Abstract
Atypical antipsychotics are a class of novel agents increasingly employed for the treatment of psychotic disorders. The pharmacodynamic properties of the atypicals appear to impact a broader spectrum of psychotic symptoms than had been appreciated with older generation antipsychotics. In addition, the atypical agents appear to have a reduced risk of neurologic side effects compared with conventional antipsychotic use. Both of these features enhance the appeal of the atypical antipsychotics and may be associated with enhanced patient compliance. The atypical antipsychotics appear to be effective for schizophrenia as well as other psychotic disorders, including schizoaffective disorder and mood disorders with psychotic features. Consequently, atypical antipsychotics are now considered to be the first-line treatment for schizophrenia, with the exception of clozapine, which is considered a second-line agent because of risks associated with its use. This review will discuss the literature on atypical antipsychotic efficacy in psychotic disorders. Issues related to antipsychotic use, dosing, adverse effects, and drug interactions are also discussed.
Primary care physicians treating patients with psychotic disorders are increasingly encountering a new armamentarium of antipsychotic medications. This article was written to inform primary care physicians about recent advances in the pharmacologic management of psychotic disorders. The intent is to provide an overview of “atypical” antipsychotics, their use and indications, dosing, adverse effects, and possibilities of drug interactions about which primary care physicians ought to be aware.
Psychosis refers to a number of symptoms reflecting impairments in reality testing. The psychotic patient is one who develops odd beliefs, speech, and/or behaviors interfering with functioning. Perceptual disturbances can occur, and the individual's ability to interpret events in the world can become distorted and idiosyncratic. Consequently, the individual experiences significant difficulties caring for self, relating with others, and maintaining academic and occupational demands and expectations.
Overt symptoms of psychosis can include hallucinations, delusions, disorganized speech and impairments in form of thought, and disorganized behavior.1 Hallucinations refer to a perception in the absence of a sensory stimulus. These can occur in the context of any of the 5 sensory modalities, e.g., auditory, such as hearing a voice(s) commenting about one's self, or tactile, such as experiencing crawling sensations on or within one's skin. Delusions refer to false beliefs defying credibility, which are characteristically inflexible, not subject to reasoning, and despite incontrovertible evidence are rigidly maintained. These are idiosyncratic to the individual and are not shared by others (as is a belief system shared by a particular faith, for example). Speech and thought form disturbances refer to the pattern of thought processes in contrast to the content of those thoughts, i.e., a delusion. Thought form disturbances are present when thoughts are so impeded as to lose goal direction, for example, overinclusion and circumstantial, tangential, and loose associations. Disorganized behaviors can take a variety of forms and reflect a lack of goal direction of action as would occur in catatonia and stereotyped behaviors, for instance.
Psychotic symptoms have been clustered in 2 groups, either or both of which can be present in a given patient: “positive” and “negative” symptoms.2 The more overt, or positive, symptoms mentioned above such as hallucinations or delusions tend to be more obvious to the clinician assessing the psychotic patient. Negative symptoms refer to a constellation of psychotic symptoms characterized by the absence of features normally present in individuals with intact reality testing. Such symptoms are subtle and therefore can be overlooked by the clinician focusing exclusively on positive symptoms or can be mistaken for depression. Examples of negative symptoms include affective flattening (absence of affect expression), alogia (absence of or significant reductions in speech/communication), and avolition (absence of motivation), among others. Such symptoms render the patient emotionally restricted, devoid of interests, lacking a sense of purpose, withdrawn, isolated, and with diminished social drive.
While characteristic of disorders such as schizophrenia and schizoaffective disorders, impairments in reality testing can occur in patients with primary mood disorders, such as major depression or bipolar disorder. Diagnosis of specific psychiatric disorders is based on the presence and pattern of psychotic symptoms, the duration of symptoms, the temporal relation (if any) to mood disturbances, and the temporal relationship to likely triggers, such as substances of abuse, withdrawal from alcohol, central nervous system (CNS) disorders, metabolic disturbances, and other disease states. Antipsychotic medications are, therefore, employed in an array of psychiatric disorders. The use of such medications is warranted when psychotic symptoms are evident and appropriately identified. However, antipsychotic use alone may not be sufficient, since the most effective treatment may involve addressing an underlying organic disturbance or substance detoxification.
