Abstract
This paper focuses on serotonin transporter 5-HTT imaging to investigate major depressive disorder (MDD) and antidepressant occupancy. Such investigations have only recently been possible as a result of major advances in ligand development. The state of the art method is [11C] DASB PET or [11C]-3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile) positron emission tomography. [11C]DASB is a breakthrough for brain imaging 5-HTT. Compared with previous radioligands, [11C]DASB offers both high selectivity and a favourable ratio of specific binding relative to free and nonspecific binding. These characteristics contribute to valid, reliable quantitation of the 5-HTT binding potential (BP). The 5-HTT BP can be viewed as an index of 5-HTT density in a medication free state, or unblocked 5-HTT density in a medication-treated state.During major depressive episodes with no other axis I comorbidity, either no difference in regional 5-HTT BP or a trend toward elevated 5-HTT BP is typically found. During major depressive episodes (of MDD) with more severe symptoms of pessimism (dysfunctional attitudes), regional 5-HTT BP is elevated. In subjects with major depressive episodes and comorbid axis I psychiatric illnesses, decreased regional 5-HTT BP is often reported. With selective serotonin reuptake inhibitor (SSRI) treatment at doses that distinguish from placebo in the treatment of major depressive episodes, 5-HTT occupancy is approximately 80%, and there is a strong relation between plasma level and occupancy that is not predictable based on affinity alone. Implications of 5-HTT imaging findings for understanding major depressive disorder and antidepressant treatment will be discussed.
Medical subject headings: serotonin, serotonin transporter, depression, antidepressant, PET, positron emission tomography
Abstract
Cet article porte sur l'utilisation de l'imagerie de la 5-HTT, transporteur de la sérotonine, pour étudier le trouble dépressif majeur (TDM) et l'occupation des récepteurs d'antidépresseurs. Une telle étude n'a que récemment été rendue possible par les grands progrès dans le domaine des ligands. La méthode de pointe dans ce domaine est la tomographie par émission de positons avec le [11C]DASB ou [11C]-3-amino-4-(2-diméthylaminométhyl-phénylsulfanyl)-benzonitrile). Le [11C]DASB représente une percée pour l'imagerie cérébrale de la 5-HTT. Comparativement aux radioligands du passé, il se caractérise tant par une haute sélectivité que par un rapport favorable entre liaison spécifique et libre liaison non spécifique. Ce sont ces caractéristiques qui contribuent à une quantification valable et sûre du potentiel de fixation (PF) de la 5-HTT. On peut voir dans ce potentiel de fixation un indice de la densité de la 5-HTT dans un état de non-médication ou de sa densité sans blocage dans un état de médication. Dans les épisodes dépressifs majeurs sans autre comorbidité axe I, il n'y a habituellement aucune différence de PF régional ou on constate ordinairement une tendance à l'élévation de ce potentiel. Dans les épisodes dépressifs majeurs (de TDM) avec des symptômes aggravés de pessimisme (attitudes dysfonctionnelles), le potentiel de fixation régional de la 5-HTT. Chez les sujets en proie à de tels épisodes dépressifs avec troubles psychiatriques de comorbidité axe I, on signale souvent une diminution du potentiel régional. Dans le traitement d'épisodes dépressifs majeurs à l'inhibiteur spécifique du recaptage de la sérotonine (ISRS) à des doses distinctes du placebo, l'occupation des récepteurs de la 5-HTT est d'environ 80 %; on note une étroite relation entre la concentration plasmatique et l'occupation qui ne saurait être prévue uniquement par l'affinité. Il sera question des conséquences de ces données d'imagerie de la 5-HTT sur le plan de la compréhension du trouble dépressif majeur et de son traitement aux antidépresseurs.
What properties of the serotonin transporter are important for major depressive disorder?
The serotonin transporter 5-hydroxy-tryptamine (5-HTT) is a 630 amino acid long receptor with 12 transmembrane domains.1,2 The human 5-HTT gene is localized on chromosome 17, centred at 17q11.2.3 Most 5-HTT are located at outer cell membranes, either perisynaptically or along axons.4 In the human brain, the density of 5-HTT varies by region: Superior and inferior raphe nuclei > hypothalamus > thalamus (depending on the nucleus) ~ amygdala > putamen > caudate ~ hippocampus > insular cortex > prefrontal cortex > white matter > cerebellar cortex (except vermis).5–7
The serotonin transporter is coupled to sodium, chlorine and potassium transport.3 However, the physiological role of interest of 5-HTT in major depressive disorder (MDD) and antidepressant treatment is its influence on extracellular serotonin levels. It is clear that many antidepressant drugs that bind to the serotonin transporter raise extracellular serotonin, and 5-HTT knockout mice have elevated extracellular serotonin, confirming the role of the serotonin transporter in modulating extracellular serotonin levels in vivo.8–14
Methods of imaging serotonin transporter in vivo
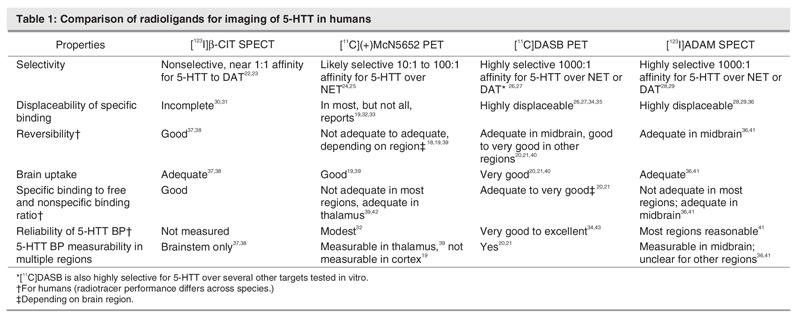
The following is a critical comparison of all 5-HTT imaging methods that have been applied in humans, with an emphasis on data relevant to humans (See Table 1). Previous comparisons have largely emphasized comparisons in baboons.15 Although this information is valuable during radiotracer development,16 it does not fully correspond to radioligand performance during human brain imaging because 5-HTT density can vary between animal species,7 and the brain pharmacokinetics of 5-HTT radiotracers can differ between baboons and humans.15,17–21
Table 1
These methods are used to derive the binding potential (BP). There are different versions of the BP but the one that is typically used is defined as follows: BP = f2 × Bmax/Kd . f2 is a fraction of free and nonspecific radiotracer that interacts with the specific binding compartment. Bmax is receptor density and Kd is the dissociation constant. BP tends to be viewed as an index of Bmax and, in the medication treated condition, it tends to be viewed as an index of receptor density not blocked by medication.
In the medication-treated state, a related measure is the 5-HTT occupancy, which can be defined as 5-HTT occupancy = (5-HTT BP1- 5-HTT BP2)/ 5-HTT BP1 × 100%. 5-HTT BP1 is the BP found in the untreated state and 5-HTT BP2 is the BP found in the treated state.
[123I]2-β-carbomethoxy-3-β-(4-iodophenyl)-tropane (β-CIT) single photon emission tomography (SPECT) was once the only technique developed for measuring the 5-HTT binding potential in humans.37,38,44 This radiotracer has almost equal affinity for the dopamine transporter, compared with the serotonin transporter.22,23 Because dopamine transporter density is high in the substantia nigra,45 one cannot determine whether any changes in specific binding in the midbrain in an experimental paradigm are due to 5-HTT binding in superior raphe nuclei or dopamine transporter binding in substantia nigra. That there are specific binding sites that are not 5-HTT is consistent with the low 5-HTT occupancy estimates for selective serotonin reuptake inhibitors found with this method,30,31 compared with 5-HTT occupancy estimates with selective 5-HTT binding radiotracers.34,35 To the best of my knowledge, there are no reliability estimates of binding potential found in the midbrain with this method. Typically, this radiotracer is used for measuring dopamine transporter BP in the striatum in humans.44
The PET radiotracer [11C](+)McN5652 (trans-1,2,3,5,6,10-β-hexahydro-6-[4-(methylthio)phenyl]-pyrrolo-[2,1-a]-isoquioline) shows greater selectivity for the serotonin transporter, compared with other monoamine transporters. It is estimated that this radiotracer has 1 or 2 orders of magnitude greater affinity for the serotonin transporter over the norepinephrine transporter and at least 2 orders of magnitude greater affinity for the serotonin transporter over the dopamine transporter.24,25 [11C](+)McN5652 has a low ratio of specific binding relative to free and nonspecific binding, which combined with modest reversibility, makes valid and reliable quantitation difficult in regions other than the thalamus, and impossible in the human cortex.18,19,32,39 Applications of this radiotracer in illness and in treatment have mostly focused on the thalamus, using the cerebellum as a reference region with noninvasive models.33,39,46 However, some investigators use arterial sampling to measure 5-HTT BP in other subcortical brain regions to obtain a total distribution volume (an index of total radiotracer binding) and use the cerebellar cortex region to obtain an index of free and nonspecific binding.32
The radiotracer [11C] 3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile (DASB) was a major advance because of its selectivity, reversibility, greater specific binding relative to free and nonspecific binding and reliability.20,21,26,27,34,35,40,43,47 This radiotracer was found to be 3 orders of magnitude more selective for the 5-HTT than for the monoamine transporters and was highly selective for the 5-HTT, compared with several other screened targets.26,27 Moreover, 92% to 95% of the specific binding to 5-HTT is displaceable by 5-HTT binding medications in animal models.26,27 In humans, [11C]DASB has good brain uptake20,40; its ratio of specific binding relative to free and nonspecific binding is good and the latter has low between–subject variability.20,21 Multiple brain regions may be assessed with noninvasive methods,20,21,26,27,34,35,40,43,47 and the reliability of regional 5-HTT BP measures is good.34,35,43,48 The 5-HTT BP measures are low in the cortex, but with standardized region of interest methods, good reliability of 5-HTT BP in the human cortex may be obtained.34,35,43,48 In summary, [11C]DASB PET imaging is the state of the art method in quantifying 5-HTT in humans.
[123I] ADAM (2-((2-((dimethylamino)methyl)-phenyl) thio)-5-iodophenylamine) SPECT is a fourth brain imaging method that has recently been applied to investigate 5-HTT BP in humans. It has a clear advantage of selectivity over [123I] β-CIT SPECT, since most of the specific binding in most brain regions is displaceable in animal models, and it is selective for the 5-HTT over several other binding sites, including other monoamine transporters.28,29 [123I] ADAM has been modelled in baboons but not yet in humans.49 The specific binding relative to free and nonspecific binding in humans is not optimal,41 likely limiting the use of this method to assessing midbrain 5-HTT BP. However, reliability in the midbrain for 5-HTT BP measurement is good.41
[11C]MADAM (11C-N,N-Dimethyl-2-(2-amino-4 methyl-phenylthio)benzylamine) is a recently developed PET radiotracer that shows excellent selectivity over other monoamine transporters in vitro and good displacability in animal models.50,51 Time activity curves presented show good reversibility potentially similar to [11C]DASB but appear to have somewhat greater variability, particularly for the raphe.20,52,53 Initial reports of reliability are also promising, although the scatter in repeated-measurement (standard deviation of percent difference in repeated-measure) appears greater than what has been reported for [11C]DASB.34,35,43,48,52,54
What is the optimal method of applying [11C]DASB PET for research protocols?
