Abstract
Objective: The possible positive effects of brochures and an audiotape containing information on efficacy and tolerance on side effects, dropout rate, and clinical outcome of treatment with venlafaxine were studied in 1048 depressed outpatients (as clinically judged by the general practitioner; 740 women and 308 men), aged 18 to 85 years.
Method: The study was of a single-blind, parallel-group design. All 4 groups were verbally informed: one group received only verbal information, a second group additionally received brochures, a third group additionally received information on audiotape, and a fourth group additionally received brochures and information on audiotape. There were 5 study visits, the first at baseline (week 0), followed by a visit at weeks 1, 2, 4, and 6. At each study visit, the Clinical Global Impressions scale, Zung Depression Scale, Quality of Life Scale, and State-Trait Anxiety Inventory were completed. The Patient's and Investigator's Subjective Ratings of Tolerance and Efficacy were completed at the final study visit.
Results: The brochures and audiotape reduced the dropout rate due to lack of efficacy (p = .01 and p = .04, respectively). In addition, the percentage of patients reporting side effects was lower in the group that received brochures than in the group that received only verbal information (p = .05). Additional information had no effect on efficacy measures.
Conclusion: Supplying patients with education in the form of brochures and/or audiotape containing information on efficacy and tolerance of the drug reduces the dropout rate due to lack of efficacy. For the reduction of side effects, brochures in particular seem suitable. Information on audiotape or written information seems to lengthen the period that patients wait for the possible beneficial action of the medication. To reduce the dropout rate, it may be recommended that patients receive, in addition to brochures, spoken information on audiotape or compact disc.
The present study investigated the effects of patient education in the form of brochures and an audiotape about efficacy and tolerance on side effects, dropout rate, and clinical outcome of treatment with venlafaxine in depressed outpatients. Providing information to patients has been proved effective in improving compliance, which in turn may enhance efficacy.1 In 1987, Haynes et al.2 described multiple intervention techniques to improve compliance with prescribed medications. He concluded that simple prescriptions and clear instructions work for all regimens. Other authors also conclude that adolescent compliance with short-term regimens can be improved by using written instructions.3 A link between compliance and recovery is made in a review article by Eraker et al.4 They state that knowledge about disease and treatment may have a positive effect on compliance. Moreover, information regarding procedures and usual experiences may yield beneficial effects on recovery.
Compliance with treatment is probably more complicated in the patient with a depressive disorder than in other therapeutic areas. A study5 of psychiatric patients showed that patients receiving antipsychotics took an average of 58% of the recommended amount of the medications, with a range of 24% to 90%. Patients receiving antidepressants took 65% of the recommended amount (range, 40%–90%), and the mean compliance rate for patients with physical disorders was 76% (range, 60%–92%).5 Factors that contribute to a low rate of compliance in depression are the slow onset of antidepressant action, the long-term nature of the disease, the characteristics of the disease itself, and the potential for relapse. Adherence to guidelines recommending 4 to 9 months of continuation therapy following remission of acute symptoms has been found to reduce the possibility of relapse or recurrence.6 Strategies to improve compliance in depressed patients include patient education and strategies for managing side effects.7 Antidepressants produce a variety of adverse effects in patients, and the occurrence of side effects is an important reason for noncompliance.8 Therefore, maintaining patient compliance requires effort on the part of the clinician. Educational approaches have become an important factor in ensuring compliance. This has been demonstrated in studies using interactions and educational meetings with the patient and family; written instructions have also improved compliance.9,10
Compliance will be improved if the clinician and patient agree on the nature and the etiology of the depressive illness and treatment possibilities. Patients should be given the opportunity to express their feelings about the illness and what it signifies. The physician should respond with feedback using the patient's concepts. The key factor in antidepressive treatment is to find a balance between the informational aspects of the illness and treatment and the affective-motivational aspects.11 Myers and Calvert10 randomly allocated 120 depressed outpatients, who were prescribed dothiepin, to 1 of 3 groups: group A was given verbal and written information about side effects; group B was given verbal and written information about beneficial effects; group C was told only that the drug was being given to treat their depression and received no written information. Results indicated that the compliance was higher in the “information” groups (groups A and B) than in the “no information” group (group C). Side effects were reported less frequently by group B, which only received information about beneficial effects of treatment. It was concluded that (1) verbal and written information about a prescribed drug improves compliance and (2) information about beneficial effects leads to fewer reports of side effects than either information about side effects or no information at all.
