Abstract
Background: New medications that enter the marketplace have been tested almost exclusively in controlled clinical trials conducted in specialty research settings. There is some concern that these carefully selected patient samples may not provide information generalizable to the “real world” clinical population. The purpose of this investigation was to compare results from a large, open-label study of sertraline in the treatment of major depression in the clinical practice setting with pooled results from 2 multicenter, double-blind, placebo-controlled studies conducted in specialty research settings.
Method: Clinical practice patients (N = 1482), aged 21 to 65 years, from 228 psychiatric clinical practice sites across the United States participated in the open-label treatment study (Clinical Practice sample). Patients who met DSM-III-R criteria for moderate-to-severe unipolar major depression (i.e., had pretreatment Hamilton Rating Scale for Depression [HAM-D] scores ≥ 18) were treated for 8 weeks with sertraline in a flexible dosing fashion (50–200 mg daily). Outcomes on the HAM-D and Clinical Global Impressions-Improvement scale (CGI-I) were compared with the pooled results from 2 previously published placebo-controlled, multicenter treatment studies of sertraline in outpatients with major depression (N = 280). The overall response to sertraline in the Clinical Practice sample was compared with the outcome from the research study patient sample (Clinical Research sample). Additionally, comparison of outcomes of patients with common depressive subtypes (double depression, anxious depression, and melancholic [“endogenous”] depression) were examined.
Results: The percentage of sertraline-treated patients rated as responders on the CGI-I was significantly higher in the Clinical Practice sample compared with the Clinical Research sample (87% vs. 73%; p < .001). Sertraline was also much better tolerated in the Clinical Practice sample than in the Clinical Research sample as evidenced by significantly lower overall reports of adverse events (9.4% vs. 13.2%; p < .05) and lower patient dropout rates (17.5% vs. 34.3%; p < .01). Among clinical practice patients, sertraline was found to be equally effective in treating endogenous/melancholic and anxious subtypes and only mildly less effective in achieving a response in patients with double depression (chronic low-grade depression with a superimposed major depression). A regression analysis identified older age and double depression as being predictors of a slower time to response. More than 70% of patients who reported nonresponse to previous treatment with fluoxetine or a tricyclic antidepressant responded to sertraline.
Conclusion: The effectiveness and tolerability of sertraline treatment was found to be significantly better in the Clinical Practice sample, suggesting that the results from controlled studies in research settings may represent an underestimate of the benefits of a drug. More effectiveness research is needed to confirm and extend these findings.
The process of bringing new drugs into the marketplace requires demonstration of efficacy. The efficacy of new antidepressants must be established on the basis of double-blind, placebo-controlled studies, which employ restrictive inclusion and exclusion criteria1,2 and which are conducted under rigorous conditions, usually in research settings. The literature suggests that patients who are recruited via advertisements and subsequently enter these clinical trials are demographically and clinically similar to patients who are seeking treatment at their own initiative, and come to a setting where entering a research study is an option.3–6 However, the methodological rigor of typical clinical trials may have the unintended consequence of limiting the research findings to patient samples that may not be representative of the depressed patients encountered in the “real world.”
Major depression is a prevalent and serious illness7 for which the practitioner welcomes newer antidepressants. Clinicians who wish to obtain practical information from treatment research—which could be applied to their clinical practices—are concerned that research patients may be different from those seen in the office. In the general psychiatric office practice, patients may be more treatment resistant, have additional psychiatric disorders,8,9 be more intolerant of medication, experience concurrent medical illnesses,10 or differ in other ways. This leaves clinicians in something of a quandary: If a medication has demonstrated efficacy, can we be certain about precisely how effective it may be in typical patients who are traditionally treated in clinics or private practice settings in the community?
We report here the results of a large study conducted among psychiatrists in clinics and private practice settings that examined the antidepressant effectiveness of sertraline in outpatients with major depression. For the purposes of comparison, we have chosen 2 studies that were conducted during approximately the same time period, but by psychiatrists working in academic and research settings—in other words, typical clinical trials.2,11
Clinical Questions Addressed by This Investigation
The questions that the current investigation addresses include the following: (1) How do the results of clinical trial research with sertraline translate into clinical practice settings? (2) Do research results overestimate or underestimate the antidepressant efficacy of sertraline? (3) Reports12 have suggested that some selective serotonin reuptake inhibitors (SSRIs) may have less efficacy in patients with severe or melancholic subtypes of depression. In light of this, how effective is sertraline at treating severe forms of depression? (4) Other reports13,14 have suggested that some SSRIs may cause treatment-emergent anxiety that may benefit from concomitant benzodiazepine therapy. How effective is sertraline in treating patients who present with anxious depression?15 (5) How does the presence of chronic low-grade depression (dysthymia) affect the efficacy of sertraline? (6) Are there specific clinical features of depression that may affect time to response?
It should be stated at the outset that the authors understand that combining patient samples from different clinical trials, no matter how methodologically similar, is not ideal. This is especially true when one of the trials is open label. However, the large sample sizes and the relative lack of information about the generalizability of research results to clinical practice suggest that the current comparison might be useful and may generate a testable hypothesis.