The consequences of psychotic illness can be severe, pervasive, and devastating.3 Some disturbances can be so severe that the terrified patient can act in such a way as to harm others or her- or himself.4–8 The long-term effects of the disorder result in impaired adaptive functioning, occupational and academic maladjustment, and declining self-efficacy.9–11 Hence, efforts have been directed at developing antipsychotics with improved efficacy, for both positive and negative symptoms, that would facilitate improved functioning and mitigate long-term consequences.
CONVENTIONAL VERSUS “ATYPICAL” ANTIPSYCHOTICS
The older generation conventional antipsychotics (also referred to as neuroleptics and hereafter referred to as conventional antipsychotics) have been the mainstay of treatment of acute and chronic psychotic states for decades. Those agents, including chlorpromazine and haloperidol among others, presumably exert their antipsychotic effect by blocking the accessibility of dopamine to the dopamine D2 receptor, present within the CNS.
Conventional antipsychotic use is associated with a number of limitations. First, while effective in managing positive symptoms, these drugs offer little for the management of the motivational and affective, i.e., negative, symptoms.12 More importantly, the reduction of positive symptoms alone has not always restored patients' functioning.9 Second, use of the conventional agents is also associated with a number of neurologic side effects, by virtue of the selectivity for the D2 receptors abundant in the basal ganglia and nigrostriatal tracts. Hence, such medications have risks of extrapyramidal side effects (EPS) and tardive dyskinesia.
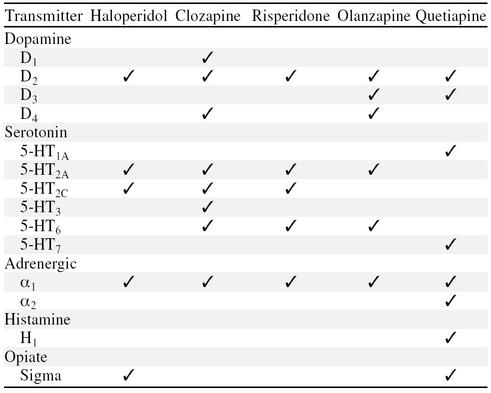
Novel antipsychotics, also referred to as the “atypicals,” include clozapine, risperidone, olanzapine, and quetiapine. The term atypical antipsychotic refers to a class of medications with a broader spectrum of neurotransmitter activity (Table 1).13 While differences exist among them, it can be said that these agents antagonize serotonin and dopamine receptors within the CNS. Both of these effects are thought to account for the ability of atypical antipsychotics to address positive and negative symptoms of schizophrenia and address mood symptoms/disorders accompanying psychosis while reducing risks of neurologic side effects.
Table 1.
Comparison of Neurotransmitter Affinities of Haloperidol and Atypical Antipsychotics
Atypical antipsychotics are now considered to be the first-line interventions for psychotic disorders. One of the atypical agents, clozapine, is reserved as a second-line treatment because of the risks associated with its use.14
EFFICACY OF ATYPICAL ANTIPSYCHOTICS
Among the psychotic disorders, schizophrenia is the most debilitating, often with high relapse rates and a chronic course.15,16 Most patients require long-term treatment. Studies assessing the efficacy of the atypical antipsychotics have thus largely focused on schizophrenic patients, although more recent studies have assessed the efficacy of atypical antipsychotics in patients with psychosis associated with mood disorders.
Atypical antipsychotics have demonstrated efficacy in reducing the severity of psychotic symptoms. As compared with conventional antipsychotics, e.g., haloperidol, chlorpromazine, and fluphenazine, double-blind studies have demonstrated the usefulness of clozapine,17–24 risperidone,25–30 olanzapine,31–34 and quetiapine35–38 in reducing both positive and negative symptoms of schizophrenia. Often, these studies have examined the treatment efficacy of the atypicals in those patients who had been poorly responsive or unresponsive to prior conventional antipsychotic treatment.18–24,27,29,33 New-onset psychosis, i.e., patients experiencing a first psychotic episode, has also been found to be responsive to atypical antipsychotics.39–41
The aforementioned studies generally employed comparisons of an atypical agent with a conventional antipsychotic and/or placebo. The duration of most studies was brief. Study designs and methods varied as well, e.g., duration of treatment and patient follow-up, measures employed to quantify psychotic symptoms, duration of prior treatment “washout” before introduction of the atypical agent, and the a priori operational definitions establishing what constituted successful treatment.42 Consequently, making statements regarding long-term efficacy of atypical antipsychotic treatment, or comparing the effectiveness of one atypical antipsychotic with another on the basis of these studies has thus been impossible.