For selecting regions of interest, my group recommends automated region of interest approaches with visual validation, such as those involving subroutines from linear transformations and/or nonlinear deformations applied in the spatial normalization procedure from statistical parametric mapping.55,56 Reliability of 5-HTT BP measurement is typically excellent when such applications are applied.35,43,54,57 For subcortical regions, manual drawing upon coregistered MRI also has excellent reliability.34
For a reference region, my group recommends selecting the posterior half of the cerebellar cortex, excluding vermis, excluding white matter and keeping at least one full width half the maximum from the venous sinuses and from occipital cortex. At a distance of one full width half maximum, spillover from the occipital cortex (which possesses specific binding) or venous sinuses is negligible. White matter is excluded because [11C]DASB has different kinetics in this tissue, compared with grey matter. The vermis is excluded because it has [11C]DASB kinetics compatible with significant specific binding. We routinely use these methods.34,35,43,58,59
For selecting models for region of interest methods, we endorse reference tissue approaches.20,21,34,35,43,58,59 By applying a linear regression between 5-HTT density and total distribution volume, we estimate that the reference tissue of posterior cerebellar cortex is composed of 93% free and nonspecific binding and 7% specific binding.6 Knowing that the true BP = distribution volume of specific binding in region of interest divided by the distribution volume of free and nonspecific binding in the cerebellar cortex, the effect of specific binding in the cerebellar cortex is quite subtle. Disease influences of even 50% magnitude on the specific binding in reference tissue translate to 3.5% changes in the distribution volume estimate of free and nonspecific binding, which ultimately results in a 3.5% bias for between–group comparisons. For occupancy studies, the nature of the occupancy equation is such that the bias from a 7% underestimate during untreated conditions is translated into a lesser bias in the overall occupancy measure (less than 2%). For example, if the striatal 5-HTT BP has a true value of 1 in the untreated condition and 0.2 in the SSRI-treated condition, the true 5-HTT occupancy is ([1–0.2]/1) = 0.8 or 80% (5-HTT occupancy = (5-HTT BP1–5-HTT BP2)/ 5-HTT BP1 × 100%). Taking into account the slight specific binding of reference tissue, the measured striatal 5-HTT BP, respectively, would be 0.93 in the untreated condition and 0.197 in the SSRI-treated condition (most of the 7% specific binding in reference tissue is blocked during treatment), leading to a measured 5-HTT occupancy of ([0.93–0.1972]/0.93) = 0.79 or 79%.
For [11C]DASB PET, arterial methods offer no advantage for identifying subcompartments of free and nonspecific binding, because [11C]DASB kinetics fit a single tissue compartment model in all regions.21,60 Arterial methods do permit measurement of total distribution volume in the cerebellum, but this value is assumed to represent free and nonspecific binding, so as to quantitate binding potential measures in other regions. Thus, when arterial sampling is done, a very similar set of assumptions as compared with reference tissue models are applied.
Among the reference tissue methods, the noninvasive logan,61 simplified reference tissue model 2 and multilinear reference tissue model 221 have excellent reliability.34,35,43,54,58,59 The latter two also avoid underestimating.21 The Logan has some underestimate but correlates highly with the ratio of the distribution volume in regions with specific binding to the distribution volume in the cerebellum.35 Moreover, with the Logan, the coefficient of variation is very low, and it has less assumptions (i.e., it does not require the single tissue compartment model).61,62 For region of interest measurement in disease processes, we favour all 3 methods, but for drug-treated conditions, we prefer the Logan (to avoid requiring the single tissue compartment assumption across different levels of 5-HTT occupancy).62
What is the key evidence for low extracellular serotonin in untreated MDD?
Direct evidence that serotonin is low in MDD is unavailable for 2 main reasons: Brain serotonin cannot be directly measured in vivo and it is likely, based on animal simulations of postmortem delay, that serotonin levels are unstable, even within 24 hours of death.63 Moreover, postmortem investigations of serotonin levels (previously listed by Mann64) have not sampled medication-free subjects with MDD in the midst of a major depressive episode (MDE).
Therefore, arguments that extracellular serotonin in the brain is likely to be low during MDE are based on the reversal of symptoms after serotonin-raising antidepressant drugs,65–68 lowering of mood during paradigms that lower brain serotonin,69–77 and changes in indices of serotonin 2 receptor density in suicide and MDD.78–90
This latter argument can be further clarified. An important property of 5-HT2 receptors is that 5-HT2 receptor density has an inverse relation to extracellular serotonin levels, such that the density of 5-HT2 receptors in the cortex increases after chronic serotonin depletion and decreases after chronically raising extracellular serotonin.91–94 Therefore, investigations of indices of 5-HT2 density would be expected to report elevations in the midst of MDE. Postmortem investigations sometimes report elevated 5-HT2 density in the prefrontal cortex of suicide victims,78–88 and several of the investigations that found elevated prefrontal 5-HT2 receptor density investigated subjects with MDD.81,84 Such studies could be considered supportive of low extracellular serotonin in the prefrontal cortex of subjects with MDD.
Studies of 5-HT2 receptors in the brain cortex usually measure 5-HT2A receptors because ligand binding to 5-HT2C receptors in the cortex is extremely low95,96 and mRNA of 5-HT2B receptors is extremely low in the cortex.97
At first review, there appears to be a contradiction between the results of postmortem and brain imaging studies of cortex 5-HT2 receptors in MDD. Most of the imaging studies listed in Table 2 report a regional decrease in 5-HT2 BP. The discrepancy can be resolved partly by the observation that most of these studies sampled subjects recently treated with serotonin-raising antidepressant drugs.
Table 2

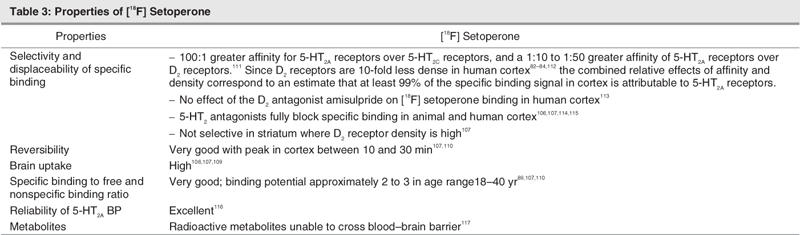
Upon further review of Table 2, the studies sampling subjects who recently had antidepressant treatment tend to report decreased regional 5-HT2 BP, whereas the 2 studies not sampling subjects who recently had antidepressant treatment find no difference between subjects with depression and healthy subjects.101,102 The study by Meyer and colleagues applied [18F]setoperone PET. [18F]setoperone is a good radioligand for imaging 5-HT2A receptors because of its specific binding in the cortex, reversibility and favourable ratio of specific binding to free and nonspecific binding106–110 (see Table 3 for properties of [18F]setoperone). It is also insensitive to acute paroxetine-induced changes in extracellular 5-HT in humans.118 The study by Meyer and colleagues sampled medication-free (> 6 mo), early-onset depression subjects with no comorbid psychiatric illnesses and found no difference in the prefrontal cortex 5-HT2 BP, compared with healthy control subjects.101 An investigation using [18F] altanserin PET in older subjects with depression who were medication free similarly found no difference in 5-HT2 BP between patients and healthy control subjects.102 After considering medication-free status, there was still a lesser discrepancy, such that 5-HT2 density was often elevated in the prefrontal cortex in postmortem studies of suicide victims, yet prefrontal 5-HT2 BP was not changed in medication-free subjects with depression.
Table 3
To resolve this lesser discrepancy, a more complicated model of low-cortex serotonin during MDEs was hypothesized. This hypothesis was that extracellular serotonin loss is heterogenous during depressive episodes and that the loss is most severe in people with a greater severity of particular symptoms.
The symptom chosen in this hypothesis was elevated pessimism (dysfunctional attitudes) observed during MDEs. (There is a modest level of dysfunctional attitudes that increase during depressive episodes.) The rationale for choosing elevated dysfunctional attitudes is that raising extracellular serotonin after administering intravenous d-fenfluramine is associated with a strong shift in dysfunctional attitudes toward optimism 1 hour later in healthy individuals.89 This suggests that, among the many roles of serotonin, one of them is to modulate dysfunctional attitudes in humans.
Dysfunctional attitudes can be measured with the dysfunctional attitudes scale (DAS), a measure sensitive for detecting negative thinking in the midst of depressive episodes119,120 that has good internal consistency (Cronbach's α = 0.85 to 0.87)121,122 and high test-retest reliability.122,123
If the hypothesis were true that extracellular serotonin in the cortex is variably reduced in the midst of depressive episodes, with the largest reductions in people with the most severe dysfunctional attitudes (pessimism), one would expect the highest prefrontal 5-HT2 BP in subjects with the most severe dysfunctional attitudes (pessimism). This is based on the finding that 5-HT2 receptor density increases after long-term serotonin depletion.92,93 In support of the hypothesis, a strong correlation was observed between severity of dysfunctional attitudes (pessimism) and elevation in cortex 5-HT2 BP.89 Moreover, cortex 5-HT2 BP was significantly elevated in subjects with severe depression with severe pessimism. For example, in the prefrontal cortex region centered on Brodman's area 9, 5-HT2 BP was elevated 29% in depression subjects with dysfunctional attitude scores higher than the median for the group (Fig. 1). Thus the 2 parts of the study combined argue that extracellular serotonin is low in depression subjects who have a greater severity of pessimism.89
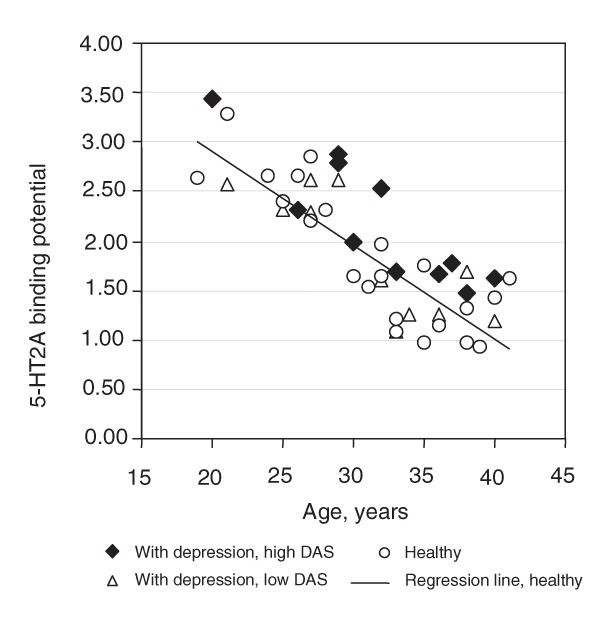
Fig. 1: 5-HT2A receptor binding potential in averaged bilateral middle frontal gyrus (Brodmann's area 9) is plotted against age to show the relation between depressed and healthy subjects. The 22 depressed patients were divided into high and low dysfunctional attitudes scale (DAS) groups, depending on whether their DAS scores were above or below the median DAS score for depression patients. This median score was 166. Patients with high DAS scores had significantly higher 5-HT2A receptor binding potential, compared with healthy subjects (ANCOVA [age covariate], diagnosis, F1,19 = 11, p = 0.003). Reprinted with permission from the American Journal of Psychiatry.
