Summary
Dickkopf-1 (DKK-1) is known inhibitor of the canonical Wnt pathway. Recent studies strongly suggested that activation of DKK-1 expression results in inhibition of cell tumorigenicity. Reduced levels of DKK-1 in melanomas were recently shown. However it is not known if DKK-1 activation in melanoma cells will inhibit cell tumorigenicity. In the present study we overexpressed DKK-1 in melanoma cell line MDA-MB435. We show that while DKK-1 did not affect cell growth in soft agar, weak but significant inhibition of tumorigenicity in nude mice in vivo was observed. Analysis of resulting tumors revealed activation of cell death. In tumors originating from cells transduced with DKK-1 tumor mass was permeated with areas of necrosis. In tumors, originated from control cells, areas of necrosis were limited to the central region, a common feature of large tumors growing in nude mice. TUNEL assay revealed that in tumors originating from cells transduced with DKK-1 apoptotic cells were detected along the border of necrotic and viable areas of the tumors indicating significant increase in apoptotic process. Thus, our results indicate that activation of DKK-1 in melanoma cells leads to activation of apoptosis in vivo and, thus, is incompatible with tumor growth in mice.
Keywords: Dickkopf-1, tumor suppressor, melanoma, cell death
Introduction
We previously described the isolation of two independent, nontumorigenic revertant clones from HeLa cervical carcinoma cell line exposed to the mutagen, ethylmethanesulfonate [1]. We subsequently demonstrated that both revertant cell lines expressed elevated levels the Dickkopf-1 (DKK-1). We further showed that DKK-1 can contribute to the inhibition of Hela cell transformation both in vitro and in vivo [2].
DKK-1 is a secreted protein, and was described as an inhibitor of canonical WNT pathway [3]. DKK-1 mediates its inhibitory effects on Wnt signaling by binding to the Kremen receptor. Frizzled, the receptor for WNT, and Kremen both use LRP5/6 as a co-receptor. As a result DKK-1 can sequester LRP5/6 away from Frizzled, thereby inhibiting Wnt-1 signaling [4–6]. Inhibition of the canonical WNT pathway results in increased GSK-3 activity. Elevated steady state levels of GSK-3 kinase activity result in increased phosphorylation of β-catenin. Targeting the latter for proteasome dependent protein degradation results in inhibition of β-catenin dependent transcription (for review see [7]).
Several components of the canonical Wnt signaling pathway have been identified as oncogenes or tumor suppressors in human cancers. Several genes involved in tumor growth, including cyclin D1, c-myc, matrilysin, are known targets of β-catenin dependent transcription. Among human colon cancers, almost 85% show loss-of-function mutations in the APC gene, an essential component in the stabilization of β-catenin and increased β-catenin-mediated transcriptional activity [8]. Mutational inactivation of AXIN1 [9] and β–catenin gene itself have also been detected in diverse human cancers (colorectal tumors, medulloblastomas, hepatoblastomas, hepatocellular carcinomas, etc.) [8, 10]. Overexpression of DKK-1 resulted in activation of apoptosis in vitro following treatment with different chemotherapeutic agents [11–13] and UV irradiation [2]. However it is not known if DKK-1 can activate apoptosis in tumor xenografts in vivo.
In the present study we tested if DKK-1 activates cell death in MDA-MB435 melanoma cells in vivo. This cell line until recently was considered as breast carcinoma cell line. However recent study [14] demonstrated that MDA-MB435 cell line is similar to the M14 melanoma cell line.
Materials and Methods
Cell Culture
Hela, MDA-MB231 breast carcinoma and MDA-MB435 melanoma cells were grown in DMEM supplemented with 5% fetal calf serum (Hyclone, Logan, UT), and 1% penicillin-streptomycin (Gibco BRL, Rockville, MD).
Retroviral Transduction
The HA-tagged form of DKK-1 cloned in LXSH, production of infectious amphotropic retrovirus have been described elsewhere [2]. Following infection, cell were selected for stable integration of viral construct with 1 mg/ml of Hygromycine.