METHOD
Clinical Practice Study
The current investigation was conducted at 228 clinical practice sites throughout the United States. Potentially eligible subjects were identified and recruited into the study over a 1-year period from among patients seeking treatment in each practice (Clinical Practice sample). Patients were eligible for study entry if they were men or women (nonpregnant, nonlactating, using a medically acceptable form of birth control) aged 21 to 65 years, inclusive. Patients were required to meet DSM-III-R16 criteria for major depressive episode with a 17-item Hamilton Rating Scale for Depression (HAM-D) score ≥ 18.17 Patients were excluded if they had a lifetime diagnosis of bipolar disorder, schizophrenia or other psychotic disorder, bulimia nervosa or anorexia nervosa, or organic mental disorder or were judged by the clinician to be a serious suicide risk. Other reasons for exclusion included a history of alcohol and/or substance abuse or dependence in the past 12 months. Patients were not permitted to enter the study if they were currently using concomitant psychotropic medication (other than p.r.n. chloral hydrate) or if they had previously failed to respond to 2 adequate trials of antidepressants. Finally, patients were excluded if they had an acute or unstable medical condition.
The study was approved by a centralized institutional review board for each of the collaborating centers. The benefits and risks of study participation were fully explained to each patient, and written informed consent was obtained.
Following a 1-week washout period, patients were begun on 8 weeks of open-label sertraline treatment initiated at a daily dose of 50 mg for the first 2 weeks. After that, flexible titration of sertraline was permitted, by 50-mg increment every 1 to 2 weeks, as clinically indicated and tolerated to a maximum daily dose of 200 mg. Compliance with study treatment was monitored by pill counts, and patients who were less than 75% compliant for 2 consecutive visits were dropped from the study.
Physicians collaborating in the study participated in a training session that included a thorough review of the DSM-III-R criteria for diagnosis of mood disorders, as well as training in the use of the assessment instruments, the HAM-D and the Clinical Global Impressions-Severity of Illness and -Improvement scales (CGI-S and CGI-I).18
Patients were evaluated for study entry by a psychiatric history and semistructured interview that included a DSM-III-R diagnostic checklist. A medical history was taken and physical examination and laboratory testing were performed. Investigator-rated assessments consisted of the 17-item HAM-D and the 7-point CGI-S and CGI-I. The patient-rated assessment consisted of the 7-point Patient-Rated Global Assessment-Improvement scale (PGI).
Safety assessments at each study visit included measurements of pulse and blood pressure, both seated and standing, and a review of adverse effects and concomitant medications. Laboratory assessments were performed and an electrocardiogram (ECG) was obtained at the screen visit and at the final study visit. Finally, adverse events were assessed by means of an open-ended inquiry.
Patients came for medication management and assessment visits at baseline and weeks 1, 2, 3, 4, 6, and 8 (or at study endpoint if the patient terminated early).
Clinical Research Sample Studies
Detailed presentation of the study design and procedures as well as the clinical and demographic characteristics of the patient samples for the 2 clinical research studies included here were presented in 2 previous reports.2,11 Briefly, both studies were conducted in psychiatric research settings. Inclusion and exclusion criteria were virtually the same. The Clinical Research sample differed from Clinical Practice sample in the use of a 1-week, single-blind, pill-placebo washout period followed by random assignment to 8 weeks of double-blind treatment with either sertraline or placebo. Both the initial dosing of sertraline and the flexible dose titration were the same for both the Clinical Research and Clinical Practice samples. The duration of study treatment (8 weeks) and the timing of the assessment periods were also the same.
Outcome Assessments
Antidepressant responder status was defined using 2 criteria: (1) ≥ 50% reduction from baseline in the HAM-D total score and (2) achievement at study endpoint of a CGI-I score of 1 (very much improved) or 2 (much improved). Remitter status was defined as achievement of a HAM-D total score ≤ 7 at study endpoint and a CGI-I score of 1 or 2.
Depressive Subtypes
To examine some of the questions stated above, subtypes of the patient samples were identified in the following manner: To define the anxious depressive subtype, the 6-item HAM-D anxiety factor was employed, which consists of HAM-D items 10, 11, 12, 13, 15, and 17. Any patient with a HAM-D anxiety factor score ≥ 7 was considered to have anxious depression at baseline. To define a melancholic subtype, patients were required to achieve a HAM-D Bech melancholia factor score19 that was above the median at pretreatment baseline. Chronic depression was defined as a current episode duration ≥ 1 year. Finally, patients with major depression superimposed on preexisting dysthymia, or “double depression,” were identified as a subgroup.
Previous Nonresponse or Intolerance to Antidepressant Treatment
When depressed patients have had an unsatisfactory treatment outcome with an SSRI, clinicians often face a clinical dilemma about what the next step in treatment should be. Specifically, does one switch to a different class of antidepressant or initiate a trial of a second agent in that class? To assess whether previous SSRI or tricyclic antidepressant (TCA) treatment failure or intolerance predicted a poor response to sertraline, convenience sample subgroups of Clinical Practice patients who reported themselves as being previously unresponsive or intolerant to fluoxetine or a TCA were identified. These 2 subgroups were compared with each other with respect to response in this study. In order to address the issue of using a second SSRI if the first one fails, the fluoxetine-treated subgroup was compared with the remainder of the Clinical Practice treatment sample.
Statistical Analyses
All statistical analyses were performed using SAS software (versions 6.11 and 6.12, SAS Institute Inc., Cary, N.C., 1996) using an intent-to-treat approach (i.e., all patients who were entered, took at least 1 dose of medication, and had at least 1 follow-up assessment are included). For those patients leaving the study before week 8, analyses were conducted using the last-observation-carried-forward (LOCF) method. Unless otherwise specified, all statistical tests were 2-tailed, with p values of less than .05 considered significant.
