Abstract
People today are living longer. Old age is the number one risk factor for dementia, which is often associated with behavioral disturbances and psychosis as well as cognitive and memory impairment. Elderly persons with dementia—particularly those who are agitated or aggressive—are often placed in nursing homes and consequently treated with antipsychotic medications. Most of the studies of antipsychotic efficacy and safety have been conducted in young schizophrenic patients, but there are differences in dosing schedules, efficacy, and compliance when these drugs are used in elderly patients with dementia and psychosis. A review of both nonpharmacologic and pharmacologic treatment is herewith presented for the treatment of elderly dementia patients, especially those living in long-term care facilities.
People today are living longer. The fastest-growing segment of the population consists of individuals over the age of 85 years.1 By the year 2000, more than 35 million people will be over 65 years old, and an additional 5 million people will be over 85 years old. The trend toward a longer life span increases the need for long-term care facilities. Currently, the 16,500 nursing homes in the United States have approximately 2 million residents. Of the 2.2 million persons who became 65 years old in 1990, almost half will enter a nursing home in their lifetime. Moreover, hospitals are releasing convalescent patients sooner, thereby forcing nursing homes and skilled nursing facilities to take a larger role in subacute care. Old age is the number one risk factor for dementia. Unfortunately, dementia often presents with psychosis; thus, many elderly patients are treated with antipsychotic medications. Most of the studies of antipsychotic efficacy and safety have been conducted in schizophrenic patients, but there are differences in dosing schedules, efficacy, and compliance in elderly patients who have dementia with psychosis. This article will discuss nonpharmacologic and pharmacologic treatment of psychosis in elderly patients with dementia, especially those living in long-term care facilities.
DEMENTIA
According to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV),2 the essential feature of dementia is the development of multiple cognitive deficits that include memory impairment and at least one of the following cognitive disturbances: aphasia, apraxia, agnosia, or a disturbance in executive functioning. However, dementia is often associated with behavioral disturbances as well, for which the DSM-IV lacks a codable specifier and for which there is no widely accepted definition. In general, these behavioral disturbances are characterized by motor restlessness, verbal and physical aggression, withdrawal, irritability, dysphoria, disturbances of sleep and other diurnal rhythms, and other troublesome behaviors such as wandering. Disturbances associated with dementia may also be psychotic in nature and may include delusions and/or hallucinations. Because of these behavioral disturbances, elderly persons—particularly those who are agitated or aggressive—are often placed in nursing homes,3 which leads to intensive and costly treatment, increased morbidity and mortality, and public health problems.
Alzheimer's type dementia is the most common form of dementia, and patients with Alzheimer's type dementia constitute half of all nursing home residents.4 An estimated 3 to 4 million people in the United States have Alzheimer's type dementia,5 and the number is expected to double by the year 2030.6 The incidence is approximately 1% of the population aged 60 years, doubling every 5 years of life, such that by age 85 years, about 1 in 3 individuals has Alzheimer's type dementia.7 Delusions and hallucinations and/or behavioral symptoms are found in 50% to 90% of patients with Alzheimer's type dementia.8 Because a substantial number of patients with Alzheimer's disease exhibit psychotic and/or behavioral disturbances, these secondary manifestations of the disease are a leading cause of caregiver distress and patient institutionalization. From a public health standpoint, the annual treatment of Alzheimer's type dementia in the United States costs an estimated $100 billion9 and is likely to cost close to $200,000 per patient over the course of the illness.10
TREATMENT PRINCIPLES
The treatment of dementia patients with behavioral or psychotic disturbances should be driven by general principles. First, any primary medical illness should be identified and treated; for example, an infection (e.g., a urinary tract infection) or medications (e.g., steroids) can induce delirium and lead to behavioral disturbances in a patient with Alzheimer's type dementia. Therefore, the initial workup should include appropriate laboratory tests. After the medical issues are resolved, realistic goals should be set and p.r.n. medications should be limited.
Nonpharmacologic approaches may aid in the management of behavioral disturbances in dementia. Caretakers should be assisted in establishing a routine for dementia patients. Like children, elderly patients with dementia often fare better if they have a routine not only for taking medications but also for waking, toileting, eating, taking walks, and sleeping. Moreover, regular toileting often improves incontinence. Physical cues placed in the patient's living space can also be helpful. For example, large “Stop” signs placed on exit doors and personalized, color-coded (rather than numbered) doors to patients' rooms may benefit those patients who tend to wander. It is also important for caregivers to approach the patient in a calm, collected manner and to explain each activity before touching the patient; for example, the caregiver should say “I'm going to wash you now.” A patient who doesn't expect to be touched may view the gesture as a threat and strike back. Just as one would deal with a small child, the caregiver should speak in a loud but calm voice and use easy one-step commands.
