Abstract
Objective
To characterize the neuropsychological profile of an extended family with unipolar depression (UPD) and other forms of affective illness.
Method
We administered a battery of neuropsychological tasks measuring various aspects of executive function and visual and verbal memory to 49 individuals in 1 extended family. Six participants had 1 lifetime episode of major depression (MDE-S), 15 were diagnosed with recurrent major depression (MDE-R), 11 had another DSM-IV diagnosis and 17 subjects were unaffected.
Results
After controlling for multiple confounding factors, including mood and medication, the MDE-R sample made significantly more errors than unaffected relatives on the Stroop Task, a measure of cognitive control.
Conclusion
There may be at least 1 subtype of UPD characterized by a state-independent deficit in cognitive control.
Medical subject headings: Afrikaner-Ancestry, affective illness, bipolar disorder, unipolar depression.
Abstract
Objectif
Caractériser le profil neuropsychologique d'une famille élargie aux prises avec la dépression unipolaire et d'autres formes de maladies affectives.
Méthode
Nous avons administré à 49 personnes d'une famille élargie une batterie de tâches neuropsychologiques permettant de mesurer divers aspects de la fonction d'exécution et de la mémoire visuelle et verbale. Six participants avaient connu un épisode de dépression majeure (MDE-S) au cours de leur vie, on a diagnostiqué une dépression majeure récurrente (MDE-R) dans 15 cas, posé un autre diagnostic DSM-IV dans 11 cas et 17 sujets n'étaient pas affectés.
Résultats
Après avoir tenu compte de multiples facteurs confusionnels, y compris l'humeur et les médicaments, les sujets de l'échantillon MDE-R ont fait beaucoup plus d'erreurs que les membres de la famille non affectés dans l'exécution de la tâche Stroop, mesure de contrôle de la cognition.
Conclusion
Il peut y avoir au moins un sous-type de dépression unipolaire caractérisé par un déficit indépendant de l'état au niveau du contrôle de la cognition.
Introduction
After many years of research, the genetic basis of psychiatric conditions like unipolar disorder (UPD) and bipolar disorder (BPD) remain largely elusive. The reasons behind this situation have been widely discussed in the genetics literature. Among the most salient of difficulties is genetic heterogeneity, the notion that what appears superficially to be the identical disease phenotype may be caused by different genetic variants in different families or population groups.1 As a response to these challenges, researchers have attempted to identify intermediate phenotypes that are closely associated with the disorder in question but are simpler to understand genetically.2 Theoretically, understanding the genetic basis of these (more) genetically homogenous endophenotypes will facilitate a greater understanding of the pathobiology of the Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV),3 disorder.
The approach in question has met some success in schizophrenia, with researchers demonstrating that genetic variants impacting on cognition, a putative endophenotype of the illness, comprise risk factors for the fully developed psychiatric condition.4 Neurocognitive dysfunction has also been suggested to be a forme fruste of BPD,5 and this is supported by evidence of neuropsychological deficits in unaffected relatives of BPD patients.6
A degree of consensus that BPD and, to a lesser extent, UPD are characterized by mood-independent neurocognitive dysfunction has been reached, with executive function and memory most commonly affected.6–9 Nonetheless, the philosophy that there are genetically distinct subtypes of putatively unitary psychiatric disorders has yet to influence psychological research where cross-sectional studies underpinned by the implicit assumption of etiological homogeneity are standard. This raises the question of whether the results of previous neuropsychological analyses of affective illness have been confounded by the presence of disease heterogeneity.
Our proposal receives some preliminary support from Altshuler and colleagues,10 who found that their BPD sample yielded a bimodal pattern of neurocognitive performance, thereby suggesting the presence of 2 subgroups, 1 with impairment and 1 with relatively normal neurocognitive functioning. Similarly, Thompson and colleagues11 reported that approximately 15%–25% of their BPD sample showed significant neuropsychological deficits — enough to result in statistically significant differences between the overall patient and control groups.
This study aims to evaluate the neurocognitive performance of a genetically, culturally and socioeconomically homogenous sample: an extended family with a high density of major depression and other psychiatric illnesses, including BPD.
