to immunotherapy is practically difficult because of the lack of an efficient way to express a costimulatory molecule in all tumor cells. An alternative to this approach is the generation of T cells that can bypass the requirement for CD28 costimulation during activation. Such an approach may allow direct activation of tumor-specific CTLs by tumor cells in the absence of costimulatory ligands, thus representing a potentially powerful therapeutic tool against cancer.
Cbl proteins are RING-finger domain–containing E3 ubiquitin ligases involved in various membrane-receptor signaling events (19–21). Previous experiments from our laboratory and others have shown that Cbl-b, a member of the Cbl family of proteins, plays a critical role in peripheral T cell activation (22, 23). Remarkably, Cblb–/– CD4+ T cells have circumvented dependence on costimulatory signals for their activation, as they proliferate vigorously and secrete large amounts of IL-2 upon TCR stimulation in the absence of CD28 costimulation. These results thus underscore the role of Cbl-b as a key regulator of CD28 costimulatory signaling and suggest that CTLs deficient in Cbl-b may respond to and mount an efficient response against tumors that lack costimulatory signals. In the present study, we have tested this hypothesis using Cblb–/– mice as a model. Our results indicate that Cblb–/– mice reject inoculated highly and poorly immunogenic tumors and that adoptive transfer of Cblb–/– CD8+ T cells is sufficient to mediate the antitumor immune response in tumor-bearing mice. These findings provide evidence that Cbl-b–ablated CD8+ T cells may be effective tools in the treatment of human cancers.
Results
CD28-independent activation of Cblb–/– CD8+ T cells.
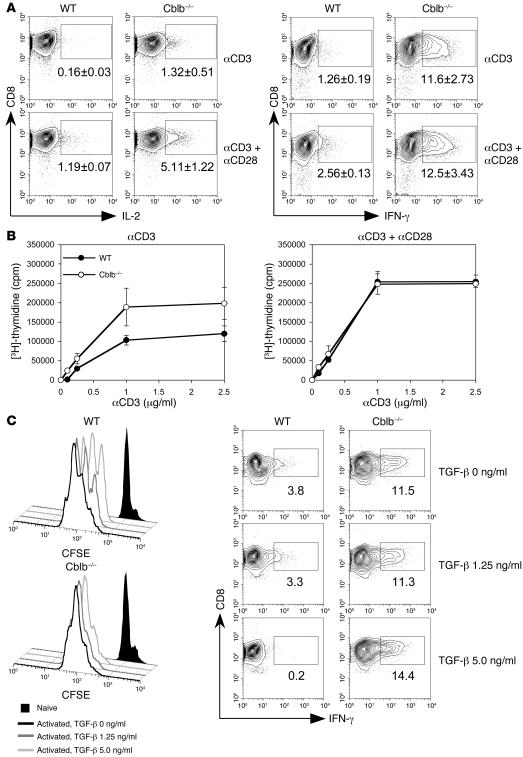
Previous results from our laboratory and others show that activation of Cblb–/– CD4+ T cells is independent of CD28 costimulation (22, 23). To determine whether Cblb–/– CD8+ T cells might also bypass dependence on CD28 signaling, we compared cytokine secretion and proliferation of purified CD8+ T cells in WT and Cblb–/– mice after stimulation through the TCR alone or costimulation through the TCR and costimulatory receptor CD28. We found that WT naive CD8+ T cells generated limited IL-2 and IFN-γ responses after stimulation with anti-CD3 antibody alone (Figure 1A). They produced approximately 6 times more IL-2 and 2–3 times more IFN-γ when CD28 costimulation was provided. In contrast, stimulation of Cblb–/– CD8+ T cells with anti-CD3 antibody alone elicited much higher levels of IL-2 (10-fold; P < 0.05) and IFN-γ (10-fold; P < 0.001). Addition of costimulatory signaling by anti-CD28 antibody also enhanced the IL-2 production by Cblb–/– CD8+ T cells 4-fold (P < 0.01); however, the level of IFN-γ was not significantly altered (Figure 1A). These results indicate that inactivation of Cbl-b largely bypasses the requirement for CD28 costimulation in these cytokine responses of CD8+ T cells, as was previously observed for CD4+ T cells (22, 23). Interestingly, when cultures containing both CD8+ and CD4+ T cells were stimulated, the production of IFN-γ but not IL-2 by WT CD8+ T cells was markedly enhanced; however, the production of IFN-γ by Cblb–/– CD8+ plus CD4+ T cells was comparable to that by purified Cblb–/– CD8+ T cells (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI29472DS1), suggesting that CD4+ T cell help does not have an additive effect on the IFN-γ response of Cblb–/– CD8+ T cells. Consistent with the effects observed on IL-2 responses, anti-CD3 antibody alone induced significantly greater proliferation of purified Cblb–/– CD8+ T cells than that of WT T cells (Figure 1B). Additionally, while the proliferative response of WT CD8+ T cells was markedly enhanced when cells were costimulated with anti-CD3 and anti-CD28, reaching a level equivalent to the response of Cblb–/– CD8+ T cells stimulated by anti-CD3 alone, the proliferation of Cblb–/– CD8+ T cells was not dramatically elevated by CD28 costimulation (Figure 1B). Based on these results, we conclude that ablation of Cbl-b renders CD8+ T cells capable of responding to TCR stimulation without a requirement for CD28 costimulation and CD4+ T cell help.