It is important that adequate time is allowed for a therapeutic trial of medication, and targeted behaviors that are expected to improve during the medication trial should be specified and quantified. In skilled nursing facilities, it is equally important to rely on feedback from the nursing staff who spend more time with patients than do the clinicians who may make only brief visits. The clinician might see a patient sitting calmly, but learn from the nursing staff that the patient was agitated earlier. Creating a checklist of behavioral disturbances that need improvement will aid the staff in quantifying the patient's improvement after treatment begins.
PHARMACOLOGIC TREATMENT
While nonpharmacologic approaches are useful in the management of behavioral disturbances, medications are also often needed. Although no medication is approved by the U.S. Food and Drug Administration specifically for the treatment of behavioral disturbances associated with dementia, many agents are used for this purpose. These agents include antipsychotics, benzodiazepines, buspirone, β-blockers, serotonergic agents, carbamazepine, lithium, and divalproex sodium; of these drugs, only antipsychotics and divalproex have demonstrated long-term efficacy and safety in elderly patients with dementia. Benzodiazepines can be effective for short-term use but may have the opposite effect of disinhibiting patients, and tolerance tends to develop. The β-blockers have inherent cardiovascular effects such as orthostatic hypotension that may possibly lead to falls and potential fractures.
Typical Antipsychotics
Typical antipsychotics are commonly used for the treatment of agitation associated with dementia.8,11 These medications often reduce psychotic as well as nonpsychotic symptoms such as excitement, hostility, restlessness, tension, agitation, anxiety, aggression, irritability, and lack of cooperation; however, they occasionally worsen these behaviors by causing akathisia, a form of motor restlessness. In addition, typical antipsychotics lack therapeutic efficacy on behaviors such as wandering, apathy, withdrawal, hypersexuality, and symptoms of executive dysfunction or other cognitive aspects of dementia.
The use of typical antipsychotics often leads to extrapyramidal or parkinsonian symptoms that include bradykinesia, rigidity, tremor, decreased postural reflexes, masked facies, drooling, gait disturbances, and decreased postural reflexes. Other side effects of typical antipsychotics are sedation, acute dystonic reactions, akathisia, tardive dyskinesia, and (rarely) neuroleptic malignant syndrome. Moreover, the increased motor restlessness associated with these extrapyramidal symptoms (EPS) often increases the behavioral disturbances that were the original targets of treatment. The risk of development of tardive dyskinesia is increased in nonschizophrenic elderly patients who are administered typical antipsychotics.12 Thus, because of the multitude of safety problems, typical antipsychotics are no longer recommended as first-line treatment for elderly patients with dementia.
Atypical Antipsychotics
The various atypical antipsychotics do not necessarily share the same pharmacologic profiles. In a review of the properties of atypical agents, Arnt and Skarsfeldt13 found that most novel antipsychotics and clozapine can be differentiated from haloperidol, particularly in regard to EPS and cognitive side effects. Among the group of novel antipsychotics studied, there were many differences in models that reflected limbic versus striatal inhibition of dopamine function: clozapine and sertindole showed the largest limbic selectivity followed by quetiapine, ziprasidone, olanzapine, and remoxipride, whereas risperidone in many respects had a profile that resembled that of haloperidol. The atypical antipsychotics—many of which are demonstrating equal efficacy in psychosis and milder side effects than conventional antipsychotics—may prove to be beneficial for treating the behavioral disturbances associated with dementia. A MEDLINE search from the National Library of Medicine database revealed few studies of atypical antipsychotic use in patients with dementia compared with those in schizophrenic patients, although some schizophrenia data relating to side effects are relevant to elderly or demented patients. Sweet and Pollock14 have noted that controlled clinical studies of atypical antipsychotics in elderly patients are urgently needed so that age-related changes in pharmacokinetics and the risks of drug-drug interactions do not have to be extrapolated from case series and studies of young patients with schizophrenia.
A recent meta-analysis15 summarized the efficacy and tolerability of the new antipsychotics risperidone, olanzapine, sertindole, and quetiapine compared with placebo and conventional antipsychotics in the treatment of global schizophrenia symptomatology. The authors concluded that (1) all of the 4 new drugs are more effective than placebo, but the magnitude of the effect is only moderate; (2) according to the studies published at the time of the meta-analysis, sertindole and quetiapine were as effective as haloperidol, and risperidone and olanzapine were slightly more effective than haloperidol; (3) with respect to negative symptoms, haloperidol (despite widespread opinion) and all of the 4 new antipsychotics were more effective than placebo; and (4) the new antipsychotics were associated with less frequent use of antiparkinsonian medications than was haloperidol, with risperidone appearing to have a slightly less favorable EPS profile than the other new drugs.
Risperidone (available, 1993), olanzapine (available, 1996), and quetiapine (available, 1997) are the only atypical antipsychotics currently approved in the United States without restrictions for the treatment of schizophrenia.