Methods
The University of Cape Town (UCT) neuropsychiatric genetics project commenced in 1997 with the recruitment of South African families with BPD and UPD. The criterion for recruitment was the presence of 2 or more affected first-degree relatives. Thereafter, more people were recruited if they provided genetically informative information. Participants were aged 18 years and over; younger participants with a psychiatric illness were included in the study.
We recruited 77 people from 1 extended, 3-generation lineage of African ancestry as part of the UCT study. Recently, 49 individuals from the family were assessed with a battery of neuropsychological tests. Reasons for sample attrition are as follows: refusal to participate (n = 11), death (n = 4), physical illness1 and residence in a remote area of the country (n = 12).
The Afrikaners originated from a small founder population of 1000–2000 Dutch settlers who arrived at the Cape of Good Hope in 1652. Geographic, social and political factors have led to approximately 15 generations of natural population growth, with minimal external contributions to the gene pool beyond the first few generations. This phenomenon has been exploited in genetic analyses of various disorders.12
Clinically trained psychiatric nurses interviewed probands and their relatives with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I).13 Then, a psychiatrist made a diagnosis for each subject on the basis of the SCID interview and on all available family and medical records. Ethics approval was obtained from the UCT, and the research participants gave informed consent.
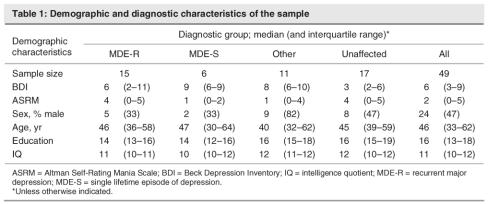
UPD was the most commonly diagnosed disorder in the family studied (n = 21), with 15 of the family members presenting with a history of recurrent major depression (MDE-R), and 6 having experienced a single lifetime episode of depression (MDE-S). Other diagnoses of interest included schizophrenia (n = 1), BPD I (n = 2), BPD not otherwise specified (n = 1), alcoholism (n = 3), generalized anxiety disorder (n = 2) and dysthymia (n = 1). The diagnosis of 1 participant was deferred. Those individuals who were not diagnosed with MDE-R or MDE-S were subsumed under the category of “other diagnosis.” Seventeen family members were unaffected. Table 1 shows the median and interquartile range for various characteristics (except sex) of the sample, stratified by diagnosis. We provide the number of males and the percentage male in that diagnostic group. The median and inter-quartile range is presented because some of the variables have skewed distributions.
Table 1
Well-validated neuropsychological tasks were used to assess the sample. Tests that measure various aspects of executive function as well as verbal and visual memory were the focus of this study, because these cognitive domains have been implicated in affective illness. The neuropsychological assessment took approximately 1 hour per person to complete and was administered in the following order: South African-Wechsler Adult Intelligence Scale (SA-WAIS) General Knowledge subtest, Digits Forward and Reverse (F+R) Controlled Oral Word Association Test (COWAT), Rey Complex Figure (RCF), Stroop Colour Word Test, Rey Auditory Verbal Learning Test (RAVLT), Wisconsin Card Sorting Test (WCST-64).
The Beck Depression Inventory (BDI) and the Altman Self-Rating Mania Scale (ASRM) were used to control for state effects. BDI scores of 10–18, 19–29 and 30 or more indicate mild, moderate and severe depression, respectively. Scores of 6 or above on the ASRM indicate mania or hypomania. Most participants were tested in their own home, whereas a small number were assessed in a counselling room at the Division of Human Genetics at UCT. Few individuals had recently been hospitalized and, in fact, numerous subjects had been stable for many years. The mean BDI score was 7.55 (standard deviation [SD] 7.85), and the mean ASRM score was 2.73 (SD 2.64). One person with MDE-R had severe depression, 2 had moderate depression and 6 suffered from mild depression, as defined by the BDI.
