Abstract
We have characterized the expression of organic anion transporter 6, Oat6 (slc22a20), in olfactory mucosa, as well as its interaction with several odorant organic anions. In situ hybridization reveals diffuse Oat6 expression throughout olfactory epithelium, yet olfactory neurons laser-capture microdissected from either the main olfactory epithelium (MOE) or the vomeronasal organ (VNO) did not express Oat6 mRNA. These data suggest that Oat6 is expressed in non-neuronal cells of olfactory tissue, such as epithelial and/or other supporting cells. We next investigated interaction of Oat6 with several small organic anions that have previously been identified as odortype components in mouse urine. We find that each of these compounds, propionate, 2- and 3-methylbutyrate, benzoate, heptanoate and 2-ethylhexanoate, inhibits Oat6-mediated uptake of a labeled tracer, estrone sulfate, consistent with their being Oat6 substrates. Previously, we noted defects in the renal elimination of odortype and odortype-like molecules in Oat1 knockout mice. The finding that such molecules interact with Oat6 raises the possibility that odorants secreted into the urine through one OAT-mediated mechanism are transported through the olfactory mucosa through another OAT-mediated mechanism. Oat6 might play a direct or indirect role in olfaction, such as modulation of the availability of odorant organic anions at the mucosal surface for presentation to olfactory neurons or facilitation of delivery to a distal site of chemosensation, among other possibilities that we discuss.
Keywords: Organic Anion Transporter, Oat, Slc22a20, olfactory mucosa, volatile organic acid, odorant
Introduction
Organic anion transporters (OATs) mediate transmembrane uptake and/or efflux of a disparate array of organic anions [1-3]. With one exception, they are mostly expressed in liver and/or kidney, the two principal excretory organs of the body [4-6]. The exception is Oat6, which is highly expressed in olfactory mucosa (with a lesser degree of expression in testis and early embryonic tissues) [7]. Moreover, while the principal site of Oat1 expression (originally identified by us as NKT [8]) is the proximal tubule of the kidney, the mRNA of Oat1 (but not of the other OATs, Oat2 – Oat5) is also detectable in olfactory mucosa [7] suggesting the importance of organic anion transport in this tissue.
OAT expression in olfactory mucosa raises the possibility that OAT-mediated transport might modulate the process of olfaction. Notably, there is evidence for a link between olfactory signaling and organic anion transport. In mice (and likely in other mammals) odortypes (individual odor ‘signatures’) appear to be specified by the relative proportion of multiple odorants, including, prominently, urinary organic acids [9, 10]. Among these latter compounds are several that are the same as or structurally similar to putative endogenous substrates of Oat1 (as identified by their increased plasma and/or decreased urinary concentration in Oat1 knockout mice [11]).
Here we report the characterization of Oat6 expression in mouse olfactory mucosa, as well as the interactions of this transporter with a set of organic anions potentially involved in odortype specification, in comparison with the interactions of those compounds with Oat1. We also discuss the possible in vivo function of the OATs in olfactory mucosa.
Materials and Methods
Organic anion transporter 6 (Oat6 and Oat1)
Capped cRNA was synthesized from a linearized plasmid DNA (mOat6, Image clone ID: 6309674; mOat1, Image clone ID: 4163278) using mMessage mMachine in vitro transcription kit (Ambion, Inc., Austin, TX).
Oat6 expression in nasal mucosa
Small sections of whole epithelia were microdissected from the main olfactory epithelium (MOE) and the vomeronasal organ (VNO) of C57BL/6 mice. RNA was extracted using a Stratagene Absolutely RNA Nanoprep Kit (La Jolla, CA). Single olfactory neurons were isolated and RNA amplified as previously described [12]. In brief, aggregates of 20-50 olfactory neurons were dissected from unfixed, 100 μm thick, frozen sections using a laser capture microscope (Arcturus Bioscience, Mountain View, CA), and RNA amplified using a TargetAmp 2-Round aRNA Amplification Kit (Epicentre Biotechnologies, Madison, WI).
