Abstract
Background: Alzheimer's disease (AD) is the most common cause of dementia in later life. It is manifested by gradual and progressive decline in cognitive function and ability to perform activities of daily living (ADL) and the development of behavioral disturbances. Progressive reduction in functional ability reduces independence and quality of life and adversely affects caregivers and society. Therefore, benefit from any AD therapy may be obtained not only from improved function but also from stabilization or reduced worsening of function.
Method: This retrospective study of pooled data from 3 randomized, placebo-controlled trials (N = 2126) compared the incidence of different levels of worsening between 2 rivastigmine treatment groups and a placebo group at week 26 for cognition, using the Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-Cog); global functioning, using the Clinicians' Interview-Based Impression of Change-Plus (CIBIC-Plus); and ADL, using the Progressive Deterioration Scale (PDS). Categories of worsening analyzed for each scale were as follows: ADAS-Cog: any decline, ≥ 4-point decline, ≥ 7-point decline; CIBIC-Plus: stabilized/worsened (rating = 4, 5, 6, or 7), any worsening (rating = 5, 6, or 7); PDS: any worsening, ≥ 10% worsening.
Results: Patients treated with rivastigmine, 6–12 mg/day, showed significantly less decline in cognition, global functioning, and ADL for all categories of worsening examined compared with patients who received placebo. The reduction in decline compared with placebo was greater in the group receiving 6–12 mg/day of rivastigmine compared with the treatment group receiving 1–4 mg/day of rivastigmine.
Conclusion: Rivastigmine reduces the amount of worsening observed in cognition, global functioning, and ADL in a 6-month trial period.
Alzheimer's disease (AD) is a relentless, progressive neurodegenerative disease affecting at least 15 million people worldwide1 and is the most common cause of dementia in later life.2 AD is manifested by progressive deterioration of memory, executive function, language, praxis, and global cognitive function. Survival from time of diagnosis to death ranges from 8 to 14 years. Most patients spend their last 2 to 5 years in a nursing home or receiving 24-hour home care.3 Disruptive agitation and other severe behavioral disturbances are common in the later stages of AD.4 The progressive nature of AD results in tremendous emotional costs for the patient, the family, and the caregiver.5
The Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-Cog) is the tool most often used in clinical drug trials to measure cognitive function objectively.6 Data collected from untreated AD patients with moderate disease have suggested that the expected annual deterioration in cognition is approximately 8 points (Figure 1).7 It also appears that ti amount of decline observed is influenced by the disease severity of the patient, with more severe patients generally declining at a faster rate than patients with mild AD.
Figure 1.
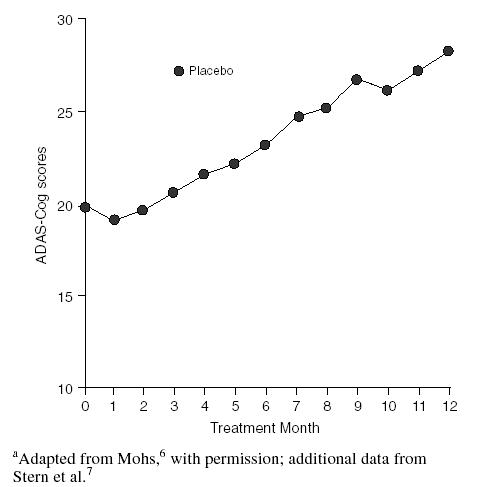
Hypothetical Data Showing Annual Deterioration of Cognition in Alzheimer's Disease Measured by the Cognitive Portion of the Alzheimer's Disease Assessment Scale (ADAS-Cog)a
Currently, cholinesterase (ChE) inhibitors are the only proven pharmacologic therapy for the symptomatic treatment of AD.1 Benefits from these medications have been judged primarily through their ability to improve cognition, global functioning, and activities of daily living (ADL) after treatment for up to 6 months. Predefined “clinically relevant” levels of improvement on various scales used in clinical trials are often the yardsticks used to establish the meaningfulness of how effective the medication is. For example, for cognition, a ≥ 4-point or ≥ 7-point improvement from baseline scores on the ADAS-Cog is thought to be required if the treatment is to be considered beneficial. However, since untreated patients can be expected to decline by 4 to 5 points every 6 months, maintaining function and cognition or simply reducing the amount of symptomatic worsening is beneficial and should be considered as such.