Northern Blots
Total RNA was isolated from confluent cells using the Trizol® Reagent (Life Technologies, Rockville, MD). Extracted RNA subjected to Northern blot analysis and hybridized to radiolabeled probes corresponding to DKK-1 or β-actin using standard procedure. To control for loading and blotting variations, ethidium bromide stained membranes were photographed.
Western Blotting
Aliquots containing 30–40 μg of total protein or 30 ul of media were boiled in sample buffer before being loaded on a 10% SDS-PAGE gel. Separated proteins were transferred on a PVDF (Immobilon-P; Millipore, Bedford, MA) in the cold transfer buffer (10mM Caps, 10% methanol, pH 11.0) for 1–2hrs under the constant current of 1 Amp. Blotted membranes were blocked and incubated with the appropriate antibody dilution as described [2]. The quality of loading and transfer was assessed by immunostaining with β-actin antibody (Santa Cruz Biotechnology, Inc., CA).
Soft Agar Assay and anchorage dependent growth assay
Soft agar growth was evaluated by scoring the cloning efficiency in 0.3% Noble agar (Difco Laboratories, Detroit, MI) with 0.5% agar underlay as described [2]. Colonies were counted and photographed 30 days later. Anchorage dependent growth assay was performed by plating 100000 cells in 10cm dish in duplicate. Cells were harvested and counted on day 2, 4 and 6. Assays were performed at least 3 times.
Tumorigenicity in nude mice
Animal experiments were performed in FHCRC vivarium. All protocols used were reviewed and approved by the Institutional Animal Care and Use Committee. Female nude mice, 4 weeks old, were purchased from Harlan-Sprague-Dawley (Indianapolis, IN) and housed in filter-capped micro-isolation cages in a barrier facility on 12-h light/dark cycles and provided food and water ad libitum. Each mouse was injected subcutaneously with the indicated number of cells, and tumor growth was measured with calipers at weekly intervals as described previously [2]. Statistical analysis of differences in tumor volumes was performed using Student's t-test.
Histological evaluation of tumors and apoptosis detection
Dissected tumors were fixed, embedded in the parafine for hematoxiline and eosine staining using standard method. Detection of apoptotic cells were performed using TUNEL assay. Briefly, sections (4- um) were deparaffinized and rehydrated and were incubated with TUNEL reaction mixtures at room temperature with addition of terminal transferase. Serial slides processed without terminal transferase, served as a control for nonspecific staining. TUNEL-positive staining was recorded when positive staining of apoptotic bodies were detected morphologically.
Results
MDA-MB435 cells express low levels of Dickkopf-1 mRNA
Previously we showed that Hela cells have low endogenous levels of DKK-1 expression and DKK-1 overexpression resulted in inhibition of cell tumorigenicity in vitro and in vivo [2]. We determined that MDA-MB435 cell line expresses endogenous levels of DKK-1 comparable to those observed in Hela cells and significantly lower than those observed in the MDA-MB231 breast carcinoma cell line (Fig. 1A). We then used same virus preparation to overexpress the Ha-tagged form of DKK-1 in Hela and MDA-MB435 cells. Overexpression and secretion of HA-tagged form of DKK-1 in the cell lysates and in the conditioned media were confirmed by Western blot analysis using HA-tag antibody (Figure 1B, C). Comparison of secreted DKK-1 in MBA-MD435 and Hela cells growing under the same conditions demonstrated that the melanoma cell line accumulated very low levels of exogenous DKK-1 in the culture media when compared to the levels in culture media conditioned by Hela cells transduced with DKK-1 (Fig. 1B). By contrast the levels of DKK-1 protein in MDA-MB435 cell lysates were significantly higher than those in Hela cell lysates. These finding suggested that secretion of DKK-1 was impaired in the melanoma cell line.
Figure 1.
