Abstract
Properly diagnosing and treating patients with anxiety, depression, or both is a challenging aspect of practicing medicine in the primary care setting. Patients often present with somatic complaints rather than classic psychiatric symptoms. In addition, there is significant overlap between anxiety and depression in this patient population. Comorbid anxiety and depression is often more resistant to pharmacologic treatment, and patients with coexisting disorders have a poorer medical prognosis than do patients with either disorder alone. Fortunately, many new therapies are available to assist the clinician in managing these patients. The newer antidepressants, in particular, are playing an increasingly important role in the treatment of both anxiety disorders alone and comorbid anxiety and depression. These new choices enable our goal of treatment to encompass not only improvement but also sustained complete remission. Of the newer agents, the selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors have been studied quite extensively in these patient populations. The specific profiles of individual agents may assist the clinician in individualizing treatment. Characteristics such as robust efficacy, speed of onset of activity, the potential for drug-drug interactions, dose response, and tolerability are important considerations in optimizing treatment.
Anxiety and depression are significant public health problems, affecting a wide segment of the general population and accounting for multibillion-dollar expenditures directly related to health care and hospitalizations and indirectly related to morbidity and mortality.1,2 In addition, these disorders are associated with significant decreases in patient well-being and social functioning and can cause considerable pain and suffering, not only for affected individuals but for their family and friends as well.1,3 Despite the availability of proven treatments, both disorders remain underrecognized and undertreated. Their diagnosis and management are complicated by the considerable overlap of symptomatology. For example, according to the National Comorbidity Survey,4 58% of those with lifetime depression were also observed to have at least one anxiety disorder. Furthermore, increased health care resource utilization and decreased productivity are more significant in patients with comorbid anxiety and depression.3
Anxiety and depression are often manifested initially as physical ailments rather than the classic symptom of altered mood; thus, it is not surprising that many of these patients turn to their primary care physicians for care. As a result, it is crucial for the generalist physician to be well versed in recognizing and managing such cases. Now more than ever, new, more user-friendly pharmacologic options demonstrate robust efficacy; simplified treatment with monotherapy can provide the necessary tools to manage anxiety and depression in the primary care setting both efficaciously and cost effectively. Toward this end, this article reviews these clinical disorders and summarizes current information on the drug therapies available to treat them.
ANXIETY
Definition and Prevalence
All of the different types of recognized anxiety disorders are characterized by the presence of clinically significant degrees of chronic anxiety. These disorders are very common in patients seen by primary care physicians. In the early 1990s, the National Comorbidity Survey5 indicated that the 1-year prevalence of all anxiety disorders in the general population was 17.2%, and the lifetime prevalence was 24.9%. In this survey, Kessler et al.5 found that social phobia was the most common type of anxiety disorder, with a lifetime prevalence of 13.3%. Simple phobias were next, at 11.3%, and generalized anxiety disorder (GAD) followed with a lifetime prevalence of 5.1% and a 1-year prevalence of 3.1%. Panic disorder had a lifetime prevalence of 3.5%.5 Other less common anxiety disorders include obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and acute stress disorder. With the exception of OCD, anxiety disorders generally occur more frequently in women than in men and usually have their first onset during adolescence or young adulthood.6
DSM-IV Classification of Anxiety Disorders
The American Psychiatric Association (APA) has sought to clarify diagnostic categories and procedures for diagnosing anxiety for the primary care physician and has published an anxiety algorithm in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), Primary Care Version.7 Somatic manifestations of anxiety complicate the clinical picture of patients with anxiety disorders; anxiety may manifest in ways such as gastrointestinal symptoms, headaches, chronic pain, chest pain, shortness of breath, and dizziness. Therefore, one of the first objectives in determining the appropriate diagnosis for a patient with anxiety is to rule out the common medical conditions that can cause anxiety, e.g., hyperthyroidism, supraventricular arrhythmias, high caffeine use, drug abuse, the misuse of prescription medications (Table 1).7
Table 1.
Initial Steps in the Anxiety Algorithma
Some anxiety disorders occur when anxiety is experienced during discrete periods (e.g., panic disorder) or in specific situations (e.g., social phobia). Patients with panic disorder regularly experience acute episodes of intense fear and discomfort that are associated with symptoms of autonomic arousal, such as tachycardia, sweating, dizziness, shortness of breath, and chest pain. These patients may also suffer from agoraphobia as a result of their fear of places and situations from which they cannot escape in the event of a panic attack.6 Therefore, they tend to avoid things such as crowds, elevators, buses, trains, and bridges. Patients with social phobia have a fear of having to interact with unfamiliar people (or people in authority) and being scrutinized by them (e.g., while writing, eating at a party, speaking to a boss or store clerk). On the other hand, patients with specific phobias experience anxiety when confronted by a specific object or situation (e.g., snakes, thunderstorms).
In OCD, patients are afflicted with chronic, recurrent thoughts, impulses, or images that cause marked anxiety, and they compulsively engage in ritualistic acts or behaviors in response to these obsessions. Patients with GAD experience anxiety that is more unfocused than in these other disorders, and they worry excessively (daily) about events and situations of everyday life. Such patients traditionally have been identified as “lifetime worriers.”6
Management of Anxiety in the Primary Care Setting
Benzodiazepines and buspirone.
The use of the benzodiazepines (BZDs) in the treatment of anxiety disorders has a long history. For many years, diazepam was the most widely prescribed BZD, but alprazolam is currently the most commonly used drug of this class because of its lower incidence of sedation.8 Alprazolam often significantly reduces panic attacks within a few days at the recommended daily dosage of 1.5 to 10 mg, although dosages as low as 0.25 mg 3 times daily may be effective in some patients.8–11 In more chronic forms of anxiety, such as GAD, typical maintenance dosages of alprazolam are generally lower (0.75 to 4.0 mg/day) than those used in panic disorder.