Risperidone.
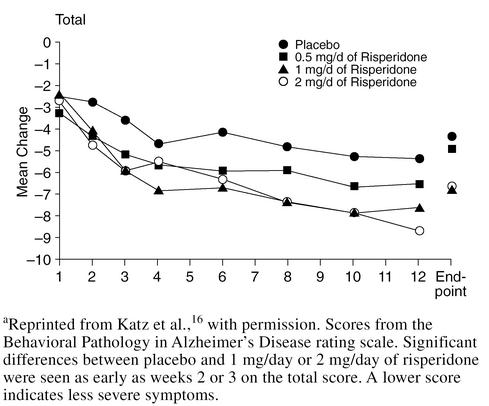
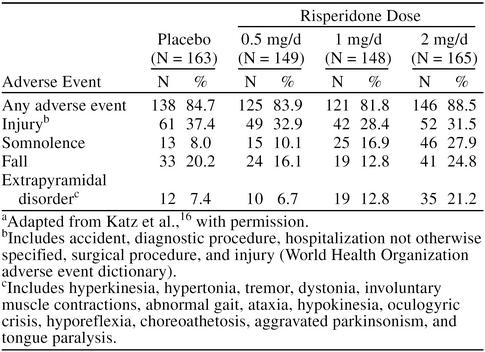
Although risperidone was initially developed to treat schizophrenia, a large double-blind, placebo-controlled study16 evaluating the efficacy and safety of risperidone in the treatment of psychotic and behavioral symptoms in elderly institutionalized patients with dementia has recently been reported. The 625 patients (mean age = 82.7 years) had DSM-IV diagnoses of Alzheimer's disease, vascular dementia, or mixed dementia, and patients with significant psychotic and behavioral symptoms were included in the trial. Patients were randomly assigned placebo or 0.5, 1, or 2 mg/day of risperidone for 12 weeks. At endpoint, significantly greater reduction in Behavioral Pathology in Alzheimer's Disease rating scale total scores and psychosis and aggressiveness subscale scores were seen in patients receiving risperidone, 1 and 2 mg/day, than placebo (p = .005 and p = .001, respectively) (Figure 1). However, more adverse events were reported by patients receiving 2 mg/day than by those patients receiving 1 mg/day of risperidone (Table 1), with the most common dose-related adverse events being EPS, somnolence (possibly leading to gait disturbances and falls), and peripheral edema. The frequency of EPS in patients receiving 1 mg/day was not significantly greater than that found with placebo; therefore, the authors suggested that 1 mg/day of risperidone was an appropriate dose for most elderly patients with dementia.
Figure 1.
Risperidone vs. Placebo in Dementiaa
Table 1.
Patients Reporting Any Adverse Event and Adverse Events Reported by at Least 10% of Patients in Any Treatment Groupa
A smaller, 9-week trial17 of risperidone in dementia had similar results. In all 15 patients, agitation remitted and aggression improved early in treatment with a risperidone dose of 0.5 mg/day. However, 8 patients experienced EPS, and cognitive skills declined in 3 patients. The results indicated that although risperidone is effective for treatment of agitation in elderly patients with dementia, adverse extrapyramidal or cognitive effects may occur even with low doses.
Olanzapine.
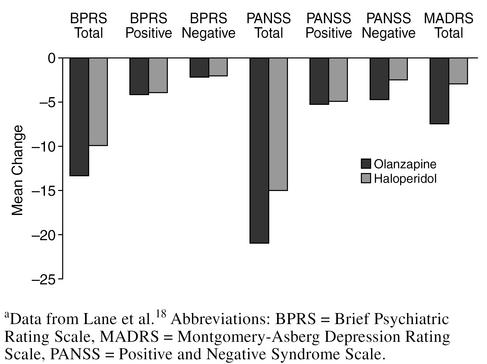
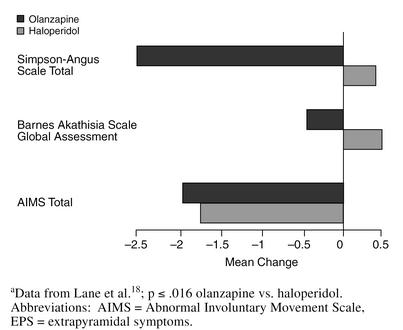
Olanzapine, like risperidone, has been studied more extensively in the treatment of patients with schizophrenia than dementia; however, a 6-week study18 has examined olanzapine treatment in geriatric patients. In 59 patients aged 65 years or older with schizophrenia, schizophreniform disorder, or schizoaffective disorder, the mean olanzapine dose was approximately 12 mg/day, similar to the dosage used in schizophrenic patients (geriatric or otherwise). The mean haloperidol dose was 8 mg/day, which is lower than the 11-mg/day dosage generally used in young adults with schizophrenia. The mean changes in the total scores on the Brief Psychiatric Rating Scale (BPRS), the Positive and Negative Syndrome Scale, and the Montgomery-Asberg Depression Rating Scale (MADRS) were greater for the olanzapine-treated than the haloperidol-treated patients (Figure 2). There was no incidence of EPS with the olanzapine-treated patients, unlike with the haloperidol-treated patients, whose total Simpson-Angus Scale and Barnes Akathisia Scale (BAS) Global Assessment item scores worsened significantly (p ≤ .016) (Figure 3).
