Abstract
Background: Insomnia is a very common symptom, particularly in the elderly. Thus, all hypnotic medications should be carefully evaluated in the elderly population. Zaleplon, a new nonbenzodiazepine hypnotic with a short elimination half-life (approximately 1 hour), was evaluated in the current study.
Method: This multicenter, randomized, placebo-controlled outpatient study evaluated the efficacy and safety of zaleplon, 5 and 10 mg, in elderly patients with insomnia (as defined by DSM-IV); zolpidem, 5 mg, was the active comparator. Sleep was assessed in 549 elderly patients (≥ 65 years old) by using morning questionnaires completed after each of 7 baseline nights during which placebo was given, 14 nights of double-blind treatment, and 7 nights of placebo after discontinuation of active treatment.
Results: Zaleplon, 10 mg, and zolpidem, 5 mg, significantly reduced sleep latency during both weeks of the study. Zaleplon, 5 mg, reduced sleep latency only during week 2. Sleep duration was increased with zolpidem, 5 mg, during weeks 1 and 2 and with zaleplon, 10 mg, during week 1. No clinically significant rebound insomnia was observed after discontinuation of treatment with zaleplon, whereas evidence of rebound effects was seen with zolpidem. There was no significant difference between either zaleplon dose and placebo in the frequency of any central nervous system adverse events.
Conclusion: Zaleplon is effective in reducing latency to sleep without evidence of undesired effects in elderly patients with insomnia.
The prevalence of insomnia increases with age. As many as 50% of elderly people report increases in time to initiate sleep, disruption of sleep maintenance, and an overall dissatisfaction with the quality of sleep.1–4 These sleep disturbances are associated with daytime napping and drowsiness, poor daytime functioning, and reduced quality of life.1,5,6 Given the high prevalence of insomnia in the elderly, it is not surprising that the use of hypnotics increases with age7 and nearly half of the prescriptions for hypnotics are written for this age group.8–10 Insomnia in the elderly can be a consequence of numerous factors, such as physical or psychiatric conditions or social changes.2,8,11,12 Once underlying physical or psychiatric causes of insomnia are addressed and attention has been paid to improving sleep hygiene,8,13 treatment with hypnotic agents can be effective in promoting sleep and improving sleep quality.14–16
All pharmacologic treatment of insomnia in the elderly must be evaluated relative to the possibility of undesired effects, such as residual sedation, increased accident rates, and rebound insomnia.17–19 Recently, there has been a trend toward development and use of shorter-acting benzodiazepine and nonbenzodiazepine hypnotics that represent a safety advantage over longer-acting hypnotics, particularly with respect to residual effects.14,19–21 However, shorter-acting hypnotics are more likely to produce rebound insomnia when discontinued.
Zaleplon is a new pyrazolopyrimidine hypnotic that selectively binds to the benzodiazepine type 1 site on the γ-aminobutyric acid subtype A (GABAA) receptor/chloride-ion channel complex.22 It has a rapid onset of action and a peak plasma concentration and elimination half-life of approximately 1 hour each.23 Zolpidem, an imidazopyridine hypnotic, is also selective for the benzodiazepine type 1 site.24 It has a time to peak plasma concentration of approximately 1.5 hours and an elimination half-life of 2.8 hours in elderly persons.25 In this multicenter, double-blind, randomized, placebo-controlled outpatient study, the effectiveness and safety of zaleplon, 5 and 10 mg, were compared with those of placebo, using zolpidem, 10 mg, as active comparator, for a treatment period of 14 days in elderly outpatients with primary insomnia.
METHOD
Patients
This study was conducted with elderly (65 years of age or older) men and women who had at least a 3-month history of primary insomnia as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)26 at study entry. This history must have included a usual sleep latency of 30 minutes or more and either 3 or more awakenings per night on average or a usual total sleep time of ≤ 6.5 hours. Patients were excluded if they had a preexisting medical condition that would affect the study results or if the raw scores on the Zung Self-Rating Anxiety27 and Depression28 Scales administered during screening were ≥ 50. Patients were also excluded if they had sleep apnea or restless legs syndrome, if their sleep complaint was considered to be secondary to nicotine use, or if the study physician judged that results of physical examinations or routine clinical laboratory assessments included a clinically important abnormality.