Comparisons between practice settings were done using a 1-way analysis of variance (ANOVA) model for continuous measures; for categorical measures, a chi-square test or Fisher exact test was utilized. Comparisons between subgroups and between depressive subtypes for the clinical practice setting were performed using a chi-square test. The significance of change from baseline between settings was determined using the least-square means from a 1-way analysis of covariance (ANCOVA) model having the baseline measure as a covariate. Some analyses included only patients completing all 8 weeks (completers).
For patients in the Clinical Practice sample, a group of clinically relevant baseline predictors of time to CGI response and time to HAM-D remission was identified. To identify predictors of the treatment outcome, a stepwise-regression selection procedure was applied to select sets of covariates, which most highly correlated with time to response and time to remission to fit an optimal proportional hazards (Cox) regression. This procedure included a covariate that was associated (at significance p < .15) with the treatment outcome in the context of covariates previously selected into the model and retained the covariate if the coefficient in the final model had p < .20. Significance was based on a Wald chi-square test.
RESULTS
Patient Characteristics
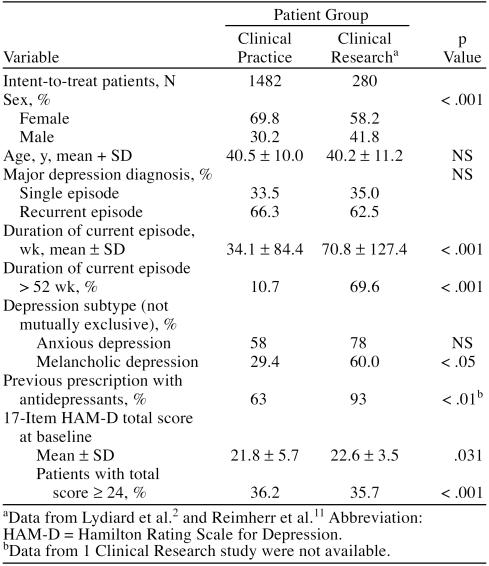
The Clinical Practice group consisted of 1482 patients who met study entry criteria; these patients constituted the intent-to-treat sample. Two hundred eighty patients received double-blind treatment with sertraline in the pooled Clinical Research sample. Table 1 summarizes demographic and clinical features of both groups. The main differences between the 2 patient samples were a higher proportion of females, a shorter duration of illness, and a lower proportion of melancholic depression subtype in the Clinical Practice sample, as well as a lower percentage of these patients having prior antidepressant treatment.
Table 1.
Demographic and Clinical Information for Patients From 2 Practice Settings Treated With Sertraline
Study Treatment, Tolerability, and Disposition
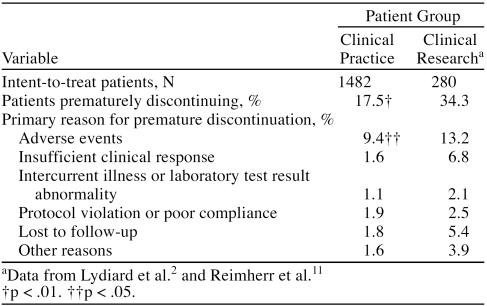
The mean ± SD daily dose of sertraline in the Clinical Practice sample at endpoint was 102.0 ± 54.0 mg and in the Clinical Research sample, 141.7 ± 61.1 mg (p < .001). The likelihood of completing acute treatment was significantly higher among the Clinical Practice patients (p < .01) (Table 2). Sertraline was well tolerated in both patient groups; patients in the Clinical Practice sample tended to report a significantly lower rate of most adverse events (Table 3), with the exception of headache and insomnia, which were significantly higher in this group. In the Clinical Research sample, dry mouth, drowsiness, tremor, and dizziness were significantly more frequent. This overall better tolerability is reflected in a significantly lower tendency among Clinical Practice patients to discontinue sertraline because of adverse events (p < .05) in the Clinical Practice sample.
Table 2.
Patient Disposition During 8 Weeks of Treatment for the 2 Study Samples
Table 3.
Treatment-Related Adverse Events Occurring at a Rate ≥ 10% for the 2 Study Samples
Efficacy of Sertraline Treatment: Comparison of Clinical Practice and Research Samples
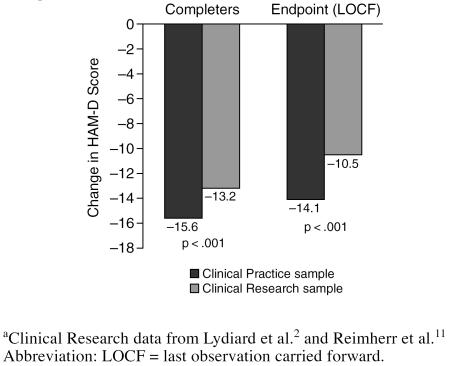
Sertraline treatment resulted in clinical improvement in a substantial percentage of both patient groups. In both the completer analysis as well as in the more conservative LOCF endpoint analysis, in which severity scores of patients who dropped out were carried forward, significantly more improvement in HAM-D scores was noted in the Clinical Practice patient group (Figure 1).
Figure 1.