Summarizing the findings mentioned above, information about clinical features of the illness, embracing severity, duration, characteristics, and rationale for the medication, including side effects, is crucial in obtaining compliance. Enhancing compliance can increase the efficacy of the treatments being delivered.12 In the present study, the effects of additional information on efficacy and, secondly, compliance were evaluated. The main attention was directed to efficacy because compliance is more difficult to assess. Moreover, efficacy, not compliance, is the ultimate goal of pharmacologic treatment.
If informing patients about beneficial effects decreases the number of reported side effects, it may be assumed that patients who are aware of the benefits and side effects of an antidepressant will report the side effects that are actually related to the medication. At present, it is not known yet whether one type of information yields better results than the other. The present study, therefore, was designed to compare different types of information with respect to the efficacy of venlafaxine. It was hypothesized that the brochures and audiotape would improve the clinical outcome of treatment with venlafaxine and reduce the number of dropouts and reported side effects.
METHOD
Patients
A total of 1048 depressed (as clinically judged by the general practitioner to require antidepressant treatment) outpatients were enrolled by 238 general practitioners, all from different sites. These sites were situated in all parts of the Netherlands and were mainly urban and suburban. The patient group consisted of 740 women, aged 18 to 85 years (mean ± SD age = 46 ± 15 years) and 308 men, aged 19 to 89 years (mean ± SD age = 46 ± 13 years). Of this group, 479 patients (46%) had suffered from prior depression and 418 patients (40%) had used antidepressants in the past. The inclusion criteria were an age of 18 years or greater, outpatient status, the presence of depressive symptoms for at least 2 weeks, the need for antidepressant treatment as judged by the general practitioner, and provision of written consent. By design, the participating doctors were given no specific training in recognizing depression, thus making it possible to generalize the results to the usual daily situation of a majority of general practitioners who are not specifically trained in recognizing depression. Women of childbearing potential could be included if they were using a medically acceptable method of contraception throughout the study. Medically acceptable methods of contraception were sterilization, oral contraceptives, and intrauterine device.
The exclusion criteria were hypersensitivity to venlafaxine, clinically significant renal or hepatic disease or any other medical disease that might compromise the study, seizure disorder (with the exception of a single childhood febrile seizure), a recent history of myocardial infarction or unstable heart disease (within 6 months of baseline), known or suspected pregnancy (if considered appropriate and necessary by the general practitioner, a pregnancy test was performed), breastfeeding, use of a monoamine oxidase inhibitor (MAOI) within 14 days of baseline, or use of any investigational drug or antipsychotic drug within 30 days of baseline.
The study protocol was approved by the Foundation for Therapeutic Evaluation of Drugs (Stichting Therapeutische Evaluatie Geneesmiddelen [STEG], Duivendrecht, the Netherlands), an independent scientific and ethical committee for the evaluation of research proposals. All patients gave written informed consent.
Material
Written information/audiotape.
The written information and the audiotape were created in such a way that the patient would become clearly aware of the action of the antidepressant, the beneficial effects, and the side effects. The information regarding the action and side effects of venlafaxine was based on the standard patient leaflet. The brochure was developed in an earlier stage by the manufacturer (Wyeth-Lederle, Hoofddorp, the Netherlands) to distribute among patients using venlafaxine. The information on tape and the written information had a similar content.