Each patient discontinued the following central nervous system (CNS)-active medications for 1 to 3 weeks before the baseline period, depending on the half-life and usage of the prestudy CNS medication: antipsychotics, antidepressants, anxiolytics, lithium, or other psychotropic drugs; over-the-counter sleeping aids; antihistamines with known sedative effects (except for occasional daytime use for cold/allergy symptoms); theophylline; corticosteroids; and diet pills. Use of other concomitant medications was acceptable provided the patient had been receiving a stable dose for at least 3 months before the start of the week of the placebo run-in phase and was expected to continue taking the drug without dose changes throughout the study. Patients were not enrolled if their usual intake of caffeine-containing beverages was ≥ 5 cups per day or if they drank caffeine-containing beverages late in the day, but they were allowed to continue a usual intake of < 5 cups per day during the study period. They were advised not to eat large meals or to consume alcohol within 3 hours of dose administration, to abstain from routine daytime napping, and not to make significant changes in lifestyle or exercise habits.
All patients provided written consent to participate in the trial after the procedures and potential side effects had been explained to them. Study protocols and amendments were approved by the institutional review board of each institution. All studies were conducted according to the Declaration of Helsinki and its amendments.
Study Design and Treatments
This multicenter, randomized, placebo-controlled, parallel-group study in elderly outpatients was conducted at 35 centers in the United States. After the initial screening visit, weekly study visits occurred at the end of each of 4 weeks: a screening/baseline week during which single-blind placebo was given; treatment week 1; treatment week 2; and the week after treatment discontinuation during which single-blind placebo was given. The 7-day placebo screening/baseline period was used to confirm patient eligibility and to obtain baseline data with postsleep questionnaires. Specifically, for a patient to be included in the double-blind phase of the study, sleep questionnaires during the screening/baseline week must have documented the following on 4 or more nights: a sleep latency ≥ 30 minutes and either ≥ 3 awakenings per night or a total sleep time ≤ 6.5 hours. Patients who met all eligibility criteria were randomly assigned to one of the 4 double-blind treatment groups: zaleplon, 5 mg; zaleplon, 10 mg; zolpidem, 5 mg; or placebo. There was a 3-to-2 ratio of assignment to the zaleplon groups versus the placebo or zolpidem groups.
Throughout the 14-night double-blind treatment phase, patients completed daily sleep questionnaires. After double-blind treatment, patients received single-blind placebo for at least 7 nights while continuing to complete daily sleep questionnaires. At completion of the discontinuation phase, patients returned for a follow-up visit, during which the sleep questionnaires were collected and final assessments were completed. Throughout the study, regular bedtimes and rise times were maintained and medication was taken at bedtime.
Efficacy Assessments
Efficacy variables were collected from the daily postsleep questionnaires, which were completed each morning approximately 30 minutes after awakening, and included subjective sleep latency, subjective total sleep time, number of awakenings, and sleep quality. Subjective sleep latency and subjective total sleep time were estimated in minutes. Sleep quality was rated on a scale from 1 (excellent) to 7 (extremely poor).
Safety and Discontinuation Effect Assessments
Safety assessments were based on reports of adverse events and the results of routine physical examinations, laboratory determinations, recording of vital signs, and electrocardiograms (ECGs). Vital signs recorded included sitting blood pressure, body temperature, and pulse rate. Treatment-emergent adverse events were defined as new events that occurred after the start of double-blind treatment or, if the date of onset preceded the start of treatment, events that worsened during double-blind treatment. The possible occurrence of rebound insomnia was assessed from sleep data derived from postsleep questionnaires completed after the first placebo discontinuation night.
Statistical Methods
Statistical comparisons between treatments for demographic data were made by using 1-way analyses of variance (ANOVAs) for continuous data and chi-square tests for categorical data.
Efficacy analyses were performed on data from all patients who took at least 1 dose of double-blind study drug and for whom baseline and at least 1 day of treatment sleep questionnaire results were available. The baseline value for each of the efficacy variables was defined as the mean of the 7 baseline night values for each patient. The values for weeks 1 and 2 were the mean of each patient's values from treatment nights 1 through 7 and 8 through 14, respectively.
Because the assumptions of normality were not satisfied, subjective sleep latency, subjective total sleep time, number of awakenings, and sleep quality were analyzed by nonparametric analysis of covariance (ANCOVA) on the ranked data, with treatment and center as factors and baseline value as the covariate. The interaction of treatment and center was not statistically significant; thus, data from all centers were pooled and an interaction term was not included in the ANCOVA.
Comparisons between zaleplon and placebo were the planned primary comparisons. Pairwise comparisons between each zaleplon dose group and the placebo group were performed on the adjusted means of the rank-transformed data from the ANCOVA by using the Dunnett test, which controls for the number of comparisons. All secondary pairwise comparisons were performed using F tests, without controlling for the number of comparisons.