Figure 2.
Mean Change in Efficacy Scale Scores: Haloperidol vs. Olanzapine in Geriatric Schizophreniaa
Figure 3.
Mean Change in EPS Scale Scores: Haloperidol vs. Olanzapine in Geriatric Schizophreniaa
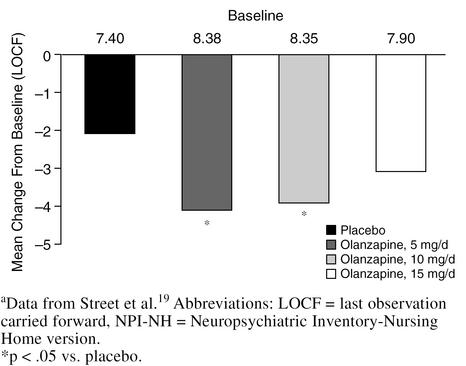
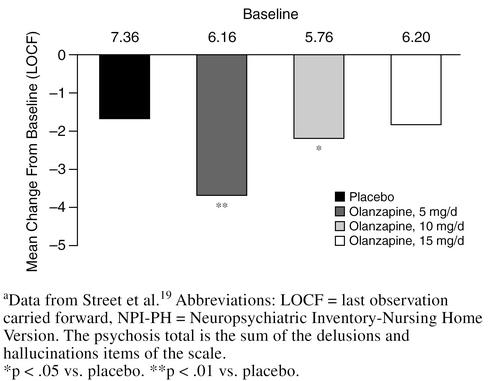
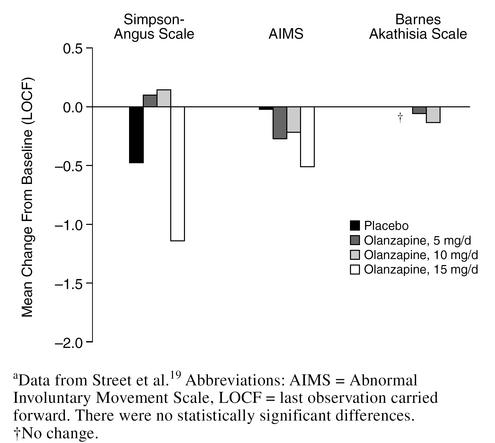
In a 6-week, double-blind, multicenter study,19 3 different doses of olanzapine (5, 10, and 15 mg/day) were compared with placebo in 206 nursing home patients with agitation, aggression, and psychosis associated with moderate-to-severe dementia. Significantly greater improvements on the agitation/aggression and the delusions and hallucinations items of the Neuropsychiatric Inventory-Nursing Home version (NPI-NH) were observed in patients treated with 5 or 10 mg/day of olanzapine compared with placebo or olanzapine, 15 mg/day (p < .05) (Figures 4 and 5). Changes in EPS were no different for olanzapine-treated patients at any dose compared with placebo as measured by the Simpson-Angus Scale, the Abnormal Involuntary Movement Scale, or the BAS (Figure 6). The most substantial improvement in the delusions and hallucinations item of the NPI-NH was seen in patients treated with olanzapine, 5 mg/day; patients in the 15-mg/day group did not respond as well as patients treated with lower doses. Thus, for elderly patients with psychosis associated with dementia, 5 mg/day of olanzapine seems to be the most efficacious dose, as opposed to the 12-mg/day dose commonly used for elderly schizophrenic patients. When treating agitated patients with dementia, olanzapine dosage should be started at 5 mg/day and increased to 10 mg/day if necessary. Frail patients should be started at 2.5 mg/day and the dose slowly increased as needed.
Figure 4.
Agitation/Aggression Item of the NPI-NH: Olanzapine vs. Placebo in Dementiaa
Figure 5.
Psychosis Total on the NPI-NH: Olanzapine vs. Placebo in Dementiaa
Figure 6.
Acute Extrapyramidal Symptoms: Olanzapine vs. Placebo in Dementiaa
Quetiapine.
