Abstract
Neonatal studies suggest elevated arousal can negatively influence perceptual and cognitive processes during early development. The authors explored this issue during the prenatal period by pharmacologically elevating physiological arousal in bobwhite quail (Colinus virginianus) embryos during exposure to a maternal call, then assessing preference for the familiar call following hatching. Embryos receiving norepinephrine showed a prenatal elevation in heart rate and failed to demonstrate a preference for the familiar call following hatching. Embryos not receiving norepinephrine showed no elevation in heart rate and demonstrated a preference for the familiar call. These results indicate elevated arousal can interfere with perceptual learning during the prenatal period and provide additional evidence for an optimal window of arousal necessary to foster species-typical perceptual functioning during early development.
Keywords: physiological arousal, prenatal learning, perceptual learning, norepinephrine, avian embryos
On the basis of his work with newly hatched domestic chicks, Gray (1990) proposed that young organisms’ attention and perceptual responsiveness to sensory stimulation is directly influenced by their current state of arousal. To evaluate the effects of arousal on auditory responsiveness in young chicks, Gray used chicks’ rates of vocalizations (peeping) as a measure of prestimulus activity and chicks’ delays in ongoing vocalizations as a measure of their auditory responsiveness. Results showed increased responsiveness (as measured by cessation of vocalizations) to pure tones at intermediate levels of prestimulus activity and reduced responsiveness during periods of high and low activity.
Research with infant mammals, including humans, has indicated a similar relationship between arousal and sensitivity or attention to sensory stimulation during early development. Lewkowicz and Turkewitz (1980) found that prestimulation of human newborns with a pulse of white noise resulted in infants’ increased looking at a dimmer light and decreased looking at a brighter light. Likewise, infants exposed to auditory stimulation immediately preceding or concurrently with visual stimuli preferred less intense visual stimulation than did unstimulated control infants (Lewkowicz & Turkewitz, 1981). Gardner and Karmel (1995) also reported that either altering the infant’s overall arousal or altering the nature of available sensory experience could modify the attentional value of specific sensory stimulation. Full-term and high-risk preterm infants both preferred less stimulating visual events when highly aroused and more stimulating visual events when less aroused. This arousal-modulated selective attention was seen both when infants are endogenously more aroused (before feeding) or less aroused (after feeding) as well as when they are exogenously more or less aroused as a result of the presence of increased or decreased amounts of sensory stimulation.
Similar results have been obtained from nonhuman animal-based research. Gottlieb (1993) modified both ducklings’ arousal and their ability to learn a heterospecific (chicken) maternal call by manipulating the sensory stimulation available to ducklings immediately following hatching. Ducklings that were denied normal amounts of tactile stimulation from broodmates following hatching did not learn to prefer a chicken maternal call over a conspecific mallard maternal call, whereas ducklings allowed normal amounts of postnatal tactile experience strongly preferred the chicken call over the mallard maternal call following familiarization with the chicken call. These outcomes appeared to be determined by the ducklings’ arousal during exposure to the chicken call. Ducklings denied normal postnatal tactile experience demonstrated more behavioral arousal (as indexed by distress vocalizations and time spent awake) than did ducklings not deprived of tactile contact. Birds not permitted tactile contact slept less and were highly aroused before, during, and after presentation of the chicken call. In contrast, socially reared ducklings slept more and were less aroused than the isolates, suggesting elevated arousal interfered with ducklings’ learning of the heterospecific call.
Lickliter and Lewkowicz (1995) explored the role of attenuated prenatal tactile and vestibular sensory stimulation on attention and perceptual learning in bobwhite quail embryos. In that study, quail eggs were incubated in individual opaque plastic tubs during the last 24–36 hr prior to hatching. This physical isolation from other eggs in their clutch during the hatching stage of prenatal development prevented embryos from receiving the normally occurring vestibular and tactile stimulation typically provided from brood-mates during the hatching process. Following hatching, these chicks failed to show typical patterns of postnatal responsiveness to both maternal auditory and visual stimulation seen in communally incubated embryos. In addition, embryos denied normal amounts of tactile and vestibular stimulation from broodmates failed to demonstrate auditory learning of an individual maternal call, a behavior reliably seen in unmanipulated, communally incubated embryos. These results suggest that the species-typical environment provides the developing embryo or neonate with an optimal range of stimulation, thereby suggesting the existence of an effective range of arousal for early perceptual learning and development (see Lickliter, 2000; Radell and Gottlieb, 1992, for further discussion). Deviations above or below an optimal window of stimulation can modify arousal and could potentially modulate the young organism’s ability to learn and consolidate available sensory information.
For example, Radell and Gottlieb (1992) demonstrated that duckling embryos fail to learn an individual maternal call when exposed concurrently to increased amounts of vestibular stimulation but do show auditory learning when the amount of vestibular stimulation provided embryos is reduced to more closely approximate their normal range of prenatal stimulation. Similarly, Sleigh and Lickliter (1997) found that bobwhite quail chicks exposed to substantially augmented amounts of prenatal auditory stimulation as embryos show modified species-typical perceptual development. Chicks receiving increased amounts of auditory stimulation as embryos continued to respond to maternal auditory cues into later stages of postnatal development and failed to respond preferentially to maternal visual cues at ages when normally reared chicks reliably exhibit this species-specific preference. Further, embryos receiving increased amounts of prenatal auditory stimulation showed an increase in behavioral arousal (as measured by rate of embryonic vocalizations) when compared with control embryos. Taken together, these results further support the notion that stimulation that falls within some optimal range maintains normal patterns of arousal and perceptual responsiveness during early development, whereas stimulation beyond the range of the species norm can modify arousal and interfere with species-typical patterns of early perceptual functioning.
To more directly examine the link between prenatal arousal and perceptual learning, the present study pharmacologically manipulated the physiological arousal of bobwhite quail embryos and assessed their capacity for perceptual learning of an individual bobwhite maternal call in the period prior to hatching. We reasoned that if changes in normal prenatal arousal can interfere with embryos’ ability to learn or consolidate information present in the immediate environment, then pharmacologically elevating physiological arousal during the late prenatal period should result in changes in perceptual learning compared with unmanipulated controls. To evaluate this hypothesis, we injected groups of quail embryos with a single dose of norepinephrine or saline early in the last day of incubation. This adrenergic stimulant is widely known to elevate heart rate in young birds, including embryos (Cheung, Gilbert, Bruyere, Ishikawa, & Hodach, 1977; Culver & Fischman, 1977; Girard, 1973; Oppenheim, Pittman, Gray, & Maderdrut, 1978; Petery & Van Mierop, 1977; Sedlacek, 1977; Tayo, 1984). Embryos in an additional control condition experienced the same egg opening and handling but did not receive an injection. Following these manipulations, embryos in all three conditions were exposed to an individual maternal call for 15 min/hr for the last 16 hr of incubation. After hatching, all chicks were tested in a simultaneous choice between the familiar maternal call and a novel maternal call. We predicted that the increased physiological arousal induced by the norepinephrine would interfere with embryos’ perceptual learning during their prenatal exposure to the maternal call, thereby limiting their subsequent ability to remember and prefer that familiar maternal call in the postnatal period.
Experiment 1: Norepinephrine Dose Response in Bobwhite Quail Embryos
To our knowledge, no studies have investigated the effects of an intramuscular injection of norepinephrine on heart rate in bobwhite quail embryos. Thus, the aims of this experiment were to establish the lowest concentration at which peripherally administered nor-epinephrine would result in a significant increase in heart rate in bobwhite quail embryos and to explore whether embryos’ heart rate would remain elevated several hours after administration.