Change From Baseline in HAM-D Score (adjusted): Comparison of Clinical Practice and Clinical Research Samplesa
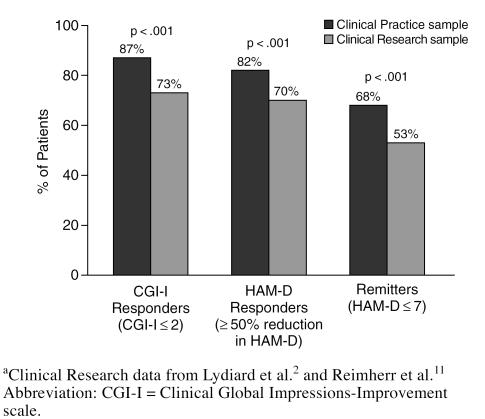
As noted earlier, different criteria were employed to define treatment response. Patients were rated by their physician as being “much” or “very much” improved on the CGI-I. In addition, those patients who achieved at least a 50% reduction from baseline in their HAM-D total score were considered responders. A more stringent definition was used to define a subgroup of treatment remitters: HAM-D total score ≤ 7 at study endpoint, suggesting minimal-to-no residual depressive symptoms. By all 3 criteria (Figure 2), sertraline appeared to be significantly more effective in achieving a favorable antidepressant outcome among Clinical Practice patients compared with Clinical Research patients (p < .001).
Figure 2.
Clinical Outcome at Study Endpoint: Comparison of Clinical Practice and Clinical Research Samplesa
Depression Subtypes and Prior Treatment Failures: Clinical Practice Sample
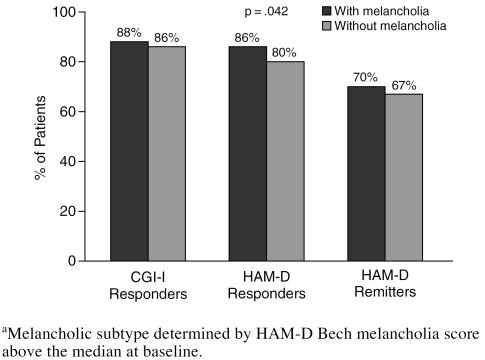
In the Clinical Practice sample, common and clinically relevant depressive subtypes were identified, and response to sertraline treatment for patients with the depressive subtype was compared with that for the remainder of the group. Sertraline treatment was comparably effective in patients with and without melancholic depression, both by the CGI-I response and the HAM-D remission criteria (Figure 3). By the HAM-D response criteria, a modest but statistically significantly larger proportion of the melancholic patients (86% vs. 80%) were responders.
Figure 3.
Effect of Melancholia on Response to Sertraline Among Clinical Practice Patientsa
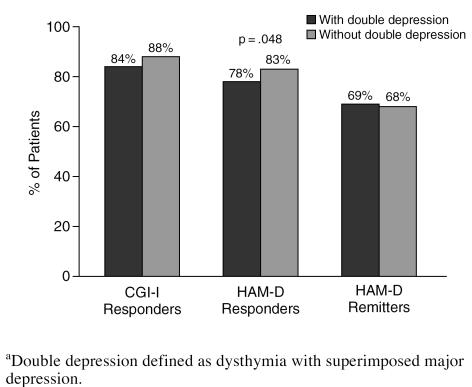
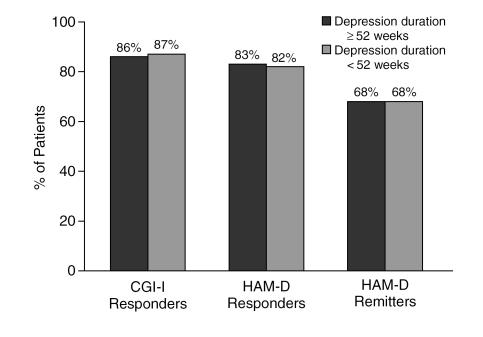
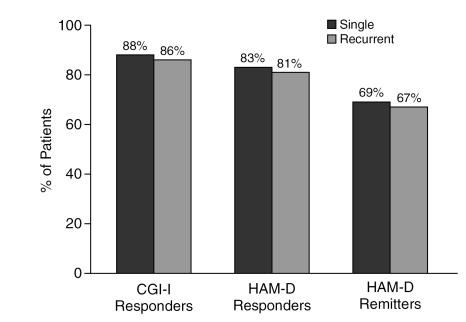
Patients with double depression appeared to respond to treatment as well as those without (Figure 4). A significantly lower percentage of patients with double depression (78%) achieved more than 50% reduction in HAM-D total score than of patients without double depression (83%), although the difference was modest. Neither the duration of the current episode (Figure 5) nor a history of previous episodes of depression influenced response or remission rates (Figure 6).
Figure 4.
The Effect of Double Depression on Response to Sertraline Among Clinical Practice Patientsa
Figure 5.
Response to Sertraline Among Clinical Practice Patients With Major Depression of at Least 52 Weeks Versus Less Than 52 Weeks in Duration
Figure 6.