PCR was carried out on cDNA generated using the Invitrogen SuperScript III system (Carlsbad, CA), with 80-100 pg RNA per reaction. Oat6 primers: 5′CGGGAGAACTCTATCCCACA3′ and 5′CTTCCCCTTCCTGAGCTTTC3′, Olfactory Marker Protein (OMP) primers: 5′AGCTAGCAACAGTGATGTCCCTG3′ and 5′CGGATCCGAGTGAGGCAGAGTTG3′, and β3-Tubulin primers: 5′GAGGAGGAGGGGGAGATGTA3′ and 5′CCCCCGAATATAAACACAACC3′. PCR conditions were: 94°C for 5 min, followed by 30 cycles of 94°C, 60°C and 72°C, each for 1 min (except for OMP amplification, when the annealing temperature was 65°C instead of 60°C), and then 72°C for 10 min.
In situ hybridization
Oat6 and OMP sense and antisense riboprobes were synthesized from plasmids encoding the full-length cDNAs using digoxigenin-UTP. Ten- μm coronal cryosections were prepared from the nasal mucosae of pregnant ICR mice (following fixation in 4% PFA, decalcification in 10% EDTA, and embedding in OCT). Sections were fixed 10 minutes in 4% PFA then rinsed 2 × 3 minutes in PBT (0.1% Tween-20 in PBS). Proteinase K (Sigma) digestion was carried out for 8 minutes at 10 μg/ml in dPBT followed by before fixing 5 minutes in 4% PFA with 0.2% ProtectRNA (Sigma). Two and 2 × 3 minute rinsingesrinses in PBT with 0.2% ProtectRNA followed. Sections were then incubated for at least 2 hours in hybridization solution: (50% deionised formamide (Ambion), 25% 20x SSC, 2% blocking powder (Roche), 0.1% Tween-20, 0.5% CHAPS (Sigma), 1 mg/ml Yeast RNA (Sigma), 0.5 M EDTA, 0.05 mg/ml heparin (Sigma), and 0.2% ProtectRNA). RNA probes were heated for 3 minutes to 80°C before applying to the sections at a final concentration of approximately 250 ng/ml in hybridisation solution. Hybridization was carried out at 60°C overnight. Subsequently, sections were washed 2 × 15 minutes in 2x SSC and 2 × 15 minutes in 0.2x SSC, all at 60°C, then equalised with TBST (TBT TBS and 0.1% Tween-20), and blocked for 1hr in TBST with 10% sheep serum (Sigma) and 2% bovine serum albumin (Sigma). Sections were next transferred to 1:2000 alkaline phosphatase-conjugated anti-DIG antibody (Roche) in blocking solution for 1 hr, then rinsed 2 × 15 minutes in TBST followed by 2 × 5 minute washes in NMT (100 mM NaCl, 50 mM MgCl2, and 100 mM Tris-Cl, (pH 9.5), 50mM MgCl2) with 0.1% Tween-20. Color development was carried out in the dark at 4°C using 17 μl/ml NBT/BCIP solution (Roche) in NMT, and sections were fixed in cold methanol before mounting on slides with Eukitt (Electron Microscopy Sciences). Images were obtained using a Nikon Eclipse TE300 microscope.
Organic anions (OAT substrates and inhibitors)
[3H]-labeled estrone sulfate ([3H]-ES, specific activity 57 Ci/mmol) and para-aminohippurate ([3H]-PAH, specific activity 4.2 Ci/mmol) were obtained from Perkin-Elmer Life Sciences (Boston, MA). Unlabeled organic anions (ES, PAH, propionate, 2-methylbutyrate, 3-methylbutyrate, benzoate, heptanoate, and 2-ethylhexanoate) were obtained from Sigma-Aldrich as free acids or sodium salts. Stock solutions of the unlabeled organic anions (100 mM) were prepared by dissolving them in water (for those purchased as salts) or in water with a calculated amount of NaOH (for free acids) and adjusted to pH 7.4.