Further significant treatment differences on sleep quality scores were examined by performing an additional analysis to compare the numbers of patients who showed improvement in sleep quality. Improvement in sleep quality was determined for each patient by subtracting the median value (of 7 daily scores) for each week of treatment from the median value of the baseline scores. Improved sleep quality was indicated if the resulting score was > 0. Medians were used to conserve the categorical nature of the data. An odds ratio and respective 95% confidence intervals compared the number of patients who showed improved sleep quality in each treatment group relative to the number who showed improvement in the placebo group. Odds ratio values that were greater than 1.0 signified an advantage of active treatment over placebo. Dose effects on improved sleep quality were assessed with a chi-square analysis, which is used to determine whether an odds ratio is different from 1.
Subjective sleep latency, subjective total sleep time, and number of awakenings for discontinuation night 1 were analyzed by ANCOVA as described above. A secondary analysis was performed to further examine rebound insomnia. Results for the efficacy variables were considered to indicate rebound insomnia if the values on discontinuation night 1 for subjective sleep latency or number of awakenings were greater than, or if those for subjective total sleep time were less than, the worst baseline night value. The number of patients who showed rebound insomnia for a given variable were compared between the active treatment groups and the placebo group using the Fisher exact test.
The Fisher exact test was also used to compare pairs of treatments for the incidence of treatment-emergent adverse events, regardless of the overall significance. Laboratory data and vital signs were analyzed by ANCOVA with treatment as a factor and baseline value as a covariate.
All tests of hypotheses were 2-sided, and results were considered to be statistically significant at the level of p ≤ .05.
RESULTS
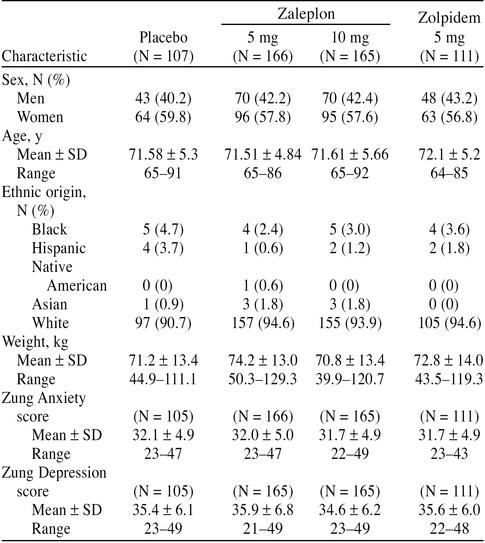
A total of 1224 patients entered the initial screening phase. Five hundred fifty-one of these patients met all entry criteria, qualified during the screening/baseline week, and were randomly assigned to 1 of the 4 treatment groups. Of the 551 patients who completed the placebo run-in phase, 549 patients received at least 1 dose of double-blind study medication and were included in all safety and efficacy analyses. The other 2 patients were randomly assigned to the zaleplon, 5 mg, treatment group and did not provide any data after baseline; 1 required steroids for a stiff neck and never took any test medication and the other did not return after the run-in phase. Baseline demographic characteristics by treatment group for the 549 patients are shown in Table 1. There were no significant differences between the treatment groups in sex, age, weight, ethnic origin, or scores on the Zung Anxiety or Zung Depression Scales.
Table 1.
Demographic and Baseline Characteristics: All Patients Included in the Safety and Efficacy Analyses
Efficacy
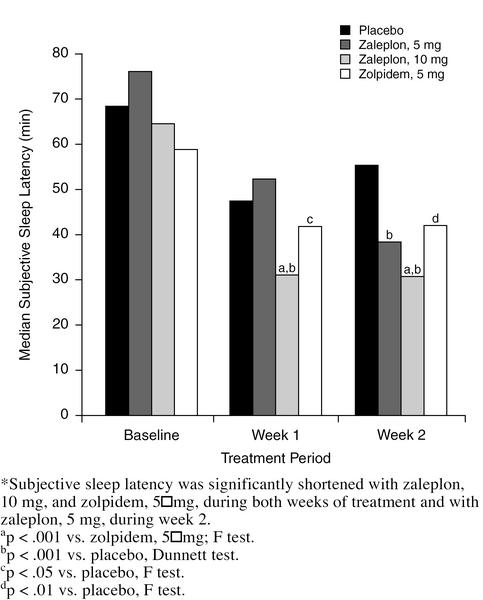
Figure 1 shows that subjective sleep latency was significantly shorter with zaleplon, 10 mg, than with placebo during both weeks of treatment (p < .001, Dunnett test). The difference in subjective sleep latency between zaleplon, 5 mg, and placebo was not statistically significant during week 1; however, during week 2, median sleep latency was significantly shorter with zaleplon, 5 mg, than with placebo (39 vs. 56 minutes, p < .001, Dunnett test). Zolpidem, 5 mg, significantly reduced sleep latency compared with placebo during weeks 1 and 2 (p < .05 and p < .01, respectively; F test). Zaleplon, 10 mg, was also statistically superior to zolpidem in reducing sleep latency during both weeks (p < .001 both weeks, F test).