Patients are often treated with variable courses of the BZDs, depending on the character of their underlying anxiety disorder. Some patients with episodic panic attacks or with social or a specific phobia may be treated successfully with intermittent therapy, whereas patients with more persistent symptoms, such as those of GAD, may benefit from longer, more sustained courses of treatment.8 Clonazepam is widely used, and its long half-life makes it well suited for intermittent use in appropriate patients. Because of the risk of addiction with these agents, their use should be reserved for patients who do not have a history of drug abuse.10 Typical adverse effects of treatment include sedation, ataxia, and difficulties with concentration, memory, and balance.8 Additionally, when BZDs are used in combination with other sedatives, such as alcohol, they may be associated with significant cognitive impairment. Older patients may be particularly vulnerable to this adverse effect, even without the use of other sedatives.9
Unlike BZDs, buspirone, a member of the azapirone class of nonbenzodiazepine anxiolytics, does not cause sedation or psychomotor and cognitive impairment and, in general, has a more favorable adverse effect profile. Adverse effects of treatment include headache, light-headedness, nausea, and restlessness.8 Buspirone also has a low abuse potential, so it may be a suitable alternative to BZDs in addictive patients.
At dosages of ≥ 20 mg/day, buspirone is effective in GAD but not in panic disorder or OCD, for which it has shown little effect as a sole agent.12 However, buspirone has a slow onset of action, and it can take 3 to 4 weeks to provide substantial relief of symptoms.9
Despite wide use in treating depressed patients in general medical practice, neither the BZDs nor buspirone has any clinically significant antidepressant effects at usual dosages; as a result, they are not recommended as monotherapy in anxious patients with coexisting depression.
Newer antidepressants for treatment of anxiety.
Some of the newer antidepressants that have been investigated in anxiety disorders are shown in Table 2. Many of the anxiolytic effects of antidepressants are believed to be modulated by inhibition of serotonin reuptake. Thus, the selective serotonin reuptake inhibitors (SSRIs) have been investigated widely in anxiety disorders, including panic disorder, OCD, GAD, and social phobia.13–17 Although these agents have a lag in their onset of action, much like that of the older tricyclic antidepressants (TCAs) and buspirone, they are generally more tolerable than the TCAs. The SSRIs are generally regarded as the treatments of first choice, certainly for OCD, more recently for panic disorder, and most recently for social phobia.17 However, complex drug-drug interactions caused by inhibition of the cytochrome P450 enzyme system can occur in some patients taking multiple concomitant medications.
Table 2.
Pharmacologic Options for Treatment of Anxiety Disorders
Nefazodone and mirtazapine have also been evaluated in depressed patients with anxiety.10,18,19 Clinical trials have shown that nefazodone is an effective antidepressant, similar to imipramine,20 that reportedly lacks some of the notable side effects associated with the use of TCAs and SSRIs. Drug-drug interactions with alprazolam and triazolam, caused by cytochrome P450 inhibition, complicate somewhat the use of nefazodone in combination with these BZDs.9 The antidepressant efficacy of mirtazapine is comparable to that of the TCAs and SSRIs, but it is associated with fewer of the adverse effects commonly attributed to agents of both these classes.19 However, its use is frequently associated with sedation and weight gain,19 typically undesirable side effects that can be useful in some patients whose depression is accompanied by insomnia and anorexia. Data on mirtazapine use in non-comorbid anxiety disorders are limited.
Venlafaxine extended release (XR) is the only agent with a dual indication for the treatment of depression and GAD. In addition to demonstrating efficacy in GAD,21–26 some data suggest it may be useful in social phobia,27 panic disorder,28 and OCD.29 Other reports suggest it is highly effective in the treatment of patients with mixed anxiety and depression.30,31 Venlafaxine XR has a low potential for drug-drug interactions,32 which makes it suitable as a first-line agent in these patients, particularly in those in whom such interactions are more likely.
DEPRESSION
Definition and Prevalence
Depression is a serious clinical disorder that is classically associated with disturbances in mood, loss of interest or pleasure in previously enjoyed activities (anhedonia), and feelings of profound sadness and hopelessness. Depressed patients may also present with alterations in sleep patterns, sexual interest and activity, appetite and weight, and the ability to concentrate.6,33 These symptoms of depression are often so severe that they significantly interfere with social, familial, and occupational functioning and cause considerable distress in the affected individual. In the primary care setting, it is not uncommon for patients with depression, such as those with comorbid anxiety, to have a variety of physical ailments that mimic other medical diseases or conditions, rather than the classic symptoms.34 Somatic manifestations of depression commonly include gastrointestinal disturbances, chronic pain, dizziness, and fatigue. These patients often have been seen repeatedly by a health care practitioner without a firm diagnosis for their physical complaints.