Studies investigating heart rate function in the prenatal and early postnatal periods in precocial avian species have indicated that embryos increasingly rely on β–adrenergic functioning for maintenance of optimal oxygenation while the embryo transfers from reliance on the chorioallantoic membrane (the avian placenta) to lung respiration (Crossley & Altimiras, 2000). The aim of this study was to examine the effects of elevated physiological arousal on attention and learning in the prenatal period. Given that our exposure paradigm required delivery of a conspecific maternal call across many hours, we were particularly interested in whether embryos’ increased reliance on β-adrenergic functioning during the late stages of incubation would result in longer responsivity to β-adrenergic agonists such as norepinephrine than typically seen in adult animals, allowing us to coincide embryos’ elevated heart rate with prenatal exposure to an individual maternal call.
Method
Subjects
Subjects were 35 bobwhite quail (Colinus virginianus) embryos obtained as fertilized eggs and incubated in an automatic turning, forced-air Grumbach BSS-160 incubator Grumbach, Munich, Germany. Bobwhite quail typically hatch after 23 days of incubation. Externally pipped embryos were selected on E21 or E22 of incubation. Pipping refers to the appearance of a small hole in the top portion of the eggshell, resulting from the embryo having broken through the amnion with its egg tooth and moved into the airspace to begin breathing. We chose externally pipped eggs because previous studies have indicated that chicken and Japanese quail embryos show transient heart rate decelerations concomitant with the first stages of breaking through the internal amnion (Moriya, Pearson, Burggren, Ar, & Tazawa, 2000; Pearson, Tsudzuki, Nakane, Akiyama, & Tazawa, 1998), and we wanted to minimize the presence of these oscillations during our heart rate measurements.
Apparatus, data acquisition, and signal processing
Data acquisition was conducted in a Hova-Bator S7501 portable incubator Hova-Bator, Savannah, GA set at 37° C. A single egg was placed in a shielded metal enclosure to eliminate electrical noise from the heating elements in the hatcher, the data acquisition computer, and the room. Before placement of the embryo into the metal enclosure, a 22-gauge needle was used to bore approximately 2-mm diameter holes into the eggshell. To approximate Einthoven’s triangle for maximum signal strength, two holes were placed on opposing sides in the lower portion of the egg, with the airspace facing up. The holes were made deep enough to penetrate the egg shell membrane into the amnion, without disturbing the embryo (Moriya et al., 2000). Once the holes were made in the eggshell, the egg was placed on the foam support inside the metal enclosure. The two copper electrodes were bent at a 90° angle 2 mm from the ends, and these ends were placed into the egg holes. Once the shielded metal enclosure and hatcher were closed, the embryo was given a 5-min settling period to adjust to the ambient temperature in the hatcher prior to recording.
A Coulbourn S73-22 impedance pneumograph coupler (Coulbourn Instruments, Allentown, PA) sent a 50-kHz sine wave through the egg to detect instantaneous heart rate. Changes in the sine wave were then amplified via a Coulbourn S7501 bioamplifier (Coulbourn Instruments, Allentown, PA) at 40,000× gain and further amplified via a BIOPAC (BIOPAC Systems, Goleta, CA) MP100A amplifier at 2,000× gain. After amplification, the BIOPAC System converted the signal from analog to digital, which permitted the use of Acknowledge 3.5.7 software (BIOPAC Systems, 2005) to digitally apply a 3–10-kHz bandpass filter to the signal, eliminating noise and normalizing the size of the signal.
Reynolds and Lickliter (2003) and our own lab observations indicate that bobwhite quail embryos’ heart rate ranges from approximately 150–350 beats-per-minute (BPM) following external pipping. This is concordant with findings that heart rate is approximately 240–350 BPM in chicken embryos the day before hatching and 350–400 BPM in Japanese quail (Aubert et al., 2004; Aubert, Leribaux, Beckers, Ramaekers, & Berckmans, 2000; Crossley & Altimiras, 2000; Moriya et al., 2000; Pearson et al., 1998). According to the Nyquist frequency, the minimum sampling rate necessary to obtain all spectral features of a signal is twice the highest rate of the signal (in this case, 800 samples/min or 13.333/s). Because embryos’ heart rate can be quite variable just prior to hatching, and because injections of norepinephrine were used to elevate heart rate potentially well above the normal range, we collected measures of instantaneous BPM at a sampling rate much higher than that required by the Nyquist frequency (i.e., at 100 samples/s).
Injections
A Hamilton 710 Luer-Lock 100 μl syringe (Hamilton Company, Reno, NV) with a 22.5 gauge needle administered injections of norepinephrine (Sigma-Aldrich, A9512). Regardless of concentration, all injections administered a 5-μl intramuscular bolus to embryos. During the perinatal period, embryos’ backs are located just under the airspace, on the opposing side from the pip. The nature of embryos’ positioning in the egg at E21–E22 allowed experimenters to administer injections into the back muscle by using the pip in the outer eggshell as an orienting cue.
Design and procedure
All embryos’ baseline instantaneous BPM was recorded for 5–10 min, depending on signal quality. Following baseline data collection, embryos received one of six injection concentrations: 0.001 mole (M), 0.003 M, 0.01 M, 0.03 M, or 0.1 M norepinephrine or saline only (0.0 M norepinephrine). In each condition, there were 5 to 6 embryos; thus, variations in effects of different norepinephrine concentrations were compared between subjects. Following injection, the embryo was returned to the rearing incubator. Embryos’ heart rate was then recorded 2 hr after injection. Thus, data were collected both between- and within-subjects: 6 between-subject dosages and 2 within-subject periods (baseline and 2 hr after treatment).
Data analysis
Signal quality varied widely across subjects. For example, at times the signal was obscured completely by movement artifact. In other instances, the signal was clean and reliable, but R-wave amplitude varied considerably depending on the position of the embryo relative to the recording electrodes. In addition, there were times during the recording window when movement artifact pushed the electrode out of contact with the amnion, preventing signal recording. These are common issues observed in studies that use noninvasive recording methods for avian embryos in ovo (Altimiras & Crossley, 2000; Sakamoto, Haque, Ono, Pearson, & Tazawa, 1995; Tazawa, Hiraguchi, Asakura, Fujii, & Whittow, 1989). To minimize these potential confounds, spectral analyses with Hamming fast Fourier transformations (FFTs) were performed to extract the relevant frequency from the data on 5–10 s of reliable signal for each subject. FFT samples were obtained from the latest section of the recording window possible. In no instance were FFTs performed on the first half of the recording window. If files contained fewer than 5 consecutive seconds of reliable signal, FFTs were performed on multiple windows 1–4.9 s long, together totaling 5–10 s. These smaller FFTs were consistent within subjects. When ambiguities arose, analyses were performed in the time domain and compared with spikes in dominant frequency obtained in the spectral domain.
Results and Discussion
Figure 1 shows the cumulative percentage responding based on different dosages of norepinephrine delivered to embryos. Data points in the graph reflect the percentage of embryos whose change in heart rate from baseline to 2 hr after treatment met or exceeded a 0.2 proportion increase. Mean proportion change from baseline and standard deviations for each concentration are indicated along the abscissa. Whereas half the embryos showed at least a 0.2 proportion increase in heart rate in response to up to 0.01 M norepinephrine, more than two thirds showed this increase at 0.03 M. A three-parameter logistic regression indicated that norepinephrine dosage explained a significant proportion of the variance in this increase, R2 = 0.999, F(2, 4) = 1574.368, p = .000.
Figure 1.