More recently, long-term trials and studies comparing the efficacy and safety of atypical agents have been undertaken. Long-term trials have demonstrated the efficacy and tolerability of atypicals as compared with conventional agents.23,43 Further, relapse and rehospitalization rates for psychotic disorders are reduced with the use of atypical antipsychotics as compared with conventional agents.44–47
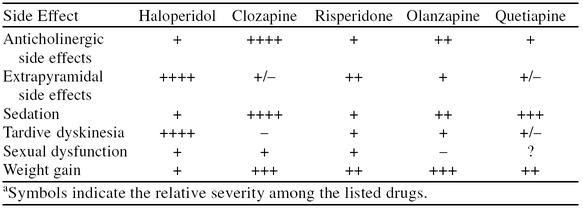
Studies comparing the atypical antipsychotics have revealed only subtle differences.48–51 For example, risperidone and clozapine were demonstrated to have comparable efficacy,48,50 although risperidone may have a faster treatment onset.48 Comparative studies involving olanzapine and risperidone demonstrated comparable reductions in psychosis ratings, but are conflicted as to which agent more effectively reduces positive and negative symptoms.49,51 The differences observed in treatment effectiveness are likely accounted for by the dosages of the atypical agents employed,52 i.e., low-dose risperidone tends to be more effective than low-dose olanzapine, whereas high-dose olanzapine is more effective than high-dose risperidone. The limiting factor with risperidone use may lie with the tolerability of side effects: higher doses were apt to produce intolerable adverse effects, thereby limiting their usefulness as compared with higher doses of olanzapine. However, higher doses of olanzapine may be required for clinical efficacy.52 The comparative studies consistently demonstrated differences in side effect profiles between the 2 atypical agents (Table 2).
Table 2.
Comparison of Common Side Effects Associated With Antipsychotic Usea
Atypical antipsychotics have likewise demonstrated efficacy in psychotic mood disorders and schizoaffective disorder.43,53–57 Depressive symptoms and clinical depression can accompany schizophrenia, but have been reduced with olanzapine treatment.58 In addition, both risperidone and olanzapine have been effective in acute mania.59,60 Olanzapine was as effective as lithium in the management of bipolar disorder.61 The atypicals hold promise for the treatment of psychosis associated with mood disorders. Controversy attends the role of atypical agents in mood disorders, however. One case series62 revealed that atypical antipsychotics can exacerbate or precipitate mania and hypomania in patients with bipolar affective disorder. In addition, the “depressive symptoms” accompanying schizophrenia may reflect mislabeled negative symptoms or previously unrecognized parkinsonism brought on by conventional antipsychotic use.63 Hence, improvements in “depression” by an atypical agent might actually reflect the improved efficacy in reducing negative symptoms or reduction in parkinsonism rather than an actual antidepressant effect. Thus, the role of the atypical antipsychotics for patients with mood disorders has yet to be clarified by further investigation.
EXTRAPYRAMIDAL SYMPTOMS, TARDIVE DYSKINESIA, AND THE ATYPICAL ANTIPSYCHOTICS
EPS, or neuroleptic-induced movement disorders,1 are frequently encountered with conventional antipsychotic use and can take a number of forms. The most common of these is akathisia, an inner sense of restlessness that may be accompanied by overt restlessness and fidgeting. Often, this fidgeting includes such behaviors as pacing, rocking in one's chair, frequent movement, and so on. Dystonia is characterized by involuntary muscle spasm of large muscles, usually neck (torticollis), limbs, and trunk. Lastly, there is parkinsonism, similar to idiopathic Parkinson's disease, which characteristically comprises bilateral tremor of the extremities, rigidity, gait instability with shuffling, and bradykinesia. EPS can be disabling, are often troubling to patients and their families, and can be anxiety-provoking, perhaps more so than the original psychotic symptoms prompting treatment. The neuroleptic-induced movement disorders are thought to be due to the blockade of the dopamine D2 receptors in the nigrostriatal tract.64
Apart from EPS is a dyskinetic movement disorder occurring late in the course of treatment, hence called tardive dyskinesia. This refers to a set of involuntary movements, usually involving the face, neck, and extremities. The dyskinesia often has a rhythmic quality, such as blepharospasm, involuntary tongue protrusions and vermicular (“worm-like”) movements, and lip sucking and lip smacking. In addition to being disfiguring, such disturbances can become so severe as to interfere with swallowing and self-care. The risk of tardive dyskinesia increases with treatment duration and appears to be a higher risk for elderly patients, patients with head injuries/CNS insults, and those with significant mood disorders.65–67 The mechanism underlying tardive dyskinesia is less well understood, but may be related to heightened sensitivity developing among the remaining unblocked dopamine D2 receptors in the nigrostriatal tract arising after chronic antipsychotic medication exposure.64,65