The somatization of depression is particularly challenging to primary care physicians, given their role as gatekeepers and continued pressures from managed care organizations to contain health care expenditures. Studies show that most mental health visits in the United States are to primary care physicians.35 Depression is, perhaps, the most common problem seen by primary care physicians, accounting for 11% to 12% of visits.36 According to data from the National Comorbidity Survey,6,37 the prevalence of current major depression is estimated to be 4.9% and that of lifetime major depression, 17.1%. The prevalence of depression among women is approximately double that among men. The Epidemiologic Catchment Area study determined that the first presentation usually occurs in young adulthood, with a mean age at onset of 27.4 years,33 although increasing numbers of depressed adolescents and young adults are seen in clinical practice.38 The prevalence rate in older patients (≥ 65 years), however, has reportedly decreased dramatically.37 The presentation of depression in older patients may differ from that typically seen in younger patients, often occurring as a consequence of an underlying physical illness or its treatment or in association with the loss of a spouse.39
DSM-IV Classification of Depressive Disorders
Unfortunately, owing in large part to the phenomenon of somatization, many depressed patients are not identified, or their disorder is misdiagnosed.40 The appropriate diagnosis for the depressed mood may be complicated by the presence of concomitant illnesses, alcohol or substance use or abuse, or inadequate knowledge or experience on the part of the physician. The APA has presented useful algorithms for distinguishing depressive states on the basis of clinical characteristics, duration of symptoms, and associations with medical illnesses, bereavement, or substance use.41
The initial steps in diagnosing a patient who complains of depressed mood or has more general medical complaints that cannot be readily explained are shown in Table 3.41 The objective of the algorithm is to assist clinicians in identifying first those conditions that have more immediate treatment considerations. These include determining whether the patient has an underlying general medical condition that may be causing the depression, whether it is related to substance use (e.g., prescription medications, alcohol, illegal drugs), or whether the patient is experiencing an acute episode of major depressive disorder (MDD).41 The latter condition may be present if the patient has had persistent symptoms of depression or anhedonia for 2 weeks or longer. In general, untreated episodes of MDD continue for a mean duration of 6 to 9 months.39
Table 3.
Initial Steps in the Depressed Mood Algorithma
In patients with MDD, it is also important to establish whether there is a history of abnormally elevated, expansive, or euphoric mood; such patients probably have bipolar I or II disorder. Although patients with bipolar I disorder may have depressive symptoms, the hallmark of this disorder is mania that requires hospitalization.39 In contrast, patients with bipolar II disorder generally do not present with full-blown mania. Rather, they may experience manic-like symptoms (called hypomania), often before or after a major depressive episode.
After these initial categories are ruled out, the clinician should review whether the current depression is part of a more chronic condition, such as recurrent MDD (possibly in partial remission) or dysthymic disorder. Dysthymic disorder is primarily characterized by chronicity (depressed mood more often than not lasting at least 2 years in adults and 1 year in children) and symptoms that are not as severe or disabling as in MDD.6 These patients experience symptoms of depression most but not all days, and the disorder, which often begins in early adulthood, may worsen as the patient ages.39
Alternatively, some patients who do not meet the criteria for the disorders noted above might have more specific conditions that are associated with depressive features. These could include bereavement (depression of < 2 months' duration that is associated with the death of a loved one) or adjustment disorder (depression occurring within 3 months in response to an identifiable psychosocial stressor that does not meet criteria for other disorders). Finally, many patients may be suffering from unspecified but disabling forms of depression that occur even more frequently than MDD, such as premenstrual dysphoric disorder.
Management of Depression in the Primary Care Setting
The aggressive and appropriate management of depression in the primary care setting is crucial, not only from a clinical perspective, but from a health economics perspective as well. Untreated or inadequately treated patients are more likely to have negative medical consequences of their depression, including a substantial risk of suicide and longer, more treatment-resistant episodes of depression.42 Such patients will continue to use valuable health care resources inappropriately, including significantly more general medical services.43
Thus, the challenge for clinicians is to make a rapid and accurate diagnosis and then to ensure adequate and effective treatment. Not surprisingly, a study from the early 1990s showed that only 30% of depressed patients seen at a tertiary care center were given any antidepressant medication, and as many as 50% were treated incorrectly with anxiolytics rather than antidepressants.44 Furthermore, when evaluating the results of the Medical Outcomes Study in depressed patients given minor tranquilizers and antidepressants, Wells et al.45 noted that more patients used minor tranquilizers and that of those who were taking antidepressants, 39% were taking inappropriately low dosages.
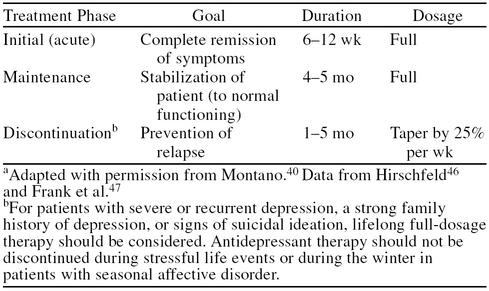
The benefits of treating depression aggressively cannot be overemphasized, particularly during the acute-phase trial (6 to 8 weeks) of antidepressant therapy. The primary goal of treatment is the complete remission of depressive symptoms, not merely a partial response to therapy (Table 4).40,46,47 After remission of clinical symptoms, maintenance therapy should follow, using full dosages for an additional 4 to 5 months to prevent relapse of depression. In some patients, a gradual discontinuation (by 25% per week) of the antidepressant may be a suitable course of action if they have been free of both mild and severe symptoms during the maintenance period. If treatment is discontinued prematurely, full recovery may be compromised by relapse in most patients (≥ 60%). This means that a successful course of antidepressant treatment for many patients is 7 to 9 months, when acute treatment (3 months) is followed by maintenance treatment (4 to 6 months). In addition, in other patients, particularly those with severe or recurrent depression, a strong family history of depression, or those who presented with suicidal ideation, lifelong treatment may be required.40
Table 4.
Guidelines for Treatment of an Initial Episode of Major Depressiona
Therapeutic options.
Until recently, drug therapy for depression was limited to the TCAs and the monoamine oxidase inhibitors (MAOIs). Unfortunately, both these classes of agents are associated with significant adverse effects and toxicity that limit their usefulness in primary care patients, and they have been, for the most part, relegated to second- and third-line options in this population.34,40 The TCAs are associated with anticholinergic side effects, such as dry mouth, blurred vision, constipation, and urinary retention, and antihistaminergic effects, such as sedation and weight gain.13,42 More deleterious, however, is the narrow therapeutic index of these agents, which makes even small amounts (e.g., a 1-week supply) potentially lethal.40 Using MAOIs to treat depression provides equally challenging dilemmas. Among the most distinctive of their adverse effects is the potential for hypertensive crisis caused by interactions with tyramine-containing foods and drinks34,44 and certain medications (e.g., sympathomimetics).