Efficacy was assessed using a variety of rating scales, which were completed by both general practitioners and patients. The Clinical Global Impressions scale (CGI)13 was selected for the clinician-rated measure. This scale is the standard global scale of the Early Clinical Drug Evaluation Unit (ECDEU system). Its universal format makes it suitable for all types of psychiatric patients, including depressed patients. In addition, the administration is not time consuming. Based on the combined results of the CGI and the Investigator's Subjective Rating of Tolerance,the presence of a clinically good outcome can be established. Patients completed the State-Trait Anxiety Inventory (STAI),14,15 the Zung Depression Scale,16,17 the Quality of Life Scale (QLS)18 and a Subjective Rating of Efficacy and Tolerance. The depression and anxiety scales were selected to evaluate the efficacy of venlafaxine specifically with regard to depression and anxiety. The other scales make it possible to evaluate the efficacy of venlafaxine with respect to improvement in general well-being and to establish a clinically good outcome from the viewpoint of the patient.
The scales were selected because they are frequently used in clinical psychopharmacology studies. Moreover, by using such a diversity of scales it was possible to make a broad evaluation of the efficacy of venlafaxine.
Rating scales for general practitioners.
The CGI13 is completed by the general practitioner and consists of subscales assessing the severity of the illness (completed at weeks 0, 1, 2, 4, and 6) and global improvement (completed at weeks 1, 2, 4, and 6). The severity ranges from 1 (normal, not at all ill) to 7 (extremely ill). The global improvement ranges from 1 (very much improved) to 7 (very much worse). For the Investigator's Subjective Rating of Tolerance, the general practitioner has to assess the patient's tolerance for the study medication on a scale ranging from 1 (not any side effects) to 4 (significant side effects that outweighed the benefits of the drug).
Rating scales for patients.
For the Patient's Subjective Rating of Efficacy and Tolerance, the patient has to indicate the total improvement during the course of the study on a scale ranging from 1 (very much improved) to 7 (very much worse). In addition, the patient rates the tolerance for the study medication on a scale ranging from 1 (not any side effects) to 4 (significant side effects that outweighed the benefits of the drug). A clinically good outcome was defined as either a score of 1 or 2 for Improvement plus a score of either 1 or 2 for Tolerance.
The STAI14,15 consists of a “state anxiety” and a “trait anxiety” scale. State anxiety refers to situational anxiety. Trait anxiety is conceived as a relatively stable personality characteristic. The scale ranges from 1 (not at all) to 4 (very much).
The Zung Depression Scale16,17 is a self-rated scale that consists of 20 items and has response categories that range from 1 (seldom or never) to 4 (nearly always).
The QLS18 is a positive scale evaluating how good people feel about relationships, eating and sleeping, and social achievements. It is a simple 10-item scale with response categories “hardly satisfied” to “very much satisfied.”
Recording of side effects.
An open-ended questionnaire was used by the general practitioner to record the side effects that were reported by the patient at each of the study visits.
Medication.
Venlafaxine is a racemic compound, designated as (R/S)-1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl] cyclohexanol hydrochloride. The mechanism of venlafaxine's antidepressant action in humans is believed to be associated with the inhibition of the neuronal uptake of both serotonin and norepinephrine and, to a lesser degree, dopamine reuptake. It does not possess monoamine oxidase inhibitory activity and shows virtually no affinity for rat brain muscarinic cholinergic, histaminergic, or adrenergic receptors.19
In randomized, double-blind comparisons of venlafaxine compared with imipramine, fluoxetine, trazodone, and placebo in depressed patients, venlafaxine was more effective than placebo in relieving the symptoms of depression and as good as or better than the comparative drugs.20–23 Based on the results of 4- to 6-week placebo-controlled clinical trials, nausea, somnolence, dry mouth, and dizziness were the most frequent complaints.24
Dosage and administration.
The patients initially received venlafaxine, 75 mg/day (37.5 mg b.i.d.). Based on the patient's clinical response and the clinical judgment of the general practitioner, this dose was increased after 2 to 3 weeks of treatment to 150 mg/day (75 mg b.i.d.) if needed. The total study period was 6 weeks. A significant response to the drug was expected within this treatment period. Patients receiving 150 mg/day at the end of the study period (6 weeks on treatment), and in whom the general practitioner decided not to continue the treatment beyond the study period, had their dosage tapered over a 1-week period. All study medication was administered orally and with meals. Venlafaxine was supplied as tablets by Wyeth-Lederle, the Netherlands. The tablets contained 37.5 mg or 75 mg of venlafaxine hydrochloride.