The most recently approved atypical antipsychotic is quetiapine. Studies with quetiapine have not been completed in patients with dementia. Peuskens and Link20 compared the efficacy of quetiapine and chlorpromazine in 100 hospitalized patients with schizophreniform disorder or acute exacerbation of chronic schizophrenia. The mean daily dosages at study end were 407 mg of quetiapine and 384 mg of chlorpromazine. Both medications were effective in the treatment of positive and negative symptoms, with a trend toward superior efficacy for quetiapine. Levels of parkinsonian symptoms as measured by the Simpson-Angus Scale total score were low in both treatment groups; however, patients in the quetiapine group suffered less in this regard as fewer quetiapine-treated patients with a baseline score of 0 were found to have worsened by study day 42 than patients in the chlorpromazine group (5% vs. 32%). On the Global Assessment item of the BAS, quetiapine was associated with statistically significantly lower levels of akathisia. Finally, the quetiapine group had 5% fewer patients who required anti-EPS medication than the chlorpromazine group (10% vs. 15%).
Arvanitis et al.21 compared 5 fixed doses of quetiapine (75, 150, 300, 600, or 750 mg/day) with haloperidol (12 mg/day) and placebo in order to evaluate efficacy, tolerability, and the dose-response relationship in schizophrenic patients. At endpoint, significant (p < .05) differences in adjusted mean changes from baseline were identified between the 4 highest doses of quetiapine and placebo on the BPRS total score, BPRS positive symptom cluster, and Clinical Global Impressions-Severity of Illness item scores and also between quetiapine, 300 mg/day, and placebo for the Scale for the Assessment of Negative Symptoms summary score. Differences between quetiapine and haloperidol were not significant. No significant safety problems were identified with dosage increases of quetiapine, and the drug did not differ from placebo across the dose range in terms of EPS incidence.
ADVERSE EVENTS
Tardive Dyskinesia
The potential of a drug to cause EPS may be linked to its risk for tardive dyskinesia.22 Jeste et al.23 found the cumulative annual incidence of tardive dyskinesia among schizophrenia patients over 45 years of age to be 26%, which is 5 to 6 times that found in young patients. Just as elderly individuals are more susceptible to tardive dyskinesia than young adults, patients with dementia may be at a higher risk for tardive dyskinesia than schizophrenic patients. Since the atypical antipsychotics have a lower incidence of EPS compared with typical agents, they may also carry a lower risk of tardive dyskinesia. In a study of young schizophrenic patients,24 the 1-year incidence of tardive dyskinesia in olanzapine-treated patients (N = 513) was 0.52% while the incidence in haloperidol-treated patients (N = 114) was 7.45% (p = .002). Tollefson et al.25 reported that the incidence of newly emergent tardive dyskinesia was significantly lower among olanzapine-treated patients (N = 707) than in haloperidol-treated patients (N = 197). An open-label clinical study26 of 2 patients with Parkinson's disease showed that treatment with quetiapine successfully controlled the psychotic symptoms without worsening their motor disability.
More EPS have been reported with risperidone, particularly at high doses, than with olanzapine or quetiapine.15 In a report of 6 psychotic patients with akinetic-rigid syndromes treated with risperidone,27 5 patients experienced intolerable exacerbation of parkinsonism. Four patients subsequently improved with clozapine treatment; only 1 patient, the youngest, responded well to risperidone. The authors concluded that risperidone should be used with caution in the treatment of psychosis in parkinsonian patients.
Other Adverse Events
Because olanzapine is a muscarinic blocking agent, there has been some concern about anticholinergic effects leading to cognitive impairment in dementia patients treated with this agent. However, Street et al.19 suggested that olanzapine does not worsen the cognitive functioning of elderly patients with dementia. Cognitive function was measured using the Mini-Mental State Examination in patients taking olanzapine in doses of 5, 10, or 15 mg/day. Patients in the 5-mg/day treatment group performed slightly better than those in the other groups, but there was no statistically significant worsening of cognition in any of the olanzapine-treated patients versus placebo. Additionally, no significant differences were found between placebo and the 3 olanzapine treatment groups in terms of other anticholinergic side effects such as constipation, fecal impaction, intestinal obstruction, dry mouth, urinary retention, or blurred vision. These findings may indicate that olanzapine has low in vivo anticholinergic activity. Although anticholinergic activity can be demonstrated in the test tube, anticholinergic side effects or worsening of cognition are seldom seen in humans treated with olanzapine. The mechanism of the discordance between in vivo and in vitro anticholinergic activity is unclear; however, it may involve olanzapine having greater affinity for muscarinic M2 or M6 receptors than M1 receptors, possibly leading to greater cholinergic activity.