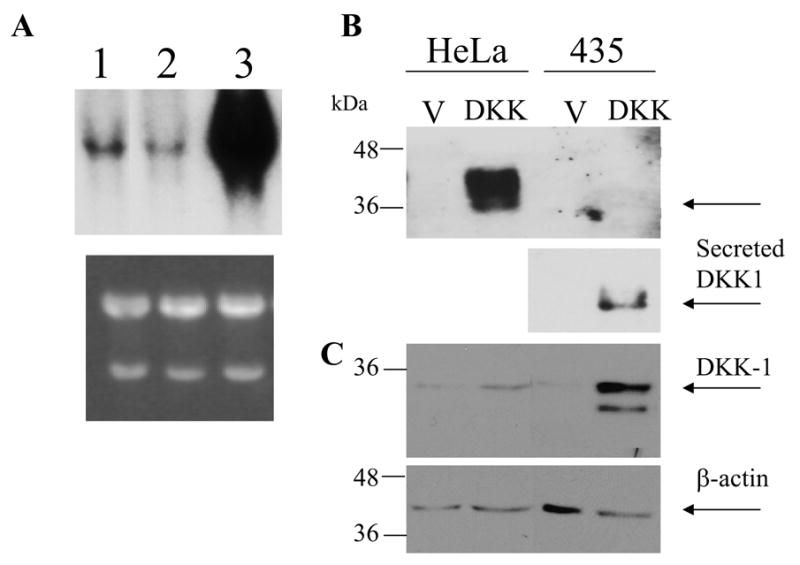
(A) Comparison of endogenous levels of DKK-1 expression in Hela (1), MDA-MB435 (2) cells, MDA-MB231 cells (3). Northern blot was probed with DKK-1 cDNA. Lower panel is Ethidium bromide stained gel. (B) MDA-MB435 and Hela cells were transduced with HA-tagged form of DKK-1. Secretion of DKK-1 was detected in the conditioned media collected after 48 hours of incubation of confluent culture. Because low levels of DKK-1 secretion in MDA-MB435 cells, membrane was re-exposed following application of higher concentration of chemoluminescence reagent for a longer time. Fragment of the membrane is shown. C. Expression of DKK-1 in cellular lysates was detected by Western blotting using HA-tag antibody. β-actin was used as a loading control (lower panel).
Inhibition of MDA-MB-435 tumor cell growth in vivo
We next assessed the effect of DKK-1 on anchorage independent growth of melanoma cells using the soft agar culture assay. As a positive control for inhibition of soft agar growth, we used HeLa cells transfected with DKK-1, which shows dramatically reduced growth in soft agar as compared to HeLa cells transduced with empty vector [2]. However, ectopic expression of DKK-1 did not affect the ability of MDA-MB435 cells to form anchorage independent colonies in soft agar (Fig.2 A, B, C, D).
Figure 2.
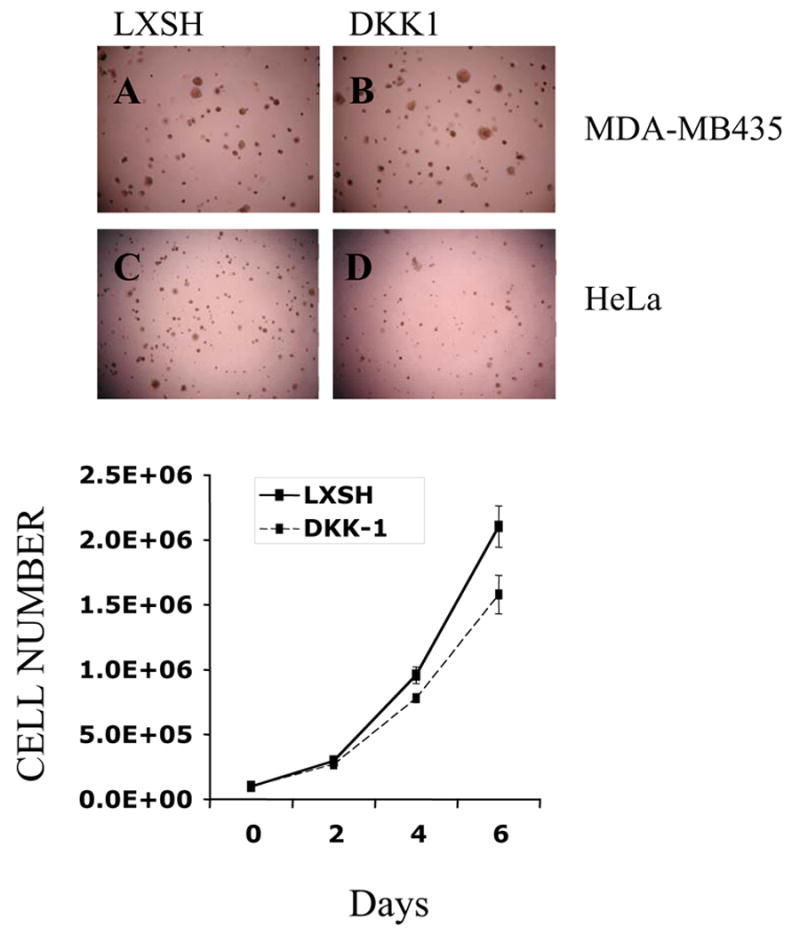
DKK-1 overexpression does not affect anchorage independent growth of MDA-MB435 melanoma cells in soft agar. MDA-MB435 cells expressing empty vector (A) or the HA-tagged form of DKK-1 (DKK-1) (B) were seeded in soft agar in duplicate. Colonies were scored 30 days later and photographed using phase contrast microscope. Hela cells transduced with LXSH (C) and DKK-1 (D) were described previously and were used as a positive control for inhibition of soft agar growth. DKK-1 overexpression inhibits anchorage dependent growth of MDA-MB435 cells on plastic. (Lower panel).