Figure 1. CD28-independent proliferation and cytokine production by Cblb–/– CD8+ T cells.
(A) IL-2 and IFN-γ production. Purified CD8+ T cells from WT and Cblb–/– mice were stimulated with either plate-bound anti-CD3 or plate-bound anti-CD3 plus soluble anti-CD28 antibodies. IL-2– and IFN-γ–producing cells were visualized by intracellular staining and analyzed by flow cytometry. Shown are contour plots of intracellular staining for IL-2 and IFN-γ expression in CD8+ T cells. Percentages of IL-2– and IFN-γ–producing cells are indicated in the plots as mean ± SD from 3 independent experiments. The boxed regions indicate the gates used for calculation of the percentage of CD8+ T cells staining positive for IFN-γ. (B) TCR-induced proliferative response. Purified CD8+ T cells from WT and Cblb–/– mouse lymph nodes and spleens were stimulated with various concentrations of anti-CD3 antibodies in the presence or absence of anti-CD28 antibodies. Cell proliferation was determined by [3H]-thymidine incorporation and presented as mean ± SD for triplicate samples. Shown are representatives of 3 independent experiments. (C) Resistance of Cblb–/– CD8+ T cells to TGF-β suppression. Histograms (left) show CSFE intensities of labeled Cblb–/– and WT CD8+ T cells after 3 days of anti-CD3 and anti-CD28 stimulation. Cells were cultured in the absence or presence of different concentrations of TGF-β as indicated in the figure. Contour plots (bottom) show the IFN-γ production in the absence or presence of TGF-β. Percentages of IFN-γ+ cells are indicated in the plots.
A recent report shows that Cblb–/– CD4+ T cells are less sensitive than WT cells to TGF-β suppression (24). To determine whether Cblb–/– CD8+ T cells are resistant to TGF-β inhibition, we measured TCR-induced proliferation and cytokine production by Cblb–/– CD8+ T cells in the presence of TGF-β. While presence of TGF-β markedly suppressed the proliferation and IFN-γ production of WT CD8+ T cells, the same TGF-β exerted little effect on Cblb–/– CD8+ T cells (Figure 1C). This result indicates that susceptibility to TGF-β suppression is compromised in Cblb–/– CD8+ T cells.
Efficient rejection of transplanted tumors in Cblb–/– mice.
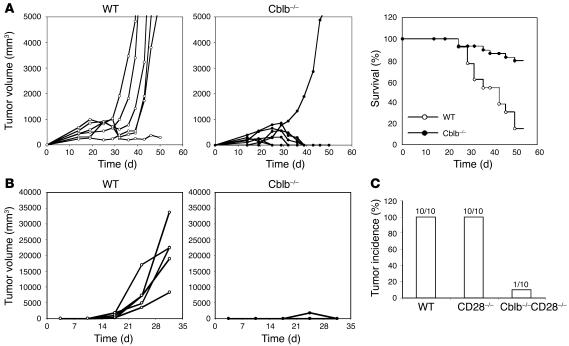
Loss of the dependence on CD28 signaling as well as increased resistance to inhibition by TGF-β for Cblb–/– CD8+ T cell activation suggested that Cblb–/– T cells might be activated by tumor cells in the absence of costimulation. We therefore predicted that Cblb–/– mice might mount an efficient immune response against tumor cells lacking expression of B7 costimulatory molecules. To explore this possibility, we examined whether Cblb–/– mice are resistant to inoculated murine EL4 and E.G7 thymomas. EL4 cells are derived from a T-lineage lymphoma that developed in a C57BL/6 mouse treated with 9,10-dimethyl-1,2-benzanthracene and represent a tumor model with weak immunogenicity as well as reported TGF-β secretion by EL4 cells (25). By contrast, E.G7 cells are EL4 transfectants that express a transgene encoding chicken OVA and are therefore considered to be highly immunogenic tumors in which OVA serves as the tumor-specific antigen (26). Importantly, both EL4 and E.G7 lymphomas do not express cell surface B7.1 or B7.2 and grow progressively in C57BL/6 mice after subcutaneous inoculation, while in contrast B7-transfected