When compared with matched subjects treated with conventional antipsychotics, atypical antipsychotics produced fewer EPS.32,35,43,68,69 Nevertheless, EPS are still associated with atypical antipsychotic use. As depicted in Table 2, risperidone may be more apt to produce EPS as compared with the other atypical antipsychotics.70 This trend toward EPS with risperidone use appears to be dose related, yet is less than that associated with conventional antipsychotic use, even at high doses.28,64 In risperidone-treated patients, positron emission tomography (PET) studies demonstrated a positive correlation between the extent of dopamine D2 receptor occupancy and the daily dose of risperidone, thus supporting the dose-EPS relationship observed clinically.71 The extent of D2-receptor occupancy by risperidone was demonstrated even at doses within recommended ranges. Similarly, PET revealed low levels of D2-receptor occupancy with the use of either clozapine or olanzapine at usual clinical doses.72–74 It has been speculated that both clozapine and olanzapine exert greater serotonin (5-HT) antagonism at 5-HT2 receptors as compared with risperidone and that this serotonin antagonism may offset some of the risk for EPS.73,75
Lower rates of tardive dyskinesia were reported among atypical antipsychotic–treated patients compared with those treated with conventional antipsychotics.76,77 Interestingly, 2 cases of tardive dyskinesia were noted in clozapine-treated patients.77 The authors argued that the 2 afflicted patients had some symptoms of tardive dyskinesia from previous antipsychotic exposure in the 12 and 25 years of treatment, respectively, prior to the initiation of the clozapine. Thus, it remains unclear if clozapine treatment precipitated the tardive dyskinesia in those 2 individuals. Other reports suggested that tardive dyskinesia from prior neuroleptic exposure can, in fact, be mitigated by clozapine treatment.78–82 As a consequence, patients experiencing tardive dyskinesia from conventional antipsychotic use might instead be considered for clozapine treatment.
COMPLIANCE WITH CONVENTIONAL VERSUS ATYPICAL ANTIPSYCHOTICS
Antipsychotic medications will not be effective if they are not taken appropriately and reliably.83,84 Treatment noncompliance contributes to the severity of the disorder and is present in as many as 80% of patients.85 Antipsychotic-associated side effects appear to be related to high rates of noncompliance; specifically, EPS appear to be most troublesome.86 Consequently, it can be appreciated that such untoward effects associated with conventional antipsychotic medications have met with patient resistance, despite efficacy in controlling some psychotic symptoms. Enhanced compliance, with improved long-term maintenance of psychosis, would be expected to restore adaptive functioning.
By circumventing many of the problems associated with EPS and tardive dyskinesia, the atypical antipsychotics have significantly reduced the morbidity associated with conventional antipsychotic treatment. Compliance with atypical antipsychotics has been demonstrated to be higher than that with conventional antipsychotics.23 In double-blind studies, lower dropout rates were noted among atypical antipsychotic–treated patients compared with those treated with conventional agents.29,34,43
NEUROLEPTIC MALIGNANT SYNDROME
A rare disorder, neuroleptic malignant syndrome (NMS) is a potentially life-threatening condition. Associated with conventional antipsychotic use, NMS can evolve rapidly at any time during the course of treatment, and symptoms can include fever, rigidity, myoclonus, tachycardia, hypertension, autonomic instability, diaphoresis, and sialorrhea. Laboratory investigations often reveal elevations in white blood cell (WBC) counts and liver function test results, and pronounced elevations in creatine kinase. Renal failure can result from temperature elevations and the creatine kinase release arising from muscle rigidity and hypermetabolism. Management is aggressive, involving discontinuation of the offending agent, hydration, muscle relaxants, and dopamine agonists along with monitoring of the patient in the intensive care setting. Dialysis may be required until renal function is restored, but may become permanent if irreversible renal dysfunction results.