Our previous studies showed that ectopic DKK-1 inhibited anchorage dependent growth of Hela cells [2]. We therefore asked if DKK-1 had a similar effect on the melanoma cell line. Our results indicated that despite its inability to inhibit anchorage independent growth, DKK-1 did reduce anchorage dependent growth of the melanoma cell line.
Tumor growth in nude mice correlates with loss of transgene expression
We next investigated the effect of DKK-1 on in vivo tumorigenicity. Athymic nude mice were subcutaneously injected with either MDA-MB435 control cells or cells overexpressing exogenous DKK-1. The increase in tumor volume was monitored over time. Modest (33%) but significant (p<0.05) reduction in overall tumor volume was observed when animals were euthanized 30 days after injection and tumors were excised (Fig. 3A). In our previous studies, we demonstrated that in tumors arising after injection of cells expressing ectopic DKK-1, the expression of transgene was frequently lost, suggesting that there was a strong selection against DKK-1 expression during tumorigenic growth [2]. Therefore, we analysed the levels of transgene expression in the tumors arising after injection of the melanoma cells expressing ectopic DKK-1. As expected, Northern blot analysis revealed dramatic change in transgene expression in tumor cells as compared to the levels in MDA-MB-435 tumor cells before injection (Figure 3B). However in contrast to our previous study, we also further observed significant loss of the Hygromycine resistance gene in tumors originating from DKK-1 overexpressing cells as compared to tumors originated from vector transduced cells. The latter observation suggested that in the melanoma cell line ectopic DKK-1 transcriptions was not being silenced epigenetically. This result indicated that cells that do not loose the entire retroviral insert are eliminated from the tumor cell population. We therefore asked if tumors arising from DKK-1 transduced melanoma cells showed higher levels of apoptosis when compared to those with the control vector.
Figure 3.