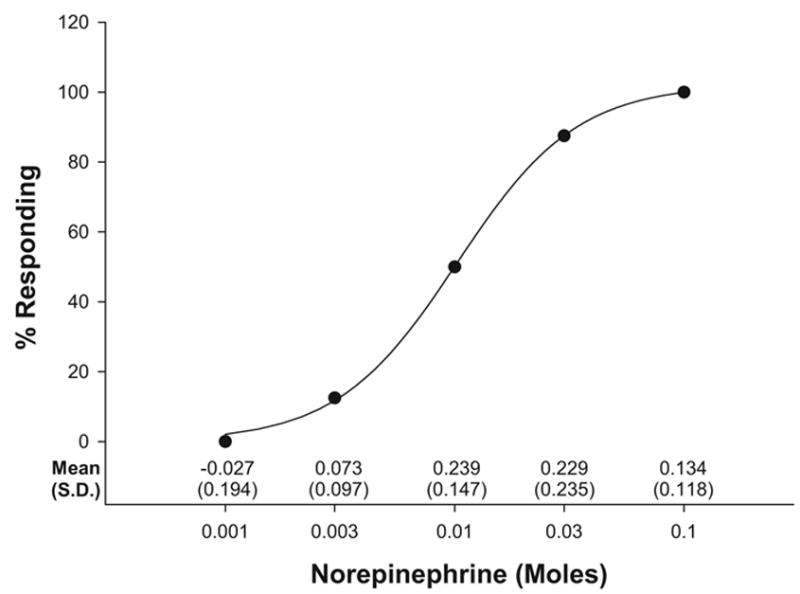
Cumulative percentage of embryos showing a ≥.2 proportion beat-per-minute increase from baseline 2 hr after injection. Solid circles represent actual cumulative percentage data and are fit with a logistic regression line. Proportion change from baseline means and standard deviations for each of the norepinephrine concentrations are shown along the abscissa.
Because of the wide variability in heart rate between embryos and the relatively few subjects exposed to each dose, we collapsed the data from embryos receiving the two lowest (including saline alone), two middle, and two highest dosages. This resulted in three groups: embryos receiving a maximum of 0.001 M, 0.01 M, and 0.1 M, respectively. We then calculated change scores for all embryos between instantaneous heart rate recorded during baseline and 2 hr after injection (see Figure 2). An omnibus one-way analysis of variance (ANOVA) confirmed there was a statistically significant difference between groups, F(2, 32) = 3.801, p = .033. Follow-up tests with Tukey’s least significant difference demonstrated a significant difference in change scores between embryos receiving the lowest and middle dosages ( p = .032) as well as between those receiving the lowest and highest dosages ( p = .018). Change scores between the middle and high dosages were not significantly different. Taken together, these data indicate that a 5-μl bolus of 0.01–0.1 M norepinephrine results in at least a 0.2 proportion increase in bobwhite quail embryonic heart rate 2 hr after injection and that increases in heart rate for birds receiving doses in this range are significantly greater than those for birds receiving doses below this range.
Figure 2.
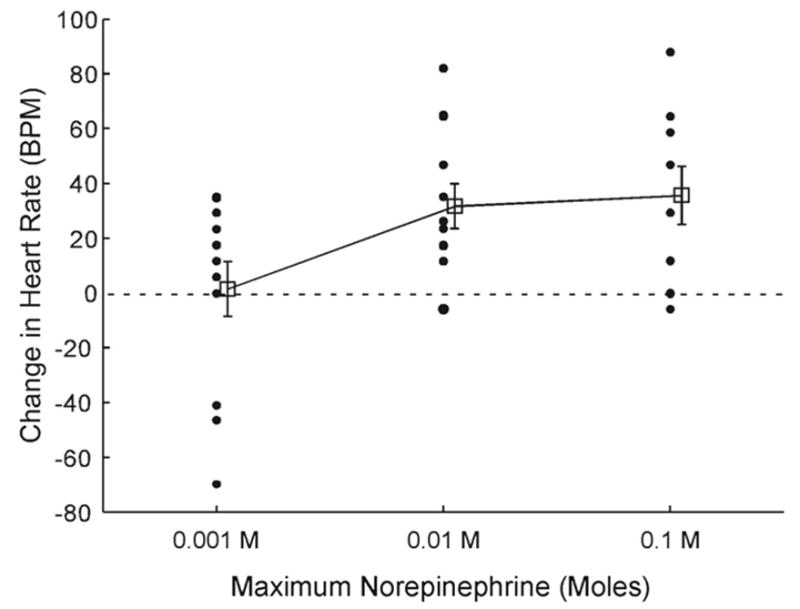
Change in embryonic mean heart rate as a function of norepinephrine dosage. All scores were obtained from different embryos. Solid circles represent individual difference scores between baseline and treatment times of measurement. Open squares represent the mean difference score for each group. The abscissa indicates the maximum dosage birds received in each group. BPM = beats per minute.
Experiment 2: Effects of Norepinephrine on Heart Rate in the Late Prenatal and Early Postnatal Periods
Experiment 1 revealed the dosage range that would reliably elevate bobwhite quail embryos’ heart rate over a period of hours in order to examine the effects of physiological arousal on perceptual learning in the prenatal period. However, Experiment 1 did not reveal whether embryos’ heart rate was elevated immediately after injection or whether it would remain elevated into the post-natal period. Because a goal of this study was to examine whether elevated physiological arousal can interfere with perceptual learning at the time of heightened arousal (and not with the ability to perform in the postnatal choice tests used to assess such effects), we needed to determine whether prenatally administered norepinephrine could have enduring effects on physiological arousal into the early postnatal period (when we planned to conduct behavioral stests). Thus, in this experiment we administered a norepinephrine injection within the effective range identified in Experiment 1 and examined the effect of the norepinephrine on heart rate within subjects immediately after injection, 2 hr after norepinephrine delivery, and 12 hr after hatching. We predicted embryos receiving norepinephrine injections would show an increase in heart rate immediately after delivery and that this effect would be sustained 2 hr after delivery but would not be evident in the period following hatching.
Method
Subjects were 36 bobwhite quail embryos. All apparatus used for this experiment and all procedures were identical to those used in Experiment 1, with the exception that heart rate sampling was extended from injection through the postnatal period.
Embryos were exposed to one of three conditions: 0.03 M norepinephrine (n = 12), saline (n = 12), or control (n = 12). Heart rate was recorded from each subject for 15 min at four different points in time: baseline, treatment, after treatment, and postnatal. Baseline data were collected immediately prior to injection. Treatment data were collected immediately after injection and only for those birds receiving injections (those in the norepinephrine and saline conditions). After treatment data were collected either 2 hr after injection or 2 hr after baseline recordings (control group). Postnatal data collection occurred 12 hr after hatching. Postnatal heart rate sampling was accomplished by placing the two copper wire electrodes used for recording in Experiment 1 in a bilateral position underneath both wings, where there are relatively few feathers on hatchlings. Hatchlings were restrained gently by hand to reduce movement artifact. Heart rate was recorded after a 5-min rest period and only if the hatchling was not emitting distress vocalizations.
Results and Discussion
The means and standard deviations for the dependent variable for all data collected in Experiment 2 are presented in Table 1. Figure 3 shows the mean BPM for subjects in each group, along with the standard error of the mean. To confirm that injections of norepinephrine resulted in an increase in heart rate immediately after delivery, we compared heart rate between those groups receiving injections (norepinephrine and saline) at two points in time (baseline and treatment). A univariate two-way repeated measures ANOVA was conducted, with time of measurement as the within-subjects factor, drug as the between-subjects factor, and BPM as the dependent variable. The ANOVA confirmed presence of an interaction, F(1, 22) = 16.205, p = .001. There was a significant main effect for time, F(1, 22) = 5.460, p = .029, and the main effect for drug approached significance, F(1, 22) = 3.438, p = .077, which is understandable considering a difference should have only been present for the embryos receiving norepinephrine injections and only at one point in time (treatment). Two follow-up paired-samples t tests confirmed there was no difference between groups at baseline, t(11) = 0.732, p = .479, but there was a difference between groups at Treatment, t(11) = 3.235, p = .008. These results indicate that there was no difference in heart rate between the groups receiving norepinephrine and saline before injection and only embryos receiving an injection of norepinephrine showed a statistically significant increase in heart rate immediately after delivery.