Figure 1.
Median Subjective Sleep Latency, in Minutes, for Each Group During Baseline and Treatment Weeks*
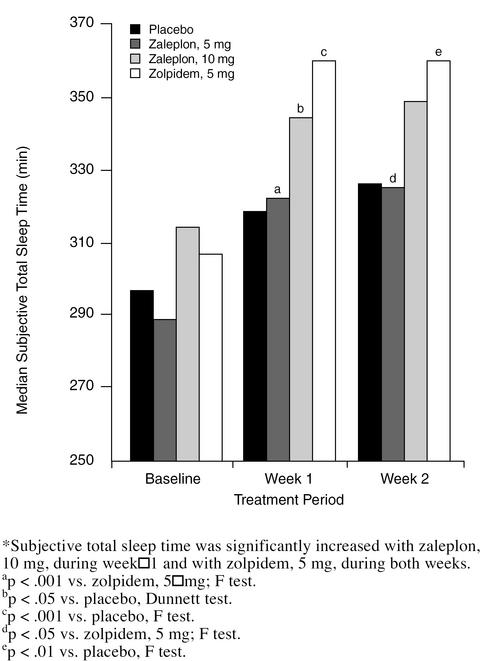
Subjective total sleep time (Figure 2) was significantly longer with zaleplon, 10 mg, than with placebo during week 1 (345 vs. 318 minutes, p < .05, Dunnett test). There was no significant difference from placebo in subjective total sleep time with zaleplon, 10 mg, for week 2 or with zaleplon, 5 mg, for either week. Subjective total sleep time was significantly longer with zolpidem, 5 mg, than with placebo during both weeks (360 vs. 318 minutes week 1, p < .001; 360 vs. 326 minutes week 2, p < .01; F test). Number of awakenings was significantly lower with zolpidem, 5 mg, during week 1 (1.7, p < .01) and week 2 (1.6, p < .05) than with placebo (2.0 and 1.9, respectively), but the difference between zaleplon, 5 mg (week 1 = 1.8; week 2 = 1.9) or 10 mg (week 1 = 1.8; week 2 = 1.7), and placebo was not significant.
Figure 2.
Median Subjective Total Sleep Time, in Minutes, for Each Group During Baseline and Treatment Weeks*
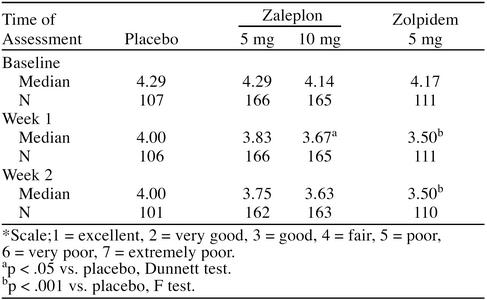
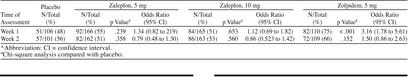
Median sleep quality (Table 2) was significantly better with zaleplon, 10 mg, than with placebo for the first week (p < .05, Dunnett test) and with zolpidem, 5 mg, than with placebo for both weeks of treatment (p < .001, F test). The number of patients showing improved sleep quality was significantly greater with zolpidem, 5 mg, than with placebo during week 1 of treatment (p < .001), but not with either dose of zaleplon (Table 3). There was no difference in the number of patients showing improved sleep quality between any active treatment and placebo during week 2.
Table 2.
Median Sleep Quality Scores*
Table 3.