Uptake in Xenopus oocytes
Xenopus oocyte isolation and uptake assays were performed as described previously [11]. Oocytes were isolated and maintained in Barth's buffer (88 mM NaCl, 1 mM KCl, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, 2.4 mM NaHCO3 and 10 mM HEPES, pH 7.4) supplemented with 5% fetal horse serum, 0.05 mg/ml gentamycin sulfate and 2.5 mM sodium pyruvate. The day after oocyte isolation, cRNA solution (mOat6 or mOat1, 0.5 μg/μl) was injected into oocytes (23 nl/oocyte) using Nanoliter 2000 nanoinjector (World Precision Instruments, Sarasota, FL).
Two days after injection, oocytes were washed in serum-free Barth's buffer, and experimental groups of 20 to 30 oocytes each were incubated at 22°C for 1 hr in 1 ml of Barth's buffer containing 1 μCi/ml of a [3H]-labeled tracer ion (17 nM [3H]-ES or 238 nM [3H]-PAH). In uptake inhibition experiments, the incubation medium for each experimental group also contained a certain concentration of an unlabeled organic anion (tested as tracer uptake inhibitor), with a control group incubated in the absence of inhibitors. Subsequently, oocytes were washed three times with ice-cold Barth's buffer, each experimental group was divided into four samples of 5 to 8 oocytes and radioactivity measured by scintillation counting.
The transport activity was calculated as clearance of [3H]-labeled tracer ion from the incubation medium (CL = Vtransport/S, μl/oocyte/hr), by dividing the amount of tracer absorbed per oocyte per incubation time (Vtransport, cpm/oocyte/hr) by the tracer concentration in the incubation medium (S, cpm/μl). In all experiments, the background (non-OAT-mediated) tracer uptake was measured in uninjected oocytes (in preliminary experiments, uptake in water-injected and uninjected oocytes was not found to be significantly different). This background uptake (probably combining non-saturable tracer diffusion and endogenous transport expressed by the oocytes) was subtracted from total uptake measured in all other samples, to calculate the Oat6-mediated uptake component. In a typical experiment, the control (uninhibited) transporter-mediated tracer clearance was 0.35 to 0.9 μl/oocyte/hr for [3H]-ES in Oat6-injected oocytes and 0.7 to 2.0 μl/oocyte/hr for [3H]-PAH in Oat1-injected oocytes.
Calculations and statistics
In each experimental group, tracer clearance was calculated as Mean ± SE of quadruplicate samples (with exception of control group comprising eight samples). In uptake inhibition experiments, the OAT-mediated clearance (i.e., difference between the cRNA-injected and uninjected oocytes) in the presence of an inhibiting organic anion was expressed as per cent of the mean transporter-mediated clearance in the control group.
To determine potencies of the organic anions (tested as tracer uptake inhibitors), tracer uptake was measured in the presence of increasing inhibitor concentrations. For each organic anion, a series of 2 to 4 concentrations was tested with successive 10-fold increments. For some of the organic anions, duplicate experiments were performed, and for a given inhibitor concentration, the clearance percent difference between duplicate experiments typically did not exceed 15%. The clearance-vs.-Log[inhibitor] data was fit in the one-site competition equation incorporated in Prism software (GraphPad Inc., San Diego, CA), and Log(IC50) was calculated as Mean ± SE. Based on these results, IC50 was calculated as 10Mean and standard error for IC50 as SEIC50 = 10Mean − 10Mean−SE.
Results
Oat6 expression in nasal mucosa
Previously it was shown that Oat6 is expressed in nasal mucosa [7], but its localization in the tissue was unclear. To clarify this issue, we performed in situ hybridization of coronal sections through the nasal mucosa with an Oat6 probe and a control Olfactory Marker Protein (OMP) probe. As expected, an intense OMP-specific signal was noted throughout the olfactory epithelium. By comparison, we found the Oat6 signal to be weaker but with a similar distribution throughout the olfactory epithelium (Figure 1).
Figure 1.

Expression of Oat6 and OMP in olfactory epithelium. Coronal sections through the nasal mucosa were hybridized to antisense (A) or sense (B) Oat6 probes, or to an antisense OMP probe (C). A diffuse Oat6-specific signal was observed throughout the olfactory epithelium. A more intense signal with a similar distribution was observed for OMP. Scale bar =100μm.