We used multiple linear regression to compare neuropsychological task scores across the various diagnostic groups. We entered the covariates age, sex, WAIS general knowledge, medication used and self-rated depression and mania scores into each model. We used the self-report questionnaires as an additional control for residual symptomatology and included medication as 3 dichotomous factors: antidepressant drugs, lithium and mood stabilizer other than lithium. Where significant differences were detected among the diagnostic groups, we assessed the differences between each group and the unaffected relatives, using independent 2-sided t tests and adjusting for all other groups and covariates. No participants were taking benzodiazepines or antipsychotic drugs at the time of testing. We report Spearman's rank-order correlations because the distributions of both the number of hospitalizations and the age of onset were skewed to the right.
Results
For each neuropsychological task score, Table 2 shows the median and interquartile range of each diagnostic group. It also shows the independent F statistic for the test of differences between diagnostic groups. The numerator and denominator degrees of freedom and p values are also given for each F test.
Table 2
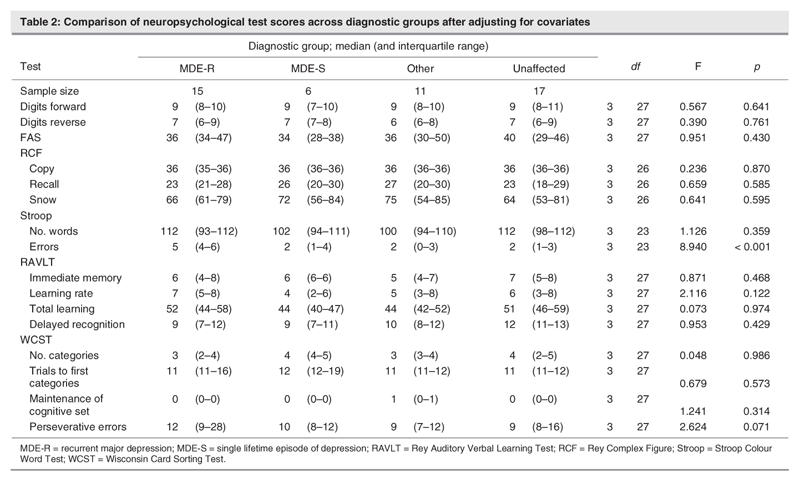
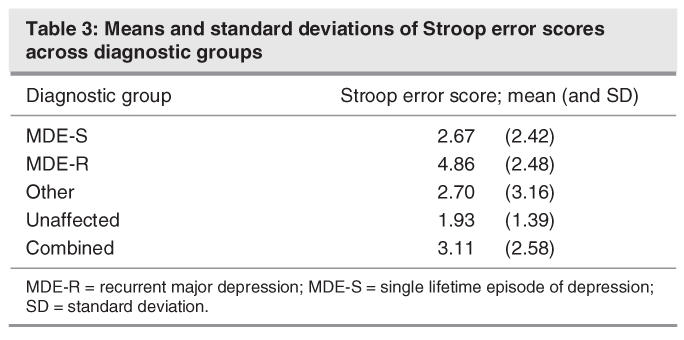
The p value for the comparison of Stroop errors between diagnostic groups is 0.00004, which will remain significant even after a conservative Bonferroni adjustment of multiplying by 11 (that is, the number of regression models assessed). The MDE-R group made significantly more errors on the Stroop Colour Word test than did their unaffected family members (estimated effect = 2.26, t = 3.16. df 23; p < 0.001). This result is independent after adjusting for all other covariates and intergroup comparisons, and it remained significant after a Bonferroni correction for multiple testing that yielded a new threshold alpha value of 0.003. The mean and SDs of the number of errors made on the Stroop task are listed in Table 3.
Table 3
Two people were admitted to hospital for mania: 1 patient was admitted once and 1 was admitted on 5 occasions; both were in the “other” diagnostic group. Five subjects with MDE-R and 2 patients in the “other” category were hospitalized for depression. Concerning MDE-R, 1 subject was hospitalized on 20 occasions, 1 person on 6 occasions and 3 on 2 occasions each. In the “other” group, 1 person was admitted 4 times and another was hospitalized twice. The Spearman correlation between the number of errors made on the Stroop Tast and the number of hospitalizations was negative (r = -0.13, p = 0.689) but not statistically significant.