EL4 cells are rejected, demonstrating a critical role of costimulation in the antitumor response of WT mice (18). To determine whether Cblb–/– mice reject E.G7 tumors, we subcutaneously injected E.G7 cells (1 × 106 cells per mouse) into the flanks of WT C57BL/6 and Cblb–/– mice and then monitored tumor growth (Figure 2A, left panels). While tumors grew progressively and led to death in 85% of WT mice (n = 13), 79% of Cblb–/– mice (n = 29) either did not develop tumors or exhibited tumor regression after initial growth (Figure 2A, right panel). Similarly, when a low dose of EL4 cells (5 × 104 cells per mouse) was injected, tumors grew rapidly and killed 85% of WT (including Cblb+/+ and Cblb+/–) mice (n = 20). By contrast, the same dose of tumor cells was completely rejected in Cblb–/– mice (Figure 2B). Tumor rejection was indeed independent of CD28 because Cblb–/–CD28–/– double-mutant mice also efficiently rejected the inoculated EL4 tumors (Figure 2C). Inoculation of a high dose of EL4 cells (2.5 × 105 cells per mouse) resulted in tumor growth in all WT mice (n = 10); however, the same high dose of tumors grew at a much slower rate in 7 and did not grow at all in 3 Cblb–/– mice (data not shown). Based on these results, we conclude that ablation of Cbl-b confers the ability to reject or attenuate the growth of tumors that express either strong or weak tumor antigens in vivo.
Figure 2. Eradication of inoculated tumors in Cblb–/– mice.
(A) Growth rates of inoculated E.G7 tumors and survival of tumor-bearing mice. After 106 E.G7 cells were inoculated into the flanks of WT or Cblb–/– mice by s.c. injection, tumor growth was documented as total volume of tumor size. Left and middle: growth rates of E.G7 tumors in 1 representative experiment of 5 or more independent experiments. Each curve represents 1 mouse. Right: percentages of surviving mice (WT, n = 13; Cblb–/–, n = 29) during the course of tumor growth. When the tumor volume reached approximately 5,000 mm3, the mice were euthanized and recorded as dead. (B) Growth rates of EL4 tumors in Cblb–/– and WT mice. EL4 cells (5 × 104 cells/mouse) were injected s.c. into the flanks of 5 WT and 5 Cblb–/– mice, and tumor growth was monitored over time. WT mice included both Cblb+/+ (n = 16) and Cblb+/– (n = 4) mice. The data presented are representative of 4 independent experiments. (C) Rejection of EL4 tumors by Cblb–/–CD28–/– mice. EL4 cells (5 × 104 cells/mouse) were injected, and tumor growth was monitored as described in B. The number of mice with tumor growths is indicated at the top of each column.
Efficient eradication of syngenic tumors by Cblb–/– CD8+ T cells.
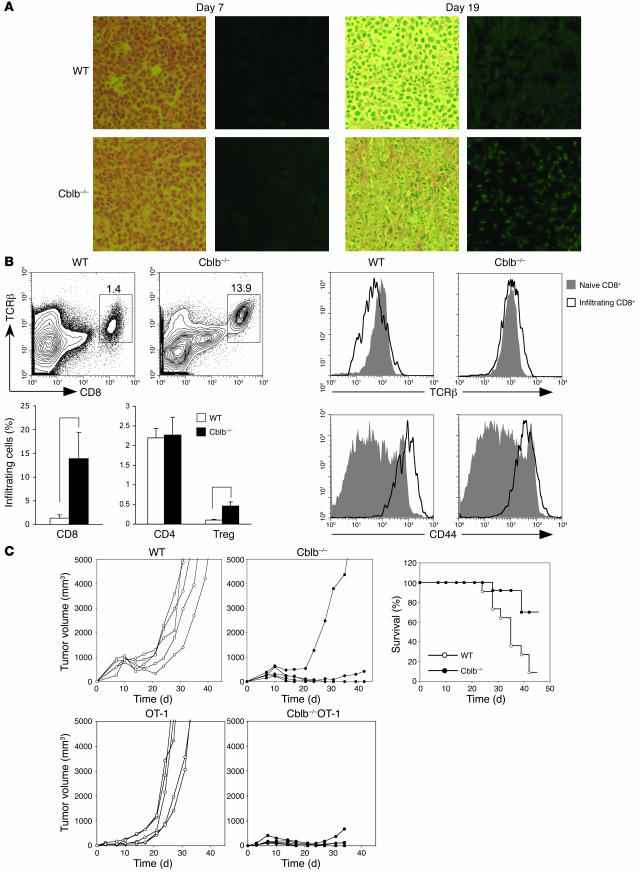
Since Cblb–/– CD8+ T cells may respond to antigen stimulation independent of CD28 costimulation and CD4+ T cell help and are resistant to TGF-β suppression, we hypothesized that the observed resistance of Cblb–/– mice to inoculated tumors was primarily mediated by CD8+ T cells. To determine whether this was the case, we first examined infiltration of CD8+ T cells in tumor tissues by immunohistology (Figure 3A). We inoculated 1 × 106 E.G7 cells into the flanks of C57BL/6 mice and Cblb–/– mice by subcutaneous injection. Seven days after inoculation, we found no detectable CD8+ cell infiltrates in tumor tissues in either WT or Cblb–/– mice. However, 2 weeks after inoculation, a