The Effect of Prior Episodes on Response to Sertraline Among Depressed Clinical Practice Patients
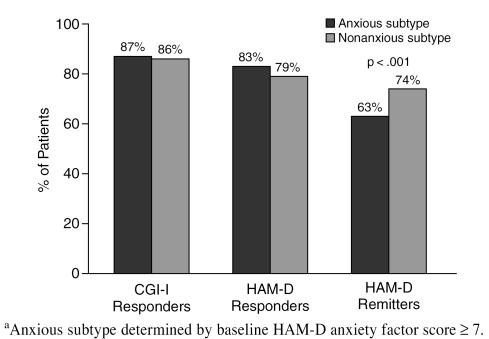
Clinical Practice patients presenting with anxious depression (defined by a HAM-D anxiety factor score ≥ 7) showed a similar endpoint response rate compared with those not presenting with prominent baseline anxiety (Figure 7). There was, however, a lower remission rate for anxious-depressed patients than for patients without prominent baseline anxiety.
Figure 7.
The Effect of Baseline Anxiety on Response to Sertraline Among Clinical Practice Patientsa
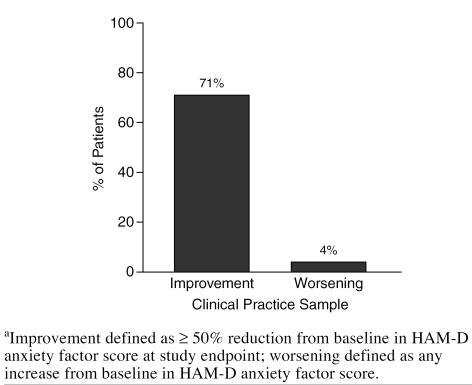
Among Clinical Practice patients presenting with anxious depression, sertraline treatment resulted in a notable reduction in the severity of anxiety (as measured by the HAM-D anxiety factor) that was significantly greater than was observed in the Clinical Research sample (−5.49 and −4.30, respectively; p < .001). In the Clinical Practice sample, sertraline treatment led to greater than 50% reduction in baseline anxiety severity, whereas only a small proportion of patients reported treatment-emergent anxiety (Figure 8).
Figure 8.
Effect of Sertraline on Baseline Anxiety Among Clinical Practice Patients (LOCF analysis)a
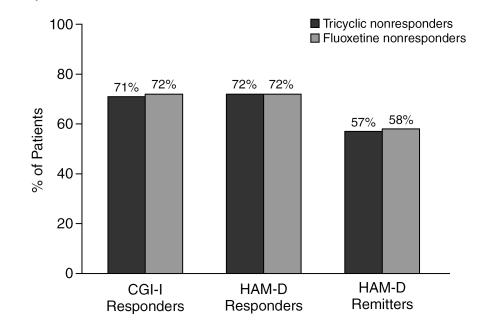
Finally, the subsets of Clinical Practice patients who rated themselves as either intolerant of or nonresponders to previous treatment with either fluoxetine or a TCA were evaluated separately as to their response to current treatment with sertraline. Previous antidepressant nonresponders as a group were found to have a good response to sertraline treatment (Figure 9).
Figure 9.
The Effect of Prior Treatment Failure on Response to Sertraline Among Clinical Practice Patients (LOCF analysis)
Predictors of Time to Response and Time to Remission
A stepwise procedure was employed, using a clinically identified list of candidate baseline variables to fit an optimal proportional hazards model to the dependent variable, time to CGI-I response. The stepwise selection procedure resulted in a model with 2 variables that predicted a longer time to response: (1) older age (Wald χ2 = 5.14, p < .023) and (2) presence of concurrent dysthymia (Wald χ2 = 6.71, p < .001).
A stepwise regression analysis was also employed to identify predictors of time to remission from among a list of clinically identified baseline variables. The stepwise selection procedure resulted in a model with 3 variables that predicted earlier achievement of remission: (1) younger age (Wald χ2 = 4.73, p < .030); (2) lower baseline depression severity (HAM-D score < 24; Wald χ2 = 15.74, p < .001); and (3) absence of agitated depression (defined as a HAM-D item 8 score ≤ 2; Wald χ2 = 5.11, p < .024).
DISCUSSION
The current investigation reports the results of a large, open-label study conducted in clinical practice settings that examined the antidepressant effectiveness of sertraline. Results for the Clinical Practice study group were compared with pooled results from 2 traditional placebo-controlled sertraline treatment studies conducted in psychiatry specialty research clinics.
Perhaps the most important finding from this exploratory analysis of pooled samples of clinical practice and research patients with major depression is the higher rates of therapeutic response in the Clinical Practice compared with the Clinical Research patients (see Figure 1). These findings are encouraging since they suggest that findings from clinical research may be generalized to the real-world clinical practice. The current results also raise the possibility that response rates in research studies may be conservative (i.e., represent the minimal level of likely improvement in terms of efficacy; see Figures 1 and 2). There was a significantly (p < .001) greater rate of response to sertraline among depressed patients treated in the Clinical Practice sample compared with those in the Clinical Research sample by all 3 response criteria, with a 12% to 14% margin. Similarly, more stringent remission criteria were achieved by 68% of patients in the Clinical Practice group, compared with 53% in the Clinical Research group (p < .001).