Patients With Improvement in Sleep Quality Relative to Baseline*
Discontinuation Effects
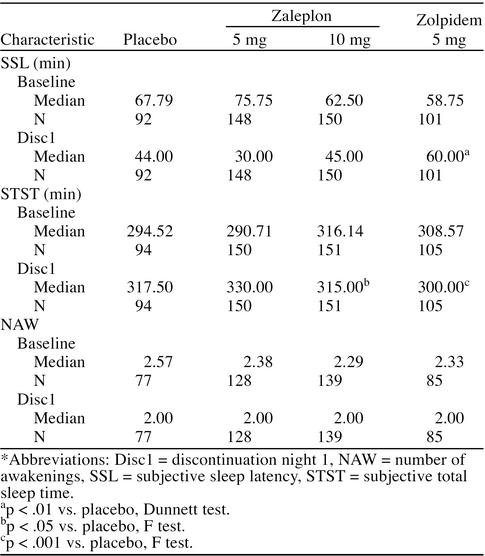
Results from the Dunnett test showed that median values for subjective sleep latency and number of awakenings with either zaleplon dose were not significantly different from the value with placebo on discontinuation night 1 (Table 4). Median subjective total sleep time with zaleplon, 10 mg, on that night was significantly different from that with placebo (p < .05), although only 2.5 minutes shorter. The ANCOVA showed that on discontinuation night 1, subjective sleep latency with zolpidem, 5 mg, was significantly longer than with placebo by 16 minutes (p < .01), while subjective total sleep time was significantly shorter by 17.5 minutes (p < .001).
Table 4.
Sleep Latency, Sleep Duration, and Number of Awakenings for Discontinuation Night 1*
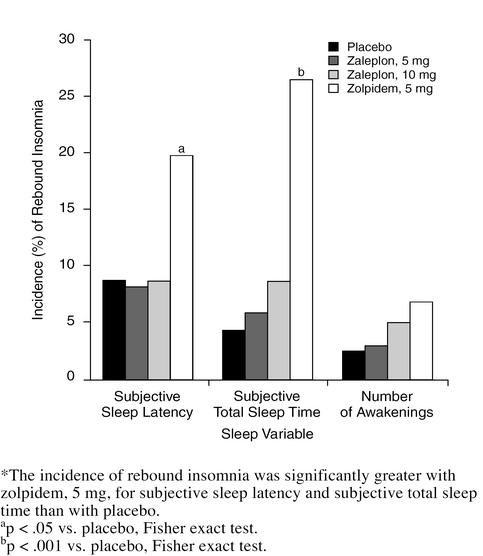
In a secondary analysis, the numbers of patients who showed evidence of rebound insomnia in each treatment group were compared using the Fisher exact test. The incidence of rebound insomnia with zaleplon, 5 and 10 mg, was not significantly different from that with placebo on discontinuation night 1 for subjective sleep latency, subjective total sleep time, or number of awakenings (Figure 3). In contrast, the incidence of rebound insomnia with zolpidem, 5 mg, was significantly higher for subjective sleep latency (20 patients [20%], p < .05) and subjective total sleep time (28 patients [27%], p < .001) compared with placebo (8 patients [9%] for subjective sleep latency and 4 patients [4%] for subjective total sleep time).
Figure 3.
Percentage of Patients in Each Treatment Group Meeting Study Criteria for Rebound Insomnia on the First Night After Discontinuation of 2 Weeks of Treatment*
Safety
The frequency of treatment-emergent adverse events was similar in the 4 treatment groups (zaleplon, 5 mg, 56%; zaleplon, 10 mg, 59%; zolpidem, 5 mg, 63%; placebo, 56%). The most common treatment-emergent adverse events for the 4 treatment groups were headache, pain, somnolence, and rhinitis. There was no significant difference between either zaleplon dose and placebo in the frequency of total CNS adverse events or of individual CNS adverse events. The frequency of total CNS adverse events was significantly greater with zolpidem, 5 mg, than with placebo (25% vs. 14%, p < .05). The incidence of somnolence in particular was significantly greater (p < .05) with zolpidem, 5 mg (10%) than with placebo (2%) or zaleplon, 5 mg (4%).
There were no clinically important physical or neurologic examination findings. The incidence of clinically important changes in laboratory results, vital signs, or ECGs was similar across groups, and there were no clinically important differences in mean results.
DISCUSSION
These results demonstrate that zaleplon significantly reduces sleep latency in elderly patients with insomnia. Sleep latency was significantly reduced with zaleplon, 10 mg, and zolpidem during both weeks of treatment and with zaleplon, 5 mg, during week 2, compared with placebo. Furthermore, sleep latency with zaleplon, 10 mg, was significantly shorter than with zolpidem, 5 mg. In addition, there was improvement in sleep duration with zaleplon, 10 mg, during week 1 and with zolpidem during both weeks. On discontinuation of treatment, minimal evidence of rebound insomnia was noted with zaleplon, 10 mg, and no evidence was noted with zaleplon, 5 mg. Evidence of rebound insomnia was noted with zolpidem, 5 mg.