Rivastigmine is a centrally selective ChE inhibitor that demonstrates brain-region selectivity for the hippocampus and cortex8 due to its preferential inhibition of the G1 isoenzyme of acetylcholinesterase, the predominant form found in the hippocampus and cortex of AD patients. Thus, rivastigmine acts on the areas of the brain that are most adversely affected by AD. The results of 2 double-blind, 6-month, placebo-controlled studies together with a pooled analysis involving 3 studies with rivastigmine in patients with mild to moderately severe AD have previously been reported.9–11 In both studies and the pooled analysis, patients treated with high-dose rivastigmine (6–12 mg/day) demonstrated clinical improvement on all outcome measures, including cognition, global assessment of change, ADL, and disease severity. Treatment with rivastigmine has not been associated with significant changes on electrocardiogram, cardiovascular vital signs, or laboratory parameters. As with other ChE inhibitors, the adverse events reported in the rivastigmine clinical trials were primarily gastrointestinal in nature (e.g., nausea, vomiting, anorexia) and were mild to moderate in intensity and transient.9,10
This article describes the effects of rivastigmine on reducing the amount of worsening observed on cognition, global function, and ADL over the 6-month trial period using a pooled analysis.
METHOD
The results described are from a pooled analysis of 3 double-blind, placebo-controlled, 26-week studies, B352, B303, and B351, which have been described previously.9–11 A total of 2126 patients were randomized in the 3 studies. Only selected aspects of the study methods are described below. This report describes the results of an analysis conducted evaluating the level of worsening observed in each treatment group on the ADAS-Cog,12 the Clinicians' Interview-Based Impression of Change-Plus (CIBIC-Plus),13 and the Progressive Deterioration Scale (PDS).14
Patient Population
Patients were at least 50 years of age (mean = 73.5 years) and of nonchildbearing potential, fulfilled American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)15 criteria for Alzheimer's type dementia, and had probable AD according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA),16 with mild to moderately severe impairment based on a Mini-Mental State Examination (MMSE) score between 10 and 26 (both inclusive).17 Patients with coexistent diseases were enrolled in the studies unless their medical condition was severe and/or unstable.9–11
The 3 groups (rivastigmine, 6–12 mg/day and 1–4 mg/day, and placebo) were comparable with respect to the mean age (73.2, 73.6, and 73.8 years, respectively) and the proportion of patients who were older than 75 years (43%, 46%, and 45%, respectively). In addition, the percentage of women in each group was also similar (61%, 56%, and 58%, respectively).
Study Design
The studies (B352, B303, and B351) were 26 weeks in duration and implemented a double-blind, placebo-controlled, parallel-group design. All of the studies had a titration phase and a maintenance phase. Details of each trial with respect to rivastigmine dose in the titration phase and maintenance phase are provided in Table 1. Studies B351 and B352 were conducted in U.S. centers. Study B303 was conducted in both U.S. and European centers. Study B351 utilized a fixed-dose forced titration design in which no dose adjustments were permitted. Studies B352 and B303 evaluated flexible dosing within 2 nonoverlapping dose ranges of rivastigmine versus placebo: 1–4 mg/day (low-dose group) and 6–12 mg/day (high-dose group). Doses (2 capsules twice daily with food) were titrated weekly during the first 12 weeks within 1 of 2 dose ranges; the low-dose group received 1–4 mg/day and the high-dose group received 6–12 mg/day. Patients were pushed to their maximum tolerated dose within their dose range. No dose reductions were permitted during the titration phase. However, doses could be held at a level for a maximum of 2 weeks, with only 3 such opportunities permitted at different dose levels during titration. Flexibility within the assigned dose range was permitted during the maintenance phase. Efficacy evaluations were performed at baseline and weeks 12, 18, and 26 or early termination and included the ADAS-Cog, CIBIC-Plus, and PDS.