To further investigate cellular localization of the transporter in olfactory tissue, we used laser capture microdissection to examine whether Oat6 is expressed in neuronal cells (easily identifiable morphologically) or in other cells of two nasal olfactory organs in mice, the main olfactory epithelium (MOE) and the vomeronasal organ (VNO). Figure 2 demonstrates that Oat6 mRNA is present in total RNA extracts of microdissected epithelia from both mouse MOE and VNO, but not in a comparable amount of RNA from individual olfactory neurons laser-captured from either MOE (three representative samples are shown of a total of 18 MOE neurons) or VNO (three of a total of 6 neurons). In the same samples, expression of β3-tubulin was readily detected, indicating cDNA integrity, as was expression of OMP, indicating neuronal cell identity [13]. These data suggest that Oat6 is expressed in non-neuronal cells of olfactory tissue (possibly epithelial and/or other supporting cells immediately adjacent to neurons).
Figure 2.

Amplification from comparable quantities of cDNA prepared from laser captured MOE sensory neurons (1-3), VNO sensory neurons (4-6), whole MOE epithelium (7), and whole VNO epithelium (8), with no template control (9). (A) Oat6 amplification (100bp ladder illustrates 492bp amplicon). (B) β3-Tubulin amplification (illustrates cDNA integrity). (C) Olfactory Marker Protein (OMP) amplification (illustrates neuronal cell identity).
Inhibition of [3H]-labeled tracer uptake in Oat6- and Oat1-injected oocytes by organic anions
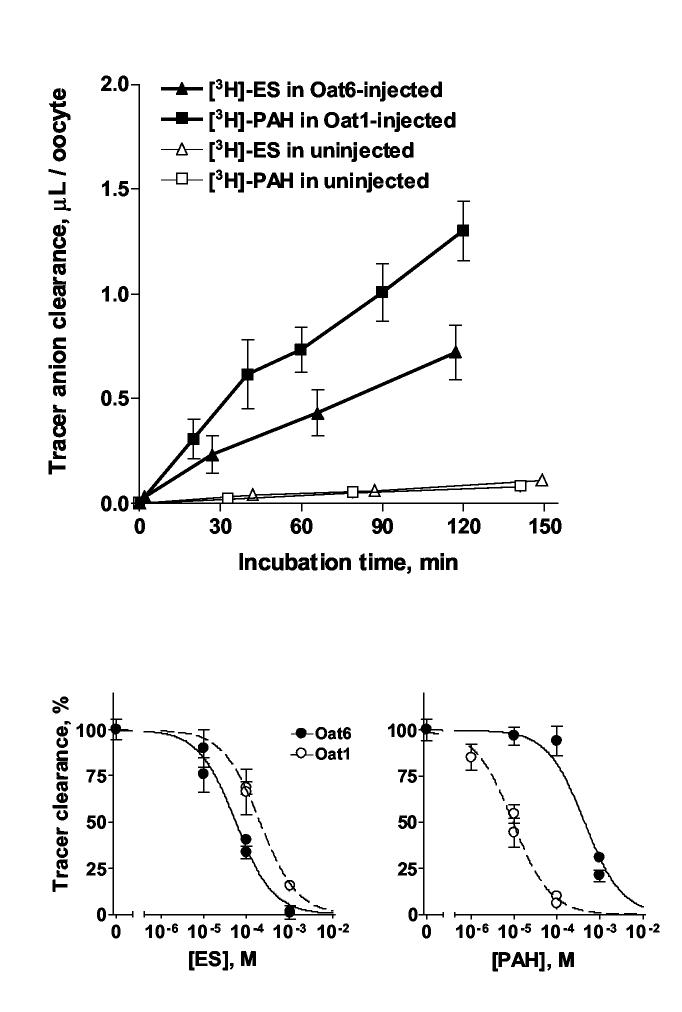
To characterize the transport function of Oat6 in comparison to that of Oat1, we initially measured uptake of the known Oat6 substrate, [3H]-ES [14], and of the known Oat1 substrate, [3H]-PAH [1], in Oat1- and Oat6-expressing oocytes respectively (Figure 3a). In these experiments, transporter-mediated uptake of [3H]-labeled tracer considerably exceeded (typically 10-fold or more) the background uptake in uninjected oocytes.