The results of a similar 2-week study in elderly patients with insomnia showed that subjective sleep latency was significantly reduced with zaleplon, 5 and 10 mg, for both weeks.29 A shorter subjective sleep latency, as well as a shorter polysomnographically recorded sleep latency, was observed with zaleplon, 5 and 10 mg, compared with placebo in a sleep laboratory study in elderly patients with insomnia.30
Patients in the present study were instructed to discontinue CNS-active medications, refrain from naps, avoid large meals or alcohol close to bedtime, and maintain regular bedtimes and rise times. Compliance with these instructions probably contributed to the improvement in sleep seen with placebo. Regression to the mean is also likely to have contributed. However, the significant improvements in sleep latency and sleep duration with zaleplon and zolpidem treatment compared with placebo are evidence that pharmacotherapy with these hypnotics provides additional benefits.
The results of the present study and others31,32 suggest that zaleplon and zolpidem may be differentiated on the basis of their pharmacokinetic profiles. The rapid onset and short half-life of zaleplon probably account for reduced subjective sleep latency and less effect on subjective total sleep time, whereas zolpidem's longer half-life results in more reliably increased subjective total sleep time, as well as reduced subjective sleep latency. The shorter half-life of zaleplon also probably accounts for the absence of residual effects in this study. Residual sedation was significantly more frequent with zolpidem, but not with zaleplon, 5 or 10 mg, than with placebo.
Rebound insomnia may be observed after treatment with short-acting hypnotics, especially at higher doses.33 In the present study, on discontinuation night 1, subjective sleep latency was significantly longer and subjective total sleep time was significantly shorter with zolpidem, 5 mg, compared with placebo. Data from individual patients also indicated that the percentage of patients who experienced rebound insomnia was significantly higher with zolpidem, 5 mg, than with placebo. Neither dose of zaleplon showed evidence of clinically significant rebound insomnia in either the group data analysis or the individual patient analysis.
The pharmacokinetic profile of hypnotics often changes in elderly adults; therefore, lower doses are often recommended.14,17,18,34 For example, the peak plasma concentration and area under the plasma concentration-time curve with zolpidem are increased by over 50% in elderly persons, and therefore a dose reduction is recommended.25 However, the pharmacokinetic profile of zaleplon in elderly subjects does not differ significantly from that in young subjects.35 Further, the incidence of adverse events with zaleplon, 10 mg, was not significantly higher than that with placebo or zaleplon, 5 mg, in the present study. Similarly, another study in the elderly showed no overall dose effect of zaleplon, 5 or 10 mg, on the incidence of common treatment-emergent adverse events.29
In summary, zaleplon, 10 mg, was shown to consistently shorten subjective sleep latency in elderly patients with insomnia. Zaleplon, 5 mg, was effective in decreasing subjective sleep latency only on week 2. Subjective total sleep time tended to increase with the 10-mg dose during the 2 weeks of treatment with zaleplon, but no change was seen with the lower dose. There was no evidence of side effects, and rebound insomnia was minimal. Treatment with zolpidem, 5 mg, decreased subjective sleep latency and increased subjective total sleep time, but residual and discontinuation effects were more frequent than with placebo.
Drug names: theophylline (Theo-Dur and others), zaleplon (Sonata), zolpidem (Ambien).
Acknowledgment: Zaleplon Clinical Study Group