Sertraline appeared to be better tolerated in the real-world Clinical Practice setting than in the research setting (see Tables 2 and 3). The aggregate adverse event rate was significantly lower in the Clinical Practice setting, as was attrition due to adverse events (9.4% vs. 13.2%; p < .05) and overall attrition (17.5% vs. 34.3%; p < .01). Dose titration schedules required in research studies may contribute to the reduced tolerability in these settings. The limited existing literature suggests that research patients recruited through advertising are quite similar in important demographic and clinical characteristics to private patients entered into clinical trials.4–7 However, the findings suggestive of superior efficacy and fewer adverse effects in the Clinical Practice setting may be due to other factors. One possibility is that patients in the Clinical Practice group were aware that they were receiving active medication. Additionally, patients receiving treatment from a trusted clinician, with whom they have a working therapeutic alliance, may confer additional confidence in the treatment process, and perhaps less vigilance regarding adverse events—or more willingness to tolerate them. Another consideration is that patients in the Clinical Research group may have had a somewhat lower response rate to active medication because they believed that they were receiving placebo. The placebo response rate in the Clinical Practice group may actually have been higher, since all of these patients knew that they were receiving active medication, a phenomenon that may partially explain the usually higher response rate observed in open-label versus double-blind, placebo-controlled studies. Therefore, it is reasonable to assume that observed differences in outcome discussed below are likely a result of a combination of pharmacologic, interpersonal, and intrapsychic factors.
From Research to Clinical Practice
Treatment research has 2 main purposes: to establish with reasonable certainty that a particular agent is both effective and safe for the treatment of a particular medical condition and to bring better treatments into the clinical setting. To accomplish these sometimes conflicting goals, studies are carefully designed and usually exclude complicated patients (e.g., those who are medically ill or have more than one significant psychiatric disorder). The study designs utilized to achieve the goal of establishing efficacy and safety may raise a concern in physicians that patients participating in research studies may be sufficiently different from patients in their own practices that confident inferences cannot be made concerning the efficacy of a drug.
The current investigation identified some significant differences between the Clinical Practice and Clinical Research groups with respect to gender, severity and duration of current episode, and other features. It may be that these baseline differences contributed to the statistically significant differences in clinical outcome (see Figure 1). The limited existing literature, however, suggests that research patients recruited through advertising are usually quite similar in important demographic and clinical characteristics to private patients entered into clinical trials.4–7 Although the current results are somewhat at variance with these previously published findings, whether these differences account for the differences in clinical outcome is uncertain. It is possible that the superior efficacy and tolerability seen in the Clinical Practice patients may be due to other factors. Patients receiving treatment from a trusted clinician with whom they have a working therapeutic alliance may have more confidence in the treatment process and perhaps less vigilance regarding adverse events or more willingness to tolerate them. Again, these differences quite likely result from medication-related, interpersonal, and intrapsychic factors.
Treatment of Depression Subtypes
One of the goals of the current investigation was to assess whether sertraline treatment was as effective in treating patients with various depression subtypes that commonly present in clinical psychiatric settings. Our results found sertraline to be a highly effective antidepressant across a range of clinical presentations. Melancholic patients were found to have a modestly higher response rate, by 2% to 6%, when compared with nonmelancholic patients (see Figure 3). Another common subtype is chronic depression, which typically presents in 1 of 2 ways: either as a chronic major depression, defined by a duration of 2 years or longer,20 or as double depression,21 defined as a chronic low-grade depression (i.e., dysthymic disorder) upon which is superimposed an acute episode of major depression. Patients with double depression are especially vulnerable to depression recurrences.21 We analyzed outcome for patients reporting major depression for over 1 year (rather than 2 years).20 Our analysis found sertraline to be equally effective in patients whose depression was longer or shorter than 52 weeks (see Figure 5). In contrast, sertraline was somewhat less effective (with a 5% lower response rate by HAM-D criteria) in patients with double depression (see Figure 4). Those with and without double depression exhibited a HAM-D remission rate of approximately 70%.
Response to sertraline in this study was not affected by whether the current depression was single or recurrent (see Figure 6). High levels of anxiety or anxiety disorders often accompany major depression22 and may confer relative treatment resistance.8,15 In this study, patients who presented with anxious depression responded as well to sertraline as those without (see Figure 7). Sertraline also was found to be effective in reducing overall symptoms of anxiety, with 71% of Clinical Practice patients exhibiting a 50% or greater reduction in anxiety by study endpoint (LOCF). This finding is consistent with a growing body of research that finds that SSRIs such as sertraline are effective for anxiety disorders23–26 as well as depression.2,3,11,14
Predictors of Outcome
Despite meaningful improvement in both depression and anxiety symptoms, patients with anxious depression were significantly less likely to have achieved a remission by the end of acute treatment—a finding consistent with the published literature.15 A regression analysis, using HAM-D score ≤ 7 at endpoint as the dependent variable, confirmed that agitated depression (i.e., representing the more severe end of the anxious-depressive spectrum) was a significant independent predictor of lower remission rates. Higher baseline depression severity was the other variable identified by the regression analysis as a significant predictor of lower 8-week remission rates. Many patients with a clinically meaningful response to an antidepressant may still have modest, but significant, residual depressive symptoms.27 Achieving remission is important for 2 reasons: first, because remission is associated with a return to normal levels of functioning and quality of life3,28; and second, because the presence of residual depressive symptoms significantly increases the risk of depression relapse.27,29–31
In the present study, the results of a second regression analysis, using a CGI-I score of 2 (much improved) or 1 (very much improved) as the dependent variable, found older age and the presence of double depression to be significant predictors of a slower time to response. Previous research has consistently found older age to be associated with a slower time to response.32 Double depression has also been associated with both a slower time to response, as well as a lower overall antidepressant response rate after acute treatment.21
Prior Treatment Failure or Intolerance
We found that sertraline treatment was highly effective in inducing response and remission in the subset of patients who reported nonresponse to previous trials of either a TCA or fluoxetine.33,34 Response rates were somewhat lower in this subgroup, but still were approximately 70%. Contrary to conventional wisdom, nonresponse or intolerance to one SSRI may not necessarily mean that a physician should immediately switch to a different class of antidepressant. In such patients, a longer trial may be indicated, especially if, as suggested above, patients are over the age of 50 years or are suffering from double depression. If any treatment period results in an adequate response, however, then use of a second SSRI may be appropriate. This recommendation is supported not only by the results of the current study, but also by results from 2 naturalistic switch studies.33,34
It should be noted that the response rates were somewhat higher in this subgroup than is seen in patients with documented treatment resistance (i.e., patients selected have failed at least 2 previous antidepressant trials that have been confirmed as adequate). In the current trial, the classification as treatment failure was based on patient report, and many of the patients had failed to respond or were intolerant to only a single agent.