Procedure
To qualify for the study, patients must have had symptoms of depression for at least 2 weeks and been judged by the general practitioner to require antidepressant treatment. The presence of all inclusion criteria and the absence of all exclusion criteria was verified by the general practitioner on the case report form. Each patient had a prestudy baseline evaluation that included a medical and psychiatric history and a complete physical examination. The medical and psychiatric history included the following information about each patient: age, sex, current and past illnesses, current and prior treatments, history and course of prior psychiatric illness, description of prior treatments for depression, duration of the current episode of depression, duration of the past episode(s) of depression, and the presence or absence of precipitating factors for the current episode.
The study was of a single-blind (the general practitioner was not aware of the particular experimental group to which a patient belonged), parallel design, comparing groups of patients. At week 0, patients were randomly assigned to 1 of 4 experimental groups. Group 1 received verbal information given by the general practitioner as usual (standard group). In addition to the usual information, group 2 received written information (brochure and the text of audiotape), group 3 received information on audiotape, and group 4 received written information (brochure and text of audiotape) as well as an audiotape. The assignment was accomplished by the general practitioner giving sealed envelopes, containing no information, written information, information on tape, or a combination tape/written information, to each patient at week 0. As these envelopes were provided only with a number, the general practitioner was not aware of the content of each envelope. To ensure the single-blind procedure, each envelope was supplied with a cassette box (with or without an audiotape).
There were 5 study visits, the first at baseline (week 0), followed by a visit at weeks 1, 2, 4, and 6. Since packs of medication contain 30 tablets (for 15 days), the interval between 2 visits could not be more than 2 weeks. The first 3 visits were separated by only 1 week to assess the side effects of the medication more frequently at the start of the study. At each study visit the CGI, Zung Depression Scale, QLS, and STAI were completed. The Patient's and Investigator's Subjective Ratings of Tolerance and Efficacy were completed at the final study visit. To evaluate safety, at each study visit the general practitioner recorded vital signs, sitting pulse, and blood pressure. In addition, weight was determined at week 0 and week 6, whereas study events were assessed at each visit. Study medication was dispensed at each visit except the final one. All unused study medication was returned by the patient.
Data Analysis
The effects of the 4 different types of information were analyzed by means of chi-square tests (on categorical data) and multivariate analyses of variance (MANOVA) and covariance (MANCOVA). MANCOVAs were used with group (type of information) as independent factor and visit as repeated-measures factor. The scores at week 0 served as covariate to eliminate the influence of possible baseline differences. Clinical outcome measures were analyzed only for patients who were available at each study visit. The more conservative last-observation-carried-forward (LOCF) analysis was also performed, in which last available clinical scores of dropouts are carried forward for analysis. Tests regarding questions related to the a priori hypotheses were 1-tailed. Additional analyses were 2-tailed. All calculations were performed with the Statistical Package for the Social Sciences, SPSS/PC+ (Chicago, Ill).
RESULTS
Since the patients did not return the unused study medication accurately, it was not possible to determine the compliance by counting the returned pills. Therefore, patients were defined as good compliers or poor compliers according to the judgment of the general practitioner. Disregarding the effects of information, the compliance was quite good. The general practitioners considered 94.6% of the patients to have good compliance and 5.4%, poor compliance. The total number of dropouts during the study period was 222 (21%). Side effects were the main reason, followed by insufficient efficacy (Table 1). At week 1 the dropout rate was 11%, which was mainly the consequence of side effects (78.5%). The dropout rate was reduced to about 3% at each of the remaining visits.
Table 1.
Discontinuation by Primary Reason
The Investigator's Subjective Rating of Tolerance, which the general practitioner completed at week 6, indicated that the study medication produced no or minor side effects in the majority of the patients. The Patient's Subjective Rating of Tolerance also indicated that the study medication produced no or minor side effects in the majority of the patients, although the absence of side effects was reported by a lower percentage of patients. The percentage of patients with side effects according to the Investigator's and Patient's Ratings of Tolerance are shown in Table 2. Side effects with an incidence of more than 5% were nausea (13.9%), headache (5.8%), dizziness (5.5%), and sweating (5.1%).