The authors gratefully acknowledge the contributions of the following members of the Zaleplon Clinical Study Group: R. Allen, Ph.D., Johns Hopkins University Sleep Disorder Center, Baltimore, Md.; A. Beck, M.D., University Hospital Sleep Disorder Center, Albuquerque, N.M.; D. Berkowitz, M.D., The Center for Sleep Disorders, Cincinnati, Ohio; D. Bliwise, M.D., Emory University School of Medicine, Atlanta, Ga.; W. Brown, M.D., Clinical Programs Ltd., Providence, R.I.; A. D. Chediak, M.D., Mount Sinai Medical Center, Miami Beach, Fla.; J. Claghorn, M.D., Claghorn-Lesem Research Clinic, Inc., Bellaire, Tex.; L. Cohen, M.D., Clinical Studies (Sarasota), Sarasota, Fla.; M. Cohn, M.D., Clinical Studies (SW Florida), Naples; C. W. Erwin, M.D., Duke University Medical Center, Durham, N.C.; M. Farmer, M.D., Clinical Studies (Florida), St. Petersburg; J. Fry, Ph.D., Medical College of Pennsylvania at EPPI, Philadelphia; J. T. Hartford, M.D., Hartford Research Group, Dayton, Ohio; P. Hauri, Ph.D., Mayo Foundation for Medical Education and Research, Rochester, Minn.; A. F. Jacobson, Ph.D., University of Miami School of Medicine, Miami, Fla.; A. Khan, M.D., Northwest Psychiatric Institute, Inc., Kirkland, Wash.; J. A. Miller, Jr., M.D., Clinical Studies (Melbourne), Melbourne, Fla.; R. Pascualy, M.D., Providence Medical Center, Seattle, Wash.; G. Richardson, M.D., Brigham & Women's Hospital, Boston, Mass.; P. Ripley, M.D., Clinical Studies Ltd., South Yarmouth, Mass.; R. Rosen, Ph.D., UMDNJ-Robert Wood Johnson Medical School, Piscataway, N.J.; A. Schneider, M.D., Pharmacology Research Institute, Northridge, Calif.; R. K. Shrivastava, M.D., Eastside Comprehensive Medical Services, New York, N.Y.; G. Silverman, M.D., Doctor's Clinic, Vero Beach, Fla.; E. J. Stepanski, Ph.D., University of Illinois Hospital, Chicago; A. Strauss, M.D., Clinical Studies (Boynton Beach), Boynton Beach, Fla.; G. W. Vogel, M.D., Sleep Research Laboratory, Atlanta, Ga.; R. Weisler, M.D., Raleigh, N.C.; T. Williams, M.D., Clinical Studies (Arizona), Peoria; A. Winokur, M.D., Ph.D., University of Pennsylvania Medical Center, Philadelphia; V. Wooten, M.D., Senatara Norfolk General Hospital, Norfolk, Va.; G. Zammit, Ph.D., St. Luke's Roosevelt Hospital Center, New York, N.Y.; J. Zuniga, M.D., Palm Beach Neurological Group, West Palm Beach, Fla.
The Zaleplon Clinical Study Group members associated with Wyeth-Ayerst Research include Madhavi Gidh-Jain, Ph.D.; James Gurr, Ph.D.; Michael Mack, Ph.D.; Leona Masukawa, Ph.D.; Bennie Nelson, M.A.; Timothy Whitaker, M.D.; and Carmen Wickland, Ph.D.
Footnotes
Supported by Wyeth-Ayerst Research, Radnor, Pa.
REFERENCES
- Dement WC, Miles LE, Carskadon MA. “White paper” on sleep and aging. J Am Geriatr Soc. 1982;30:25–50. doi: 10.1111/j.1532-5415.1982.tb03700.x. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment: prevalence and correlates. Arch Gen Psychiatry. 1985;42:225–232. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation survey I. Sleep. 1999;22(suppl 2):S347–S353. [PubMed] [Google Scholar]
- Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation survey I. Sleep. 1999;22(suppl 2):S354–S358. [PubMed] [Google Scholar]
- Hatoum HT, Kong SX, Kania CM, et al. Insomnia, health-related quality of life and health care resource consumption: a study of managed care organization enrollees. Pharmacoeconomics. 1998;14:629–637. doi: 10.2165/00019053-199814060-00004. [DOI] [PubMed] [Google Scholar]
- Morgan K, Dallosso H, Ebrahim S, et al. Prevalence, frequency, and duration of hypnotic drug use among the elderly living at home. BMJ. 1988;296:601–602. doi: 10.1136/bmj.296.6622.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MG, Thompson TL, Nies AS. Sleep disorders in the elderly. Am J Psychiatry. 1988;155:1369–1378. doi: 10.1176/ajp.145.11.1369. [DOI] [PubMed] [Google Scholar]
- Balestrieri M, Bortolomasi M, Galleta M, et al. Patterns of hypnotic drug prescription in Italy. Br J Psychiatry. 1997;170:176–180. doi: 10.1192/bjp.170.2.176. [DOI] [PubMed] [Google Scholar]
- Rayon P, Serrano-Castro M, del Barrio H, et al. Hypnotic drug use in Spain: a cross-sectional study based on a network of community pharmacies. Pharmacoepidemiology. 1996;30:1092–1100. doi: 10.1177/106002809603001005. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S. Sleep problems in older adults: putting myths to bed. Geriatrics. 1997;52:20–30. [PubMed] [Google Scholar]
- Vitiello M. Sleep disorders and aging: understanding the causes. J Gerontol A Biol Sci Med Sci. 1997;52A:M189–M191. doi: 10.1093/gerona/52a.4.m189. [DOI] [PubMed] [Google Scholar]