Table 1.
Mean Instantaneous Heart Rate Scores for Exposure to Saline and Norepinephrine at Baseline and Immediately After Injection (Treatment), and for Embryos in the Control, Saline, and Norepinephrine Groups at Baseline, 2 hrs After Baseline (After Treatment), and 12 hrs After Hatching (Postnatal)
| Control
|
Saline
|
Norepinephrine
|
||||
|---|---|---|---|---|---|---|
| Time | M | SD | M | SD | M | SD |
| Univariate repeated measures ANOVA | ||||||
| Baseline | 226 | 24.130 | 215 | 44.107 | ||
| Treatment | 210 | 44.341 | 275 | 50.524 | ||
|
| ||||||
| Multivariate repeated measures ANOVA | ||||||
| Baseline | 195 | 29.206 | 226 | 24.130 | 215 | 44.107 |
| After treatment | 225 | 34.239 | 214 | 37.112 | 292 | 40.746* |
| Postnatal | 146 | 30.809 | 135 | 21.223* | 135 | 2.567* |
Note. ANOVA = analysis of variance.
p < .01 (paired-samples t tests with Bonferroni adjustment, as necessary).
Figure 3.
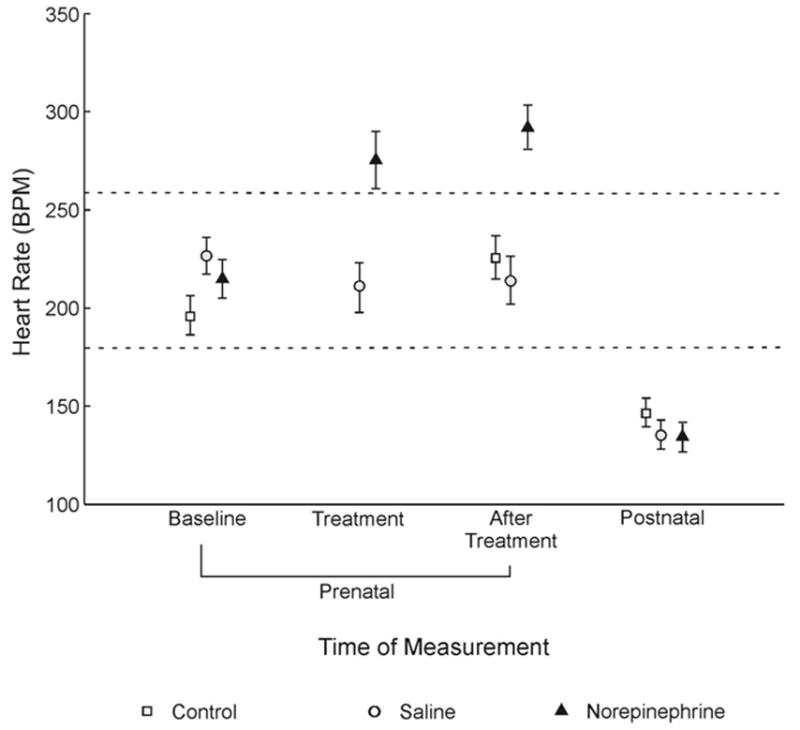
Mean beats per minute (BPM) for three groups of quail receiving saline, norepinephrine, or no injection. Open circles, open squares, and solid triangles reflect the mean for each group, and stems reflect the standard error of the mean. Data were recorded within subjects at four different times: immediately prior to injection (baseline), immediately after injection (treatment), 2 hr after baseline or injection (after treatment), and 12 hr after hatching (postnatal). No control group data were collected during treatment.
We then conducted tests to examine differences for all groups between baseline and the remaining times of measurement. Specifically, we conducted a multivariate two-way repeated measures ANOVA, again with time of measurement as the within-subjects factor (baseline, after treatment, and postnatal), drug as the between-subjects factor (norepinephrine, saline, or control), and BPM as the dependent variable.
Mauchly’s test revealed there was not sufficient evidence to reject the sphericity assumption, indicating homogeneity of variance among the groups. The multivariate Wilk’s lambda criterion confirmed a significant overall interaction, Λ = 0.382, F(4, 64) = 9.3896, p = .000, and a main effect for time of measurement, Λ = 0.110, F(2, 32) = 129.862, p = .000. The univariate tests for drug also revealed a significant main effect, F(2, 33) = 4.379, p = .021. Follow-up paired-sample t tests with a Bonferroni adjustment showed a statistically significant difference between baseline and postnatal times of measurement, t(35) = 10.073, p = .000; between the norepinephrine group and the two groups not exposed to norepinephrine after treatment, t(11) = 4.043, p = .002; and between the two groups not exposed to norepinephrine after treatment and at the postnatal time of measurement, t(23) = 10.739, p = .000. Scores collected during baseline did not differ from those collected from embryos not exposed to norepinephrine at the after treatment time of measurement, t(23) = 1.023, p = .317. These analyses demonstrate that embryos’ heart rate increased from baseline to after treatment only for those subjects receiving norepinephrine injections and that this induced elevation in heart rate did not persist into the postnatal period. All embryos’ heart rate decreased below baseline during the postnatal period and there were no differences in heart rate between groups during the post-natal recording period. Thus, a prenatal injection of norepinephrine did not appear to have an enduring effect on chicks’ autonomic regulation in the period following hatching.
Taken together, these results illustrate that a 0.03 M injection of norepinephrine results in an immediate increase in heart rate that is not simply a function of receiving a 5-μl bolus injection. This increase is sustained for at least 2 hr after injection but is absent 12 hr into the postnatal period. Sensory stimulation delivered immediately after norepinephrine injections in the prenatal period should thus be coincident with heightened physiological arousal. Further, heightened physiological arousal should not confound results of postnatal behavioral tests between groups.
Experiment 3: Effects of Elevated Physiological Arousal on Prenatal Perceptual Learning
Experiments 1 and 2 provide the foundational physiological measurements necessary to begin to investigate the relationship between elevated physiological arousal and perceptual learning in the prenatal period. In this experiment, we presented an individual bobwhite maternal call to quail embryos after they had received a single injection of norepinephrine, a single injection of saline, or no prenatal injection. In keeping with our notion of an optimal window of arousal for perceptual learning, we predicted that unmanipulated embryos would successfully learn a bobwhite maternal call presented over several hours during the late prenatal period (Lickliter & Hellewell, 1992). In contrast, we predicted that embryos experiencing heightened physiological arousal during presentations of the maternal call would show deficits in their ability to learn the maternal call, as evidenced by a lack of preference for the familiar call in postnatal testing.
Method
Subjects
Quail embryos (N = 108) were divided among the three conditions: norepinephrine (n = 32), saline (n = 34), and auditory only (n = 42). After hatching, all chicks were transferred to polycarbonate rearing tubs (45 cm × 25 cm × 15 cm) and reared in groups of 10–12 to approximate natural brood conditions. The rearing room was maintained at 30° C, and all chicks were provided free access to food and water except during test sessions.