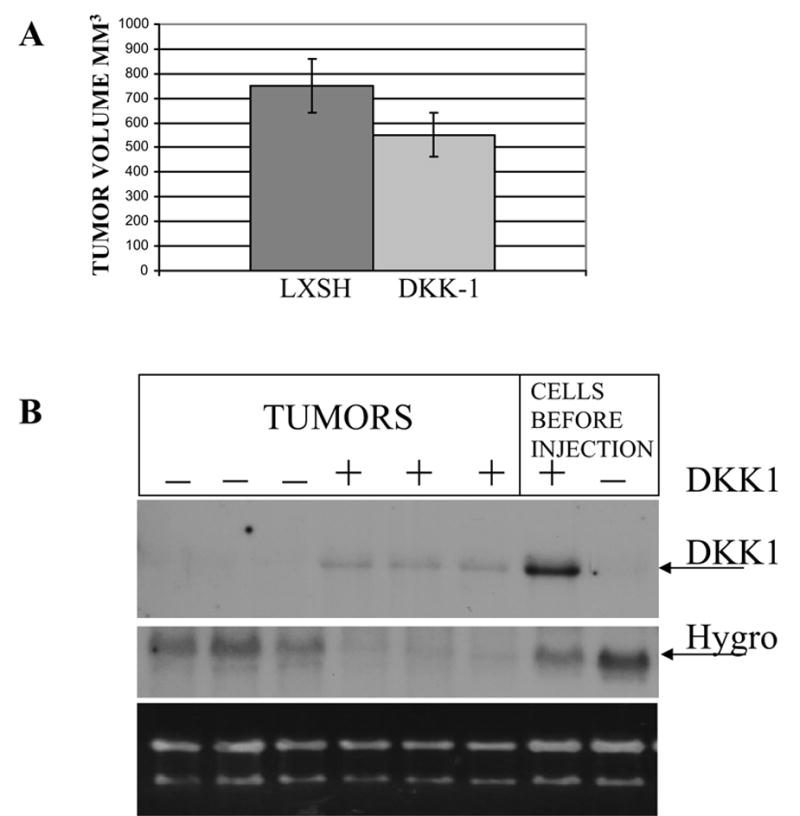
DKK-1 mediated suppression of MDA-MB435 cell tumorigenicity in vivo. (A)The 5x106 cells, expressing empty virus (LXSH) or HA-tagged form of DKK-1 were injected subcutaneously of three females nude mice following irradiation to inhibit NK activity. Tumor growth was monitored weekly. Mean tumor volumes on day of animal termination are shown. (*-p<0.05).
(B) Loss of DKK-1 and selectable marker Hygromycine phosphotransferase (Hygro) was observed in tumors originating from cells transduced with DKK-1 (+) compared to cells before injection. In tumors, originating from cells transduced with empty vector (−), loss of Hygromycine phosphotransferase expression was less pronounced, indicating that tumor growth is associated with preferential loss of DKK-1 transgene expression.
Activation of cell death in tumor cells expressing DKK-1
We first compared the histopathology of tumors arising from DKK-1 expressing and control cells. Areas of necrosis within the tumor mass were identified by their eosinophilic staining pattern and by a loss of cellular organization. In tumors originating from cells transduced with DKK-1, the tumor mass was permeated with extensive areas of necrosis (Fig. 4A), surrounded by only a rim of viable tumor cells. By contrast, areas of necrosis in tumors originating from control cells were limited to the central region, a common feature of large tumors growing in nude mice. Immunohistochemistry with antibodies aganinst the Ki67 growth specific marker did not reveal significant differences in areas unaffected by necrosis indicating comparable rates of proliferation in these regions (not shown). These findings suggested that decreased tumor volumes were probably the result of increased tumor cell death. To determine if increased tumor cell death was associated with increased apoptosis, we next used the TUNEL staining assay to compare the levels of apoptosis. In the control tumors, TUNEL staining detected was within areas of necrosis. These areas of necrosis were identified by nonspecific staining on slides without the addition of terminal transferase (Fig. 4B). By contrast, tumors originating from mice injected with DKK-1 overexpressing cells, showed distinct apoptotic bodies along the border between viable and necrotic areas, as well as within necrotic areas (Fig. 4B). These observations are consistent with the hypothesis that DKK-1 overexpression activates apoptosis in melanoma cells during in vivo growth in athymic nude mice.
Figure 4.