CONCLUSION
Because clinical research focuses on specific medical conditions, it usually excludes patients with “complicated” diagnoses—patients often seen in clinical practice—and thus renders making inferences to clinical practice difficult. In this comparative analysis of antidepressant treatment delivered under vastly different settings but with similar methods, the outcomes were at least comparable. In some cases, patients in the Clinical Practice groups fared even better than those in the Clinical Research sample. As was reported above, there were some significant differences between the Clinical Practice and Clinical Research groups with respect to gender, severity and duration of current episode, and other features such as melancholia, which was significantly more common in the Clinical Research group (60.0%) versus the Clinical Practice group (29.4%). It may be that one or more of these contributed to the usually small but statistically significant differences in outcome. We also realize that the treatment setting and open-label versus double-blind, placebo-controlled design differed. While this is clearly a weakness of comparative studies such as this one, it also provided a unique opportunity. The current results suggest that clinical trials may underestimate actual antidepressant effectiveness in clinical psychiatric settings.
In summary, sertraline was found to be a potent, broad-spectrum antidepressant with efficacy across a range of common depression subtypes in the outpatient setting. It is quite likely that this finding is not unique to sertraline and that similar results would have been obtained with the other SSRIs as well. Further research in this important area of “exportability of results” should be conducted to confirm and extend these findings to other patient populations and other psychiatric disorders. A better understanding of the correlates and causes of the differences in clinical outcome between the research “bench” and the clinical “trenches” could serve to assist the field in better understanding how research results translate into clinical practice.
Drug names: fluoxetine (Prozac), sertraline (Zoloft).
Footnotes
Supported by grants from Pfizer Inc.
Portions of this article were presented earlier at the 151st annual meeting of the American Psychiatric Association, June 1998, Toronto, Ontario, Canada, and the 21st meeting of the CINP (Collegium Internationale Neuro-Psychopharmacologicum), July 1998, in Glasgow, Scotland.
REFERENCES
- Fabre LF, Putnam HP III. A fixed-dose clinical trial of fluoxetine in outpatients with major depression. J Clin Psychiatry. 1987;48:406–408. [PubMed] [Google Scholar]
- Lydiard RB, Stahl SM, Hertzman M, et al. A double-blind, placebo-controlled study comparing the effects of sertraline versus amitriptyline in the treatment of major depression. J Clin Psychiatry. 1997;58:484–491. doi: 10.4088/jcp.v58n1104. [DOI] [PubMed] [Google Scholar]
- Bielski RJ, Lydiard RB. Therapeutic trial participants: where do we find them and what does it cost? Psychopharmacol Bull. 1997;33:75–78. [PubMed] [Google Scholar]
- Brauzer B, Goldstein BJ. Symptomatic volunteers: another patient dimension for clinical trials. J Clin Pharmacol. 1973;13:89–98. doi: 10.1002/j.1552-4604.1973.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Bialik RJ, Ravindran AV, Bakish D, et al. A comparison of placebo responders and nonresponders in subgroups of depressive disorder. J Psychiatry Neurosci. 1995;20:265–270. [PMC free article] [PubMed] [Google Scholar]
- Rapaport MH, Zisook S, Frevert T, et al. A comparison of demographic variables, symptoms profiles, and measurements of functioning in symptomatic volunteers in an outpatient population. J Clin Psychopharmacol. 1996;16:242–246. [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–18. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Gorman JM. Comorbid depression and anxiety spectrum disorders. Depress Anxiety. 1996;4:160–168. doi: 10.1002/(SICI)1520-6394(1996)4:4<160::AID-DA2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Lydiard RB. Comorbidity of panic disorder, phobia, and major depression. Eur Psychiatry. 1995;10(suppl 2):65S–66S. doi: 10.1016/0924-9338(96)80327-0. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Goldenberg DL, Pope HG Jr, et al. Comorbidity of fibromyalgia with medical and psychiatric disorders. Am J Med. 1992;4:363–367. doi: 10.1016/0002-9343(92)90265-d. [DOI] [PubMed] [Google Scholar]
- Reimherr FW, Chouinard G, Cohn CK, et al. Antidepressant efficacy of sertraline: a double-blind, placebo- and amitriptyline-controlled, multicenter comparison study in outpatients with major depression. J Clin Psychiatry. 1990;51(12, suppl B):18–27. [PubMed] [Google Scholar]