With the introduction of second-generation antidepressants (i.e., the SSRIs, serotonin-norepinephrine reuptake inhibitors [SNRIs], and others) and their improved safety profiles, the primary care clinician has an ever-wider array of treatments from which to choose. These newer drugs clearly are more suitable than the older antidepressants for use by generalists because of their wide therapeutic index, relative safety from overdosage, and more favorable adverse effect profiles. Once-daily dosing is an added feature of these newer agents—the SSRIs, venlafaxine XR, and mirtazapine—that makes them convenient to use and may help increase patients' compliance with the regimen. Compliance is a critical issue because most depressed patients in primary care fail to complete the acute and maintenance phases of antidepressant treatment.
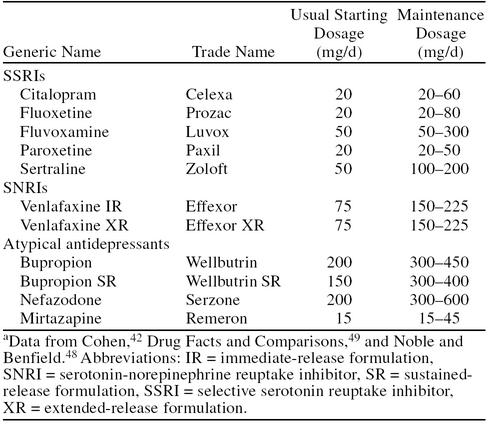
The usual starting and maintenance dosages for some of the newer antidepressants are shown in Table 5.42,48,49 Unlike the older TCAs, which have positive dose-response curves (albeit at the risk of greater adverse events), most of the newer antidepressants have relatively flat dose-response curves. For example, the SSRIs citalopram and paroxetine, when compared with clomipramine in some studies50,51 and titrated to higher dosages, did not demonstrate greater results.39,52 Venlafaxine XR, however, has been shown to produce greater antidepressant responses with increasing dosages. The labeling for venlafaxine XR recommends a maximum dosage of 225 mg/day. More severely depressed patients, however, can be treated with higher dosages if necessary.53,54 Blood pressure monitoring is recommended at these higher dosages. Most data indicate that the newer antidepressants all have efficacy rates in mild-to-moderate depression similar to those of the TCAs and are better tolerated.42 In some patients, venlafaxine XR and mirtazapine, because of their unique mechanisms of action, may be more effective than the TCAs and SSRIs.18,19,55
Table 5.
Newer Antidepressant Drugs: Starting and Maintenance Dosagesa
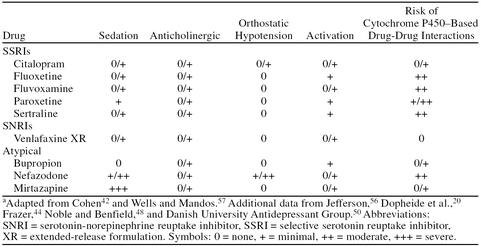
Achieving and maintaining full antidepressant dosage is facilitated by the favorable adverse event profile of the newer antidepressants. In general, these drugs lack the anticholinergic and antihistaminergic effects associated with the TCAs, although both mirtazapine and nefazodone are associated with higher rates of sedation than are venlafaxine XR, bupropion, and the SSRIs (Table 6).20,42,44,48,50,56,57
Table 6.
Side Effect and Drug-Interaction Profiles of Newer Antidepressantsa
Some of the adverse effects associated with the SSRIs include nausea, headache, sleep disturbance, and sexual dysfunction.44 Nausea is also the most notable adverse effect associated with venlafaxine XR, but it occurs more frequently at higher dosages and generally resolves within 2 weeks of continued treatment to levels seen with placebo.42 Bupropion, which is devoid of serotonergic effects, has a low rate of sexual side effects, although agitation is a frequent reason for discontinuation of treatment.10 Nefazodone also appears to lack the sexual side effects of the other antidepressants. These latter 2 agents are often used frequently after sexual side effects occur with other antidepressants because of their lower incidence of these side effects.
The potential for drug-drug interactions is an additional aspect of therapy that should be considered when individualizing therapy for the primary care patient. Interactions involving the cytochrome P450 system of metabolism are becoming more widely known with the SSRIs (and nefazodone), all of which inhibit this system to some degree (see Table 6).20,42,44,48,50,56,57 Such interactions impair the metabolism of many drugs that may be coadministered to patients taking antidepressants and increase the likelihood of adverse effects. This is a potential advantage for venlafaxine XR, which appears to have a very favorable drug-drug–interaction profile, because it does not interact with the cytochrome P450 system to any clinically significant degree.32
Therapeutic strategies for nonresponders.
In general, clinicians should not be overzealous in categorizing patients who do not respond to an initial course of treatment as being refractory to treatment. In most cases, such patients have been treated inadequately with less-than-therapeutic dosages or for too short a period. However, certain patients may not respond adequately to a particular antidepressant, despite full-dosage therapy for 6 to 8 weeks. In these cases, a second course of monotherapy with a different antidepressant is recommended. Patients do frequently respond to a second agent in the same class, but many clinicians prefer to switch to a different pharmacologic class.42 Patients who fail 2 trials of antidepressant monotherapy may be candidates for combination therapy or for augmentation therapy with an antidepressant from a different class, a neuroleptic agent, or lithium. An adequate augmentation-therapy trial should be a minimum of 3 weeks.