marked increase in CD8+ leukocyte infiltrates was found in tumors in Cblb–/– but not WT mice. The CD8+ infiltrates remained abundant in the regressing tumors in Cblb–/– mice 19 days after inoculation (Figure 3A); however, infiltration was minimal in tumors in WT mice. Flow cytometric analysis revealed that, unlike CD8+ infiltrates in tumors in WT mice, which downregulated surface TCRβ, CD8+ infiltrates in tumors from Cblb–/– mice expressed TCRαβ receptors at a level comparable to that on naive lymph node T cells (Figure 3B). These CD8+ T cells also expressed a high level of CD44 (Figure 3B), suggesting that they were activated. In addition to CD8+ T cells, tumors in both WT and Cblb–/– mice also contained CD4+ T cell and CD4+ FoxP3+ Treg infiltrates (Figure 3B and Supplemental Figure 2). Interestingly, there were significantly more Treg infiltrates in tumors in Cblb–/– mice than in WT mice (P < 0.005). The reason behind this expansion is unclear, but this might suggest that Cblb–/– Tregs mounted a stronger proliferative response to tumor antigen stimulation than did WT Tregs. Nevertheless, since tumors are efficiently rejected by Cblb–/– CD8+ T cells, it appears that these Tregs could not suppress Cblb–/– CD8+ T cell function, despite the previously reported finding that Cblb–/– Tregs are functionally normal in their ability to suppress responses of WT T cells (24).
Figure 3. Tumor rejection in Cblb–/– mice is mediated by Cblb–/– CD8+ T cells.
(A) Immunohistology of tumor-infiltrating CD8+ cells. Shown are sections of tumors stained with H&E (left) or anti-CD8 antibody (right). CD8+ cells are FITC positive (green). Tumors were from WT and Cblb–/– mice at the seventh or nineteenth day after inoculation. (B) Flow cytometric analysis of tumor infiltrates. Tumor infiltrates were prepared from tumors from WT or Cblb–/– mice. These cells were stained with anti-TCRβ, anti-CD8, anti-CD4, and anti-CD44 antibodies and analyzed on LSR II. Foxp3+ (Treg) cells were identified by intracellular staining with an anti-Foxp3 antibody according to manufacture protocol. Shown at the top left are contour plots of CD8 and TCRβ expression on tumor-infiltrating cells from 1 of 3 independent experiments. The percentages of CD8+ TCRβ+ cells are indicated in the plots. The percentages of CD8+ TCRβ+, CD4+, and Treg infiltrates in total tumor infiltrates are summarized and shown as bars (bottom left). *P < 0.001, **P < 0.005. Histograms show the levels of TCRβ and CD44 expression on the infiltrating CD8+ T cells (right). (C) Eradication of established E.G7 tumors by adoptively transferred Cblb–/– or Cblb–/– OT1 CD8+ T cells. 106 E.G7 cells were inoculated into C57BL/6 mice by s.c. injection. Seven days after the inoculation, 3 × 106 purified WT or Cblb–/– CD8+ T cells (top) or WT OT1 or Cblb–/– OT1 CD8+ T cells (bottom) were transferred into the tumor-bearing mice by i.v. injection. Shown at the left are the tumor volumes at different time points after the CD8+ T cell transfer. Genotypes of the donor cells are indicated on the top of each plot. Results are from 1 of 5 or more independent experiments. The plot in the right panel shows percentages of surviving recipient mice (WT, n = 11; Cblb–/–, n = 13) that received WT or Cblb–/– CD8+ T cells. When the tumor volume reached approximately 5,000 mm3, the mice were euthanized and recorded as dead.