- Nierenberg AA. The treatment of severe depression: is there an efficacy gap between SSRI and TCA antidepressant generations? J Clin Psychiatry. 1994;55(9, suppl A):55–59. [PubMed] [Google Scholar]
- Smith WT, Londborg PD, Glaudin V, et al. Short-term augmentation of fluoxetine with clonazepam in the treatment of depression. Am J Psychiatry. 1998;155:1339–1345. doi: 10.1176/ajp.155.10.1339. [DOI] [PubMed] [Google Scholar]
- Aguglia E, Casacchia M, Cassano GB. Double-blind study of the efficacy and safety of sertraline versus fluoxetine in major depression. Int Clin Psychopharmacol. 1993;8:197–202. doi: 10.1097/00004850-199300830-00010. [DOI] [PubMed] [Google Scholar]
- Lydiard RB, Brawman-Mintzer O. Anxious depression. J Clin Psychiatry. 1998;59(suppl 18):10–17. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised. Washington, DC: American Psychiatric Association. 1987 [Google Scholar]
- Hamilton MA. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare publication (ADM) 76-338. Rockville, Md: National Institute of Mental Health. 1976 218–222. [Google Scholar]
- Bech P, Gram LF, Dein E, et al. Quantitative rating of depressive states. Acta Psychiatr Scand. 1975;51:161–170. doi: 10.1111/j.1600-0447.1975.tb00002.x. [DOI] [PubMed] [Google Scholar]
- Keller MB, Hanks DL. The natural history and heterogeneity of depressive disorders: implications for rational antidepressant therapy. J Clin Psychiatry. 1994;55(9, suppl A):25–31. [PubMed] [Google Scholar]
- Keller MB, Hirschfeld RM, Hanks D. Double depression: a distinctive subtype of unipolar depression. J Affect Disord. 1997;45:65–73. doi: 10.1016/s0165-0327(97)00060-8. [DOI] [PubMed] [Google Scholar]
- Sanderson WC, Beck AT, Beck J. Syndrome comorbidity in patients with major depression or dysthymia: prevalence and temporal relationships. Am J Psychiatry. 1990;147:1025–1028. doi: 10.1176/ajp.147.8.1025. [DOI] [PubMed] [Google Scholar]
- Katzelnick DJ, Kobak KA, Greist JH, et al. Sertraline for social phobia: a double-blind placebo-controlled crossover study. Am J Psychiatry. 1995;152:1368–1371. doi: 10.1176/ajp.152.9.1368. [DOI] [PubMed] [Google Scholar]
- Pohl RB, Wolkow RM, Clary CM. Sertraline in the treatment of panic disorder: a double-blind multicenter trial. Am J Psychiatry. 1998;155:1189–1195. doi: 10.1176/ajp.155.9.1189. [DOI] [PubMed] [Google Scholar]
- Greist J, Chouinard G, DuBoff E, et al. Double-blind parallel comparison of three dosages of sertraline and placebo in outpatients with obsessive-compulsive disorder. Arch Gen Psychiatry. 1995;52:289–295. doi: 10.1001/archpsyc.1995.03950160039008. [DOI] [PubMed] [Google Scholar]
- Pollack MH, Otto MW, Worthington JJ, et al. Sertraline in the treatment of panic disorder: a flexible-dose multicenter trial. Arch Gen Psychiatry. 1998;55:1010–1016. doi: 10.1001/archpsyc.55.11.1010. [DOI] [PubMed] [Google Scholar]
- Cornwall PL, Scott J. Partial remission in depressive disorders. Acta Psychiatr Scand. 1997;95:265–271. doi: 10.1111/j.1600-0447.1997.tb09630.x. [DOI] [PubMed] [Google Scholar]
- Miller IW, Keitner GI, Schatzberg AF, et al. The treatment of chronic depression, part 3: psychosocial functioning before and after treatment with sertraline or imipramine. J Clin Psychiatry. 1998;59:608–619. doi: 10.4088/jcp.v59n1108. [DOI] [PubMed] [Google Scholar]
- Paykel ES. Remission and residual symptomatology in major depression. Psychopathology. 1998;31:5–14. doi: 10.1159/000029018. [DOI] [PubMed] [Google Scholar]
- Van Londen L, Molenaar RP, Goekoop JG, et al. Three- to 5-year prospective follow-up of outcome in major depression. Psychol Med. 1998;28:731–735. doi: 10.1017/s0033291797006466. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM. Long-term drug treatment of unipolar depression. Int Clin Psychopharmacol. 1996;11:211–217. doi: 10.1097/00004850-199612000-00001. [DOI] [PubMed] [Google Scholar]
- Reynolds CF. Treatment of major depression in later life: a life cycle perspective. Psychiatr Q. 1997;68:221–246. doi: 10.1023/a:1025484123244. [DOI] [PubMed] [Google Scholar]
- Thase ME, Blomgren SL, Birkett MA, et al. Fluoxetine treatment of patients with major depressive disorder who failed initial treatment with sertraline. J Clin Psychiatry. 1997;58:16–21. doi: 10.4088/jcp.v58n0103. [DOI] [PubMed] [Google Scholar]
- Brown WA, Harrison W. Are patients who are intolerant to one serotonin selective reuptake inhibitor intolerant to another? J Clin Psychiatry. 1995;56:30–34. [PubMed] [Google Scholar]