Table 2.
Patients With Side Effects According to Investigator's and Patient's Rating of Tolerance
At each visit, the CGI was completed by the general practitioner. The severity and improvement scores were significantly reduced across the 5 visits, both decrements indicating a reduction in severity of the illness (p < .001). Also, the data from the self-rated Zung Depression Scale indicated a significant decrease in depression scores across the 5 visits (p < .001). Finally, the scores on the QLS increased significantly across the 5 visits (p < .001), which indicates that the patients perceive their quality of life as improved.
Type of Information and Compliance
No significant difference was found between the 4 types of information with respect to compliance.
Type of Information and Dropouts
With respect to the number of dropouts, no significant differences were found between the 4 types of information. However, a separate analysis of dropouts due to “lack of efficacy” (as indicated by the general practitioner) yielded differences between the information groups. The percentage of dropouts caused by lack of efficacy was lower in the groups receiving additional information compared with the group receiving the standard verbal information alone. A significant difference was found between the group receiving the audiotape and the standard group (p = .04) and between the group receiving the brochure and the standard group (p = .01). The percentage for each reason for dropping out in each information group is shown in Table 3. The percentage of dropouts caused by lack of efficacy is largest in the standard group. A separate chi-square analysis of the 4 information groups within the group of dropouts regarding number of patients reporting side effects yielded no differences between groups (percentage of patients reporting side effects: standard group, 64.4%; brochure group, 53.8%; audiotape group, 56%; and brochure plus audiotape group, 62.5%). Thus, the number of dropouts as a result of side effects was not related to the type of information received.
Table 3.
Dropouts According to Type of Informationa
Type of Information and Side Effects
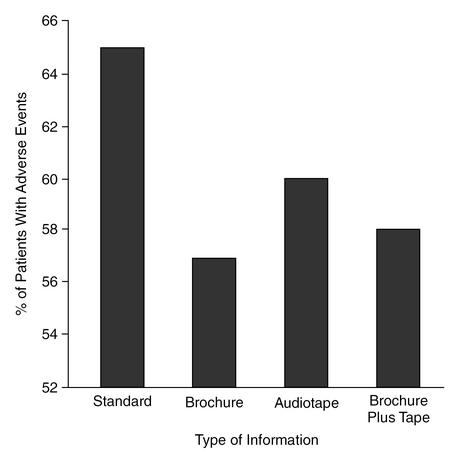
With respect to side effects that the patients reported to their general practitioner, a significant difference was found between the brochure group and the standard group (p = .05). Thus, in the group receiving additional written information on efficacy and tolerance, a significantly lower percentage of patients reported side effects than in the group that received standard information. Although the differences between the audiotape group and the brochure plus audiotape group were not significantly different from the standard group, the percentage of reported side effects in the audiotape and the brochure plus audiotape groups was lower than in the standard group (Figure 1).
Figure 1.
Percentage of Patients in the Total Study Group (N = 1048) With Adverse Events According to Type of Information
The MANCOVAs performed on CGI Severity of Illness and Global Improvement subscales did not result in any significant differences between the information groups.
DISCUSSION
One of the limitations of the present study is the rough assessment of compliance. As a consequence, differential effects of the additional information on compliance were difficult to establish. A second limitation is that the general practitioners did not assess depression with a specific depression scale. Therefore, the efficacy measurements by the general practitioners can be considered global. It might be possible that effects of type of information on efficacy would have been found if depression had been more specifically assessed by the general practitioner. However, to minimize the effort required from the general practitioners and to prevent a situation that is dissimilar to the usual practice situation, they were not instructed to complete a depression scale.