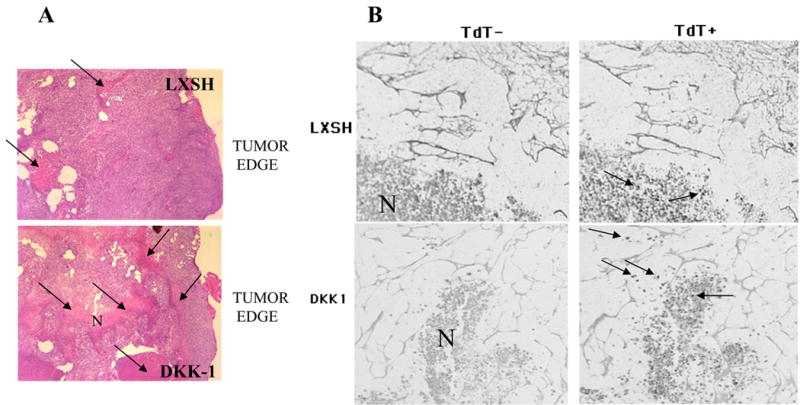
(A) Expression of DKK-1 in MDA-MB435 cells (top panel) results in significant cell death the tumors growing in nude mice. Massive areas of necrosis (N) are shown with arrows. Areas of viable cells are seen along the tumor edge. Control tumors originating from cells transduced with empty vector had significantly less and smaller necrotic areas (top panel) localized mostly closer to the center of the tumor. Similar areas relative to tumor edge were compared. The tumor edge was determined by detection of normal mouse muscle tissue or muscle tissue invaded by tumor cells along the tumor edge. Magnification X10. Hematoxylin and eosin staining. (B) DKK-1 overexpression induced apoptosis in tumor xenografts. TUNEL assay was used to detect apoptotic bodies. Slides stained without terminal transferase (TdT-) demonstrated nonspecific staining of the necrotic areas (N). Serial sections processed with in the presence of terminal transferase (TdT+) show apoptotic bodies in tumors originating from DKK-1 expressing cells, which are localized on the border of necrotic areas and viable cells as well as within necrotic areas (shown with arrows). In tumors originating from vector transduced cells apoptotic bodies with weaker signal are detected within necrotic areas only. Representative fields are shown.
Discussion
Several recent studies have also implicated members of the Dickkopf family of Wnt inhibitors in suppression of human cancer. Reduced levels of DKK-3 were found in prostate [15], lung cancers [16], renal clear cell carcinoma [17]. Overexpression of DKK-1 or DKK-3 resulted in inhibition of cell tumorigenicity, cell motility and invasiveness. We previously demonstrated inhibition of Hela cell tumorigenicity by DKK-1 overexpression [2]. Transfection of DKK-3 and dominant-negative LRP5 into p53 null Saos-2 tumor cells significantly reduced cell motility and invasiveness. Recently it was demonstrated that expression of DKK family of proteins (DKK-1, -2 and -3) is reduced in most of melanoma cell lines and most of tumor samples [18]. Overexpression of DKK-3 in melanoma cells resulted in inhibition of cell invasion and migration [18] which correlated with re-expression of E-cadherin, inhibition of Snail-1 and fibronectin expression.
Inhibition of tumorigenicity can be result of cell sensitization to apoptosis. Previous studies showed that overexpression of DKK-1 leads to activation of apoptosis after treatment with chemotherapeutic agents or UV. Sensitization to apoptosis was demonstrated in glioma cells [13], Hela cells [2], breast carcinoma cells [11], mesothelioma cells [12]. Activation of apoptosis in DKK-1 expressing cells was suggested is due to increased JNK activity. Inhibition of JNK by chemical inhibitor led to inhibition of apoptosis [12]. In glioma cells over-expression of DKK-1 sensitizes cells to apoptosis induced by ceramide in vitro affecting the BAX/BCL-2 expression ratio [13].
In the present study we generated melanoma cell line which expresses cellular levels of DKK-1 above to those observed in Hela cells. However, unexpectedly levels of DKK-1 secretion in the cell culture media were dramatically lower compare to Hela cells. Reasons for low levels of DKK-1 secretion are not known. This observation indicates that comparison of the intracellular levels of the DKK-1 expression may be misleading when functional activity depends on amount of secreted protein.
Despite the lower levels of secretion, we observed weak effect of DKK-1 on anchorage dependent growth. Thus, cells are sensitive to the relatively low levels of DKK-1 expression. DKK-1 expression did not affect MDA-MB435 cells soft agar growth. Nevertheless we found weak tumor suppressive effect in nude mice at the end of experiment. Thus, even low levels of DKK-1 secretion is incompatible with tumor growth from MDA-MB435 cells.