COMORBID ANXIETY AND DEPRESSION
Although the interrelationship between anxiety states and depression has long been recognized in psychiatry, extensive exploration of the clinical significance of this relationship has begun only recently. Data are accumulating to suggest that these 2 conditions occur together even more frequently than they do as distinct clinical entities.58 Some investigators have concluded that these disorders may reflect a continuum of the clinical expression of a single disease. Because patients with chronic anxiety often develop depressive disorders over time, it also has been hypothesized that anxiety disorders actually may be, in some cases, a prodrome for depression.59 Contrasting data suggest that GAD, in particular, is an anxiety disorder that should be considered an independent entity, rather than as a prodrome, residual, or severity marker of depression.60 Nevertheless, the coexistence of anxiety and depression in the same patient negatively affects his or her clinical outcome substantially. Such patients typically have more severe manifestations of these illnesses and respond less robustly to treatment than do patients with either disorder alone.61 Fortunately, with the wide array of new antidepressants available today that can provide robust efficacy, these patients now have access to new agents that can treat both mood and anxiety disorders effectively.
In terms of prevalence, it has been more difficult to establish the exact frequency with which these conditions coexist in the general population because of overlaps in diagnostic criteria. As many as 10% of patients in the primary care setting may have comorbid anxiety and depression.62 However, as many as 60% of patients with MDD have moderate anxiety, and 20% to 25% have more severe anxiety.4,60,61
DSM-IV Classification of Mixed Anxiety-Depressive Disorder
Many patients seen in the primary care setting with manifestations of both anxiety and depression fail to meet the full criteria for a specific anxiety or depressive disorder. However, it is now recognized that these patients may have a distinct disorder termed “mixed anxiety-depressive disorder” and that the identification of such patients would be enhanced by the development of specific diagnostic criteria. Thus, the APA has included this provisional category in DSM-IV. Mixed anxiety-depressive disorder is characterized by a persistent or recurrent dysphoric mood of ≥ 4 weeks' duration that is accompanied by symptoms of anxiety and depression. Symptoms could include difficulties with concentration or memory, sleep disturbances, fatigue or low energy, irritability, or worry, but these fail to meet criteria for a full anxiety or depressive disorder.63
Patients with elements of both anxiety and depression but with more specific distress than that exhibited by patients with mixed anxiety-depressive disorder usually can be distinguished on the basis of the predominant feature of their syndrome. For example, depressed patients with comorbid anxiety generally can be identified by the significant absence of a positive affect, with emphasis on anhedonia and hopelessness. In contrast, patients with predominant anxiety and subsymptoms of depression more often have physiologic symptoms, such as motor tension and autonomic hyperactivity.63
Treatment Considerations for Comorbid Anxiety and Depression
Resolving anxiety quickly should be the first goal of treatment in patients with anxiety and depression.61 Once this objective is accomplished, patients are much more likely to remain compliant with their antidepressant regimen and to continue their therapy for the full duration necessary to achieve complete remission of their depression.
One choice for pharmacotherapy in patients with comorbid anxiety and depression is the combination of a BZD or buspirone with an antidepressant. Alternatively, some patients may respond to antidepressant therapy alone, provided a drug that is effective in treating both disorders is used. Because the traditional anxiolytics will have little if any effect on depression, an adequate course of therapy with an antidepressant is imperative.62 On the other hand, in patients treated with an antidepressant plus an anxiolytic, the clinician may consider tapering the anxiolytic slowly, then discontinuing it when the symptoms of acute anxiety have resolved.61 Because of their well-documented withdrawal effects, the BZDs should be tapered gradually over the course of at least several weeks, depending on the dosage and duration of BZD therapy.
The choice of antidepressant therapy may be guided by data that have elucidated the efficacy of various agents in specific anxiety disorders. The older antidepressants—TCAs and MAOIs—are highly effective in patients with comorbid anxiety and depression. However, as is the case in the treatment of depression alone, adverse effects of treatment and the risk of overdosage are significant roadblocks to the use of these agents.61
A growing body of data supports the use of the newer agents—the SSRIs, the SNRI venlafaxine (both immediate-release [IR] and XR formulations), mirtazapine, and nefazodone—in patients with anxiety or coexisting anxiety and depression.18,27,30,31,62,64–67 The SSRIs have been studied more extensively in panic disorder, social anxiety disorder, and OCD,13,15,17,64,68,69 whereas venlafaxine XR has been studied extensively in GAD.21–23,25 Hackett et al.26 reported that venlafaxine XR was a safe, effective, and rapidly acting anxiolytic in a double-blind, controlled, parallel-group, multicenter study of 544 patients with GAD without MDD (DSM-IV criteria). In this study, significant advantages over placebo, as demonstrated by decreases from baseline in scores of the efficacy parameters, were seen as early as week 1 with a daily dosage of 150 mg of venlafaxine XR and starting at week 2 with a daily dosage of 75 mg. Except for 1 week, this significant advantage was maintained throughout the study.
As in the treatment of anxiety or depression alone, issues related to the speed of onset of activity, side effect profile, and drug-drug–interaction profile may be the basis for recommendation of 1 agent over another for an individual patient. For both newer and older antidepressants, most data point to a lag in the onset of activity of between 2 and 4 weeks.70 As a result, an initial course of therapy should last for at least 6 weeks and possibly even 8 weeks.40 Venlafaxine XR, however, has shown evidence in some trials of a more rapid onset of action, possibly because it exhibits dual inhibition of the reuptake of serotonin and norepinephrine at clinical dosages.42 At mean total dosages in the range of 150 to 375 mg/day, venlafaxine IR was significantly better than placebo in reducing Hamilton Rating Scale for Depression total scores as early as week 1 in severely depressed inpatients; a reduction in comorbid symptoms of anxiety was also evident.71 Similarly, in nondepressed patients with GAD, venlafaxine XR was superior to placebo as early as week 1, with significantly lower scores from baseline on the Hamilton Rating Scale for Anxiety total score and the Hospital Anxiety and Depression scale.21–26 This seemingly earlier onset of activity with venlafaxine XR makes it a particularly attractive choice in patients with GAD and in those with comorbid anxiety and depression.