Figure 3.
(A) Time course of the accumulation of [3H]-ES in Oat6-injected oocytes, of [3H]-PAH in Oat1-injected oocytes, and of both [3H]-labeled tracers in uninjected oocytes. Each data point represents Mean ± SE of triplicate samples. (B) Concentration-dependent inhibition of the clearance of tracer substrate ([3H]-ES in Oat6-injected oocytes or [3H]-PAH in Oat1-injected oocytes) by ‘cold’ (unlabeled) ES and PAH. Each data point represents Mean ± SE of quadruplicate samples. Best-fit curves and IC50 values were calculated using GraphPad Prism software.
We subsequently examined the concentration-dependent inhibition of OAT-mediated tracer uptake by organic anions for quantitative assessment of their potency. Since an approximately linear time course was observed for the accumulation of both tracers for up to two hours (Figure 3A), an incubation time of 1 hr was chosen for measurements of inhibition of uptake.
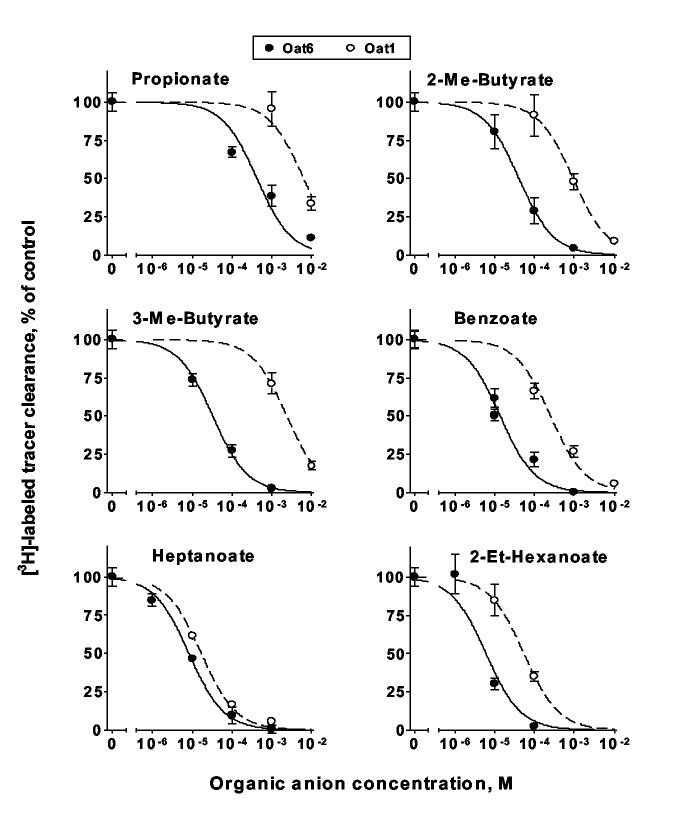
Analysis of the inhibition of tracer uptake by ‘cold’ (unlabeled) ES and PAH demonstrated higher potency of ES for Oat6 than for Oat1, whereas PAH was significantly less potent for Oat6 than for Oat1 (Figure 3B and Table 1). We then characterized interaction of the transporters with anions of volatile organic acids identified elsewhere as odortype components in mouse urine (propionate, 2- and 3-methylbutyrates, benzoate, heptanoate and 2-ethylhexanoate [9,10]). Figure 4 demonstrates that each of these compounds inhibited Oat6-mediated transport of [3H]-ES in a dose-dependent manner, with sub-millimolar to micromolar potency. Moreover, the potency of inhibition in each case was substantially greater (2- to 70-fold) than that of uptake mediated by Oat1 (Figure 4 and Table 1).
Table 1.