This investigation89 is the first imaging study to show consistency with postmortem investigations reporting increased 5-HT2 receptor density in the prefrontal cortex: The dysfunctional attitudes scale is well correlated with hopelessness, as measured with the Beck Hopelessness Scale.124–127 Given that hopelessness is a risk factor for suicide,128,129 it is plausible that investigations of suicide victims reporting increased 5-HT2 BP sampled depression subjects with a greater severity of pessimism. A recent report has replicated the relation between cortex 5-HT2A BP and severity of dysfunctional attitudes in recovered depression patients.130
Serotonin receptor binding that relates to extracellular serotonin in subcortical regions is also important. An interesting postmortem study by Stockmeier and colleagues90 found elevated [3H]OH-DPAT binding in the dorsal raphe nucleus of subjects in the midst of depressive episodes. Replication of this finding has been reported in suicide victims.131 [3H]OH-DPAT, being a 5-HT1A agonist, would be expected to have elevated binding when serotonin is low.132 In this interpretation, the result would support a hypothesis of lower extracellular serotonin in the dorsal raphe region (although this finding could also be viewed as a mechanism that enhances inhibition of the dorsal raphe by nearby extracellular serotonin90).
Possible disease models and the serotonin transporter in MDD
If extracellular serotonin is low during MDEs, with greater severity of dysfunctional attitudes, then it is logical to consider serotonin transporter function in the removal of extracellular monoamines. There are at least 4 plausible ways to understand how 5-HTT BP, an index of 5-HTT density and affinity, could be altered in a disease that lowers brain serotonin, described below. Prior to investigations of [11C]DASB imaging during depressive episodes, model 1 and model 3 were the most plausible.
Model 1
The first possible model is a lesion model. If serotonin nerve terminals were destroyed in MDD, then one could expect that there would be less release of serotonin. This model is observed in neurotoxin exposure133 and late in Parkinson's disease.134,135 If there were less nerve terminals in MDD, then one would expect a reduction in regional 5-HTT BP.
Model 2
A second model to consider would be whether a lowering of extracellular serotonin (via a process unrelated to 5-HTT sites) would have a secondary effect on 5-HTT density. Acute reductions in serotonin have repeatedly shown reductions in 5-HTT mRNA.136–138 However, longer-term reductions or elevations in serotonin typically show no influence on regional 5-HTT density.139–141 The is not comparable to other monoamine transporters. For example, for the dopamine transporter, the evidence is much stronger to support a relation between long-term reductions in extracellular dopamine and a lowering of striatal dopamine transporter density.142–145 This second model seems unlikely.
Model 3
A third model is increased clearance of serotonin via greater density of 5-HTT. There is an inverse relation between functioning 5-HTT and extracellular serotonin. For example, it has been demonstrated through antidepressant occupancy and 5-HTT knockout models that less functioning 5-HTT are associated with greater extracellular serotonin.9–14,146 It is possible that, under conditions of greater 5-HTT density, greater extracellular serotonin loss may occur. With this model, greater 5-HTT BP would be associated with more severe serotonin loss.
Model 4
A fourth model to consider is endogenous displacement. Endogenous displacement refers to the property, found in a minority of PET radiotracers under physiological conditions, to have increased binding potential measures after a reduction in endogenous neurotransmitter.147 The name originates from the initial explanation for this phenomenon, that the neurotransmitter itself prevented access of the radiotracer to receptors. For [11C]DASB, endogenous displacement may occur with large magnitude changes in extracellular 5-HT but would not be expected to occur with extracellular 5-HT changes that are physiologically relevant for humans. In an animal study with [11C]DASB, after raising extracellular serotonin with an intraperitoneal injection of 10 mg/kg of tranylcypromine, a MAO-A and MAO-B inhibitor, a reduction in 5-HTT BP was observed.148 Similar work has been replicated with similarly substantial doses of tranylcypromine.149 Notably, the rise in extracellular serotonin with high doses of tranylcypromine is enormous, with a several hundred to thousand percent rise being typical.150–152 Humans cannot tolerate 1/10 this dose of tranylcypromine, even with lengthy titrations and oral administration. Thus this magnitude of serotonin change may exceed what is physiologically relevant in humans. In 14 humans, we examined the effect of tryptophan depletion upon 5-HTT and found no effect, demonstrating that endogenous serotonin occupancy is unlikely to appreciably influence [11C]DASB43 under physiologically tolerable conditions. Talbot and colleagues reported similar results in 8 humans.153 Hence, the fourth model is unlikely to apply to PET imaging studies with [11C]DASB in humans.
Studies of the serotonin transporter in MDD
There are only 2 postmortem investigations of 5-HTT density in subjects with recent symptoms of depressive episodes. In these 2 studies, no changes in 5-HTT density were found in either the dorsal raphe or the locus coeruleus.154,155 Other postmortem studies of 5-HTT density sampled subjects with a history of a depressive episode (not necessarily recent) and typically focused on prefrontal cortex regions. These studies tend to report either decreased 5-HTT density156–159 or no difference in 5-HTT density.160–165 In several of these studies, subjects were medication free, based on clinical history and toxicological screening.158,160,161 For many of these investigations, average postmortem delays were less than a day.154–156,158,159,162
Most of these studies were recently reviewed in detail by Stockmeier and colleagues.88 However, most postmortem studies are not representative of a depressive episode, since only 2 studies of 5-HTT density sampled subjects who recently had symptoms.154,155 Further, the postmortem studies are not completely selective for MDD because all include comorbid axis I psychiatric illnesses156–162 and some also sample bipolar disorder.158,160,165 A third key issue with sampling is that all studies include both early-and late-onset MDD.156–162
The first imaging study of 5-HTT in vivo used [123I]β-CIT SPECT to measure specific binding of [123I]β-CIT in the hypothalamus-midbrain region in depressed patients and healthy control subjects.166 See Table 4 for a descriptive list of imaging studies of the 5-HTT. A reduction in the binding potential was found. Interestingly, subjects with major depression were subdivided based on recency of medication use, and this did not affect the results. The following methodological issue makes this finding difficult to interpret: Because [123I]β-CIT has similar affinity for both 5-HTT and DAT transporters166 and because both the raphe and substantia nigra are within this region sampled, it is not clear whether specific binding of β-CIT reflects 5-HTT binding or DAT binding. The DAT downregulates in striatum after long-term dopamine depletion.142–145 If DAT in substantia nigra downregulate similarly and if there is a monoamine lowering process in the midbrain during depressive episodes, it would be expected that an index of specific binding (to serotonin and dopamine transporters) would be lower.
Table 4

The next study of 5-HTT applied [11C](+)McN5652 PET to measure thalamus 5-HTT BP in 7 subjects with MDD.46 The data from these subjects were added to data from 6 subjects who had bipolar disorder. Ichimiya found that, in subjects with either MDD or bipolar disorder, 5-HTT BP in the thalamus is elevated.46 A strength of the study was that subjects were medication free, and a more selective radioligand was used. A disadvantage of the study is that it may be incorrect to assume that unipolar MDEs and bipolar MDEs have a common serotonin transporter abnormality. This study did not investigate 5-HTT BP during a current MDE because only 5 subjects with a current MDE and MDD were enrolled in the study.46
The next study was the first application of [11C]DASB PET imaging to MDD. It sampled 20 subjects with MDE and 20 healthy controls.170 This sample had a number of advantages because it was reasonably large, subjects were medication free for at least 3 months, and they had no other comorbid axis I illnesses, were nonsmoking, and had early onset depression. As a result of the technical advantages of [11C]DASB, 5-HTT BP could be reliably measured in multiple brain regions, including cortex. There was no evidence to support a hypothesis of a difference in 5-HTT BP as no difference in regional 5-HTT BP was found between MDE and healthy subjects in any brain region.170
However, in this same study of [11C]DASB PET,170 there was highly significant support for the hypothesis that greater regional 5-HTT BP would occur during MDE with severe, pessimistic dysfunctional attitudes. This hypothesis was based on the interpretation of 3 findings which argue that extracellular serotonin is lowest during MDE with severe, pessimistic, dysfunctional attitudes: The first finding is the acute shift toward optimism in humans after raising extracellular serotonin with d-fenfluramine which argues for a role of serotonin in modulating pessimism/optimism in humans.89 The second finding is that cortex 5-HT2 BP is greater during MDE with severe pessimism.89 This can occur when extracellular serotonin is low, according to the third set of findings: 5-HT2 receptor density increases when extracellular serotonin is chronically lowered92,93 (for more detail, see Key Evidence For Low Extracellular Serotonin in Untreated Major Depressive Disorder in this article). The subgroup of MDE subjects with severely pessimistic dysfunctional attitudes had significantly higher 5-HTT BP, compared with healthy subjects, in brain regions sampling serotonin nerve terminals (prefrontal cortex, anterior cingulate, thalamus, bilateral caudate, bilateral putamen) (see Fig. 2). On average, 5-HTT BP was 21% greater in these regions in MDE subjects with severely pessimistic dysfunctional attitudes (see Fig. 3). Moreover, within the MDE group, greater 5-HTT BP was strongly associated with more negativistic dysfunctional attitudes in the same brain regions. The interpretation was that serotonin transporters have an important role in influencing extracellular serotonin during MDEs: Greater regional 5-HTT BP can provide greater vulnerability to low extracellular 5-HT and symptoms of extremely negativistic dysfunctional attitudes.170

Fig. 2: Correlations between dysfunctional attitudes and serotonin transporter binding potential (5-HTT BP) in some of the larger regions in depression subjects. Highly significant correlations were found: prefrontal cortex (p = 0.0004), anterior cingulate (p = 0.002), bilateral putamen (p = 0.0002), bilateral thalamus (p = 0.001). Reprinted with permission from the Archives of General Psychiatry.

Fig. 3: Comparison of regional 5-HTT BP between 8 subjects with depression with severely negativistic dysfunctional attitudes (greater than 190) and 20 healthy subjects. For regions primarily sampling serotonergic nerve terminals (prefrontal cortex, anterior cingulate, caudate, putamen, thalamus) the 5-HTT BP was significantly greater in the depressed group (F1,26 = 5.6 to 12.2, p = 0.03 to 0.002). The midbrain 5-HTT BP was not significantly different (F1,26 = 0.5, p = 0.5). Reprinted with permission from the Archives of General Psychiatry.
It is premature to conclude upon the etiology of an elevation in 5-HTT BP in the subset of MDE subjects with more severe dysfunctional attitudes, because one could consider both genetic171 and environmental influences.172 The simplest explanation is that the subgroup with greater pessimism happened to inherit a greater 5-HTT density. Under this explanation, it would be expected that inheriting a greater 5-HTT could increase the risk of acquiring a MDD with more severe pessimism during MDEs.