In the present study, a dropout rate of 21% was found; 2% withdrew for lack of efficacy and 15.5% for adverse events. The 11% dropout rate at week 1 was the highest dropout rate, mainly caused by side effects. As the dropout rate was reduced to 3% at each of the following visits, the side effects seem to be most bothersome in the first week of treatment. A meta-analysis25 of 67 published studies showed that the pooled discontinuation rate for lack of efficacy was 7.4% for selective serotonin reuptake inhibitors (SSRIs) and 6.8% for tricyclic antidepressants (TCAs). The pooled discontinuation rates for side effects were 13.9% for SSRIs and 18.7% for TCAs.25 Thus, the dropout rate for lack of efficacy in the present study is quite low, and for side effects, similar to that seen with other antidepressants. The judgment of clinically good outcome was reported by 60% of the patients, and by 69% if tolerance was not taken into account.
For the study group as a whole, side effects were absent or minor in the majority of the patients (over 80%). The percentage of patients reporting side effects was the lowest in the group receiving brochures, and the dropout rate due to lack of efficacy was the least in the groups receiving an audiotape or a brochure and the highest in the standard group. Thus, the additional information (written or on tape) seems to give the patient a realistic view of the action of the antidepressant and thus prevents the patient from dropping out as a consequence of wrong expectations. Also, the percentage of patients that reported side effects to the general practitioner was affected by the additional information. Although the percentages of patients reporting side effects were lower in the brochure, the audiotape, and the brochure plus audiotape groups than in the standard group, only the brochure group was significantly different from the standard group. Thus, brochures in particular seem to reduce the number of patients reporting side effects. This may indicate that written information is, in particular, suitable for providing information on side effects since it can easily be read several times.
In summary, the present data indicate that written information in particular seems to be useful in reducing the number of patients reporting side effects, which may be explained by an increased knowledge of the effects that may be related to the study medication. Furthermore, supplying patients with brochures and/or audiotape containing information on efficacy and tolerance of the drug reduces the dropout rate, in particular the dropout rate caused by lack of efficacy. This finding indicates that verbal or written information lengthens the period that patients wait for the possible beneficial action of the medication. To reduce the dropout rate as much as possible, it may therefore be recommended that patients receive, in addition to brochures, spoken information on audiotape or compact disc.
Because the present study shows that additional information has a beneficial impact on the dropout rate and the reported side effects, it can be concluded that the information the general practitioner usually gives to the patient may be improved. Although brochures and audiotapes give important additional information, the instructions from the general practitioners themselves remain the most important way to educate the patient. Therefore, it can be recommended that physicians and their staff increase their effort to inform the patient clearly on all aspects of the illness and the medication. Also, questions from patients need to be taken seriously and addressed appropriately. Physicians may be recommended to (1) explain why a particular medication is prescribed, (2) pay attention to the possible side effects, (3) try to reduce the resistance of the patient against medication, and (4) emphasize the importance of complying with the regimen. In addition to such an attitude, brochures and audiotapes may help general practitioners with their educational efforts.
Drug names: fluoxetine (Prozac), trazodone (Desyrel and others), venlafaxine (Effexor).
Footnotes
Supported by a grant from Wyeth-Lederle, Hoofddorp, the Netherlands.