To determine whether Cblb–/– CD8+ T cells were responsible for the eradication of tumors, we transferred purified WT or Cblb–/– CD8+ T cells (3 × 106 cells per mouse) into E.G7 tumor–bearing mice by i.v. injection and monitored tumor growth. We observed that established E.G7 tumors, which were inoculated 7 days before CD8+ T cell transfer, were efficiently eradicated within 4–5 weeks or grew at a much slower rate in mice that received Cblb–/– CD8+ T cells compared with mice that received WT CD8+ T cells (Figure 3C, left panels). In total, 70% of mice (n = 13) adoptively transferred with Cblb–/– CD8+ T cells eradicated tumors and survived. By contrast, more than 90% of mice (n = 11) that received WT CD8+ T cells had rapid tumor growth and died (Figure 3C, right panel).
To determine whether the observed rejection of E.G7 tumors was mediated by tumor-specific CD8+ T cells, we crossed Cblb–/– mice to OT1 TCR transgenic mice, which expressed a transgenic TCR recognizing an OVA peptide in context of H-2Kb. The majority of CD8+ T cells developed in OT1 mice express this TCR transgene and are thus capable of recognizing OVA peptide expressed by E.G7 tumor (27). We found that adoptive transfer of purified WT OT1 CD8+ T cells (3 × 106 cells per mouse) into E.G7 tumor–bearing mice (n = 5) failed to prevent any tumor growth. By contrast, transfer of Cblb–/– OT1 CD8+ T cells into the tumor-bearing mice resulted in complete regression of the tumors in all of the mice (n = 5) (Figure 3C). Taken together, these data indicate that Cblb–/– CD8+ T cells can elicit an efficient immune response against established tumors in a WT host that is otherwise incapable of tumor rejection.
Prevention of spontaneous tumors in ataxia telangiectasia mutated–deficient mice by ablation of Cbl-b.
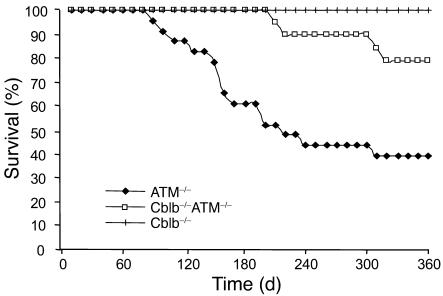
Effective recognition and elimination of transplanted E.G7 and EL4 tumors by Cblb–/– CD8+ T cells raised the possibility that the Cblb–/– mutation might also facilitate efficient surveillance and protection against spontaneous tumors. To assess this possibility, we crossed Cblb–/– mice with ataxia telangiectasia mutated–deficient (ATM–/–) mice and analyzed the incidence of T cell lymphomas in, and the life span of, the resulting double-mutant mice. Consistent with previous findings (28), we observed that approximately 50% of ATM–/– mice died by 6 months of age (Figure 4). Pathological inspection of 13 sick mice revealed that 8 of them had T cell lymphomas. In contrast, no death was observed in 21 Cblb–/–ATM–/– double-mutant mice by 6 months of age, and less than 10% (2 of 21) of the mice became moribund by 9 months of age. These results thus indicate that complete ablation of Cbl-b function may delay or even prevent the onset of at least some spontaneous tumors.
Figure 4. Tumor incidence in Cblb–/–ATM–/– double-mutant mice.
Numbers of surviving ATM–/– single-mutant (including 5 ATM–/–Cblb+/+ and 18 ATM–/–Cblb+/–) and Cblb–/–ATM–/– (n = 21) mice are shown. Cblb–/–ATM–/– double-mutant mice exhibited reduced incidence and delayed onset of spontaneous lymphoma.
Discussion
Tumor immunotherapy is a promising approach that can result in the regression of established primary and metastatic cancer in a number of experimental animal models as well as in some clinical trials. However, currently available approaches remain less successful than desired. A major obstacle that has yet to be overcome derives from the observation that generation of effective CTL responses to antigenic tumors requires costimulatory signals, while many tumors do not express the necessary costimulatory ligands. In addition, tumor sites are often enriched in TGF-β expression, so that effective CTL responses may need to be resistant to TGF-β. As a result, T cells recognizing these tumors are often nonresponsive in vivo. In this report, we find that Cblb–/– CD8+ T cells respond efficiently to antigen stimulation even without CD28 costimulation and in the presence of a high concentration of TGF-β. As a consequence, Cblb–/– mice are resistant to inoculated E.G7 and EL4 tumors, and Cblb–/–ATM–/– double-mutant mice exhibit a significantly lower incidence of spontaneous lymphomas than do mice expressing Cbl-b. Our further analysis shows that the adoptive transfer of purified polyclonal or tumor-specific monoclonal Cblb–/– CD8+ T cells is sufficient to eradicate established E.G7 tumors in WT recipient mice, thus indicating that the observed antitumor immunity is mediated by tumor-specific CD8+ T cells, despite the fact that it remains unclear whether the mice that have undergone tumor regression develop antitumor memory. Finally, our data indicate that the antitumor response is indeed independent of CD28, as Cblb–/–CD28–/– mice are also resistant to inoculated EL4 tumors, and is not inhibited by Tregs, which are present in tumor infiltrates in high numbers. Taken together, these results demonstrate that ablation of Cbl-b in T cells may be sufficient to elicit effective antitumor immunity to relatively highly or poorly immunogenic tumors by facilitating tumor-specific T cell activation even in the absence of CD28 costimulation, providing a powerful tool in cancer immunotherapy.