IC50 values calculated from the inhibition of OAT-mediated tracer uptake by various organic anions (Figures 3B and 4).
| Organic anion | IC50 (Oat6), μM | IC50 (Oat1), μM | IC50 ratio (Oat1/ Oat6) |
|---|---|---|---|
| Estrone sulfate | 58 ± 10 | 203 ± 10 | 3.50 |
| p-Aminohippurate | 446 ± 98 | 9.4 ± 1.1 | 0.02 |
| Propionate | 279 ± 147 | 8180 ± 1860 | 29.32 |
| 2-Methylbutyrate | 40.7 ± 0.5 | 920 ± 25 | 22.60 |
| 3-Methylbutyrate | 32.3 ± 3.4 | 2335 ± 174 | 72.29 |
| Benzoate | 13.8 ± 2.5 | 253 ± 55 | 18.33 |
| Heptanoate | 8.2 ± 0.8 | 16.7 ± 1.8 | 2.04 |
| 2-Ethylhexanoate | 6.6 ± 3.1 | 57 ± 21 | 8.64 |
Figure 4.
Concentration-dependent inhibition of [3H]-labeled tracer clearance in Oat6- and Oat1-injected oocytes by odorant organic anions.
Discussion
In this work, we have clarified the localization and characterized functional properties of the olfactory organic anion transporter, Oat6. In situ hybridization (Figure 1) revealed diffuse expression for Oat6 throughout the olfactory epithelium. Analysis of individual olfactory neurons laser-captured from either the main olfactory epithelium (MOE) or the vomeronasal organ (VNO) (Figure 2) indicated that Oat6 is expressed in non-neuronal cells of both these structures. All of the volatile carboxylic acids described elsewhere as odortype components and tested in this study as anions (propionate, 2- and 3-methylbutyrates, benzoate, heptanoate and 2-ethylhexanoate) showed sub-millimolar to micromolar affinity for Oat6 (Figure 4). Importantly, the IC50 values determined for the interaction of these compounds with Oat6 are significantly lower (up to 70-fold) than the respective IC50 values found for Oat1 (Table 1), suggesting that Oat6 might be specifically fit for the binding of odorant organic acids.
A significant affinity of odorant organic anions for Oat6 might indicate an important role for this transporter in olfaction, quite apart from any contribution in transport of xenobiotics (such as inhaled toxicants or nasally administered drugs).
For example, Oat6-mediated transport, whether in the direction of uptake (absorption from the mucosal surface) or efflux (secretion into the nasal space), might significantly alter the concentrations of particular odorant organic anions on the mucosal surface, thereby modulating the stimulus presented to specific olfactory neurons. Alternatively, absorption of odorants via Oat6 might serve to facilitate delivery of these molecules to a distal site of chemosensation. It has been suggested, for instance, that some pheromonal signals might exert their CNS actions via humoral delivery to the brain rather than via neuronal signaling [15,16]. Conversely, the potential ability of Oat6 to excrete odorant molecules raises the interesting possibility that the olfactory mucosa might not only function as an odor detector (odorant sensation via specific receptors expressed in neuronal cells), but also as an odor emitter (odorant secretion via Oat6 expressed in non-neuronal, e.g. epithelial cells). Such an idea might partly explain, for example, the nose-to-nose sniffing behavior of many animals when first introduced to each other. Finally, it is conceivable that some odorants might act within the cell upon intracellular receptor molecules. In such a scenario, Oat6 would essentially function as a component of an alternative pathway for olfactory sensation, wherein signal transduction occurs after the transporter-mediated cellular entry of odorants.
Acknowledgements
This work was supported by NIH grants AI057695 and HD40011 to SKN, and DK064839 and DK075486 to SAE.