Further investigation of DKK-1 transgene expression revealed significantly reduced levels of exogenous DKK-1 mRNA in all tumors. This observation was similar to those we reported in Hela cells [2]. However, unlike previous study in Hela cells, we also observed loss of Hygromycine resistant gene. Loss of transgene expression was associated with intensive cell death in tumors originated from DKK-1 expressing cells. We speculate that cell death and loss of transgene expression are related to cancer cell selection for survival. In vivo and in vitro selection for tumor cells with a more aggressive malignant phenotype is a well-documented phenomenon. Hypoxia in fast growing tumors can contribute to the selection of tumors with a more transformed phenotype. For example, murine B16 melanoma cells subjected to sequential rounds of exposure to hypoxia and confluence in vitro produced populations with significantly enhanced growth capabilities, which could establish dominance within tumors [19]. Probably, cell death due to DKK-1 expression under hypoxic conditions inside the tumor represent driving force in the process of selection for survival. Mutant or inactivated p53 found in MDA-MB435 and Hela cells facilitates process of selection for survival.
It is also known that overexpression of secreted proteins may saturate cell capacity to properly fold proteins leading to unfolded protein response (UPR) which causes cell death [20]. This is very unlikely explanation because DKK-1 overexpression did not cause any inhibition of soft agar growth or cell death under anchorage dependent conditions. This is consistent with our previous study [2] when overexpression of DKK-1 did not lead to apoptosis measured by Annexin V, unless cells are treated with UV. These observations are consistent with results published by others. However, UPR induces adaptive response which would favor cell survival due to inhibition of apoptosis [21]. We cultured cells for 2.5–3 months before injection into animals which would provide sufficient time for adaptation and inhibition of apoptosis. Therefore activation of apoptosis in tumor xenografts is not due to unfolded protein response. It rather represents consequences of changes occurring during tumor growth in the presence of functional DKK-1.
Therefore our results indicate that DKK-1 expression is incompatible with melanoma cell tumorigenicity due to activation of cell death. We can speculate that death can be result of DKK-1 mediated sensitization to hypoxic conditions observed in tumor at later stage of growth. This is reasonable explanation because MDA-MB435 cells formed very large tumor (750mm3). Size of these tumors significantly exceeded size of the tumors formed by Hela cells (290 mm3) in our previous experiments performed under similar conditions [2]. To investigate if cell death in the tumors is due to reduced vascularization, we used immunohistochemical detection of CD31 vascular marker (not shown). We identified very few vessel structures within the tumor mass indicating very poor tumor vascularization (not shown). Because of poor vessel development we were not able to determine if DKK-1 inhibited vessel formation in tumors. Thus, additional investigation are required to determine if DKK-1 may have antiangiogenic effect.
In summary, current study shows that DKK-1 overexpression is incompatible with melanoma cell tumor growth in vivo leading to activation of cell death. Even relatively low levels of DKK-1 expression are sufficient to induce cell death and inhibit tumor growth. Thus our results are consistent with accumulating evidences that reactivation of DKK-1 expression or signaling maybe a viable strategy for the therapeutic intervention in a wide spectrum of human tumors.