CONCLUSIONS
Among the issues that may help distinguish between the various available anxiolytics and antidepressants in a clinical context are (1) speed of onset of activity, (2) ease and flexibility of administration, including the ability to improve the response with higher dosages, (3) safety in a variety of patient types, such as the elderly and those with concomitant diseases or conditions, (4) compliance and tolerance, (5) cost of a course of treatment, and (6) spectrum of efficacy. The newer agents, such as the SSRIs, venlafaxine XR, nefazodone, and mirtazapine, stand apart from the older antidepressants in terms of their improved safety profiles and relative safety in overdosage. Many of these agents have shown significant efficacy in the treatment of comorbid anxiety and depression and are gaining a more prominent role both in combination with BZDs and buspirone and as monotherapy for anxiety disorders.
The goal of therapy with any antidepressant agent is full recovery, and therefore aggressive treatment is warranted. This now appears to be more achievable with the availability of the newer pharmacologic agents.
Drug names: alprazolam (Xanax and others), bupropion (Wellbutrin), buspirone (BuSpar), citalopram (Celexa), clomipramine (Anafranil and others), clonazepam (Klonopin and others), diazepam (Valium and others), fluoxetine (Prozac), fluvoxamine (Luvox), mirtazapine (Remeron), nefazodone (Serzone), paroxetine (Paxil), sertraline (Zoloft), triazolam (Halcion), venlafaxine (Effexor).
Footnotes
Supported in part by an unrestricted educational grant from Wyeth-Ayerst Laboratories.
REFERENCES
- Rice DP, Miller LS. Health economics and cost implications of anxiety and other mental disorders in the United States. Br J Psychiatry. 1998;173(suppl 34):4–9. [PubMed] [Google Scholar]
- Greenberg PE, Sisitsky T, Kessler RC, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- Souêtre E, Lozet H, Cimarosti I, et al. Cost of anxiety disorders: impact of comorbidity. J Psychosom Res. 1994;38(suppl 1):151–160. doi: 10.1016/0022-3999(94)90145-7. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Nelson CB, McGonagle KA, et al. Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. Br J Psychiatry. 1996;168(suppl 30):17–30. [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association. 1994 [Google Scholar]
- American Psychiatric Association. Anxiety algorithm. In: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Primary Care Version. Washington, DC: American Psychiatric Association. 1995 47–63. [Google Scholar]
- Shader RI, Greenblatt DJ. Use of benzodiazepines in anxiety disorders. N Engl J Med. 1993;328:1398–1405. doi: 10.1056/NEJM199305133281907. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Folks DG, McNeilly DP. Effective use of anxiolytics in older adults. Clin Geriatr Med. 1998;14:47–65. [PubMed] [Google Scholar]
- Goldberg RJ. Diagnostic dilemmas presented by patients with anxiety and depression. Am J Med. 1995;98:278–284. doi: 10.1016/S0002-9343(99)80375-1. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Burrows GD, DuPont RLJ, et al. Alprazolam in panic disorder and agoraphobia: results from a multicenter trial, 1: efficacy in short-term treatment. Arch Gen Psychiatry. 1988;45:413–422. doi: 10.1001/archpsyc.1988.01800290027004. [DOI] [PubMed] [Google Scholar]
- Sussman N. The uses of buspirone in psychiatry. J Clin Psychiatry Monograph. 1994;12(1):3–19. [Google Scholar]
- Ballenger J. Treatment of panic disorder with serotonin reuptake inhibitors (SSRIs). In: den Boer JA, Montgomery SA, eds. SSRIs in Depression and Anxiety. Chichester, NY: John Wiley & Sons. 1998 115–134. [Google Scholar]
- Katzelnick DJ, Kobak KA, Greist JH, et al. Sertraline for social phobia: a double-blind, placebo-controlled crossover study. Am J Psychiatry. 1995;152:1368–1371. doi: 10.1176/ajp.152.9.1368. [DOI] [PubMed] [Google Scholar]
- Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280:708–713. doi: 10.1001/jama.280.8.708. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Wheadon DE, Steiner M, et al. Double-blind, fixed-dose, placebo-controlled study of paroxetine in the treatment of panic disorder. Am J Psychiatry. 1998;155:36–42. doi: 10.1176/ajp.155.1.36. [DOI] [PubMed] [Google Scholar]
- Ballenger JC. Current treatments of the anxiety disorders in adults. Biol Psychiatry. 1999;46:1579–1594. doi: 10.1016/s0006-3223(99)00220-6. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Barkin RL. A meta-analysis of eight randomized, double-blind, controlled clinical trials of mirtazapine for the treatment of patients with major depression and symptoms of anxiety. J Clin Psychiatry. 1998;59:123–127. [PubMed] [Google Scholar]
- Stimmel GL, Dopheide JA, Stahl SM. Mirtazapine: an antidepressant with noradrenergic and specific serotonergic effects. Pharmacotherapy. 1997;17:10–21. [PubMed] [Google Scholar]
- Dopheide JA, Stimmel GL, Yi DD. Focus on nefazodone: a serotonergic drug for major depression. Hosp Formulary. 1995;30:205–210. 212. [Google Scholar]