In vivo nonresponsiveness of CTLs to tumors may involve active suppression of CTLs or lack of appropriate recognition of tumor cells by CTLs. Although our models suggest that the costimulation-independent activation of Cblb–/– T cells is one of the factors responsible for the observed antitumor response, mechanisms other than CD28-independent CD8+ T cell activation may also contribute to the tumor rejection. For example, massive infiltration of Cblb–/– CD8+ T cells in the tumor mass may result from altered cell adhesion and migration. Enhanced cell adhesion may also serve as a mechanism for increased costimulatory signals for T cell activation, since enhanced adhesion may strengthen immunological synapses, thus extending the duration of TCR signaling (29). Our preliminary data indicate that adhesion of Cblb–/– T cells to fibronectin is significantly enhanced compared with that of WT T cells (data not shown), thus providing support for this notion. Additionally, it has been shown that Cblb–/– T cells may escape an anergic fate under conditions that normally induce immune tolerance in vivo (30). Cblb–/– T cells may therefore be resistant to tolerance induction by tumor cells, consequently providing better immune surveillance against newly transformed or inoculated tumor cells. Finally, it has been demonstrated that the CTL response against tumors can be inhibited by TGF-β produced by tumor or CD25+ Tregs (9). CD25+ Tregs may exert immune suppression through secretion of TGF-β (31). Our data indicate that although many Tregs are found infiltrating the tumor mass, tumors are still efficiently rejected by Cblb–/– CD8+ T cells, suggesting that these Cblb–/– T cells are less sensitive to TGF-β suppression. Thus, lack of immune suppression of Cblb–/– CD8+ T cells by TGF-β–secreting tumors or CD4+ Tregs could also be a factor leading to the enhanced antitumor immune responses.
The ability of adoptively transferred Cblb–/– CD8+ T cells to reject antigenic tumors provides a new avenue in tumor immunotherapy. This approach has several advantages. Cblb–/– CD8+ T cells might be broadly effective against a large class of somatic tumors, the majority of which do not express costimulatory ligands. Additionally, the ability of Cblb–/– CD8+ T cells to produce substantial cytokine responses and to function independent of CD28 costimulation and CD4+ T cell help might avoid the need to administer large amounts of cytokines or to immunize patients with vaccines designed to enhance costimulatory signaling and helper T cell responses. It is possible that the effectiveness of current strategies, such as the clinical use of tumor-infiltrating lymphocytes in adoptive immunotherapy, could be enhanced by ablation of Cbl-b using RNAi or dominant-negative forms of the gene.
Finally, our data show that germline ablation of Cbl-b in ATM–/– mice results in a marked reduction of spontaneous tumors and a consequently increased life span. Given the observation that Cblb–/– CD8+ T cells can mount efficient immune responses to transplanted E.G7 and EL4 tumors, the reduced incidence of lymphomagenesis in Cblb–/–ATM–/– double-mutant mice may result from the improved immune surveillance of Cblb–/– CD8+ T cells against the spontaneous tumors that arise in these mice. However, given that tumorigenesis is a very complex process, we cannot exclude the possibility that the germline Cblb–/– mutation decreases tumorigenesis per se, rather than enhancing rejection of tumors arising in Cblb–/–ATM–/– mice. Overall, our results suggest that systemic inhibition of Cbl-b function may be an effective approach to reducing the incidence of tumors. Although our results suggest that systemic inhibition of Cbl-b function might be beneficial in the prevention of cancers, the potential effect of such inhibition on critical physiologic function remains a very substantial caveat to clinic translation. The enhanced susceptibility to autoimmune disease that has been observed in Cblb–/– mice is an example of potentially undesirable consequences of Cbl-b inactivation (22, 23). Thus, a thorough study on the short- and long-term physiological impact of Cbl-b ablation is necessary before this approach can be considered for cancer prevention.
Methods
Mice and cells.