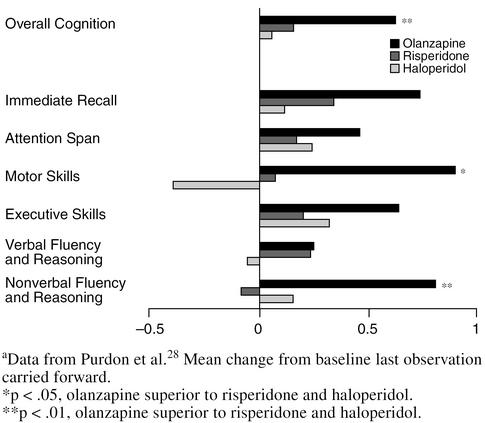
In a study of young schizophrenic patients,28 olanzapine improved cognition. The authors compared olanzapine, risperidone, and haloperidol, and overall cognition and nonverbal fluency and construction were significantly superior in the olanzapine group (p < .01) (Figure 7). Motor skills were also significantly superior with olanzapine treatment (p < .05). All other measures showed improvement (although not statistically significant) with olanzapine treatment. Olanzapine need not be avoided in dementia patients for fear of worsening cognition; in fact, the drug may improve cognition, but more studies should be performed in elderly persons to make such a determination.
Figure 7.
Cognitive Changes: Olanzapine, Risperidone, and Haloperidol in Schizophrenia on a General Cognitive Indexa
Sax et al.29 reported an improvement in attentional performance in schizophrenic patients treated with quetiapine. Prior to treatment, attentional performance in 10 schizophrenic patients was significantly (p < .01) worse than in 12 matched controls. After 2 months of quetiapine treatment, the patients with schizophrenia did not differ significantly from the controls, suggesting that quetiapine produced a significant improvement in attentional functioning.
Another potential adverse event related to antipsychotic treatment is an elevated blood prolactin level that can result in amenorrhea, gynecomastia, galactorrhea, and sexual dysfunction. Popli et al.30 reported 2 patients who developed galactorrhea associated with risperidone treatment. One patient was switched to a typical antipsychotic; the other patient was continued on risperidone treatment because of a robust response, and the galactorrhea was partially treated with bromocriptine. Shiwach and Carmody31 described a case of a male patient who developed gynecomastia and sexual difficulties while taking risperidone for chronic paranoid schizophrenia. A small study was subsequently conducted on male psychotic patients (N = 14) to compare the prolactogenic effects of risperidone with those of traditional antipsychotic medication (N = 15). The results showed a greater but nonsignificant prolactogenic effect of risperidone. The role of prolactin is unclear in relation to blood estrogen levels, but sustained prolactin elevation may lead to decreased estrogen levels in young patients. If the same situation holds true in older patients, the resultant risk is possible decreased bone mineral density and osteoporosis.
Prolactin elevation in the postpartum phase32 or in patients with prolactin-producing tumors33 may lead to depression. Thus, in patients with depression coexistent with psychosis, prolactin-sparing antipsychotics such as olanzapine or quetiapine may prove to be the most beneficial treatment. In an international multicenter trial of olanzapine versus haloperidol in patients with schizophrenia, schizophreniform disorder, or schizoaffective disorder, Tollefson et al.34 reported that the treatment effecton associative depressive symptoms revealed that olanzapine-treated patients had a 2-fold greater improvement in MADRS total scores than patients in the haloperidol group. In a comparison of olanzapine and risperidone, Tran et al.35 found no mean change in blood prolactin levels in olanzapine-treated patients at baseline, week 8, or week 28. However, risperidone-treated patients had elevated and persistent prolactin levels, and a greater proportion of risperidone-treated patients experienced treatment-emergent sexual dysfunction (abnormal ejaculation) than their olanzapine-treated counterparts. Peuskens and Link20 found that mean prolactin levels decreased markedly from baseline levels during treatment with quetiapine, while chlorpromazine was associated with small inconsistent reductions. Finally, a side effect of atypical antipsychotics that has been problematic in young patients is weight gain; however, many elderly dementia patients are underweight at baseline and may potentially benefit from weight gain.
AUGMENTATION
Augmentation strategies can be used if treatment with antipsychotics leads to only a partial response of the behavioral disturbances or psychosis associated with dementia. A cholinesterase inhibitor such as donepezil may be beneficial in reducing aggression and agitation and improving behavioral functioning while stabilizing or improving cognition in the patient with mild-to-moderate dementia; it is unlikely to have a great effect on improving cognition in a severely ill patient. Donepezil should be started at a dosage of 5 mg/day for the first 4 to 6 weeks and then increased to 10 mg/day if tolerated. Divalproex, which can be beneficial in manic patients, may also be useful as augmentation therapy; dosage should start at 125 or 250 mg twice a day and titrated to a therapeutic blood level of 50 to 100 (possibly up to 125) µg/mL if the patient tolerates the medication. Another medication, zolpidem, may help some patients to reregulate their sleep cycle. Buspirone may also be of benefit to the mildly agitated patient or as augmentation to the atypical antipsychotics. In the occasional young dementia patient, atypical antipsychotics can be combined, but research is needed to determine if this strategy should be employed routinely.