- Karacan I, Williams RL. Sleep disorders in the elderly. Am Fam Physician. 1983;27:143–152. [PubMed] [Google Scholar]
- Roger M, Attali P, Coquelin JP. Multicenter, double-blind, controlled comparison of zolpidem and triazolam in elderly patients. Clin Ther. 1993;15:127–136. [PubMed] [Google Scholar]
- Scharf MB, Mayleben DW, Kaffeman M, et al. Dose response effects of zolpidem in normal geriatric subjects. J Clin Psychiatry. 1991;52:77–83. [PubMed] [Google Scholar]
- Roehrs T, Zorick F, Wittig R, et al. Efficacy of a reduced triazolam dose in elderly insomniacs. Neurobiol Aging. 1985;6:293–296. doi: 10.1016/0197-4580(85)90006-5. [DOI] [PubMed] [Google Scholar]
- Kruse WH. Problems and pitfalls in the use of benzodiazepines in the elderly. Drug Saf. 1990;5:328–344. doi: 10.2165/00002018-199005050-00003. [DOI] [PubMed] [Google Scholar]
- Shorr RI, Robin DW. Rational use of benzodiazepines in the elderly. Drugs Aging. 1994;4:9–20. doi: 10.2165/00002512-199404010-00002. [DOI] [PubMed] [Google Scholar]
- Ray WA. Psychotropic drugs and injuries among the elderly: a review. J Clin Psychopharmacol. 1992;12:386–396. [PubMed] [Google Scholar]
- Langtry HD, Benfield P. Zolpidem: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential. Drugs. 1990;40:291–313. doi: 10.2165/00003495-199040020-00008. [DOI] [PubMed] [Google Scholar]
- Maczaj M. Pharmacological treatment of insomnia. Drugs. 1993;45:44–45. doi: 10.2165/00003495-199345010-00005. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Morel E, Perrault G. Comparison of the pharmacological profiles of the hypnotic drugs, zaleplon and zolpidem. Eur J Pharmacol. 1996;313:35–42. doi: 10.1016/0014-2999(96)00510-9. [DOI] [PubMed] [Google Scholar]
- Beer B, Ieni JR, Wu WH, et al. A placebo-controlled evaluation of single, escalating doses of Cl-284,846, a non-benzodiazepine hypnotic. J Clin Pharmacol. 1994;34:335–344. doi: 10.1002/j.1552-4604.1994.tb02002.x. [DOI] [PubMed] [Google Scholar]
- Langer SZ, Arbilla S, Scatton B, and et al. Receptors involved in the mechanism of action of zolpidem. In: Sauvanet JP, Langer SZ, Morselli PL, eds. Imidazopyridines in Sleep Disorders. New York, NY: Raven Press. 1988 55–70. [Google Scholar]
- Bianchetti G, Dubruc C, Thiercelin JF, and et al. Clinical pharmacokinetics of zolpidem in various physiological and pathological conditions. In: Sauvanet JP, Langer SZ, Morselli PL, eds. Imidazopyridines in Sleep Disorders. New York, NY: Raven Press. 1988 155–163. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association. 1994 [Google Scholar]
- Zung WWK. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- Zung WWK. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- Hedner J, Emilien G, Salinas E. Improvement in sleep latency and sleep quality with zaleplon in elderly patients with primary insomnia. J Sleep Res. 1998;7(suppl 2):115. [Google Scholar]
- Erwin CW. Zaleplon effectively and safely treats chronic insomnia in elderly patients. Presented at the annual meeting of the American Geriatric Society. 20–23May1999 Philadelphia, Pa. [Google Scholar]
- Fry J, Scharf MB, Berkowitz DV, et al. A phase III, 28-day, multicenter, randomized, double-blind comparator- and placebo-controlled, parallel-group safety, tolerability, and efficacy study of 5, 10, and 20 mg of zaleplon, compared with 10 mg of zolpidem or placebo, in adult outpatients with insomnia [abstract] Sleep. 1998;21(suppl 1):262. [Google Scholar]
- Elie R, D'Avignon M, Emilien G, et al. Zaleplon decreases sleep latency in outpatients without producing rebound insomnia after 4 weeks of treatment. J Sleep Res. 1998;7(suppl 2):76. [Google Scholar]
- Roth T, Roehrs TA. Issues in the use of benzodiazepine therapy. J Clin Psychiatry. 1992;53(6, suppl):14–18. [PubMed] [Google Scholar]
- Greenblatt DJ, Harmatz JS, Shader RI. Clinical pharmacokinetics of anxiolytics and hypnotics in the elderly: therapeutic considerations. Clin Pharmacokinet. 1991;21:165–177. 262–273. doi: 10.2165/00003088-199121030-00002. [DOI] [PubMed] [Google Scholar]
- Darwish M. The effects of age and gender on the pharmacokinetics of zaleplon. Presented at the 12th European College of Neuropsychopharmacology Congress. September1999 London, England. [Google Scholar]