Cblb–/–, OT1 TCR transgenic, and ATM–/– mice were generated as previous described (22, 28, 32). Cblb–/– mice were backcrossed to C57BL/6 mice for 12 generations. C57BL/6, Cblb–/–, Cblb–/– OT1 TCR transgenic, ATM–/–, and Cblb–/–ATM–/– double-knockout mice were maintained in specific pathogen–free conditions and all animal experiments were approved by the Animal Care and Use Committees of the NIH and Columbia University. Animals were housed at BIOQUAL Inc., the Twinbrook II Facility of the National Institute of Allergy and Infectious Diseases, and Columbia University Hammer Health Science Center (HHSC) Animal Facility. EL4 (ATCC) and E.G7 cells (generously provided by Lieping Chen, Johns Hopkins University, Baltimore, Maryland, USA) were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, and 50 μM 2-mercaptoethanol.
In vitro T cell proliferation and cytokine production assays.
Total T cells or CD8+ T cells were purified from spleen by MACS (Miltenyi Biotec) using a T cell or CD8+ T cell enrichment kit (Miltenyi Biotec). For T cell proliferation assay, purified CD8+ T cells were stimulated with various concentrations of plate-bound anti-CD3ε and soluble anti-CD28 (5 μg/ml) for 72 hours. After pulsing cells with [3H]-thymidine (1 μCi/well) for 12 hours, cells were harvested, and [3H]-thymidine uptake was determined using a β-counter. For intracellular IL-2 and IFN-γ staining, cells were stimulated for 12 hours with anti-CD3 or anti-CD3 plus anti-CD28 antibodies. After further incubation with Brefeldin A (5 μg/ml) for 6 hours, stimulated cells were surface-stained with anti-CD8 antibody, fixed and permeabilized in Cytofix/Cytoperm solution (BD Biosciences — Pharmingen), and then stained intracellularly with anti–IL-2 and anti–IFN-γ antibodies. Data were acquired on an LSR II flow cytometer system (BD Bioscience) and analyzed with FlowJo software (version 4.4.1; Tree Star Inc). Purified or fluorochrome-conjugated anti-CD3, anti-CD28, anti-CD4, anti-CD8, anti–IL-2, anti–IFN-γ, anti-TCRβ, and anti-CD44 antibodies were from BD Biosciences — Pharmingen, and anti-FoxP3 was from eBioscience. Recombinant mouse TGF-β was from R&D Systems. ELISA analysis of IL-2 production was performed according to a previous protocol (22).
Immunohistochemistry and analyses of tumor-infiltrating lymphocytes.
Seven and nineteen days after tumor inoculation, tumor tissues were collected, frozen in Tissue-Tek OCT medium (Sakura), and cryosectioned. Sections were stained with either H&E or FITC–anti-CD8 antibody. Staining results were recorded by normal or fluorescence microscopy. To analyze tumor infiltrates by flow cytometry, tumor-infiltrating lymphocytes were prepared from tumor tissues 14 days after inoculation. Tumors were excised from sacrificed mice, washed in PBS, and cut into pieces 2–3 mm in size. The resulting tumor pieces were then digested at 37°C with collagenase D (1.5 mg/ml; Sigma-Aldrich) in DMEM supplemented with 2% fetal bovine serum and 50 U/ml DNase I (Sigma-Aldrich). After digestion for 40 minutes, cells were passed through a 70-μm strainer, and flow-through cell suspension was washed with PBS, stained with different combinations of antibodies, and analyzed on an LSR II flow cytometer system. Antibodies used for staining were anti-TCRβ, anti-CD4, anti-CD8, anti-CD44, and anti-FoxP3.
In vivo experiments.
Tumor cells in log-phase growth were washed 3 times with PBS and resuspended in PBS. After shaving the right flank, subcutaneous injection with the numbers of cells indicated in the figure legends and in the Results. For adoptive transfer, at day 7 after establishing tumors in C57BL/6 mice, mice were injected with 3 × 106 purified CD8+ T cells isolated from lymph node cells of WT and Cblb–/– or Cblb–/– OT1 mice using a CD8+ T cell enrichment kit (Miltenyi Biotec). Tumor growth was then monitored twice a week using a caliper. Tumor growth in Cblb–/–ATM–/– mice was measured weekly. Tumor volumes were approximated by multiplying the measured length by the measured width by the calculated mean of the measured length and width values as described previously (27).
Statistics.
All paired comparisons were subjected to a 2-tailed Student’s t test. P ≤ 0.05 was considered statistically significant.
Acknowledgments
We thank Y.R. Zou for critical reading of the manuscript and L. Chen for E.G7 cells. This work was supported by the NIH Intramural Research Program and The Irene Diamond Fund/Professorship to H. Gu.
Footnotes
Nonstandard abbreviations used: ATM–/–, ataxia telangiectasia mutated–deficient (mice).
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:1029–1036 (2007). doi:10.1172/JCI29472
Jeffrey Y. Chiang and Ihn Kyung Jang contributed equally to this work.