Procedure
Embryos in the norepinephrine and saline groups received injections identical to those delivered in Experiments 1 and 2 and were then exposed to auditory stimulation 15–20 min later. Auditory only embryos were selected and treated identically to injected embryos but did not receive an injection prior to the onset of auditory stimulation. Auditory stimulation involved repeated playbacks of an individual bobwhite maternal assembly call for 15 min/hr, over the course of 16 hr (total exposure time = 240 min). Delivery of the auditory stimulation occurred in a portable incubator (Hova-Bator Model #1588; Hova-Bator, Savannah, GA) to ensure proper temperature and humidity. A custom computer program timed the onset and offset of call presentation each hour. The call amplitude was calibrated to 65 dB, measured by a Bruel & Kjaer (Model 2232; Marlborough, MA) sound-level meter.
Chicks in all groups received a 5-min simultaneous choice test between the familiar call and a novel variant of the bobwhite quail maternal assembly call at 16 hr following hatching. Testing took place in a circular arena 130 cm in diameter, surrounded by a wall 60 cm in height. Midrange dome-radiator speakers were located behind the curtain in each of two approach areas. Each speaker received input from a separate compact disc player. The walls of the apparatus were lined with foam to attenuate echoes and were covered by an opaque black curtain to shield speakers and other irregularities that could serve as visual cues to subjects. The floor of the arena was painted black. A video camera suspended near the ceiling in the center of the room allowed for remote observation of behavioral testing. The video camera sent a signal to a TV monitor located in an adjoining room. Two rectangles drawn on the TV monitor demarcated approach areas on opposite sides of the arena, each 15 cm × 30 cm in size. These two approach areas together represented less than 10% of the total area of the arena.
During tests the experimenter placed an individual chick in a start box equidistant from the two approach areas. All hatchlings received a 1-min settling period followed by a 5-min simultaneous choice test between the familiar and novel variants of the bobwhite maternal assembly call, one played from each speaker. These two maternal calls were recorded in the field and are similar in phrasing, repetition rate, and frequency modulation. They vary primarily in minor peaks of dominant frequency and in the temporal microstructure of rhythm and duration (Heaton, Miller, & Goodwin, 1978). Previous studies have revealed that hatchlings do not show a naive preference for either of these two variants of the bobwhite maternal call (Honeycutt & Lickliter, 2001; Lickliter & Hellewell, 1992). The sound intensity of each call was adjusted to peak at 65 dB, measured from the start box where hatchlings were introduced into the arena. The locations of the two maternal calls presented during testing were counterbalanced across trials within groups to prevent a possible side bias from affecting results.
Each hatchling was tested individually and only once. A Visual Basic computer program allowed semi-automated collection of latency and duration of response to the test stimuli. Latency was defined as the amount of time in seconds that elapsed from the onset of the trial until the hatchling entered an approach area. Duration was defined as the cumulative amount of time in seconds the hatchling remained in an approach area. Any hatchling that did not enter either approach area during a test trial or spent less than a total of 10 s in both areas was considered a nonresponder. These hatchlings were excluded from subsequent analyses. Preference for a given stimulus was scored if a hatchling stayed in an approach area for at least twice the time spent in the opposing approach area. No preference for a stimulus was scored if a hatchling approached both areas during a trial but did not spend at least twice as much time in one approach area as the other. These individual preference scores have been used in a number of prior studies of perceptual discrimination in bobwhite quail (see Lickliter & Hellewell, 1992; Lickliter & Lewkowicz, 1995, for examples) and constituted the primary dependent variable.
Data analyses
Several measures were analyzed: latency of initial approach to the familiar and novel calls was recorded for each subject and compared by the Wilcoxon matched-pairs signed-ranks test, and duration of time spent in each approach area by subjects in a group were also compared by the Wilcoxon test to determine whether duration scores for one stimulus differed from that of the other within a condition. Individual preference scores were evaluated by the chi-square goodness-of-fit test to determine whether there was a statistically significant difference from chance in the number of hatchlings preferring the familiar call compared with the novel call.
We used nonparametric tests for our analyses in the present study for several reasons. Previous studies of bobwhite quail that have used methods comparable to those used here indicate hatchlings’ latency and duration scores in the simultaneous choice test vary widely within and across experimental groups (e.g., Lickliter & Hellewell, 1992; Lickliter & Lewkowicz, 1995). The data from the present experiments show similar patterns of variance. The wide range of scores obtained makes measures of central tendency hard to interpret. Nonparametric statistics are preferable under these conditions (Siegel & Castellan, 1988).
Results and Discussion
As illustrated in Figure 4, results of postnatal testing supported our predictions. Chicks in the auditory only group showed a significant preference for the familiar call compared with a novel call, χ2(2) = 18.2, p = .001. Likewise, chicks in the saline group showed a significant preference for the familiar call, χ2(2) = 21.6, p = .001. In contrast, chicks in the norepinephrine group did not show a preference for the familiar maternal call, χ2(2) = 4.2, p = .122.
Figure 4.
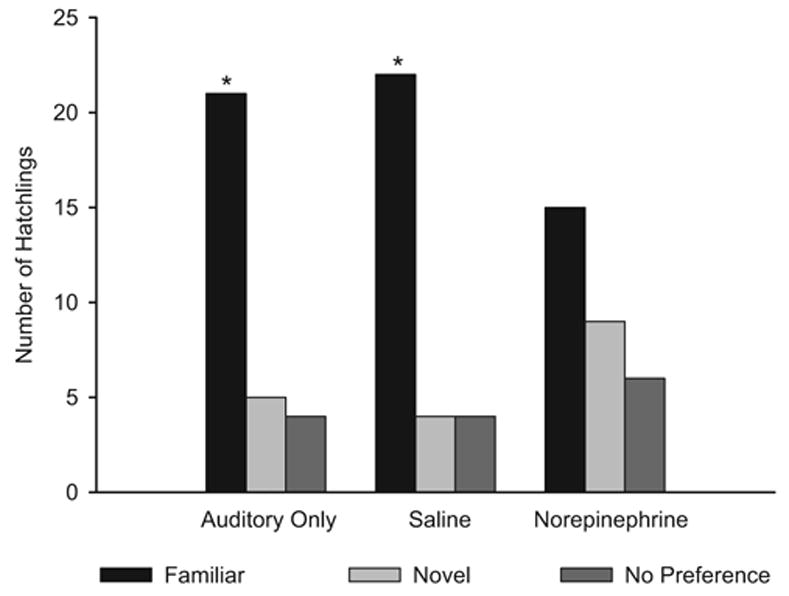
Number of quail hatchlings preferring the familiar maternal call and a novel maternal call from the auditory only, saline, and norepinephrine conditions. All chicks received repeated presentations of the familiar call as embryos. *Chi-square tests indicate a statistically significant difference between groups at p < .001.
Additional analyses of latency and duration scores by the Wilcoxon signed-ranks test further supported these results (see Table 2). Chicks in the auditory only group showed a faster response to the familiar call compared with the novel call (z = −2.540, p= .011) as did chicks in the saline group (z = −3.548, p = .000). However, chicks in the norepinephrine group showed no difference in latency of approach between the two calls (z = −1.162, p = .245). Chicks in the auditory only group also spent more time near the familiar call (z = −2.664, p = .008) as did chicks in the saline group (z = −3.322, p = .001). In contrast, chicks in the norepinephrine group did not show a significant difference in duration of response to either the familiar or novel maternal calls during test trials (z = −0.051, p = .959).
Table 2.
Median Latency of Approach and Duration Scores (in Seconds) for the Familiar and Novel Variants of the Bobwhite Maternal Call for Chicks Exposed to the Auditory Only, Saline, or Norepinephrine Conditions as Embryos
| Maternal call | Auditory only | Saline | Norepinephrine |
|---|---|---|---|
| Latency | |||
| Familiar | 33.29* | 23.18* | 58.61 |
| Novel | 119.44 | 149.36 | 83.46 |
|
| |||
| Duration | |||
| Familiar | 83.85* | 107.36* | 51.93 |
| Novel | 14.77 | 18.47 | 51.08 |
p < .05 (Wilcoxon signed-rank tests).