The relation between genotypes such as the 5-HTTLPR and/or 5-HTT LPR (LA/LG) and brain 5-HTT density or binding potential is an area of ongoing study.59,158,173–178 Some investigators interpret the genotype associated with greater 5-HTT synthesis in cell lines as being reflective of 5-HTT density in the brain. The genotype associated with greater 5-HTT synthesis is sometimes associated with greater clinical response179–182 and better long-term outcome.183 It is well known that better antidepressant responsiveness predicts long-term outcome.184–187 This body of literature does not need to be inconsistent with the finding of greater 5-HTT BP in depression subjects with more severe dysfunctional attitudes.170 From a theoretical perspective, it could certainly be that subjects with depression with the highest 5-HTT binding potential and the lowest levels of extracellular serotonin could have more severe pessimism, yet they could be more responsive to SSRI treatment and have better long-term outcomes as a result of being more responsive to SSRI treatment.
The other [11C]DASB PET imaging study in mood disorder sampled depression subjects with bipolar disorder.188 Since the idea of increased 5-HTT BP being associated with illness or severity of symptoms is still new, it is interesting that 5-HTT BP was significantly greater at the uncorrected level in 5 of 8 predefined regions of interest (with a similar trend in a sixth region). On a voxel level analysis, medial prefrontal cortex, thalamus, caudate and insula had significantly greater 5-HTT BP after accounting for multiple comparisons. No region had a significant decrease in 5-HTT BP after correcting for multiple comparisons.
A fourth study of brain 5-HTT in depression applied [123I]ADAM SPECT to study 7 subjects with MDEs and 6 healthy control subjects.167 Thalamus, striatum and midbrain regions were assessed. No difference in 5-HTT BP was found in the thalamus and striatum; however, a reduction in midbrain 5-HTT BP was reported. Limitations of the study were that 2 subjects had selective serotonin reuptake inhibitors as recently as 3 weeks previously and the small sample size.
A fifth study applied [11C](+)McN5652 PET to study depressed and healthy subjects.168 A strength of the study was that the sample size was reasonable and a subdivision of antidepressant naive subjects was gathered. The authors employed an approach of adding constants to data and applying the natural logarithm to 5-HTT binding potential values. It was reported that the 5-HTT binding potential was lower in the midbrain and amygdala, but it is unclear that this would have been the case had untransformed 5-HTT BP values been presented.
The sixth study applied [123I]ADAM SPECT169 in subjects with depression and in healthy subjects and found a trend toward increased midbrain 5-HTT BP. The main strengths of the study related to the sample of depression subjects selected: A reasonable number of medication-free subjects who had no comorbid illnesses were enrolled (n = 21) .
Summary interpretations of 5-HTT imaging studies in MDD
The following interpretations summarize key findings of 5-HTT imaging studies in MDD:
• Studies that exclude comorbid axis I illnesses (Meyer and others,170 Ichimiya and others,46 Herold and others169) tend to find either no change in regional 5-HTT BP or an increase in 5-HTT BP.
• The only study of [11C]DASB PET with rigorously collected samples found no difference in regional 5-HTT BP.170 This provides a strong argument against a degenerative model of loss of serotonin neurons (ruling out model 1 under possible disease processes section).
• The study of [11C]DASB PET found significantly greater 5-HTT BP in depression subjects with more severe pessimism.170 This finding argues that the contributing mechanism to extracellular serotonin loss is excessive 5-HTT (supporting model 3 under possible disease processes section).
• The [11C]DASB PET study by Meyer and colleagues found no regional differences in 5-HTT BP between subjects with depression and healthy subjects. This study sampled depression subjects with MDD and no comorbid axis I psychiatric illnesses. Both imaging151,172 and postmortem studies156–159 that include other comorbid axis I illnesses in their sampling found some regional decreases in 5-HTT BP. It is possible that the findings in studies that sample comorbid axis I psychiatric illnesses reflect effects of common comorbid illnesses rather than MDD alone.
Studies of serotonin transporter cccupancy
Prior to the first [11C]DASB PET study, it was generally thought that 5-HTT occupancy of current SSRI was close to 100%, given the known plasma levels and known affinity of commonly prescribed SSRIs. The problem of using plasma levels as direct, identically proportionate estimates of brain levels of antidepressant drugs is that this method assumes medications are equally and readily brain penetrant. These assumptions are tenuous because there are active transport processes that remove medication from the brain, and brain uptake is also related to lipophillicity. The initial work by Pirker and colleagues,30 which had about 50% occupancy, was assumed to be inaccurate due to binding of β-CIT to DAT. However, they did demonstrate that citalopram entered the brain and had 5-HTT occupancy in humans. See Table 5 for a descriptive list of imaging studies of 5-HTT occupancy.
Table 5
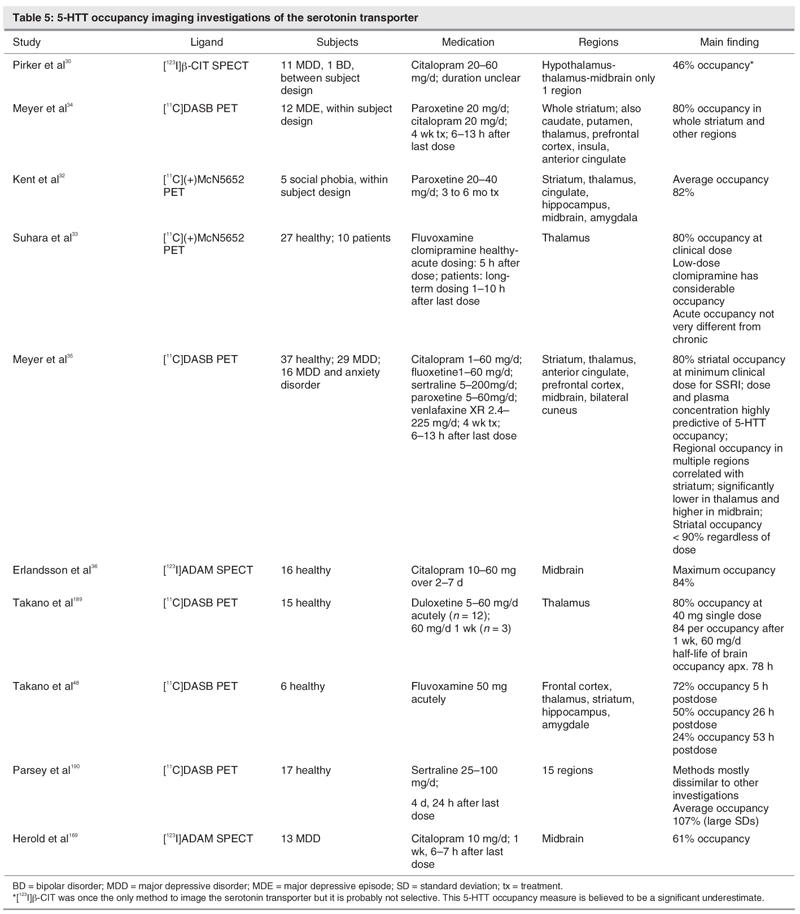
In 2001, the first SSRI occupancy study with [11C]DASB PET found an 80% occupancy in multiple regions after 4 weeks of antidepressant treatment, with doses of paroxetine and citalopram known to have clinical effects that distinguish from placebo.34 Since that time, this result has been replicated in brain regions of reasonable size with fluvoxamine33 as well as fluoxetine, sertraline and venlafaxine35 (see Fig. 4 and Fig. 5). Interestingly, an 80% striatal 5-HTT occupancy occurs at minimum clinical dose, despite the 100-fold range in affinity of the 5 SSRIs for the serotonin transporter.35 Further, the in vitro EC50 does not correlate with affinity.35 This demonstrated that, although affinity is obviously a very important property of a drug, it cannot predict occupancy, even when plasma levels are known.35

Fig. 4: 5–HTT occupancy at minimum therapeutic dose. Mean striatal serotonin transporter (5-HTT) occupancy for 5 selective serotonin reuptake inhibitors after 4 wk of minimum therapeutic dosing. The vertical ranges represent standard deviation. Subjects received citalopram 20–40 mg (n = 7), fluoxetine 20 mg (n = 4), sertraline 50 mg (n = 3), paroxetine 20 mg (n = 7), venlafaxine XR 75 mg (n = 4). Reprinted from the American Journal of Psychiatry.
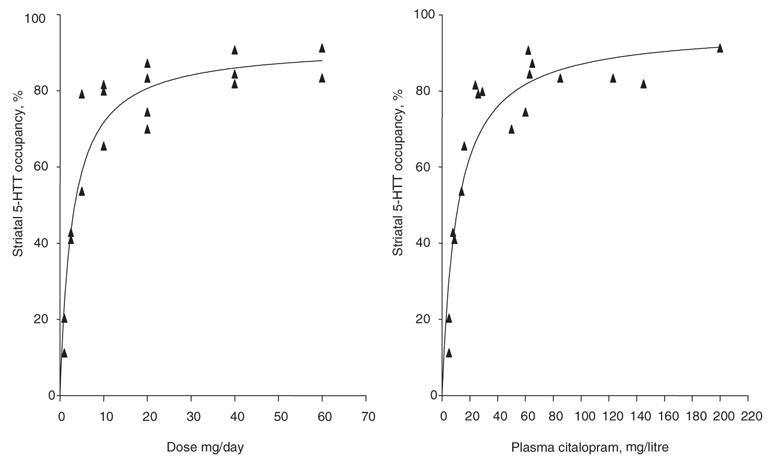
Fig. 5: Relation between striatal 5-HTT occupancy and dose* or plasma concentration† of citalopram. *†The data were fit using an equation of form f(x) = a*x/(b+x). *The relation between dose and occupancy was highly significant (f(x) = 92*x/(b+x), F1,16 = 127, p < 0.0001). †The relation between plasma level and occupancy was highly significant (f(x) = 96*x/(b+x), F1,16 = 103, p < 0.0001). Reprinted from the American Journal of Psychiatry.
Given the association between the clinically relevant dose and 5-HTT occupancy for all SSRIs, it is now generally believed that an 80% 5-HTT occupancy with an SSRI is therapeutically useful. Consequently, to develop antidepressant drugs with serotonin transporter binding, an 80% serotonin transporter occupancy is considered optimal. This practical approach can be applied to phase I trials to assess whether potential new antidepressant drugs are adequately brain penetrant and to guide dosing selection for subsequent phase II clinical trials.
The 2004 study35 also studied the relation between 5-HTT occupancy and plasma level for 5 commonly prescribed SSRIs. There was increasing occupancy with increasing plasma levels, and occupancy plateaued at the higher doses and higher plasma levels. An important result of this study was that both dose and especially plasma level, had a very strong relation to 5-HTT occupancy. This has several practical clinical applications. First, it is unlikely that inadequate 5-HTT occupancy is a barrier to therapeutic response, because one may simply raise the dose of the SSRI to obtain adequate plasma levels. Second, in clinical circumstances, to estimate 5-HTT occupancy, a 5-HTT imaging study does not need to be completed. Instead, one may use the plasma level and the table in the study to estimate the 5-HTT occupancy of any SSRI.35 Third, given the plateau of 5-HTT occupancy in the clinical dosing range, it is unlikely that 5-HTT occupancy has a strong relation to clinical response within current clinical dosing ranges (as was observed).35
In the [11C]DASB studies of 2001 and 2004, 5-HTT occupancy of SSRIs did not exceed 90%. This raises the question as to whether there is a gap in current therapeutic development, such that SSRIs with extremely high 5-HTT occupancy are not available. Suhara and colleagues reported near 100% 5-HTT occupancy with clomipramine, using [11C](+)McN5652 PET within clinical dosing ranges.33 This has clinical implications because it suggests that clomipramine may be associated with high 5-HTT occupancy within clinical dosing ranges. This result is consistent with the clinical preference for clomipramine or high-dose SSRI for obsessive compulsive disorder191 (for which greater 5-HTT occupancy is preferred).