Table 1.
Design of the Phase 3 Placebo-Controlled Studies

Analyses were performed to compare the incidence of different levels of worsening between the 6–12 mg/day of rivastigmine group and placebo group and the 1–4 mg/day of rivastigmine group and placebo group at week 26 for the ADAS-Cog, CIBIC-Plus, and the PDS. The following describes the scales utilized in the phase 3 controlled studies with rivastigmine and the categories of worsening analyzed.
ADAS-Cog
The ADAS-Cog is the cognitive subscale of the ADAS. It is an 11-item cognitive subscale that objectively measures language, memory, orientation, and praxis.12 In this analysis, worsening categories included the following: any decline, a ≥ 4-point decline, and a ≥ 7-point decline.
CIBIC-Plus
The CIBIC-Plus measures overall global functioning by taking into account cognition, behavior, and ADL. It is a 7-point rating scale (1 = markedly improved, 2 = moderately improved, 3 = minimally improved, 4 = no change, 5 = minimally worse, 6 = moderately worse, 7 = markedly worse) that is completed following an interview of both the patient and the caregiver by an independent clinician.13 Two categories of worsening were included in this analysis: stabilized or worsened (rating = 4, 5, 6, or 7) and any worsening (rating = 5, 6, or 7).
PDS
The PDS is a 29-item bipolar analog scale that assesses the ADL or quality of life of the patient and is completed by the caregiver. The PDS assesses items such as orientation, memory, time, finances, hobbies, self-care interactions, and task performance. A clinically significant improvement is defined as a 10% or greater improvement from baseline.14 In this analysis, the categories of PDS worsening were defined as any worsening and ≥ 10% worsening.
Statistical Methods
Efficacy analyses were performed on data sets for intent-to-treat (all randomized patients), last-observation-carried-forward (LOCF; randomized patients with at least one evaluation while on study drug), and observed cases (randomized patients with at least one evaluation while on study treatment at designated assessment times). Nonefficacy analyses (e.g., patient demographic characteristics and patient disposition) were performed on data sets of patients who were randomized and had taken at least one dose of the study drug. For all comparisons, the Fisher exact test was performed. All comparisons with placebo were 2-tailed, with p values < .05 being considered statistically significant. Primary analyses used for worsening included the Fisher exact test. The statistical methods have been described previously.9–11
RESULTS
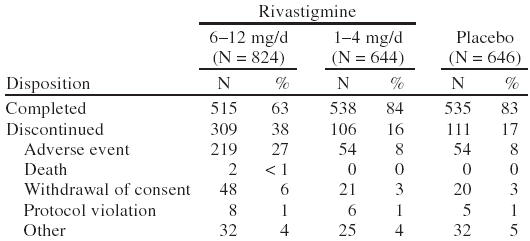
A total of 2126 patients were randomly assigned in the 3 clinical studies: 828 to the 6–12-mg/day group, 651 to the 1–4-mg/day group, and 647 to placebo. Of the 2126 randomized patients, 2114 patients received at least one dose of the study drug. Approximately 88% of patients reported at least one active medical condition at baseline (Table 2). The most common conditions were musculoskeletal (37%), cardiovascular (32%), gastrointestinal (22%), central and peripheral nervous system (21%), psychiatric (20%), and metabolic/nutritional disorders (19%). By the end of the study, the mean ± SD dose of rivastigmine was 7.0 ± 2.4 mg/day in the 6–12 mg/day group and 2.8 ± 0.7 mg/day in the 1–4 mg/day group. A summary of patient disposition is provided in Table 3.
Table 2.