These results suggest that heightened physiological arousal can interfere with perceptual learning during the prenatal period. Embryos receiving a single norepinephrine injection immediately prior to exposure to repeated presentations of an individual maternal call failed to show evidence of having learned the call, whereas embryos exposed to the call only or to saline injections and then exposed to the call significantly preferred the familiar maternal call in postnatal testing. Given that norepinephrine injections were shown to increase heart rate in embryos immediately after hatching as well as 2 hr after hatching in Experiment 2, the embryos receiving norepinephrine in the present experiment likely had an elevated heart rate during at least the first several hours of exposure to the maternal call. Unpublished observations from our lab indicate that embryos’ heart rate can remain elevated some 8 hr after a single norepinephrine injection, although not at the magnitude shown in Experiment 2. Thus, it is likely that embryos’ heart rate was elevated during most of the first half of the 16 hr of stimulation, and this elevation appeared to interfere with perceptual learning and/or memory consolidation.
It is interesting to note that these results run contrary to studies showing the enhancing effects of norepinephrine on cognitive processes during early development (for reviews, see Gibbs & Summers, 2002; McGaugh, 2004). However, it is important to note that these studies used contingent rather than perceptual (noncontingent) learning paradigms to explore the effects of norepinephrine on learning and memory and also worked with organisms older than those in the present study. To our knowledge, our results are the first to demonstrate an effect of prenatal exposure to norepinephrine on perceptual or behavioral responsiveness following birth or hatching.
Experiment 4: Effects of Elevated Arousal on Postnatal Perceptual Preferences
The results of Experiment 3 indicate that chicks receiving norepinephrine injections prenatally do not show a preference for an individual maternal call to which they are familiarized, whereas chicks receiving saline injections or no injections prenatally show a significant preference for the familiar maternal call following hatching. Of course, embryos are undergoing extensive systemic changes in many neural and physiological systems during the late stages of incubation, and it is possible that exposure to norepinephrine during this period could somehow compromise general auditory or motor function, orienting, or some combination of these processes. To assess this possibility, in the current experiment we delivered norepinephrine to a group of embryos in a manner identical to that in the prior experiments of this study, then tested whether they would prefer a conspecific call over a heterospecific call in simultaneous choice tests following hatching. Prior studies have shown that naive bobwhite chicks reliably prefer their conspecific bobwhite maternal call compared with a heterospecific chicken or scaled quail maternal call (Lickliter & Virkar, 1989; McBride & Lickliter, 1993). We thus predicted embryos receiving no injections would prefer the conspecific call compared with a heterospecific call following hatching. We also predicted that embryos receiving norepinephrine would prefer the conspecific call, thereby indicating that the failure to demonstrate learning of the maternal call observed in Experiment 3 was not due to a general deficit in auditory or motor functioning resulting from the prenatal norepinephrine injection.
Method
Sixty bobwhite quail embryos were assigned to one of two conditions: control (n = 30) or norepinephrine (n = 30). Unlike the procedures used in Experiment 3, embryos in this experiment did not receive prenatal exposure to the bobwhite maternal call. Following hatching, the naive chicks were given a 5-min choice test between a bobwhite maternal call and a heterospecific scaled quail maternal call. Rates of activity were also recorded by counting the frequency of entries into either approach area by subjects during the 5-min test.
Results and Discussion
As illustrated in Figure 5, results of postnatal testing supported our predictions. Chicks in the control group showed a significant preference for the conspecific maternal call over the heterospecific maternal call, χ2(2) = 42.25, p = .000, as did chicks in the norepinephrine group, χ2(2) = 54, p = .000. Chicks in the control group showed shorter latencies to respond to the conspecific call than the heterospecific call (z = −3.657, p = .000) as did chicks in the norepinephrine group (z = −4.469, p = .000). Chicks in the control group also spent more time in proximity to the conspecific call compared with the heterospecific call (z = −4.286, p = .000) as did chicks in the norepinephrine group (z = −4.541, p = .000). (See Table 3.)
Figure 5.
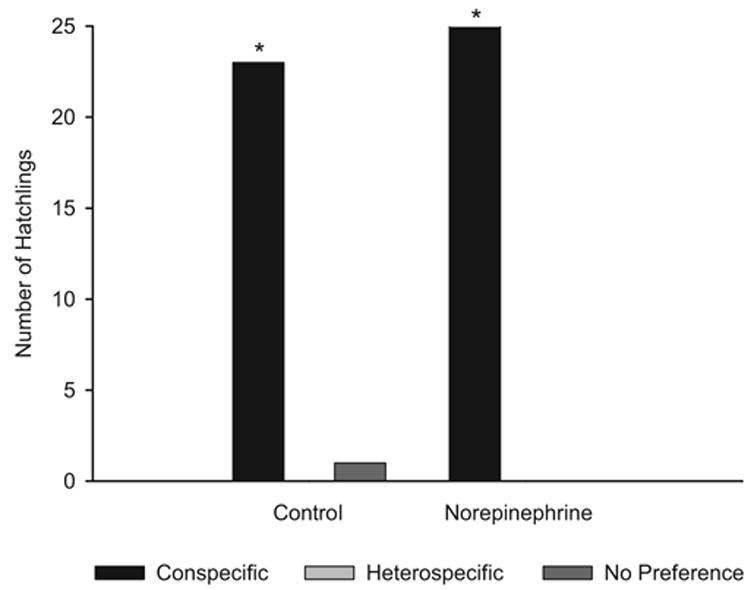
Number of quail hatchlings preferring the conspecific or heterospecific maternal call from the control and norepinephrine conditions. *Chi-square tests indicate a statistically significant difference at p < .000.
Table 3.
Median Latency of Approach and Duration Scores (in Seconds) for Conspecific and Heterospecific Maternal Calls for Chicks Exposed to the Control or the Norepinephrine Conditions as Embryos
| Maternal call | Control | Norepinephrine |
|---|---|---|
| Latency | ||
| Conspecific | 18.98 | 26.81 |
| Heterospecific | 114.44 | 167.30 |
|
| ||
| Duration | ||
| Conspecific | 82.08 | 83.31 |
| Heterospecific | 4.83 | 1.10 |
Note. For all Wilcoxon signed-ranks tests, p < .000.
We conducted a 2 × 2 ANOVA to assess whether chicks receiving norepinephrine injections prenatally showed different rates of activity during testing when compared with unmanipulated control chicks. The frequency data on entries into the two approach areas was somewhat skewed because of the large number of zeros for entries into the approach area associated with the heterospecific call. Thus, these indices were square root transformed to normalize the data. The means and standard deviations for square root transformed number of entries to each approach area are shown in Table 4. The ANOVA indicated no significant interaction between condition and maternal call, F(1, 98) = 0.35, p = .558, and a nonsignificant main effect for condition, F(1, 98) = 0.3, p = .588. The test also revealed a significant main effect for maternal call, F(1, 98) = 140.14, p = .000. These analyses further support our predictions and indicate that chicks receiving a prenatal norepinephrine injection did not display higher rates of behavioral activity than did unmanipulated chicks during postnatal testing. The physiological data from Experiment 2 indicated no differences in postnatal heart rate between hatchlings receiving norepinephrine and those not receiving norepinephrine. The results from the current experiment provide evidence that neither chicks from the treatment group nor controls differed in behavioral arousal at the time of testing. This suggests that the differences in responsivity to the familiar maternal call observed in Experiment 3 can be attributed to elevated physiological arousal at the time of exposure to the call only. Further, the observed differences were not due to general deficits in auditory or locomotor function that could have resulted from a prenatal injection of norepinephrine.