Age of onset varied from 13 to 56 years, with only 6 individuals reporting onset at age over 21 years. The correlation between Stroop performance and age of onset was not significant in any of the groups. In the MDE-R group, we observed a nonsignificant negative correlation (r = -0.27, p = 0.40).
Discussion
The main finding of this study was the general absence of cognitive deficits in our family members with UPD. The exception to this pattern was the significantly more error-ridden performance of the MDE-R cohort on the Stroop Task. This result remained highly significant (p < 0.001) even after correcting for multiple covariates including medication.
The Stroop Colour Word Test is primarily a measure of the ability to inhibit overlearnt or prepotent responses in the face of conflicting information. We are aware of 3 other studies that have reported reduced cognitive control as evinced by the Stroop Test in euthymic UPD patients.9,14,15 Other studies of remitted UPD patients have reported no evidence of neurocognitive dysfunction.16,17
Impaired performance on the Stroop Task has been reported with equal frequency in remitted BPD samples,18–20 including a group of unaffected relatives of bipolar probands.21 Conversley, several studies of euthymic patients with BPD have not presented any evidence of impaired Stroop performance.22–24 A recent meta-analysis of 11 studies confirmed the finding of poor response inhibition as measured by the Stroop Task, although the effect size was relatively small (d = 0.63).25
A possible cause of these contradictory findings is the use of case-control methodologies that include genetically and etiologically heterogeneous samples of UPD and BPD patients. For example, when we analyzed our entire cohort of 45 families, we found no significant Stroop effects. However, as in many other studies, we did find verbal memory deficits (Savitz et al., unpublished data, 2006). This could be a problem for genetic endophenotyping: the use of memory (rather than cognitive control) as an endophenotype in our large sample would likely have biased subsequent genetic analyses.
In conclusion, we suggest that there could be at least 1 subtype of UPD that displays state-and medication-independent deficits in cognitive control. We do, however, note the following caveats. First, a small number of family members were diagnosed with BPD, thus it is possible that our MDE-R sample has a BPD diathesis and that our results are specific to a BPD-related form of UPD illness. Whether previously reported UPD-associated cognitive control deficits have been obtained with bipolar spectrum UPD patients is a moot point. Comparisons with other large lineages would be useful in determining whether patients with affective illness with cognitive control deficits display subtle clinical differences than their counterparts, with and without other types of cognitive impairment. Second, it is possible that our MDE-R group would show other neurocognitive deficits, compared with an unrelated group of control subjects, a possibility that should be investigated in future studies. Nevertheless, the use of unrelated relatives as a comparison group allows for greater control of common environmental influences on neurocognition. Finally, a small number (n = 11) of people originally recruited for the project, refused to participate in the next round of the study. It is theoretically possible that these individuals differed neuropsychologically from their counterparts who agreed to undergo cognitive testing.
Acknowledgments
The support of the Medical Research Council of South Africa is acknowledged. Thank you to Elize Pietersen and Gameda Benefel for conducting psychological interviews.
Footnotes
Contributors: Drs. Savitz and Ramesar designed the study. Dr. Savitz acquired the data, which Drs. Savitz, van der Merwe and Solms analyzed. Drs. Savitz and van der Merwe wrote the article; Drs. van der Merwe, Solms and Ramesar revised the article. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. Jonathan Savitz, Division of Human Genetics, Institute of Infectious Disease and Molecular Medicine, University of Cape Town, South Africa; savitzj@mail.nih.gov
References