Summary of Frequently Occurring Concurrent Medical Conditions
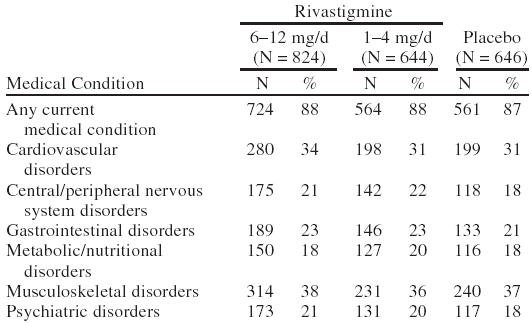
Table 3.
Patient Disposition
Efficacy
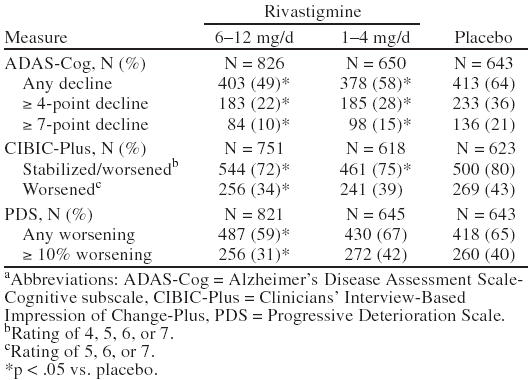
The intent-to-treat data set results at week 26 for the ADAS-Cog, CIBIC-Plus, and PDS as presented in Table 4 were significant for all definitions on each measure for the 6–12 mg/day of rivastigmine group compared with placebo. The observed cases data set results (not presented here) were also similar to the intent-to-treat results. Overall, a greater percentage of patients receiving placebo worsened on the ADAS-Cog, CIBIC-Plus, and PDS compared with the high-dose rivastigmine (6–12 mg/day) and low-dose rivastigmine (1–4 mg/day) groups. The following describes the specific results observed.
Table 4.
Patients With Decline on the ADAS-Cog, CIBIC-Plus, and PDS at Week 26 (intent-to-treat) for the Pooled Analysisa
ADAS-Cog
Statistically significant differences were found for both groups taking 6–12 mg/day of rivastigmine compared with the placebo group in all 3 categories of decline (any decline, ≥ 4-point decline, and ≥ 7-point decline). It should be noted that only 49% of patients treated with 6–12 mg/day of rivastigmine showed any decline compared with 64% of those treated with placebo. In addition, 22% of patients treated with 6–12 mg/day of rivastigmine showed ≥ 4-point decline compared with 36% of those treated with placebo.
CIBIC-Plus
Statistically significant differences were found in the group taking 6–12 mg/day of rivastigmine compared with the placebo group in both categories: stabilized or worsened (rating of 4, 5, 6, or 7) and worsened (rating of 5, 6, or 7). In addition, statistically significant differences from placebo were also found in the group taking 1–4 mg/day of rivastigmine in the stabilized or worsened category. It should be noted that only 34% of patients treated with 6–12 mg/day of rivastigmine worsened compared with 43% of patients treated with placebo.
PDS
Statistically significant differences between the group taking 6–12 mg/day of rivastigmine and the placebo group were found in the any worsening and ≥ 10% worsening categories.
DISCUSSION
Alzheimer's disease patients treated with rivastigmine worsen less on measures of cognition, global functioning, and ADL than patients treated with placebo. The reduction in decline is greater with the higher dose range of 6–12 mg/day than with the lower dose range of 1–4 mg/day. These data suggest that clinicians should include “reduced worsening” in their concept of benefit when treating patients with AD. Patients within the community may have treatment discontinued when no improvement is observed within the first few months of starting a ChE inhibitor. However, lack of improvement does not necessarily indicate lack of benefit. Patients who receive rivastigmine and show decline may well be declining at a slower rate than without treatment.