Acknowledgments
We thank Dr. P. Porter for help with pathological evaluation of tumors and advice. This research was supported in part by funding from the NIEHS sponsored Toxicogenomics Research Consortium, Grant # NIEHS U19ES011387, the U.S. Army Medical Research and Materiel Command under DAMD17-98-1-8086, and by the UW NIEHS sponsored Center for Ecogenetics and Environmental Health, Grant #: NIEHS P30ES07033.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boylan MO, Athanassiou M, Houle B, Wang Y, Zarbl H. Activation of tumor suppressor genes in nontumorigenic revertants of the HeLa cervical carcinoma cell line. Cell Growth & Differentiation. 1996;7:725–735. [PubMed] [Google Scholar]
- 2.Mikheev AM, Mikheeva SA, Liu B, Cohen P, Zarbl H. A functional genomics approach for the identification of putative tumor suppressor genes: Dickkopf-1 as suppressor of HeLa cell transformation. Carcinogenesis. 2004;25:47–59. doi: 10.1093/carcin/bgg190. [DOI] [PubMed] [Google Scholar]
- 3.Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP, Kraus MH, Aaronson SA. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. Journal of Biological Chemistry. 1999;274:19465–19472. doi: 10.1074/jbc.274.27.19465. [DOI] [PubMed] [Google Scholar]
- 4.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nature Cell Biology. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 5.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 6.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 7.Clevers H. Wnt breakers in colon cancer. Cancer Cell. 2004;5:5–6. doi: 10.1016/s1535-6108(03)00339-8. [DOI] [PubMed] [Google Scholar]
- 8.Polakis P. Wnt signaling and cancer. Genes & Development. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 9.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nature Genetics. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 10.Buendia MA. Genetics of hepatocellular carcinoma. Seminars in Cancer Biology. 2000;10:185–200. doi: 10.1006/scbi.2000.0319. [DOI] [PubMed] [Google Scholar]
- 11.Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Lee AY, He B, You L, Xu Z, Mazieres J, Reguart N, Mikami I, Batra S, Jablons DM. Dickkopf-1 antagonizes Wnt signaling independent of beta-catenin in human mesothelioma. Biochem Biophys Res Commun. 2004;323:1246–1250. doi: 10.1016/j.bbrc.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Shou J, Ali_Osman F, Multani AS, Pathak S, Fedi P, Srivenugopal KS. Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene. 2002;21:878–889. doi: 10.1038/sj.onc.1205138. [DOI] [PubMed] [Google Scholar]
- 14.Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 Melanoma cells--a loss for breast cancer, but a boon for melanoma research. Breast Cancer Research and Treatment Epub ahead of print. 2006 doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- 15.Kawano Y, Kitaoka M, Hamada Y, Walker M, Waxman J, Kypta R. Regulation of prostate cell growth and morphogenesis by Dickkopf-3. Oncogene. 2006 doi: 10.1038/sj.onc.1209661. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, Ouchida M, Tsuji T, Hanafusa H, Miyazaki M, Namba M, Shimizu N, Shimizu K. Reduced expression of the REIC/Dkk-3 gene by promoter-hypermethylation in human tumor cells. Gene. 2002;282:151–158. doi: 10.1016/s0378-1119(01)00838-1. [DOI] [PubMed] [Google Scholar]
- 17.Kurose K, Sakaguchi M, Nasu Y, Ebara S, Kaku H, Kariyama R, Arao Y, Miyazaki M, Tsushima T, Namba M, Kumon H, Huh NH. Decreased expression of REIC/Dkk-3 in human renal clear cell carcinoma. J Urol. 2004;171:1314–1318. doi: 10.1097/01.ju.0000101047.64379.d4. [DOI] [PubMed] [Google Scholar]
- 18.Kuphal S, Lodermeyer S, Bataille F, Schuierer M, Hoang B, Bosserhoff A. Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene. 2006;25:5027–5036. doi: 10.1038/sj.onc.1209508. [DOI] [PubMed] [Google Scholar]
- 19.Stackpole CW, Groszek L, Kalbag SS. Benign-to-malignant B16 melanoma progression induced in two stages in vitro by exposure to hypoxia. Journal of the National Cancer Institute. 1994;86:361–367. doi: 10.1093/jnci/86.5.361. [DOI] [PubMed] [Google Scholar]
- 20.Raden D, Hildebrandt S, Xu P, Bell E, Doyle F, Robinson A. Analysis of cellular response to protein overexpression. Syst Biol (Stevenage) 2005;152:285–289. doi: 10.1049/ip-syb:20050048. [DOI] [PubMed] [Google Scholar]
- 21.Rutkowski D, Arnold S, Miller C, Wu J, Li J, Gunnison K, Mori K, Akha A, Raden D, Kaufman R. Adaptation to ER Stress Is Mediated by Differential Stabilities of Pro-Survival and Pro-Apoptotic mRNAs and Proteins. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040374. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