REFERENCES
- Posavac EJ, Sinacore JM, Brotherton SE, et al. Increasing compliance with medical treatment regimens: a meta-analysis of program evaluation. Eval Health Prof. 1985;8:7–22. [Google Scholar]
- Haynes RB, Wang E, da Mota GM, et al. A critical review of interventions to improve compliance with prescribed medications. Patient Educ Couns. 1987;10:155–166. [Google Scholar]
- Jay S, Litt IF, Durant RH. Compliance with therapeutic regimens. J Adolesc Health Care. 1984;5:124–136. doi: 10.1016/s0197-0070(84)80012-1. [DOI] [PubMed] [Google Scholar]
- Eraker SA, Kirscht JP, Becker MH. Understanding and improving patient compliance. Ann Intern Med. 1984;100:258–268. doi: 10.7326/0003-4819-100-2-258. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv. 1998;49:196–201. doi: 10.1176/ps.49.2.196. [DOI] [PubMed] [Google Scholar]
- Melfi CA, Chawla AJ, Croghan TW, et al. The effects of adherence to antidepressant treatment guidelines on relapse and recurrence of depression. Arch Gen Psychiatry. 1998;55:1128–1132. doi: 10.1001/archpsyc.55.12.1128. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ. Introduction: compliance strategies to optimize antidepressant treatment outcomes. J Clin Psychiatry. 1995;56(suppl 1):3. [Google Scholar]
- Maddox JC, Levi M, Thompson C. The compliance with antidepressants in general practice. J Clin Psychopharmacol. 1994;8:48–53. doi: 10.1177/026988119400800108. [DOI] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Perel JM. Three outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry. 1990;47:1093–1099. doi: 10.1001/archpsyc.1990.01810240013002. [DOI] [PubMed] [Google Scholar]
- Myers ED, Calvert EJ. Information, compliance and side-effects: a study of patients on antidepressant medication. Br J Clin Pharmacol. 1984;17:21–25. doi: 10.1111/j.1365-2125.1984.tb04993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyttenaere K. Noncompliance with antidepressants: who's to blame? [review] Int Clin Psychopharmacol. 1998;13(suppl 2):s19–s25. [PubMed] [Google Scholar]
- Fawcett J. Compliance: definitions and key issues. J Clin Psychiatry. 1995;56(suppl 1):4–10. [PubMed] [Google Scholar]
- van Riezen H, Segal M. Comparative Evaluation of Rating Scales for Clinical Psychopharmacology. New York, NY: Elsevier. 1998 [Google Scholar]
- van der Ploeg HM, Defares PB, and Spielberger CD. Handleiding bij de Zelf-Beoordelings Vragenlijst. In: Een nederlandstalige Bewerking van de Spielberger State-Trait Anxiety Inventory. Lisse, the Netherlands: Swets & Zeitlinger. 1980 [Google Scholar]
- Spielberger CD. Test Manual for the State-Trait Anxiety Inventory—STAI form Y. Palo Alto, Calif: Consulting Psychologists Press. 1980 [Google Scholar]
- Dijkstra P. De zelfbeoordelings schaal van Zung. In: van Praag HM, Rooymans HGM, eds. Stemming en Ontstemming. Amsterdam, the Netherlands: De Erven Boon BV. 1974 192–209. [Google Scholar]
- Zung WWK. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- Blau TH. Quality of life, social indicators, and criteria of change. Profess Psychol. 1977;8:464–473. [Google Scholar]
- Holliday SM, Benfield P. Venlafaxine: a review of its pharmacology and therapeutic potential in depression. Drugs. 1995;49:281–294. doi: 10.2165/00003495-199549020-00010. [DOI] [PubMed] [Google Scholar]
- Clerc GE, Ruimy P, Verdeau-Paillès J. A double-blind comparison of venlafaxine and fluoxetine in patients hospitalized for major depression and melancholia. Int Clin Psychopharmacol. 1994;9:139–143. doi: 10.1097/00004850-199409000-00001. [DOI] [PubMed] [Google Scholar]
- Mendlewicz J. Pharmacologic profile and efficacy of venlafaxine. Int Clin Psychopharmacol. 1995;10(suppl 2):5–13. doi: 10.1097/00004850-199503002-00003. [DOI] [PubMed] [Google Scholar]
- Schweizer E, Feighner J, Mandos LA, et al. Comparison of venlafaxine and imipramine in the acute treatment of major depression in outpatients. J Clin Psychiatry. 1994;55:104–108. [PubMed] [Google Scholar]
- Shrivastava RK, Cohn C, Crowder J, et al. Long-term safety and clinical acceptability of venlafaxine and imipramine in outpatients with major depression. J Clin Psychopharmacol. 1994;14:322–329. [PubMed] [Google Scholar]
- Schweizer E, Weise C, Clary C, et al. Placebo-controlled trial of venlafaxine for the treatment of major depression. J Clin Psychopharmacol. 1991;11:233–236. [PubMed] [Google Scholar]
- Montgomery SA, Kasper S. Comparison of compliance between serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. Int Clin Psychopharmacol. 1995;9(suppl 4):33–40. doi: 10.1097/00004850-199501004-00005. [DOI] [PubMed] [Google Scholar]