Most patients with AD spend their last years in a nursing home. A study by Knopman et al.18 showed that after 2 years of follow-up, patients treated with a high dose of the ChE inhibitor tacrine were less likely to enter a nursing home than those patients receiving lower doses. Thus, reduced worsening in patients receiving long-term adequate doses of ChE-inhibitor therapy may have important health care implications. Unfortunately, owing to the worsening effects on liver function tests associated with this agent, tacrine has limited therapeutic use. Although no comparable data are yet available for other ChE inhibitors, a recently published report by Farlow et al.19 suggests that patients treated with rivastigmine for up to 52 weeks continue to show cognitive benefits. The cognitive benefits observed in the group receiving 6–12 mg/day of rivastigmine after 52 weeks of treatment were superior to those seen in the placebo patients, who eventually received open-label rivastigmine for at least 26 weeks.
The benefits of any AD therapy must be viewed in light of the fact that AD is a disease manifested by progressive deterioration in cognition, behavior, and function.2 Therefore, benefit from AD therapy may be obtained not only from improvement but also stabilization and reduced worsening of these parameters. These data indicate that the amount of cognitive and functional worsening in patients treated with rivastigmine is reduced significantly.
Drug names: rivastigmine (Exelon), tacrine (Cognex).
Footnotes
Supported by Novartis Pharmaceuticals Corporation, East Hanover, N.J.
REFERENCES
- Mayeux R, Sano M. Treatment of Alzheimer's disease. N Engl J Med. 1999;341:1670–1679. doi: 10.1056/NEJM199911253412207. [DOI] [PubMed] [Google Scholar]
- Katzman R. Alzheimer's disease. N Engl J Med. 1986;314:937–964. doi: 10.1056/NEJM198604103141506. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, Fiorella T, et al. The spectrum of behavioral changes in Alzheimer's disease. Neurology. 1996;46:130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- Anthony-Bergstone CR, Zarit SH, Gatz M. Symptoms of psychological distress among caregivers of dementia patients. Psychol Aging. 1988;3:245–248. doi: 10.1037//0882-7974.3.3.245. [DOI] [PubMed] [Google Scholar]
- Mohs RC. The Alzheimer's Disease Assessment Scale. Int Psychogeriatr. 1996;8:195–203. doi: 10.1017/s1041610296002578. [DOI] [PubMed] [Google Scholar]
- Stern R, Mohs RC, Davidson M, et al. A longitudinal study of Alzheimer's disease: measurement, rate, and predictors of cognitive deterioration. Am J Psychiatry. 1994;151:390–396. doi: 10.1176/ajp.151.3.390. [DOI] [PubMed] [Google Scholar]
- Polinsky R. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer's disease. Clin Ther. 1998;20:634–647. doi: 10.1016/s0149-2918(98)80127-6. [DOI] [PubMed] [Google Scholar]
- Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer's disease. Int J Geriatr Psychopharmacol. 1998;1:55–65. [Google Scholar]
- Rösler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomized controlled trial. BMJ. 1999;318:633–640. doi: 10.1136/bmj.318.7184.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Anand R, Farlow MR. Systematic review of the efficacy of rivastigmine for patients with Alzheimer's disease. Int J Geriatr Psychopharmacol. 1998;1:S26–S34. [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH. CIBIC-Plus Interview Guide. East Hanover, NJ: Novartis Pharmaceuticals Corp. 1994. [Google Scholar]
- DeJong R, Osterlund OW, Roy GW. Measurement of quality-of-life changes in patients with Alzheimer's disease. Clin Ther. 1989;11:545–554. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association. 1994. [Google Scholar]
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Knopman D, Schneider L, Davis K, et al. Long-term tacrine (Cognex) treatment: effects on nursing home placement and mortality. Neurology. 1996;47:166–177. doi: 10.1212/wnl.47.1.166. [DOI] [PubMed] [Google Scholar]
- Farlow M, Anand R, Messina J, and et al. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer's disease. Eur Neurology. In press. [DOI] [PubMed] [Google Scholar]