Table 4.
Means and Standard Deviations for Square Root Transformed Rate of Activity, Indexed as the Number of Times Chicks Entered Each Approach Area During Simultaneous Choice Tests
| Maternal call | M | SD |
|---|---|---|
| Control | ||
| Conspecific | 4.91* | 2.12 |
| Heterospecific | 1.57 | 1.23 |
|
| ||
| Norepinephrine | ||
| Conspecific | 4.93* | 1.32 |
| Heterospecific | 1.23 | 1.19 |
p < .000 (significant main effect in a 2 × 2 analysis of variance).
General Discussion
This study examined whether elevating physiological arousal during the late prenatal period would interfere with perceptual learning in bobwhite quail embryos. Results revealed that embryos receiving a single injection of norepinephrine prior to repeated exposure to an individual bobwhite maternal call failed to prefer that familiar call over a novel maternal call in postnatal testing. In contrast, embryos receiving either a saline injection or no injection prior to exposure to the individual maternal call showed a significant preference for the familiar call in postnatal choice tests (Experiment 3). Our findings parallel results from previous studies that elevated precocial birds’ arousal in the prenatal period by increasing the amount of available sensory stimulation rather than using pharmacological methods. These studies also found interference with perceptual learning (e.g., Honeycutt & Lickliter, 2001; Radell & Gottlieb, 1992; Sleigh, Columbus, & Lickliter, 1996). Taken together with the present results, this body of research provides converging evidence that increasing an embryo’s overall arousal (via either sensory stimulation or pharmacologically) over a period of hours decreases the embryo’s sensitivity to specific sensory information present in the prenatal environment, thereby interfering with species-typical perceptual learning. In the present study, embryos receiving a prenatal injection of norepinephrine showed elevated heart rate when compared with embryos receiving a saline injection both immediately after injection and 2 hrs after injection (but not during postnatal measurement). These aroused embryos failed to show evidence of learning an individual bobwhite maternal call, an ability seen in both saline and control embryos.
These findings stand in contrast to studies showing that norepinephrine delivery can result in enhancement rather than impairment of cognitive processes (Gibbs & Summers, 2002, 2005; LaLumiere, Buen, & McGaugh, 2003; McGaugh, 2004). Gibbs and Summers (2002) and several others have demonstrated that the effects that norepinephrine has on learning vary according to several factors, including but not limited to developmental age, learning process, species, and neural region of interest. As a case in point, virtually all studies showing an enhancement in cognitive functioning resulting from norepinephrine activation have used associative learning paradigms. In contrast, we used a perceptual learning paradigm not involving contingent arousal. It may be that episodic and contingent arousal is necessary for memory enhancement to occur; otherwise, tonic physiological arousal may facilitate sensitivity to all endogenous and exogenous stimulation across a longer period of time, resulting in some form of “sensory overload” that can prevent the young animal from attending to the specific features of events. For example, footshocks paired with a light and the resulting norepinephrine endogenously secreted by the fearful event, may facilitate attending to the concomitant light and may suppress attending to other stimulation occurring at adjacent points in time (indeed, in vivo microdialysis and high-performance liquid chromatography experiments have shown that norepinephrine increases systematically in the amygdala with the level of footshock (Galvez, Mesches, & McGaugh, 1996; Quirarte, Galvez, Roozendaal, & McGaugh, 1998). It seems likely that changes in amounts of endogenous norepinephrine are relatively phasic, and therefore physiological arousal subsides to at least some degree until the next pairing, creating temporal contiguity between changes in physiological arousal with changes in a conditioned stimulus such as a light. By comparison, our stimulation paradigm involves exogenously delivered norepinephrine, which resulted in tonic physiological arousal paired with an episodically presented maternal call. Thus, there was a lack of temporal contiguity between changes in physiological arousal and the auditory stimulus to be learned.
Adrenoceptor activation points to another possibility. Several studies have shown that if enough β-adrenoceptors are activated, excess norepinephrine may bind probabilistically and thereby activate α1-adrenoceptors, and such activation has been shown to inhibit cognitive processes (Gibbs & Summers, 2001, 2002, 2005). Developmental studies indicate that, unlike adult organisms, young organisms have an immature parasympathetic nervous system, preventing them from effectively down regulating physiological arousal via this mechanism (Crossley & Altimiras, 2000). Thus, it is possible that young organisms have a lowerβ-adrenoceptor/α1-adrenoceptor ratio to compensate for this lack of parasympathetic control, resulting in a need for less norepinephrine to activate the cognition-inhibiting α1-adrenoceptor subtype. This has important implications for the effects of physiological arousal in the prenatal and early postnatal periods, and future research is needed to explore this possibility. As such, it seems likely that the relationships between physiological arousal and perceptual learning are somewhat different for younger organisms than they are for adults. Additional research is needed to further the understanding of the nature of these differences.
In a more general sense, the present results provide additional evidence that there is a range of arousal that is optimal for responding to perceptual information during early development. Deviations above or below this optimal range of arousal can negatively affect the young organism’s ability to learn available sensory input, at least during the prenatal period (see Carlsen & Lickliter, 1999; Lickliter & Lewkowicz, 1995; Radell & Gottlieb, 1992, for behavioral evidence in support of this notion). Whether these effects are transient or long lasting and whether elevations in prenatal arousal can impair postnatal perceptual learning are important questions to pursue. The nature of the links between arousal, attention, and learning remain of enduring interest in the psychological sciences and in cognitive and behavioral neuroscience. Systematic comparison of effects of modified arousal on attention, learning, memory consolidation, and memory reconsolidation across similar test situations is needed to advance the knowledge of the specific experiential features underlying early perceptual and cognitive functioning in the prenatal and early postnatal periods.
Footnotes
Rebecca G. Markham, Gabriella Toth, and Robert Lickliter, Infant Development Research Center, Department of Psychology, Florida International University.
This research was supported by National Institute of Mental Health Grant RO1 MH 62225 and National Institute of Child Health and Human Development Grant RO1 HD 048423.