To date, it seems that 5-HTT occupancy values after short-term dosings largely resemble the findings after 4 weeks of SSRI dosing.33–36,48,169 This has implications for antidepressant development, because it is often desirable to study single-dose occupancy in early phase I investigations, before multiple dosing studies. Results by Parsey and colleagues190 are similar in subcortical regions but may be discrepant in other regions; this may require further study.
Future 5-HTT occupancy investigations are likely to focus on novel antidepressant drugs that bind to both the serotonin transporter and other targets with high affinity.189 An interesting question for future research will be whether an 80% occupancy in reasonably large brain structures is necessary for therapeutic effect when antidepressants target additional therapeutic sites.
Main Findings and Implications
The following are the key findings and/or implications from studies of 5-HTT occupancy:
• An 80% regional 5-HTT occupancy, particularly in striatum, typically occurs at doses of SSRIs known to distinguish from placebo in clinical trials of MDD.33–35
• Affinity values, even with accompanying blood plasma drug levels, cannot predict 5-HTT occupancy.34,35 5-HTT imaging methods are essential to predict 5-HTT occupancy.
• Inadequate 5-HTT occupancy alone cannot adequately explain treatment refractoriness, because 5-HTT occupancy is most strongly related to dose and plasma levels.34,35
• 5-HTT occupancy will be a useful tool for antidepressant development, either to develop antidepressant drugs for first-line treatment (ideally with near 80% 5-HTT occupancy34,35) or to develop antidepressant drugs with higher 5-HTT occupancy values33 for treatment refractory depression.
Acknowledgments
I would like to thank Alan Wilson who discovered (first to synthesize and characterize) the [11C]DASB compound. I would like to also thank Nathalie Ginovart and Sylvain Houle for their modeling efforts with [11C]DASB. I would also like to thank members of the Toronto PET centre who have actively contributed to studies of serotonin receptors in depression or supporting technology, especially Armando Garcia, Sandra Sagrati, Anahita Carbonneau, Anna Carella, Verdell Goulding, Terry Bell, Ted Harris-Brandts, Alvina Ng and Doug Hussey. Funding support from the Canadian Institutes of Health Research, NARSAD and Eli Lilly Canada was also appreciated.
Footnotes
2005 CCNP Young Investigator Award Paper
Competing interests: Dr. Meyer has acted as a paid consultant to Lundbeck and GlaxoSmithKline.
Correspondence to: Dr. Jeffrey Meyer, Neurochemical Imaging Program in Mood Disorders, PET Centre, Centre for Addiction and Mental Health, 250 College St., Toronto ON M5T 1R8; fax 416 979-4656; jeff.meyer@camhpet.ca
References
- 1.Lesch KP, Wolozin BL, Estler HC, et al. Isolation of a cDNA encoding the human brain serotonin transporter. J Neural Transm Gen Sect 1993;91:67-72. [DOI] [PubMed]
- 2.Lesch KP, Wolozin BL, Murphy DL, et al. Primary structure of the human platelet serotonin uptake site: identity with the brain serotonin transporter. J Neurochem 1993;60:2319-22. [DOI] [PubMed]
- 3.Blakely R, Ramamoorthy S, Qian Y, et al. Regulation of Antidepressant-Sensitive Serotonin Transporters. In: Reith M, editor. Neurotransmitter Transporters: Structure, Function, and Regulation. Totowa: Humana Press, 1997. p. 29-72.
- 4.Zhou FC, Tao-Cheng JH, Segu L, et al. Serotonin transporters are located on the axons beyond the synaptic junctions: anatomical and functional evidence. Brain Res 1998;805:241-54. [DOI] [PubMed]
- 5.Cortes R, Soriano E, Pazos A, et al. Autoradiography of Antidepressant Binding Sites in the Human Brain: Localization Using [3H]Imipramine and [3H]Paroxetine. Neuroscience 1988;27:473-96. [DOI] [PubMed]
- 6.Kish SJ, Furukawa Y, Chang LJ, et al. Regional distribution of serotonin transporter protein in postmortem human brain: is the cerebellum a SERT-free brain region? Nucl Med Biol 2005;32:123-8. [DOI] [PubMed]
- 7.Laruelle M, Vanisberg MA, Maloteaux JM. Regional and subcellular localization in human brain of [3H] paroxetine binding, a marker of serotonin uptake sites. Biol Psychiatry 1988;24:299-309. [DOI] [PubMed]
- 8.Blier P, De Montigny C. Electrophysiological Investigations On The Effect of Repeated Zimelidine Administration on Serotonergic Neurotransmission in the Rat. J Neurosci 1983;3:1270-8. [DOI] [PMC free article] [PubMed]
- 9.Bel N, Artigas F. Fluvoxamine preferentially increases extracellular 5-hydroxytryptamine in the raphe nuclei: an in vivo microdialysis study. Eur J Pharmacol 1992;229:101-3. [DOI] [PubMed]
- 10.Bel N, Artigas F. Chronic Treatment With Fluvoxamine Increases Extracellular Serotonin in Frontal Cortex but Not in Raphe Nuclei. Synapse 1993;15:243-5. [DOI] [PubMed]
- 11.Dreshfield LJ, Wong DT, Perry KW, et al. Enhancement of fluoxetine-dependent increase of extracellular serotonin (5-HT) levels by (-)-pindolol, an antagonist at 5-HT1A receptors. Neurochem Res 1996;21:557-62. [DOI] [PubMed]
- 12.Moret C, Briley M. Effects of acute and repeated administration of citalopram on extracellular levels of serotonin in rat brain. Eur J Pharmacol 1996;295:189-97. [DOI] [PubMed]
- 13.Bel N, Artigas F. In vivo evidence for the reversible action of the monoamine oxidase inhibitor brofaromine on 5-hydroxytryptamine release in rat brain. Naunyn Schmiedebergs Arch Pharmacol 1995;351:475-82. [DOI] [PubMed]
- 14.Mathews TA, Fedele DE, Unger EL, et al. Effects of serotonin transporter inactivation on extracellular 5-HT levels, in vivo microdialysis recovery, and MDMA-induced release of serotonin and dopamine in mouse striatum. Soc Neurosci Abstr 2000;30:624.
- 15.Huang Y, Hwang DR, Narendran R, et al. Comparative evaluation in nonhuman primates of five PET radiotracers for imaging the serotonin transporters: [11C]McN 5652, [11C]ADAM, [11C]DASB, [11C]DAPA, and [11C]AFM. J Cereb Blood Flow Metab 2002;22:1377-98. [DOI] [PubMed]
- 16.Frankle WG, Huang Y, Hwang DR, et al. Comparative evaluation of serotonin transporter radioligands 11C-DASB and 11C-McN 5652 in healthy humans. J Nucl Med 2004;45:682-94. [PubMed]
- 17.Szabo Z, Kao P, Scheffel U, et al. Positron Emission Tomography Imaging of Serotonin Transporters in the Human Brain Using [11C](+)McN5652. Synapse 1995;20:37-43. [DOI] [PubMed]
- 18.Buck A, Gucker PM, Schonbachler RD, et al. Evaluation of serotonergic transporters using PET and [11C](+)McN-5652: assessment of methods. J Cereb Blood Flow Metab 2000;20:253-62. [DOI] [PubMed]
- 19.Parsey RV, Kegeles LS, Hwang DR, et al. In vivo quantification of brain serotonin transporters in humans using [11C]McN 5652. J Nucl Med 2000;41:1465-77. [PubMed]
- 20.Ginovart N, Wilson AA, Meyer JH, et al. Positron emission tomography quantification of [(11)C]-DASB binding to the human serotonin transporter: modeling strategies. J Cereb Blood Flow Metab 2001;21:1342-53. [DOI] [PubMed]
- 21.Ichise M, Liow JS, Lu JQ, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 2003;23:1096-112. [DOI] [PubMed]
- 22.Carroll FI, Kotian P, Dehghani A, et al. Cocaine and 3 beta-(4'-substituted phenyl)tropane-2 beta-carboxylic acid ester and amide analogues. New high-affinity and selective compounds for the dopamine transporter. J Med Chem 1995;38:379-88. [DOI] [PubMed]
- 23.Laruelle M, Giddings SS, Zea-Ponce Y, et al. Methyl 3 beta-(4-[125I]iodophenyl)tropane-2 beta-carboxylate in vitro binding to dopamine and serotonin transporters under “physiological” conditions. J Neurochem 1994;62:978-86. [DOI] [PubMed]
- 24.Shank RP, Vaught JL, Pelley KA, et al. McN-5652: a highly potent inhibitor of serotonin uptake. J Pharmacol Exp Ther 1988;247:1032-8. [PubMed]
- 25.Kung MP, Hou C, Oya S, et al. Characterization of [(123)I]IDAM as a novel single-photon emission tomography tracer for serotonin transporters. Eur J Nucl Med 1999;26:844-53. [DOI] [PubMed]
- 26.Wilson AA, Ginovart N, Schmidt M, et al. Novel radiotracers for imaging the serotonin transporter by positron emission tomography: synthesis, radiosynthesis, in vitro and ex vivo evaluation of [11]C-labeled 2-(phenylthio)araalkylamines. J Med Chem 2000;43:3103-10. [DOI] [PubMed]
- 27.Wilson AA, Ginovart N, Hussey D, et al. In vitro and in vivo characterisation of [11C]-DASB: a probe for in vivo measurements of the serotonin transporter by positron emission tomography. Nucl Med Biol 2002;29:509-15. [DOI] [PubMed]
- 28.Choi SR, Hou C, Oya S, et al. Selective in vitro and in vivo binding of [(125)I]ADAM to serotonin transporters in rat brain. Synapse 2000;38:403-12. [DOI] [PubMed]