COMPLIANCE
Use of nonpharmacologic treatment, such as gaining a daily routine for the patient, is important for optimizing therapeutic efficacy among patients with behavioral disturbances associated with dementia. Because elderly patients are sensitive to side effects, compliance may improve if they are given medications at dosages that provide therapeutic efficacy but have gentle adverse event profiles. The atypical antipsychotics have shown efficacy over placebo in the treatment of dementia and are safer than typical antipsychotic medications in terms of a lower risk of EPS and tardive dyskinesia. The atypical agents also appear to avoid worsening cognition in elderly dementia patients. Using a medication dosage that provides the best efficacy:safety ratio will lead to the greatest treatment outcome. To avoid side effects in the frail patient, the starting dose of an antipsychotic may need to be lower than the usual therapeutic dose; the dose can be increased as needed but should not reach the usual dosage for nonelderly schizophrenic patients.
CONCLUSION
In addition to memory impairment and cognitive disturbances, dementia is often associated with behavioral disturbances and psychosis. While nonpharmacologic approaches may aid in management of the behavioral disturbances, treatment with antipsychotic medications is often necessary. Since elderly patients are sensitive to side effects, compliance may improve if they are given medications with gentle adverse event profiles at dosages that provide therapeutic efficacy. Because of the multitude of safety problems, typical antipsychotics are no longer recommended as first-line treatment for elderly patients with dementia. Atypical antipsychotics are demonstrating equal efficacy and milder side effects than conventional antipsychotics; however, most studies of antipsychotic efficacy and safety have been conducted in schizophrenic patients. Controlled clinical studies of atypical antipsychotics in elderly patients are urgently needed so that age-related changes in pharmacokinetics and the risks of drug-drug interactions do not have to be extrapolated from case series and studies of young patients with schizophrenia.
Drug names: bromocriptine (Parlodel and others), buspirone (BuSpar), carbamazepine (Tegretol and others), chlorpromazine (Thorazine and others), clozapine (Clozaril and others), divalproex sodium (Depakote), donepezil (Aricept), haloperidol (Haldol and others), olanzapine (Zyprexa), quetiapine (Seroquel), risperidone (Risperdal), zolpidem (Ambien).
REFERENCES
- Hobbs FB. 65+ In the United States. US Department of Commerce: Economic and Statistics Administration. Washington, DC: US Bureau of the Census. 1996 [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association. 1994 [Google Scholar]
- Ferris SH, Steinberg G, Shulman E, et al. Institutionalization of Alzheimer's patients: reducing precipitating factors through family counseling. Arch Found Thanatol. 1985;12:7. doi: 10.1300/J027v08n01_03. [DOI] [PubMed] [Google Scholar]
- Forsyth E, Ritzline PD. An overview of the etiology, diagnosis, and treatment of Alzheimer disease. Phys Ther. 1998;78:1325–1331. doi: 10.1093/ptj/78.12.1325. [DOI] [PubMed] [Google Scholar]
- Reisberg B. Alzheimer's disease. In: Sadavoy J, ed. Comprehensive Review of Geriatric Psychiatry II. 2nd ed. Washington, DC: American Psychiatric Press. 1996 401–458. [Google Scholar]
- Brumback RA, Leech RW. Alzheimer's disease: pathophysiology and the hope for therapy. J Okla State Med Assoc. 1996;87:103–111. [PubMed] [Google Scholar]
- Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand. 1987;76:465–479. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- Tariot PN. Treatment strategies for agitation and psychosis in dementia. J Clin Psychiatry. 1996;57(suppl 14):21–29. [PubMed] [Google Scholar]
- Snow C. Medicare HMOs develop plan for future of Alzheimer's disease programming. Modern Healthcare. 1996;26(39):67–70. [PubMed] [Google Scholar]
- Schumock GT. Economic considerations in the treatment and management of Alzheimer's disease. Am J Health Syst Pharm. 1998;55(suppl 2):S17–S21. doi: 10.1093/ajhp/55.suppl_2.S17. [DOI] [PubMed] [Google Scholar]
- Verma SD, Davidoff DA, Kambhampati KK. Management of the agitated elderly patient in the nursing home: the role of the atypical antipsychotics. J Clin Psychiatry. 1998;59(suppl 19):50–55. [PubMed] [Google Scholar]
- Saltz BL, Woerner MG, Kane JM, et al. Prospective study of tardive dyskinesia incidence in the elderly. JAMA. 1991;266:2402–2406. [PubMed] [Google Scholar]
- Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? a review of the evidence. Neuropsychopharmacology. 1998;18:63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Pollock BG. New atypical antipsychotics: experience and utility in the elderly. Drugs Aging. 1998;12:115–127. doi: 10.2165/00002512-199812020-00004. [DOI] [PubMed] [Google Scholar]
- Leucht S, Pitschel-Walz G, Abraham D, et al. Efficacy and extrapyramidal side effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo: a meta-analysis of randomized controlled trials. Schizophr Res. 1999;35:51–68. doi: 10.1016/s0920-9964(98)00105-4. [DOI] [PubMed] [Google Scholar]
- Katz IR, Jeste DV, Mintzer JE, et al. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psychiatry. 1999;60:107–115. doi: 10.4088/jcp.v60n0207. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Sultzer D. A structured trial of risperidone for the treatment of agitation in dementia. Am J Geriatr Psychiatry. 1998;6:127–135. [PubMed] [Google Scholar]
- Lane LM, Burns PR, Sanger TM, and et al. Olanzapine in the treatment of elderly patients with schizophrenia and related psychotic disorders. Presented at the 11th Congress of the European College of Neuropsychopharmacology (ECNP); Oct 31–Nov 4, 1998; Paris, France. [Google Scholar]
- Street J, Clark WS, Mitan S, and et al. Olanzapine in the treatment of psychosis and behavioral disturbances associated with Alzheimer's disease. Arch Gen Psychiatry. In press. [Google Scholar]
- Peuskens J, Link CGG. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Psychiatr Scand. 1997;96:265–273. doi: 10.1111/j.1600-0447.1997.tb10162.x. [DOI] [PubMed] [Google Scholar]
- Arvanitis LA, Miller BG. and the Seroquel Trial 13 Study Group. Multiple fixed doses of Seroquel (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. Biol Psychiatry. 1997 42:233–246. [DOI] [PubMed] [Google Scholar]
- Barnes TRE, McPhillips MA. Novel antipsychotics, extrapyramidal side effects and tardive dyskinesia. Int Clin Psychopharmacol. 1998;13(suppl 3):S49–S57. doi: 10.1097/00004850-199803003-00009. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Eastham JH, Lacro JP, et al. Management of late-life psychosis. J Clin Psychiatry. 1996;57(suppl 3):39–45. [PubMed] [Google Scholar]
- Beasley CM Jr, Dellva MA, Tamura RN, et al. Randomized double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. Br J Psychiatry. 1999;174:23–30. doi: 10.1192/bjp.174.1.23. [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Beasley CM Jr, Tamura RN, et al. Blind, controlled, long-term study of the comparative incidence of treatment-emergent tardive dyskinesia with olanzapine or haloperidol. Am J Psychiatry. 1997;154:1248–1254. doi: 10.1176/ajp.154.9.1248. [DOI] [PubMed] [Google Scholar]
- Parsa MA, Bastani B. Quetiapine (Seroquel) in the treatment of psychosis in patients with Parkinson's disease. J Neuropsychiatry Clin Neurosci. 1998;10:216–219. doi: 10.1176/jnp.10.2.216. [DOI] [PubMed] [Google Scholar]
- Rich SS, Friedman JH, Ott BR. Risperidone versus clozapine in the treatment of psychosis in six patients with Parkinson's disease and other akinetic-rigid syndromes. J Clin Psychiatry. 1995;56:556–559. [PubMed] [Google Scholar]
- Purdon SE, Jones BDW, Stip E, and et al. for The Canadian Collaborative Group for Research on Cognition in Schizophrenia. Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. Arch Gen Psychiatry. 2000 57:249–258. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM, Keck PE Jr. Attentional improvement following quetiapine fumarate treatment in schizophrenia. Schizophr Res. 1998;33:151–155. doi: 10.1016/s0920-9964(98)00067-x. [DOI] [PubMed] [Google Scholar]
- Popli A, Gupta S, Rangwani SR. Risperidone-induced galactorrhea associated with a prolactin elevation. Ann Clin Psychiatry. 1998;10:31–33. doi: 10.1023/a:1026150629199. [DOI] [PubMed] [Google Scholar]
- Shiwach RS, Carmody TJ. Prolactogenic effects of risperidone in male patients: a preliminary study. Acta Psychiatr Scand. 1998;98:81–83. doi: 10.1111/j.1600-0447.1998.tb10046.x. [DOI] [PubMed] [Google Scholar]
- Fava M, Fava GA, Kellner R, et al. Psychosomatic aspects of hyperprolactinemia. Psychother Psychosom. 1983;40:257–262. doi: 10.1159/000287773. [DOI] [PubMed] [Google Scholar]
- Cohen AJ. Bromocriptine for prolactinoma-related dissociative disorder and depression [letter] J Clin Psychopharmacol. 1995;15:144–145. doi: 10.1097/00004714-199504000-00015. [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Beasley CM Jr, Tran PV, et al. Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry. 1997;154:457–465. doi: 10.1176/ajp.154.4.457. [DOI] [PubMed] [Google Scholar]
- Tran PV, Hamilton SH, Kuntz AJ, et al. Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol. 1997;17:407–418. doi: 10.1097/00004714-199710000-00010. [DOI] [PubMed] [Google Scholar]