- Rickels K, Pollack MH, Sheehan DV, et al. Efficacy of extended-release venlafaxine in nondepressed outpatients with generalized anxiety disorder. Am J Psychiatry. 2000;157:968–974. doi: 10.1176/appi.ajp.157.6.968. [DOI] [PubMed] [Google Scholar]
- Davidson JRT, DuPont RL, Hedges D, et al. Efficacy, safety, and tolerability of venlafaxine extended release and buspirone in outpatients with generalized anxiety disorder. J Clin Psychiatry. 1999;60:528–535. doi: 10.4088/jcp.v60n0805. [DOI] [PubMed] [Google Scholar]
- Haskins JT, Rudolph R, Aguiar L, and et al. Venlafaxine XR is effective for the short- and long-term treatment of generalized anxiety disorder [poster]. Presented at the 11th World Congress of Psychiatry; Aug 6–11, 1999; Hamburg, Germany. [Google Scholar]
- Hackett D, Desmet A, Salinas EO. Dose-response efficacy of long-term treatment of venlafaxine extended-release in generalized anxiety disorder [abstract] Eur Neuropsychopharmacol. 1999;9(suppl 5):S315. [Google Scholar]
- Haskins JT, Rudolph R, and Aguiar L. et al. for the Venlafaxine XR 218 Study Group. Venlafaxine XR is an efficacious short- and long-term treatment for generalized anxiety disorder. Presented at the 11th annual congress of the European College of Neuropsychopharmacology; Oct 31–Nov 4, 1998; Paris, France. [Google Scholar]
- Hackett D, Desmet A, and Salinas EO. Dose-response efficacy of venlafaxine XR in GAD [poster]. Presented at the 11th World Congress of Psychiatry; Aug 6–11, 1999; Hamburg, Germany. [Google Scholar]
- Kelsey JE. Venlafaxine in social phobia. Psychopharmacol Bull. 1995;31:767–771. [PubMed] [Google Scholar]
- Geracioti TD Jr. Venlafaxine treatment of panic disorder: a case series. J Clin Psychiatry. 1995;56:408–410. [PubMed] [Google Scholar]
- Rauch SL, O'Sullivan RL, Jenike MA. Open treatment of obsessive-compulsive disorder with venlafaxine: a series of ten cases [letter] J Clin Psychopharmacol. 1996;16:81–84. doi: 10.1097/00004714-199602000-00017. [DOI] [PubMed] [Google Scholar]
- Khan A, Upton GV, Rudolph RL, et al. The use of venlafaxine in the treatment of major depression and major depression associated with anxiety: a dose-response study. J Clin Psychopharmacol. 1998;18:19–25. doi: 10.1097/00004714-199802000-00004. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Entsuah AR, McPherson MK. Efficacy of once-daily venlafaxine extended release (XR) for symptoms of anxiety in depressed outpatients. J Affect Disord. 1998;47:55–62. doi: 10.1016/s0165-0327(97)00109-2. [DOI] [PubMed] [Google Scholar]
- Ereshefsky L. Drug-drug interactions involving antidepressants: focus on venlafaxine. J Clin Psychopharmacol. 1996;16(3, suppl 2):37S–53S. doi: 10.1097/00004714-199606002-00009. [DOI] [PubMed] [Google Scholar]
- Council on Scientific Affairs, American Medical Association. The etiologic features of depression in adults. Arch Fam Med. 1993;2:76–84. doi: 10.1001/archfami.2.1.76. [DOI] [PubMed] [Google Scholar]
- De Wester JN. Recognizing and treating the patient with somatic manifestations of depression. J Fam Pract. 1996;43(suppl 6):S3–S15. [PubMed] [Google Scholar]
- Regier DA, Narrow WE, Rae DS, et al. The de facto US mental and addictive disorders service system: Epidemiologic Catchment Area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50:85–94. doi: 10.1001/archpsyc.1993.01820140007001. [DOI] [PubMed] [Google Scholar]
- Ormel J, VonKorff M, Ustun TB, et al. Common mental disorders and disability across cultures: results from the WHO Collaborative Study on Psychological Problems in General Health Care. JAMA. 1994;272:1741–1748. doi: 10.1001/jama.272.22.1741. [DOI] [PubMed] [Google Scholar]
- Blazer DG, Kessler RC, McGonagle KA, et al. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151:979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- Cross-National Collaborative Group. The changing rate of major depression: cross-national comparisons. JAMA. 1992;268:3098–3105. doi: 10.1001/jama.1992.03490210080039. [DOI] [PubMed] [Google Scholar]
- Dunner DL. Therapeutic considerations in treating depression in the elderly. J Clin Psychiatry. 1994;55(12, suppl):48–58. [PubMed] [Google Scholar]
- Montano CB. Recognition and treatment of depression in a primary care setting. J Clin Psychiatry. 1994;55(12, suppl):18–34. [PubMed] [Google Scholar]
- American Psychiatric Association. Depressed mood algorithm. In: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Primary Care Version. Washington, DC: American Psychiatric Association. 1995 35–45. [Google Scholar]
- Cohen LJ. Rational drug use in the treatment of depression. Pharmacotherapy. 1997;17:45–61. [PubMed] [Google Scholar]
- Simon G, Ormel J, VonKorff M, et al. Health care costs associated with depressive and anxiety disorders in primary care. Am J Psychiatry. 1995;152:352–357. doi: 10.1176/ajp.152.3.352. [DOI] [PubMed] [Google Scholar]
- Frazer A. Antidepressant drugs. Depression. 1994;2:1–19. [Google Scholar]