- 29.Oya S, Choi SR, Hou C, et al. 2-((2-((dimethylamino)methyl) phenyl)thio)-5-iodophenylamine (ADAM): an improved serotonin transporter ligand. Nucl Med Biol 2000;27:249-54. [DOI] [PubMed]
- 30.Pirker W, Asenbaum S, Kasper S, et al. beta-CIT SPECT demonstrates blockade of 5HT-uptake sites by citalopram in the human brain in vivo. J Neural Transm Gen Sect 1995;100:247-56. [DOI] [PubMed]
- 31.Tauscher J, Pirker W, de Zwaan M, et al. In vivo visualization of serotonin transporters in the human brain during fluoxetine treatment. Eur Neuropsychopharmacol 1999;9:177-9. [DOI] [PubMed]
- 32.Kent JM, Coplan JD, Lombardo I, et al. Occupancy of brain serotonin transporters during treatment with paroxetine in patients with social phobia: a positron emission tomography study with 11C McN 5652. Psychopharmacology (Berl) 2002;164:341-8. [DOI] [PubMed]
- 33.Suhara T, Takano A, Sudo Y, et al. High levels of serotonin transporter occupancy with low-dose clomipramine in comparative occupancy study with fluvoxamine using positron emission tomography. Arch Gen Psychiatry 2003;60:386-91. [DOI] [PubMed]
- 34.Meyer JH, Wilson AA, Ginovart N, et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry 2001;158:1843-9. [DOI] [PubMed]
- 35.Meyer JH, Wilson AA, Sagrati S, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry 2004;161:826-35. [DOI] [PubMed]
- 36.Erlandsson K, Sivananthan T, Lui D, et al. Measuring SSRI occupancy of SERT using the novel tracer [123I]ADAM: a SPECT validation study. Eur J Nucl Med Mol Imaging 2005;32:1329-36. [DOI] [PubMed]
- 37.Brucke T, Kornhuber J, Angelberger P, et al. SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT. Binding kinetics in the human brain. J Neural Transm Gen Sect 1993;94:137-46. [DOI] [PubMed]
- 38.Kuikka JT, Bergstrom KA, Vanninen E, et al. Initial experience with single-photon emission tomography using iodine-123-labelled 2 beta-carbomethoxy-3 beta-(4-iodophenyl) tropane in human brain. Eur J Nucl Med 1993;20:783-6. [DOI] [PubMed]
- 39.Ikoma Y, Suhara T, Toyama H, et al. Quantitative analysis for estimating binding potential of the brain serotonin transporter with [11 C]McN5652. J Cereb Blood Flow Metab 2002;22:490-501. [DOI] [PubMed]
- 40.Houle S, Ginovart N, Hussey D, et al. Imaging the serotonin transporter with positron emission tomography: initial human studies with [11C]DAPP and [11C]DASB. Eur J Nucl Med 2000;27:1719-22. [DOI] [PubMed]
- 41.Catafau AM, Perez V, Penengo MM, et al. SPECT of serotonin transporters using 123I-ADAM: optimal imaging time after bolus injection and long-term test-retest in healthy volunteers. J Nucl Med 2005;46:1301-9. [PubMed]
- 42.Frankle WG, Lombardo I, New AS, et al. Brain serotonin transporter distribution in subjects with impulsive aggressivity: a positron emission study with [11C]McN 5652. Am J Psychiatry 2005;162:915-23. [DOI] [PubMed]
- 43.Praschak-Rieder N, Wilson AA, Hussey D, et al. Effects of tryptophan depletion on the serotonin transporter in healthy humans. Biol Psychiatry 2005;58:825-30. [DOI] [PubMed]
- 44.Innis RB, Seibyl JP, Scanley BE, et al. Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease. Proc Natl Acad Sci U S A 1993;90:11965-9. [DOI] [PMC free article] [PubMed]
- 45.Ciliax BJ, Drash GW, Staley JK, et al. Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol 1999;409:38-56. [DOI] [PubMed]
- 46.Ichimiya T, Suhara T, Sudo Y, et al. Serotonin transporter binding in patients with mood disorders: a PET study with [11C](+)McN5652. Biol Psychiatry 2002;51:715-22. [DOI] [PubMed]
- 47.Meyer JH, Houle S, Sagrati S, et al. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry 2004;61:1271-9. [DOI] [PubMed]
- 48.Takano A, Suhara T, Ichimiya T, et al. Time course of in vivo 5-HTT transporter occupancy by fluvoxamine. J Clin Psychopharmacol 2006;26:188-91. [DOI] [PubMed]
- 49.Acton PD, Choi SR, Hou C, et al. Quantification of serotonin transporters in nonhuman primates using [(123)I]ADAM and SPECT. J Nucl Med 2001;42:1556-62. [PubMed]
- 50.Chalon S, Tarkiainen J, Garreau L, et al. Pharmacological characterization of N,N-dimethyl-2-(2-amino-4-methylphenyl thio)benzylamine as a ligand of the serotonin transporter with high affinity and selectivity. J Pharmacol Exp Ther 2003;304:81-7. [DOI] [PubMed]
- 51.Halldin C, Lundberg J, Sovago J, et al. [(11)C]MADAM, a new serotonin transporter radioligand characterized in the monkey brain by PET. Synapse 2005;58:173-83. [DOI] [PubMed]
- 52.Lundberg J, Halldin C, Farde L. Measurement of serotonin transporter binding with PET and [(11)C]MADAM: A test-retest reproducibility study. Synapse 2006;60:256-63. [DOI] [PubMed]
- 53.Lundberg J, Odano I, Olsson H, et al. Quantification of 11C-MADAM binding to the serotonin transporter in the human brain. J Nucl Med 2005;46:1505-15. [PubMed]
- 54.Kim JS, Ichise M, Sangare J, et al. PET Imaging of Serotonin Transporters with [11C]DASB: Test-Retest Reproducibility Using a Multilinear Reference Tissue Parametric Imaging Method. J Nucl Med 2006;47:208-14. [PubMed]
- 55.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp 1999;7:254-66. [DOI] [PMC free article] [PubMed]
- 56.Ashburner J, Neelin P, Collins DL, et al. Incorporating prior knowledge into image registration. Neuroimage 1997;6:344-52. [DOI] [PubMed]
- 57.Rusjan P, Mamo D, Ginovart N, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res 2006;147:79-89. [DOI] [PubMed]
- 58.Meyer J, Wilson A, Ginovart N, Houle S. Misunderstandings about how to choose a reference region. Biol Psychiatry 2007. In press. [DOI] [PubMed]
- 59.Praschak-Rieder N, Kennedy J, Wilson A, et al. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: An [11C] DASB Positron Emission Tomography study. Biol Psychiatry. In press. [DOI] [PubMed]
- 60.Ginovart N, Wilson A, Meyer J, et al. PET Quantification of [11C]-DASB binding to the serotonin transporter in humans, in Abstracts of the 48th Annual Meeting of the Society of Nuclear Medicine. Reston: SNM; 2001. p. 62.
- 61.Logan J, Fowler JS, Volkow ND, et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 1996;16:834-40. [DOI] [PubMed]
- 62.Ichise M, Meyer JH, Yonekura Y. An introduction to PET and SPECT neuroreceptor quantification models. J Nucl Med 2001;42:755-63. [PubMed]
- 63.Kontur PJ, al-Tikriti M, Innis RB, et al. Postmortem stability of monoamines, their metabolites, and receptor binding in rat brain regions. J Neurochem 1994;62:282-90. [DOI] [PubMed]
- 64.Mann J, Underwood M, Arango V. Postmortem Studies of Suicide Victims, in Biology of Schizophrenia and Affective Disorders. In: Watson SJ, editor. Biology of Schizophrenia and Affective Disease. Washington: American Psychiatric Press; 1996 p. 197-221.
- 65.Pare CM. Iproniazid in the treatment of depression. Proc R Soc Med 1959;52:585-7. [PubMed]
- 66.Owens MJ, Morgan WN, Plott SJ, et al. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther 1997;283:1305-22. [PubMed]
- 67.Tatsumi M, Groshan K, Blakely RD, et al. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol 1997;340:249-58. [DOI] [PubMed]
- 68.Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. Am J Psychiatry 2000;157(4 Suppl):1-45. [PubMed]
- 69.Quetsch RM, Achor RW, Litin EM, et al. Depressive reactions in hypertensive patients; a comparison of those treated with Rauwolfia and those receiving no specific antihypertensive treatment. Circulation 1959;19:366-75. [DOI] [PubMed]
- 70.Delgado PL, Charney DS, Price LH, et al. Serotonin function and the mechanism of antidepressant action: reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry 1990;47:411-8. [DOI] [PubMed]
- 71.Delgado PL, Price LH, Miller HL, et al. Serotonin and the neurobiology of depression. Effects of tryptophan depletion in drug-free depressed patients. Arch Gen Psychiatry 1994;51:865-74. [DOI] [PubMed]
- 72.Leyton M, Young SN, Benkelfat C. Relapse of depression after rapid depletion of tryptophan [letter; comment]. Lancet 1997;349:1840-1. [DOI] [PubMed]
- 73.Smith KA, Fairburn CG, Cowen PJ. Relapse of depression after rapid depletion of tryptophan. Lancet 1997;349:915-9. [DOI] [PubMed]
- 74.Benkelfat C, Ellenbogen MA, Dean P, et al. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry 1994;51: 687-97. [DOI] [PubMed]
- 75.Young SN, Smith SE, Pihl RO, et al. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology (Berl) 1985;87:173-7. [DOI] [PubMed]
- 76.Smith K, Clifford E, Hockney R, et al. Effect of tryptophan depletion on mood in male and female volunteers: a pilot study. Hum Psychopharmacol Clin Exp 1997;12:111-7.