References
- 1.Boon T., et al. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 2.Houghton A.N., Gold J.S., Blachere N.E. Immunity against cancer: lessons learned from melanoma. Curr. Opin. Immunol. 2001;13:134–140. doi: 10.1016/s0952-7915(00)00195-3. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg S.A. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg S.A. A new era of cancer immunotherapy: converting theory to performance. CA Cancer J. Clin. 1999;49:70–73. doi: 10.3322/canjclin.49.2.70. [DOI] [PubMed] [Google Scholar]
- 5.Boon T., van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J. Exp. Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berzofsky J.A., Ahlers J.D., Belyakov I.M. Strategies for designing and optimizing new generation vaccines. Nat. Rev. Immunol. 2001;1:209–219. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- 7.Groh V., Wu J., Yee C., Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 8.Chambers C.A., Kuhns M.S., Egen J.G., Allison J.P. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 9.Somasundaram R., et al. Inhibition of cytolytic T lymphocyte proliferation by autologous CD4+/CD25+ regulatory T cells in a colorectal carcinoma patient is mediated by transforming growth factor-beta. Cancer Res. 2002;62:5267–5272. [PubMed] [Google Scholar]
- 10.Terabe M., et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 11.Restifo N.P., et al. Defective presentation of endogenous antigens by a murine sarcoma. Implications for the failure of an antitumor immune response. J. Immunol. 1991;147:1453–1459. [PMC free article] [PubMed] [Google Scholar]
- 12.Ramarathinam L., Castle M., Wu Y., Liu Y. T cell costimulation by B7/BB1 induces CD8 T cell-dependent tumor rejection: an important role of B7/BB1 in the induction, recruitment, and effector function of antitumor T cells. J. Exp. Med. 1994;179:1205–1214. doi: 10.1084/jem.179.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz R.H. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 14.Townsend S.E., Allison J.P. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 15.Chen L., et al. Tumor immunogenicity determines the effect of B7 costimulation on T cell-mediated tumor immunity. J. Exp. Med. 1994;179:523–532. doi: 10.1084/jem.179.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallin J.J., Liang L., Bakardjiev A., Sha W.C. Enhancement of CD8+ T cell responses by ICOS/B7h costimulation. J. Immunol. 2001;167:132–139. doi: 10.4049/jimmunol.167.1.132. [DOI] [PubMed] [Google Scholar]
- 17.Liu X., et al. B7H costimulates clonal expansion of, and cognate destruction of tumor cells by, CD8(+) T lymphocytes in vivo. J. Exp. Med. 2001;194:1339–1348. doi: 10.1084/jem.194.9.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu X., Abe R., Hodes R.J. The role of B7-CD28 co-stimulation in tumor rejection. Int. Immunol. 1998;10:791–797. doi: 10.1093/intimm/10.6.791. [DOI] [PubMed] [Google Scholar]
- 19.Lupher M.L., Jr., Rao N., Eck M.J., Band H. The Cbl protooncoprotein: a negative regulator of immune receptor signal transduction. Immunol. Today. 1999;20:375–382. doi: 10.1016/s0167-5699(99)01484-x. [DOI] [PubMed] [Google Scholar]
- 20.Joazeiro C.A., et al. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y.C. Ubiquitin ligases and the immune response. Annu. Rev. Immunol. 2004;22:81–127. doi: 10.1146/annurev.immunol.22.012703.104813. [DOI] [PubMed] [Google Scholar]
- 22.Chiang Y.J., et al. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 23.Bachmaier K., et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 24.Wohlfert E.A., Gorelik L., Mittler R., Flavell R.A., Clark R.B. Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-beta sensitivity in vitro and in vivo. J. Immunol. 2006;176:1316–1320. doi: 10.4049/jimmunol.176.3.1316. [DOI] [PubMed] [Google Scholar]
- 25.Gorer P.A. Studies in antibody response of mice to tumour inoculation. Br. J. Cancer. 1950;4:372–379. doi: 10.1038/bjc.1950.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore M.W., Carbone F.R., Bevan M.J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 27.Helmich B.K., Dutton R.W. The role of adoptively transferred CD8 T cells and host cells in the control of the growth of the EG7 thymoma: factors that determine the relative effectiveness and homing properties of Tc1 and Tc2 effectors. J. Immunol. 2001;166:6500–6508. doi: 10.4049/jimmunol.166.11.6500. [DOI] [PubMed] [Google Scholar]
- 28.Barlow C., et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 29.Naramura M., et al. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat. Immunol. 2002;3:1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- 30.Jeon M.S., et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Kronenberg M., Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. doi: 10.1038/nature03725. [DOI] [PubMed] [Google Scholar]
- 32.Hogquist K.A., et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]