- Wells KB, Katon W, Rogers B, et al. Use of minor tranquilizers and antidepressant medications by depressed outpatients: results from the Medical Outcomes Study. Am J Psychiatry. 1994;151:694–700. doi: 10.1176/ajp.151.5.694. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RMA. Guidelines for the long-term treatment of depression. J Clin Psychiatry. 1994;55(12, suppl):61–69. [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Perel JM, et al. Comparison of full-dose versus half-dose pharmacotherapy in the maintenance treatment of recurrent depression. J Affect Disord. 1993;27:139–145. doi: 10.1016/0165-0327(93)90001-z. [DOI] [PubMed] [Google Scholar]
- Noble S, Benfield P. Citalopram: a review of its pharmacology, clinical efficacy and tolerability in the treatment of depression. CNS Drugs. 1997;8:410–431. [Google Scholar]
- Central nervous system, drugs. In: Drug Facts and Comparisons, 1999 ed. St. Louis, Mo: Facts and Comparisons. 1998 1603. [Google Scholar]
- Danish University Antidepressant Group. Citalopram: clinical effect profile in comparison with clomipramine: a controlled multicenter study. Psychopharmacology. 1986;90:131–138. doi: 10.1007/BF00172884. [DOI] [PubMed] [Google Scholar]
- Danish University Antidepressant Group. Paroxetine: a selective serotonin reuptake inhibitor showing better tolerance, but weaker antidepressant effect than clomipramine in a controlled multicenter study. J Affect Disord. 1990;18:289–299. doi: 10.1016/0165-0327(90)90081-i. [DOI] [PubMed] [Google Scholar]
- Kelsey JE. Dose-response relationship with venlafaxine. J Clin Psychopharmacol. 1996;16(suppl 2):21S–28S. doi: 10.1097/00004714-199606002-00005. [DOI] [PubMed] [Google Scholar]
- Feighner JP. The role of venlafaxine in rational antidepressant therapy. J Clin Psychiatry. 1994;55(9, suppl A):62–70. [PubMed] [Google Scholar]
- Guelfi JD, White C, Hackett D, et al. Effectiveness of venlafaxine in patients hospitalized for major depression and melancholia. J Clin Psychiatry. 1995;56:450–458. [PubMed] [Google Scholar]
- Thase ME. Antidepressant options: venlafaxine in perspective. J Clin Psychopharmacol. 1996;16(3, suppl 2):10S–20S. doi: 10.1097/00004714-199606002-00003. [DOI] [PubMed] [Google Scholar]
- Jefferson JW. Drug interactions: friend or foe? J Clin Psychiatry. 1998;59(suppl 4):37–47. [PubMed] [Google Scholar]
- Wells BG, Mandos LA. Depressive disorders. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. Stamford, Conn: Appleton & Lange. 1997 1395–1417. [Google Scholar]
- Brown C, Schulberg HC, Madonia MJ, et al. Treatment outcomes for primary care patients with major depression and lifetime anxiety disorders. Am J Psychiatry. 1996;153:1293–1300. doi: 10.1176/ajp.153.10.1293. [DOI] [PubMed] [Google Scholar]
- Schapira K, Roth M, Kerr TA, et al. The prognosis of affective disorders: the differentiation of anxiety states from depressive illnesses. Br J Psychiatry. 1972;121:175–181. doi: 10.1192/bjp.121.2.175. [DOI] [PubMed] [Google Scholar]
- Kessler RC, DuPont RL, Berglund P, et al. Impairment in pure and comorbid generalized anxiety disorder and major depression at 12 months in two national surveys. Am J Psychiatry. 1999;156:1915–1923. doi: 10.1176/ajp.156.12.1915. [DOI] [PubMed] [Google Scholar]
- Gorman JM. Comorbid depression and anxiety spectrum disorders. Depress Anxiety. 1996;4:160–168. doi: 10.1002/(SICI)1520-6394(1996)4:4<160::AID-DA2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kuzel RJ. Treating comorbid depression and anxiety. J Fam Pract. 1996;43(6, suppl):S45–S53. [PubMed] [Google Scholar]
- Keller MB, Hanks DL. Anxiety symptom relief in depression treatment outcomes. J Clin Psychiatry. 1995;56(suppl 6):22–29. [PubMed] [Google Scholar]
- Lepola UM, Wade AG, Leinonen EV, et al. A controlled prospective, 1-year trial of citalopram in the treatment of panic disorder. J Clin Psychiatry. 1998;59:528–534. doi: 10.4088/jcp.v59n1006. [DOI] [PubMed] [Google Scholar]
- Koponen H, Lepola U, Leinonen E, et al. Citalopram in the treatment of obsessive-compulsive disorder: an open pilot study. Acta Psychiatr Scand. 1997;96:343–346. doi: 10.1111/j.1600-0447.1997.tb09927.x. [DOI] [PubMed] [Google Scholar]
- Pollack MH, Worthington JJ III, Otto MW, et al. Venlafaxine for panic disorder: results from a double-blind, placebo-controlled study. Psychopharmacol Bull. 1996;32:667–670. [PubMed] [Google Scholar]
- Hedges DW, Reimherr FW, Strong RE, et al. An open trial of nefazodone in adult patients with generalized anxiety disorder. Psychopharmacol Bull. 1996;32:671–676. [PubMed] [Google Scholar]
- Greist JH, Jefferson JW, Kobak KA, et al. Efficacy and tolerability of serotonin transport inhibitors in obsessive-compulsive disorder: a meta-analysis. Arch Gen Psychiatry. 1995;52:53–60. doi: 10.1001/archpsyc.1995.03950130053006. [DOI] [PubMed] [Google Scholar]
- Jefferson JW. Antidepressants in panic disorder. J Clin Psychiatry. 1997;58(suppl 2):20–25. [PubMed] [Google Scholar]
- Quitkin FM, McGrath PJ, Stewart JW, et al. Chronological milestones to guide drug change: when should clinicians switch antidepressants? Arch Gen Psychiatry. 1996;53:785–792. doi: 10.1001/archpsyc.1996.01830090031005. [DOI] [PubMed] [Google Scholar]
- Ballenger JC. Clinical evaluation of venlafaxine. J Clin Psychopharmacol. 1996;16(suppl 2):29S–36S. doi: 10.1097/00004714-199606002-00007. [DOI] [PubMed] [Google Scholar]