- 77.Neumeister A, Konstantinidis A, Stastny J, et al. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry 2002;59:613-20. [DOI] [PubMed]
- 78.Arango V, Ernsberger P, Marzuk PM, et al. Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry 1990;47:1038-47. [DOI] [PubMed]
- 79.Arango V, Underwood M, Mann J. Alterations in monoamine receptors in the brain of suicide victims. J Clin Psychopharmacol 1992;12:8S-12S. [PubMed]
- 80.Arora RC, Meltzer HY. Serotonergic measures in the brains of suicide victims: 5-HT2 binding sites in the frontal cortex of suicide victims and control subjects. Am J Psychiatry 1989;146:730-6. [DOI] [PubMed]
- 81.Hrdina PD, Vu TB. Chronic fluoxetine treatment upregulates 5-HT uptake sites and 5-HT2 receptors in rat brain: an autoradiographic study. Synapse 1993;14:324-31. [DOI] [PubMed]
- 82.Mann JJ, Stanley M, McBride PA, et al. Increased serotonin2 and beta-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry 1986;43:954-9. [DOI] [PubMed]
- 83.Stanley M, Mann JJ. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet 1983;1:214-6. [DOI] [PubMed]
- 84.Yates M, Leake A, Candy JM, et al. 5HT2 receptor changes in major depression. Biol Psychiatry 1990;27:489-96. [DOI] [PubMed]
- 85.Stockmeier CA, Dilley GE, Shapiro LA, et al. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology 1997;16:162-73. [DOI] [PubMed]
- 86.Turecki G, Briere R, Dewar K, et al. Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am J Psychiatry 1999;156:1456-8. [DOI] [PubMed]
- 87.Pandey GN, Dwivedi Y, Rizavi HS, et al. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry 2002;159:419-29. [DOI] [PubMed]
- 88.Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res 2003;37:357-73. [DOI] [PubMed]
- 89.Meyer JH, McMain S, Kennedy SH, et al. Dysfunctional Attitudes and 5-HT(2) Receptors During Depression and Self-Harm. Am J Psychiatry 2003;160:90-9. [DOI] [PubMed]
- 90.Stockmeier CA, Shapiro LA, Dilley GE, et al. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci 1998;18:7394-401. [DOI] [PMC free article] [PubMed]
- 91.O'Regan D, Kwok RP, Yu PH, et al. A behavioural and neurochemical analysis of chronic and selective monoamine oxidase inhibition. Psychopharmacology (Berl) 1987;92:42-7. [DOI] [PubMed]
- 92.Roth BL, McLean S, Zhu XZ, et al. Characterization of two [3H]ketanserin recognition sites in rat striatum. J Neurochem 1987;49:1833-8. [DOI] [PubMed]
- 93.Stockmeier CA, Kellar KJ. In vivo regulation of the serotonin-2 receptor in rat brain. Life Sci 1986;38:117-27. [DOI] [PubMed]
- 94.Todd KG, McManus DJ, Baker GB. Chronic administration of the antidepressants phenelzine, desipramine, clomipramine, or maprotiline decreases binding to 5-hydroxytryptamine2A receptors without affecting benzodiazepine binding sites in rat brain. Cell Mol Neurobiol 1995;15:361-70. [DOI] [PubMed]
- 95.Hoyer D, Pazos A, Probst A, et al. Serotonin receptors in the human brain. II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Res 1986;376:97-107. [DOI] [PubMed]
- 96.Marazziti D, Rossi A, Giannaccini G, et al. Distribution and characterization of [3H]mesulergine binding in human brain postmortem. Eur Neuropsychopharmacol 1999;10:21-6. [DOI] [PubMed]
- 97.Schmuck K, Ullmer C, Engels P, et al. Cloning and functional characterization of the human 5-HT2B serotonin receptor. FEBS Lett 1994;342:85-90. [DOI] [PubMed]
- 98.D'haenen H, Bossuyt A, Mertens J, et al. SPECT imaging of serotonin2 receptors in depression. Psychiatry Res 1992;45:227-37. [DOI] [PubMed]
- 99.Biver F, Wikler D, Lotstra F, et al. Serotonin 5-HT2 receptor imaging in major depression: focal changes in orbito-insular cortex. Br J Psychiatry 1997;171:444-8. [DOI] [PubMed]
- 100.Attar-Levy D, Martinot J-L, Blin J, et al. The cortical serotonin2 receptors studied with positron emission tomography and [18F]-setoperone during depressive illness and antidepressant treatment with clomipramine. Biol Psychiatry 1999;45:180-6. [DOI] [PubMed]
- 101.Meyer JH, Kapur S, Houle S, et al. Prefrontal cortex 5-HT2 receptors in depression: a [18F] setoperone PET imaging study. Am J Psychiatry 1999;156:1029-34. [DOI] [PubMed]
- 102.Meltzer CC, Price JC, Mathis A, et al. PET imaging of serotonin type 2A receptors in late-life neuropsychiatric disorders. Am J Psychiatry 1999;156:1871-8. [DOI] [PubMed]
- 103.Yatham LN, Liddle PF, Shiah IS, et al. Brain serotonin2 receptors in major depression: a positron emission tomography study. Arch Gen Psychiatry 2000;57:850-8. [DOI] [PubMed]
- 104.Messa C, Colombo C, Moresco RM, et al. 5-HT(2A) receptor binding is reduced in drug-naive and unchanged in SSRI-responder depressed patients compared to healthy controls: a PET study. Psychopharmacology (Berl) 2003;167:72-8. [DOI] [PubMed]
- 105.Mintun MA, Sheline YI, Moerlein SM, et al. Decreased hippocampal 5-HT2A receptor binding in major depressive disorder: in vivo measurement with [18F]altanserin positron emission tomography. Biol Psychiatry 2004;55:217-24. [DOI] [PubMed]
- 106.Blin J, Pappata S, Kiyosawa M, et al. [18F] setoperone: a new high-affinity ligand for positron emission tomography study of the serotonin - 2 receptors in baboon brain in vivo. Eur J Pharmacol 1988;147:73-82. [DOI] [PubMed]
- 107.Blin J, Sette G, Fiorelli M, et al. A method for the in vivo investigation of the serotonergic 5-HT2 receptors in the human cerebral cortex using positron emission tomography and 18F-labeled setoperone. J Neurochem 1990;54:1744-54. [DOI] [PubMed]
- 108.Petit-Taboue MC, Landeau B, Osmont A, et al. Estimation of neocortical serotonin-2 receptor binding potential by single-dose fluorine-18-setoperone kinetic PET data analysis. J Nucl Med 1996;37:95-104. [PubMed]
- 109.Maziere B, Crouzel C, Venet M, et al. Synthesis, affinity and specificity of 18F-setoperone, a potential ligand for in-vivo imaging of cortical serotonin receptors. Int J Rad Appl Instrum B 1988;15:463-8. [DOI] [PubMed]
- 110.Fischman AJ, Bonab AA, Babich JW, et al. Positron emission tomographic analysis of central 5-hydroxytryptamine2 receptor occupancy in healthy volunteers treated with the novel antipsychotic agent ziprasidone. J Pharmacol Exp Ther 1996;279:939-47. [PubMed]
- 111.Seeman P. Receptor tables vol. 2: Drug dissociation constants for neuroreceptors and transporters. Toronto: SZ Research; 1993.
- 112.De Keyser J, Clayes A, DeBacker J-P, et al. Autoradiographic localization of D1 and D2 dopamine receptors in the human brain. Neurosci Lett 1988;91:142-7. [DOI] [PubMed]
- 113.Trichard C, Paillere-Martinot ML, Attar-Levy D, et al. Binding of antipsychotic drugs to cortical 5-HT2A receptors: a PET study of chlorpromazine, clozapine, and amisulpride in schizophrenic patients. Am J Psychiatry 1998;155:505-8. [DOI] [PubMed]
- 114.Kapur S, Zipursky RB, Remington G, et al. 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry 1998;155:921-8. [DOI] [PubMed]
- 115.Mamo D, Kapur S, Shammi CM, et al. A PET study of dopamine D2 and serotonin 5-HT2 receptor occupancy in patients with schizophrenia treated with therapeutic doses of ziprasidone. Am J Psychiatry 2004;161:818-25. [DOI] [PubMed]
- 116.Kapur S, Jones C, Da Silva J, Wilson A, et al. Reliability of [18F] setoperone in humans. Nucl Med Commun 1997;18:395-9. [DOI] [PubMed]
- 117.Blin J, Crouzel C. Blood-cerebrospinal fluid and blood-brain barriers imaged by 18F-labeled metabolites of 18F-setoperone studied in humans using positron emission tomography. J Neurochem 1992;58:2303-10. [DOI] [PubMed]
- 118.Meyer JH, Cho R, Kennedy S, et al. The effects of single dose nefazodone and paroxetine upon 5-HT2A binding potential in humans using [18F]-setoperone PET. Psychopharmacology (Berl) 1999;144:279-81. [DOI] [PubMed]
- 119.Dohr KB, Rush AJ, Bernstein IH. Cognitive Biases and Depression. J Abnorm Psychol 1989;98:263-7. [DOI] [PubMed]
- 120.Simons AD, Garfield SL, Murphy GE. The process of change in cognitive therapy and pharmacotherapy for depression. Changes in mood and cognition. Arch Gen Psychiatry 1984;41:45-51. [DOI] [PubMed]
- 121.Cane DB, Olinger LJ, Gotlib IH, et al. Factor structure of the dysfunctional attitude scale in a student population. J Clin Psychol 1986;42:307-9.
- 122.Oliver JM, Baumgart EP. The dysfunctional attitude scale: psychometric properties and relation to depression in an unselected adult population. Cognit Ther Res 1985;9:161-7.
- 123.Weissman A. The Dysfunctional Attitude Scale: A validation study. Dissertation Abstracts International 1979;40:1389B-1390B.
- 124.Bouvard M, Charles S, Guerin J, et al. [Study of Beck's hopelessness scale. Validation and factor analysis]. Encephale 1992;18:237-40. [PubMed]
- 125.Cannon B, Mulroy R, Otto MW, et al. Dysfunctional attitudes and poor problem solving skills predict hopelessness in major depression. J Affect Disord 1999;55:45-9. [DOI] [PubMed]
- 126.DeRubeis RJ, Evans MD, Hollon SD, et al. How does cognitive therapy work? Cognitive change and symptom change in cognitive therapy and pharmacotherapy for depression. J Consult Clin Psychol 1990;58:862-9. [DOI] [PubMed]
- 127.Norman WH, Miller W, Dow MG. Characteristics of depressed patients with elevated levels of dysfunctional cognitions. Cognit Ther Res 1988;12:39-51.
- 128.Beck AT, Steer RA, Kovacs M, et al. Hopelessness and eventual suicide: A 10-year prospective study of patients hospitalized with suicidal ideation. Am J Psychiatry 1985;142:559-63. [DOI] [PubMed]
- 129.Beck AT, Brown G, Steer RA. Prediction of eventual suicide in psychiatric inpatients by clinical ratings of hopelessness. J Consult Clin Psychol 1989;57:309-10. [DOI] [PubMed]
- 130.Bhagwagar Z, Hinz R, Taylor M, et al. Increased 5-HT2A Receptor Binding in Euthymic Medication Free Patients Recovered from Depression: A Positron Emission Tomography Study With [11C] MDL 100,907. Am J Psychiatry 2006;163:1580-7. [DOI] [PubMed]
- 131.Boldrinin M, Underwood MD, Martini A, et al. Brainstem raphe nucleus changes in suicide victims. Psychiatr Danub 2006; 18 (Suppl 1):120.