References
- Altimiras J, Crossley DA., II Control of blood pressure mediated by baroreflex changes of heart rate in the chicken embryo (Gallus gallus) American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2000;278:980–986. doi: 10.1152/ajpregu.2000.278.4.R980. [DOI] [PubMed] [Google Scholar]
- Aubert AE, Beckers F, Ramaekers D, Verheyden B, Leribaux C, Aerts JM, et al. Heart rate and heart rate variability in chicken embryos at the end of incubation. Experimental Physiology. 2004;89:199–208. doi: 10.1113/expphysiol.2003.027037. [DOI] [PubMed] [Google Scholar]
- Aubert AE, Leribaux C, Beckers F, Ramaekers D, Berckmans D. Noninvasive measurement of heart rate from chicken embryos in the egg. Computers in Cardiology. 2000;27:227–230. [Google Scholar]
- BIOPAC Systems. Acknowledge for PC/Windows, Version 3.5.7 [Computer software] Santa Barbara, CA: Author; 2005. [Google Scholar]
- Carlsen R, Lickliter R. Augmented prenatal tactile and vestibular stimulation alters postnatal auditory and visual responsiveness in bobwhite quail chicks. Developmental Psychobiology. 1999;35:215–225. [PubMed] [Google Scholar]
- Cheung MO, Gilbert EF, Bruyere HJ, Jr, Ishikawa S, Hodach RJ. Chronotropism and blood flow patterns following teratogenic doses of catecholamines in 5-day-old chick embryos. Teratology. 1977;16:327–343. doi: 10.1002/tera.1420160312. [DOI] [PubMed] [Google Scholar]
- Crossley D, Altimiras J. Ontogeny of cholinergic and adrenergic cardiovascular regulation in the domestic chicken (Gallus gallus) American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2000;279:1091–1098. doi: 10.1152/ajpregu.2000.279.3.R1091. [DOI] [PubMed] [Google Scholar]
- Culver NG, Fischman DA. Pharmacological analysis of sympathetic function in the embryonic chick heart. American Journal of Physiology. 1977;232:116–123. doi: 10.1152/ajpregu.1977.232.3.R116. [DOI] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiology of Learning and Memory. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Karmel BZ. Development of arousal-modulated visual preferences in early infancy. Developmental Psychology. 1995;31:473–482. [Google Scholar]
- Gibbs ME, Summers RJ. Stimulation of alpha1-adrenoceptors inhibits memory consolidation in the chick. European Journal of Neuroscience. 2001;14:1369–1376. doi: 10.1046/j.0953-816x.2001.01742.x. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Summers RJ. Role of adrenoceptor subtypes in memory consolidation. Progress in Neurobiology. 2002;67:345–391. doi: 10.1016/s0301-0082(02)00023-0. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Summers RJ. Contrasting roles for beta1, beta2 and beta3-adrenoceptors in memory formation in the chick. Neuroscience. 2005;131:31–42. doi: 10.1016/j.neuroscience.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Girard H. Adrenergic sensitivity of circulation in the chick embryo. American Journal of Physiology. 1973;224:461–469. doi: 10.1152/ajplegacy.1973.224.2.461. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Social induction of malleability in ducklings: Sensory basis and psychological mechanism. Animal Behavior. 1993;45:707–719. [Google Scholar]
- Gray L. Activity level and auditory responsiveness in neonatal chickens. Developmental Psychobiology. 1990;23:297–308. doi: 10.1002/dev.420230402. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Miller DB, Goodwin DG. Species-specific auditory discrimination in bobwhite quail neonates. Developmental Psychobiology. 1978;11:13–21. doi: 10.1002/dev.420110106. [DOI] [PubMed] [Google Scholar]
- Honeycutt H, Lickliter R. Order-dependent timing of unimodal and multimodal stimulation affects prenatal auditory learning in bobwhite quail embryos. Developmental Psychobiology. 2001;38:1–10. doi: 10.1002/1098-2302(2001)38:1<1::aid-dev1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Buen TV, McGaugh JL. Posttraining intrabasolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. Journal of Neuroscience. 2003;23:6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowicz DJ, Turkewitz G. Cross-modal equivalence in early infancy: Auditory–visual intensity matching. Developmental Psychology. 1980;16:597–607. [Google Scholar]
- Lewkowicz DJ, Turkewitz G. Intersensory interaction in newborns: Modification of visual preferences following exposure to sound. Child Development. 1981;52:827–832. [PubMed] [Google Scholar]
- Lickliter R. Atypical perinatal sensory stimulation and early perceptual development: Insights from developmental psychobiology. Journal of Perinatology. 2000;20:45–54. doi: 10.1038/sj.jp.7200450. [DOI] [PubMed] [Google Scholar]
- Lickliter R, Hellewell TB. Contextual determinants of auditory learning in bobwhite quail embryos and hatchlings. Developmental Psychobiology. 1992;25:17–31. doi: 10.1002/dev.420250103. [DOI] [PubMed] [Google Scholar]
- Lickliter R, Lewkowicz DJ. Intersensory experience and early perceptual development: Attenuated prenatal sensory stimulation affects postnatal auditory and visual responsiveness in bobwhite quail chicks (Colinus virginianus) Developmental Psychology. 1995;31:609–618. [Google Scholar]
- Lickliter R, Virkar P. Intersensory functioning in bobwhite quail chicks: Early sensory dominance. Developmental Psychobiology. 1989;22:651–667. doi: 10.1002/dev.420220702. [DOI] [PubMed] [Google Scholar]
- McBride TC, Lickliter R. Social experience with siblings fosters species specific responsiveness to maternal visual cues in bobwhite quail chicks. Journal of Comparative Psychology. 1993;107:320–327. doi: 10.1037/0735-7036.107.3.320. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Moriya K, Pearson JT, Burggren WW, Ar A, Tazawa H. Continuous measurements of instantaneous heart rate and its fluctuations before and after hatching in chickens. Journal of Experimental Biology. 2000;203:895–903. doi: 10.1242/jeb.203.5.895. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Pittman R, Gray M, Maderdrut JL. Embryonic behavior, hatching and neuromuscular development in the chick following a transient reduction of spontaneous motility and sensory input by neuromuscular blocking agents. Journal of Comparative Neurology. 1978;179:619–640. doi: 10.1002/cne.901790310. [DOI] [PubMed] [Google Scholar]
- Pearson JT, Tsudzuki M, Nakane Y, Akiyama R, Tazawa H. Development of heart rate in the precocial king quail Coturnix chinensis. Journal of Experimental Biology. 1998;201(Pt 7):931–941. doi: 10.1242/jeb.201.7.931. [DOI] [PubMed] [Google Scholar]
- Petery LB, Jr, Van Mierop LH. Evidence for the presence of adrenergic receptors in 3-day-old chick embryo. American Journal of Physiology. 1977;232:H250–254. doi: 10.1152/ajpheart.1977.232.3.H250. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Research. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Radell PL, Gottlieb G. Developmental intersensory interference: Augmented prenatal sensory experience interferes with auditory learning in duck embryos. Developmental Psychology. 1992;28:795–803. [Google Scholar]
- Reynolds GD, Lickliter R. Effects of redundant and nonredundant bimodal sensory stimulation on heart rate in bobwhite quail embryos. Developmental Psychobiology. 2003;43:304–310. doi: 10.1002/dev.10138. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Haque MA, Ono H, Pearson J, Tazawa H. Two-dimensional cardiogenic ballistic movements of avian eggs. Medical and Biological Engineering and Computing. 1995;33:611–614. doi: 10.1007/BF02522522. [DOI] [PubMed] [Google Scholar]
- Sedlacek J. Development of spontaneous motility in chick embryos. Sensitivity to aminergic transmitters. Physiologia Bohemoslovaca. 1977;26:425–433. [PubMed] [Google Scholar]
- Siegel S, Castellan NJ. Choosing an appropriate statistical test. In: Anker JD, editor. Nonparametric statistics for the behavioral sciences. 2. Boston: McGraw-Hill; 1988. pp. 19–36. [Google Scholar]
- Sleigh MJ, Columbus RF, Lickliter R. Type of prenatal sensory experience affects prenatal auditory learning in bobwhite quail (Colinus virginianus) Journal of Comparative Psychology. 1996;110:233–242. doi: 10.1037/0735-7036.110.3.233. [DOI] [PubMed] [Google Scholar]
- Sleigh MJ, Lickliter R. Augmented prenatal auditory stimulation alters postnatal perception, arousal, and survival in bobwhite quail chicks. Developmental Psychobiology. 1997;30:201–212. [PubMed] [Google Scholar]
- Tayo FM. Are there alpha-adrenoceptors in the young chick atria? British Journal of Pharmacology. 1984;81:289–291. doi: 10.1111/j.1476-5381.1984.tb10077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa H, Hiraguchi T, Asakura T, Fujii H, Whittow GC. Noncontact measurements of avian embryo heart rate by means of laser speckle: Comparison with contact measurements. Medical and Biological Engineering and Computing. 1989;27:580–586. [Google Scholar]