List of nonstandard abbreviations used in the paper
- OAT
organic anion transporter
- ES
estrone sulfate
- PAH
p-aminohippurate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burckhardt BC, Burckhardt G. Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev Physiol Biochem Pharmacol. 2003;146:95–158. doi: 10.1007/s10254-002-0003-8. [DOI] [PubMed] [Google Scholar]
- 2.Dantzler WH, Wright SH. The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochimica et Biophysica Acta (BBA) -Biomembranes. 2003;1618:185. doi: 10.1016/j.bbamem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Eraly SA, Bush KT, Sampogna RV, Bhatnagar V, Nigam SK. The molecular pharmacology of organic anion transporters: from DNA to FDA? Mol Pharmacol. 2004;65:479–487. doi: 10.1124/mol.65.3.479. [DOI] [PubMed] [Google Scholar]
- 4.Eraly SA, Monte JC, Nigam SK. Novel slc22 transporter homologs in fly, worm, and human clarify the phylogeny of organic anion and cation transporters. Physiol. Genomics. 2004;18:12–24. doi: 10.1152/physiolgenomics.00014.2004. [DOI] [PubMed] [Google Scholar]
- 5.Robertson EE, Rankin GO. Human renal organic anion transporters: Characteristics and contributions to drug and drug metabolite excretion. Pharmacology & Therapeutics. 2006;109:399. doi: 10.1016/j.pharmthera.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Shitara Y, Horie T, Sugiyama Y. Transporters as a determinant of drug clearance and tissue distribution. European Journal of Pharmaceutical Sciences. 2006;27:425. doi: 10.1016/j.ejps.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Monte JC, Nagle MA, Eraly SA, Nigam SK. Identification of a novel murine organic anion transporter family member, OAT6, expressed in olfactory mucosa. Biochem Biophys Res Commun. 2004;323:429–436. doi: 10.1016/j.bbrc.2004.08.112. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Nieto CE, You G, Bush KT, Barros EJ, Beier DR, Nigam SK. Molecular cloning and characterization of NKT, a gene product related to the organic cation transporter family that is almost exclusively expressed in the kidney. J Biol Chem. 1997;272:6471–6478. doi: 10.1074/jbc.272.10.6471. [DOI] [PubMed] [Google Scholar]
- 9.Singer AG, Beauchamp GK, Yamazaki K. Volatile signals of the major histocompatibility complex in male mouse urine. PNAS. 1997;94:2210–2214. doi: 10.1073/pnas.94.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willse A, Belcher AM, Preti G, Wahl JH, Thresher M, Yang P, Yamazaki K, Beauchamp GK. Identification of Major Histocompatibility Complex-Regulated Body Odorants by Statistical Analysis of a Comparative Gas Chromatography/Mass Spectrometry Experiment. Anal. Chem. 2005;77:2348–2361. doi: 10.1021/ac048711t. [DOI] [PubMed] [Google Scholar]
- 11.Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, Monte JC, Rieg T, Truong DM, Long JM, Barshop BA, Kaler G, Nigam SK. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knockout mice. J Biol Chem. 2005 doi: 10.1074/jbc.M508050200. [DOI] [PubMed] [Google Scholar]
- 12.Matsunami H, Buck LB. A Multigene Family Encoding a Diverse Array of Putative Pheromone Receptors in Mammals. Cell. 1997;90:775. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- 13.Berghard A, Buck LB, Liman ER. Evidence for distinct signaling mechanisms in two mammalian olfactory sense organs. PNAS. 1996;93:2365–2369. doi: 10.1073/pnas.93.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnabolk GW, Youngblood GL, Sweet DH. Transport of Estrone Sulfate by the Novel Organic Anion Transporter Oat6 (Slc22a20) Am J Physiol Renal Physiol. 2006 doi: 10.1152/ajprenal.00497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefanczyk-Krzymowska S, Krzymowski T, Grzegorzewski W, sowska BW, Skipor J. Humoral pathway for local transfer of the priming pheromone androstenol from the nasal cavity to the brain and hypophysis in anaesthetized gilts. Exp Physiol. 2000;85:801–809. doi: 10.1111/j.1469-445x.2000.02056.x. [DOI] [PubMed] [Google Scholar]
- 16.Stefanczyk-Krzymowska S, Krzymowski T, Wasowska B, Jana B S, J l. Intramuscular injections of male pheromone 5 alpha-androstenol change the secretory ovarian function in gilts during sexual maturation. Reproductive Biology. 2003;3:241–257. [PubMed] [Google Scholar]