- 132.Compan V, Segu L, Buhot MC, et al. Differential effects of serotonin (5-HT) lesions and synthesis blockade on neuropeptide-Y immunoreactivity and 5-HT1A, 5-HT1B/1D and 5-HT2A/2C receptor binding sites in the rat cerebral cortex. Brain Res 1998;795:264-76. [DOI] [PubMed]
- 133.McCann UD, Szabo Z, Seckin E, et al. Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology 2005; 30: 1741-50. [DOI] [PMC free article] [PubMed]
- 134.Bernheimer H, Birkmayer W, Hornykiewicz O. [Distribution of 5-hydroxytryptamine (serotonin) in the human brain and its behavior in patients with Parkinson's syndrome.] Klin Wochenschr 1961;39:1056-9. [DOI] [PubMed]
- 135.Kish SJ. What is the evidence that Ecstasy (MDMA) can cause Parkinson's disease? Mov Disord 2003;18:1219-23. [DOI] [PubMed]
- 136.Linnet K, Koed K, Wiborg O, et al. Serotonin depletion decreases serotonin transporter mRNA levels in rat brain. Brain Res 1995;697:251-3. [DOI] [PubMed]
- 137.Xiao Q, Pawlyk A, Tejani-Butt SM. Reserpine modulates serotonin transporter mRNA levels in the rat brain. Life Sci 1999;64:63-8. [DOI] [PubMed]
- 138.Yu A, Yang J, Pawlyk AC, et al. Acute depletion of serotonin down-regulates serotonin transporter mRNA in raphe neurons. Brain Res 1995;688:209-12. [DOI] [PubMed]
- 139.Benmansour S, Cecchi M, Morilak D, et al. Effects of Chronic Antidepressant Treatments on Serotonin Transporter Function, Density and mRNA level. J Neurosci 1999;19:10494-501. [DOI] [PMC free article] [PubMed]
- 140.Dewar KM, Grondin L, Carli M, et al. [3H]paroxetine binding and serotonin content of rat cortical areas, hippocampus, neostriatum, ventral mesencephalic tegmentum, and midbrain raphe nuclei region following p-chlorophenylalanine and p-chloroamphetamine treatment. J Neurochem 1992;58:250-7. [DOI] [PubMed]
- 141.Graham D, Tahraoui L, Langer SZ. Effect of chronic treatment with selective monoamine oxidase inhibitors and specific 5-hydroxytryptamine uptake inhibitors on [3H]paroxetine binding to cerebral cortical membranes of the rat. Neuropharmacology 1987;26:1087-92. [DOI] [PubMed]
- 142.Gordon I, Weizman R, Rehavi M. Modulatory effect of agents active in the presynaptic dopaminergic system on the striatal dopamine transporter. Eur J Pharmacol 1996;298:27-30. [DOI] [PubMed]
- 143.Han S, Rowell PP, Carr LA. D2 autoreceptors are not involved in the down-regulation of the striatal dopamine transporter caused by alpha-methyl-p-tyrosine. Res Commun Mol Pathol Pharmacol 1999;104:331-8. [PubMed]
- 144.Ikawa K, Watanabe A, Kaneno S, et al. Modulation of [3H]mazindol binding sites in rat striatum by dopaminergic agents. Eur J Pharmacol 1993;250:261-6. [DOI] [PubMed]
- 145.Kilbourn MR, Sherman PS, Pisani T. Repeated reserpine administration reduces in vivo [18F]GBR 13119 binding to the dopamine uptake site. Eur J Pharmacol 1992;216:109-12. [DOI] [PubMed]
- 146.Blier P, De Montigny C. Electrophysiological investigations on the effect of repeated zimelidine administration on serotonergic neurotransmission in the rat. J Neurosci 1983;3:1270-8. [DOI] [PMC free article] [PubMed]
- 147.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 2000;20:423-51. [DOI] [PubMed]
- 148.Ginovart N, Wilson AA, Meyer JH, et al. [11C]-DASB, a tool for in vivo measurement of SSRI-induced occupancy of the serotonin transporter: PET characterization and evaluation in cats. Synapse 2003;47:123-33. [DOI] [PubMed]
- 149.Lundquist P, Wilking H, Hoglund AU, et al. Potential of [11C]DASB for measuring endogenous serotonin with PET: binding studies. Nucl Med Biol 2005;32:129-36. [DOI] [PubMed]
- 150.Celada P, Artigas F. Monoamine oxidase inhibitors increase preferentially extracellular 5-hydroxytryptamine in the midbrain raphe nuclei. A brain microdialysis study in the awake rat. Naunyn Schmiedebergs Arch Pharmacol 1993;347:583-90. [DOI] [PubMed]
- 151.Malyszko J, Urano T, Serizawa K, et al. Serotonergic measures in blood and brain and their correlations in rats treated with tranylcypromine, a monoamine oxidase inhibitor. Jpn J Physiol 1993;43:613-26. [DOI] [PubMed]
- 152.Ferrer A, Artigas F. Effects of single and chronic treatment with tranylcypromine on extracellular serotonin in rat brain. Eur J Pharmacol 1994;263:227-34. [DOI] [PubMed]
- 153.Talbot PS, Frankle WG, Hwang DR, et al. Effects of reduced endogenous 5-HT on the in vivo binding of the serotonin transporter radioligand 11C-DASB in healthy humans. Synapse 2005;55:164-75. [DOI] [PubMed]
- 154.Bligh-Glover W, Kolli TN, Shapiro-Kulnane L, et al. The serotonin transporter in the midbrain of suicide victims with major depression. Biol Psychiatry 2000;47:1015-24. [DOI] [PubMed]
- 155.Klimek V, Roberson G, Stockmeier CA, et al. Serotonin transporter and MAO-B levels in monoamine nuclei of the human brainstem are normal in major depression. J Psychiatr Res 2003;37:387-97. [DOI] [PubMed]
- 156.Perry EK, Marshall EF, Blessed G, et al. Decreased imipramine binding in the brains of patients with depressive illness. Br J Psychiatry 1983;142:188-92. [DOI] [PubMed]
- 157.Crow TJ, Cross AJ, Cooper SJ, et al. Neurotransmitter receptors and monoamine metabolites in the brains of patients with Alzheimer-type dementia and depression, and suicides. Neuropharmacology 1984;23(12B):1561-9. [DOI] [PubMed]
- 158.Mann JJ, Huang YY, Underwood MD, et al. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry 2000;57:729-38. [DOI] [PubMed]
- 159.Austin MC, Whitehead RE, Edgar CL, et al. Localized decrease in serotonin transporter-immunoreactive axons in the prefrontal cortex of depressed subjects committing suicide. Neuroscience 2002;114:807-15. [DOI] [PubMed]
- 160.Hrdina PD, Foy B, Hepner A, et al. Antidepressant binding sites in brain: autoradiographic comparison of [3H]paroxetine and [3H]imipramine localization and relationship to serotonin transporter. J Pharmacol Exp Ther 1990;252:410-8. [PubMed]
- 161.Lawrence KM, De Paermentier F, Cheetham SC, et al. Brain 5-HT uptake sites, labelled with [3H]paroxetine, in antidepressant-free depressed suicides. Brain Res 1990;526:17-22. [DOI] [PubMed]
- 162.Little KY, McLauglin DP, Ranc J, et al. Serotonin transporter binding sites and mRNA levels in depressed persons committing suicide. Biol Psychiatry 1997;41:1156-64. [DOI] [PubMed]
- 163.Arango V, Underwood MD, Boldrini M, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology 2001;25:892-903. [DOI] [PubMed]
- 164.Hendricksen M, Thomas AJ, Ferrier IN, et al. Neuropathological study of the dorsal raphe nuclei in late-life depression and Alzheimer's disease with and without depression. Am J Psychiatry 2004;161:1096-102. [DOI] [PubMed]
- 165.Leake A, Fairbairn AF, McKeith IG, et al. Studies on the serotonin uptake binding site in major depressive disorder and control post-mortem brain: neurochemical and clinical correlates. Psychiatry Res 1991;39:155-65. [DOI] [PubMed]
- 166.Malison RT, Price LH, Berman R, et al. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 1998;44:1090-8. [DOI] [PubMed]
- 167.Newberg AB, Amsterdam JD, Wintering N, et al. 123I-ADAM binding to serotonin transporters in patients with major depression and healthy controls: a preliminary study. J Nucl Med 2005;46:973-7. [PubMed]
- 168.Parsey RV, Hastings RS, Oquendo MA, et al. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry 2006;163:52-8. [DOI] [PubMed]
- 169.Herold N, Uebelhack K, Franke L, et al. Imaging of serotonin transporters and its blockade by citalopram in patients with major depression using a novel SPECT ligand [(123)I]-ADAM. J Neural Transm 2006;113:659-70. [DOI] [PubMed]
- 170.Meyer JH, Houle S, Sagrati S, et al. Brain serotonin transporter binding potential measured with [11C]DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry 2004;61(12):1271-9. [DOI] [PubMed]
- 171.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996;274:1527-31. [DOI] [PubMed]
- 172.Filipenko ML, Beilina AG, Alekseyenko OV, et al. Increase in expression of brain serotonin transporter and monoamine oxidase a genes induced by repeated experience of social defeats in male mice. Biochemistry (Mosc) 2002;67:451-5. [DOI] [PubMed]
- 173.Little KY, McLaughlin DP, Zhang L, et al. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry 1998;155:207-13. [DOI] [PubMed]
- 174.Heinz A, Jones DW, Mazzanti C, et al. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry 2000;47:643-9. [DOI] [PubMed]
- 175.Willeit M, Stastny J, Pirker W, et al. No evidence for in vivo regulation of midbrain serotonin transporter availability by serotonin transporter promoter gene polymorphism. Biol Psychiatry 2001;50:8-12. [DOI] [PubMed]
- 176.Shioe K, Ichimiya T, Suhara T, et al. No association between genotype of the promoter region of serotonin transporter gene and serotonin transporter binding in human brain measured by PET. Synapse 2003;48:184-8. [DOI] [PubMed]
- 177.Parsey RV, Hastings RS, Oquendo MA, et al. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry 2006;163:48-51. [DOI] [PubMed]
- 178.Lim JE, Papp A, Pinsonneault J, et al. Allelic expression of serotonin transporter (SERT) mRNA in human pons: lack of correlation with the polymorphism SERTLPR. Mol Psychiatry 2006;11:649-62. [DOI] [PubMed]
- 179.Kim DK, Lim SW, Lee S, et al. Serotonin transporter gene polymorphism and antidepressant response. Neuroreport 2000;11:215-9. [DOI] [PubMed]
- 180.Zanardi R, Benedetti F, Di Bella D, et al. Efficacy of paroxetine in depression is influenced by a functional polymorphism within the promoter of the serotonin transporter gene. J Clin Psychopharmacol 2000;20:105-7. [DOI] [PubMed]
- 181.Pollock BG, Ferrell RE, Mulsant BH, et al. Allelic variation in the serotonin transporter promoter affects onset of paroxetine treatment response in late-life depression. Neuropsychopharmacology 2000;23:587-90. [DOI] [PubMed]
- 182.Kraft JB, Slager SL, McGrath PJ, et al. Sequence analysis of the serotonin transporter and associations with antidepressant response. Biol Psychiatry 2005;58:374-81. [DOI] [PubMed]
- 183.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386-9. [DOI] [PubMed]
- 184.Faravelli C, Ambonetti A, Pallanti S, et al. Depressive relapses and incomplete recovery from index episode. Am J Psychiatry 1986;143:888-91. [DOI] [PubMed]
- 185.Georgotas A, McCue RE, Cooper TB, et al. How effective and safe is continuation therapy in elderly depressed patients? Factors affecting relapse rate. Arch Gen Psychiatry 1988;45:929-32. [DOI] [PubMed]
- 186.Paykel ES, Ramana R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med 1995;25:1171-80. [DOI] [PubMed]
- 187.Paykel ES, Scott J, Teasdale JD, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry 1999;56:829-35. [DOI] [PubMed]
- 188.Cannon DM, Ichise M, Fromm SJ, et al. Serotonin transporter binding in bipolar disorder assessed using [11C]DASB and positron emission tomography. Biol Psychiatry 2006;60:207-17. [DOI] [PubMed]
- 189.Takano A, Suzuki K, Kosaka J, et al. A dose-finding study of duloxetine based on serotonin transporter occupancy. Psychopharmacology (Berl) 2006;185:395-9. [DOI] [PubMed]
- 190.Parsey RV, Kent JM, Oquendo MA, et al. Acute occupancy of brain serotonin transporter by sertraline as measured by [11C]DASB and positron emission tomography. Biol Psychiatry 2006;59:821-8. [DOI] [PubMed]
- 191.Fineberg NA, Gale TM. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol 2005;8:107-29. [DOI] [PubMed]