- 1.Terwilliger JD, Goring HHH. Gene mapping in the 20th and 21st centuries: statistical methods, data analysis and experimental design. Hum Biol 2000;72:63-132. [PubMed]
- 2.Gottesman II, Gould TD. the endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 2003;160:636-45. [DOI] [PubMed]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 4.Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A 2001;98:6917-22. [DOI] [PMC free article] [PubMed]
- 5.Savitz JB, Solms M, Ramesar RS. Neurocognitive function as an endophenotype for genetic studies of bipolar affective disorder. Neuromolecular Med 2005;7:275-86. [DOI] [PubMed]
- 6.Savitz J, Solms M, Ramesar R. Neuropsychological deficits in bipolar affective disorder: a critical opinion. Bipolar Disord 2005;7:216-35. [DOI] [PubMed]
- 7.Ferrier IN, Thompson JM. Cognitive impairment in bipolar affective disorder: implications for the bipolar diathesis. Br J Psychiatry 2002;180:293-5. [DOI] [PubMed]
- 8.Clark L, Goodwin GM. State and trait-related deficits in sustained attention in bipolar disorder. Eur Arch Psychiatry Clin Neurosci 2004;254:61-8. [DOI] [PubMed]
- 9.Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. J Affect Disord 2005;89:125-35. [DOI] [PubMed]
- 10.Altshuler LL, Ventura J, van Gorp WG, et al. Neurocognitive function in clinically stable men with bipolar i disorder or schizophrenia and normal control subjects. Biol Psychiatry 2004;56:560-9. [DOI] [PubMed]
- 11.Thompson JM, Gallagher P, Hughes JH, et al. Neurocognitive impairment in euthymic patients with bipolar affective disorder. Br J Psychiatry 2005;186:32-40. [DOI] [PubMed]
- 12.Abecasis GR, Burt RA, Hall D, et al. Genomewide scan in families with schizophrenia from the founder population of Afrikaners reveals evidence for linkage and uniparental disomy on chromosome1. Am J Hum Genet 2004;74:403-17. [DOI] [PMC free article] [PubMed]
- 13.First MB, Spitzer RL, Gibbon M, et al. (1996). Structured Clinical Interview for the DSM-IV, Patient Edition. SCID-I/P (Version 2.0). Modified for the Norvatis Bipolar Disorder Genetic Study: January 7, 1998.
- 14.Trichard C, Martinot JL, Alagille M, et al. Time course of prefrontal lobe dysfunction in severely depressed in–patients: a longitudinal neuropsychological study. Psychol Med 1995;25:79-85. [DOI] [PubMed]
- 15.Paradiso S, Lamberty GJ, Garvey MJ, et al. Cognitive impairment in the euthymic phase of chronic unipolar depression. J Nerv Ment Dis 1997;185:748-54. [DOI] [PubMed]
- 16.Liu SK, Chiu CH, Chang CJ, et al. Deficits in sustained attention in schizophrenia and affective disorders: Stable versus state-dependent markers. Am J Psychiatry 2002;159:975-82. [DOI] [PubMed]
- 17.Biringer E, Lundervold A, Stordal K, et al. Executive function improvement upon remission of recurrent unipolar depression. Eur Arch Psychiatry Clin Neurosci 2005;255:373-80. [DOI] [PubMed]
- 18.Ali SO, Denicoff KD, Altshuler LL, et al. A preliminary study of the relation of neuropsychological performance to neuroanatomic structures in bipolar disorder. Neuropsychiatry Neuropsychol Behav Neurol 2000;13:20-8. [PubMed]
- 19.Zubieta JK, Huguelet P, O'Neil RL, et al. Cognitive function in euthymic bipolar I disorder. Psychiatry Res 2001;102:9-20. [DOI] [PubMed]
- 20.Martinez-Aran A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry 2004;161:262-70. [DOI] [PubMed]
- 21.Zalla T, Joyce C, Szoke A, et al. Executive dysfunctions as potential markers of familial vulnerability to bipolar disorder and schizophrenia. Psychiatry Res 2004;121:207-17. [DOI] [PubMed]
- 22.van Gorp WG, Altshuler L, Theberge DC, et al. Cognitive impairment in euthymic bipolar patients with and without prior alcohol dependence. Arch Gen Psychiatry 1998;55:41-6. [DOI] [PubMed]
- 23.Krabbendam L, Honig A, Wiersma J, et al. Cognitive dysfunctions and white matter lesions in patients with bipolar disorder in remission. Acta Psychiatr Scand 2000;101:274-80. [PubMed]
- 24.Cavanagh JTO, Van Beck M, Muir W, et al. Case-control study of neurocognitive function in euthymic patients with bipolar disorder: an association with mania. Br J Psychiatry 2002;180:320-6. [DOI] [PubMed]
- 25.Robinson LJ, Thompson JM, Gallagher P, et al. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. In press. [DOI] [PubMed]