Atypical antipsychotics may be associated with lower risks of NMS. To date, 30 cases of NMS have been related to clozapine use; 26 involved risperidone; 8 involved olanzapine; and 1 involved quetiapine.87,88 The extent of the association between NMS and atypical antipsychotic use has, as yet, to be borne out from long-term studies of clinical use.
NEUROLOGIC DISORDERS
Psychotic symptoms can emerge in the context of neurologic conditions, e.g., Parkinson's disease and Huntington's disease, and can pose a vexing problem regarding management. This is particularly true in the management of Parkinson's disease, in which use of conventional antipsychotics such as haloperidol or fluphenazine can further compromise motor disturbances. Because of the reduced risk of associated EPS, the atypical antipsychotics may offer particular advantages in the management of psychosis associated with Parkinson's disease. Initial investigations have suggested a role for clozapine,89–91 risperidone,92,93 olanzapine,94,95 and quetiapine.96 However, the adverse effects associated with worsening of parkinsonism led to substantial rates of medication discontinuation in some investigations.90,91,93,94,96
Huntington's disease, by contrast, has traditionally been treated with conventional antipsychotics, e.g., haloperidol and pimozide. These agents have been effective in reducing the choreiform movements characteristic of the disorder. There may be a role for risperidone in Huntington's disease as well,97–100 although its efficacy relative to conventional agents has, as yet, to be clarified. Nonetheless, it may prove to be a viable alternative in those patients unable to tolerate conventional antipsychotics.
DOSING, SIDE EFFECTS, AND DRUG INTERACTIONS
Clozapine
Clozapine was the first atypical antipsychotic marketed in the United States. Since its initial use in 1990, it has demonstrated clinical utility for patients with schizophrenia unresponsive to conventional antipsychotics, patients with marked negative symptoms, patients with severe untoward effects on conventional antipsychotics, and patients with tardive dyskinesia. It is available in tablet form from Novartis Pharmaceuticals Corp. Dosing is generally begun at 12.5 mg/day, increased to 25 mg on day 2, then to 25 mg b.i.d. on day 3, then increased an initial 25 mg every 3 days thereafter. The recommended dose range is 300 to 900 mg/day.
Some of the side effects associated with clozapine use are summarized in Table 2. Sedation is most common, particularly with the onset of treatment and/or dose increases, although it tends to be mild and transient. Tolerance appears to develop to sedation.101 If the bulk of the daily dose is administered at bedtime, the sedative effects may enhance sleep. Reduction in doses of, or elimination of, coadministered CNS depressants and sedative-hypnotics may reduce the risk of any additive sedative effects. Another common adverse effect associated with clozapine use is increased salivation. Patients report awakening from sleep with a wet pillow.102 Sialorrhea can be reduced by minimizing administered doses of clozapine or by administering a clonidine patch.103
Despite its ability to trigger hypersalivation, clozapine is strongly anticholinergic. Consequently, its use results in nausea, vomiting, constipation, bloating, and abdominal pain.104 Patients should be advised to ensure that they drink adequate amounts of fluids, supplement their diet with high-fiber foods, and use bulk-forming agents or stool softeners as needed. Weight gain can be particularly problematic.104,105
The most serious side effects associated with clozapine use include agranulocytosis, cardiorespiratory arrest, and seizures. The risk of agranulocytosis has been estimated to be between 1% and 2%.106 From 1990 to 1994, the death rate associated with clozapine-induced agranulocytosis in the United States was approximately 3%. The risk of agranulocytosis appears to be highest in the first 3 months of treatment, increases with age, and appears to be higher in women.107,108 Consequently, patients treated with clozapine are required to have weekly complete blood count testing. The WBC count must be maintained at or above 3000 mm3, and the absolute neutrophil count must be maintained at or above 1500/mm3. If patients maintain acceptable WBC and neutrophil parameters, blood sampling can be reduced to once every 2 weeks after the first 6 months of continuous clozapine therapy. Clozapine availability is, therefore, contingent upon compliance with a distribution system that ensures WBC testing prior to the delivery of the patient's supply of medication for the following week.109
Clearly, patients who are treated with clozapine need to be monitored for signs of infection continuously. The presence of fever, chills, sore throat, urinary frequency or burning, or upper respiratory tract infection would immediately warrant a WBC count and differential to rule out agranulocytosis. Evidence of significant neutropenia would warrant discontinuation of clozapine, admission to the hospital, and antibiotic treatment. Patients who have significant agranulocytosis, WBC count below 3000 mm3, and/or absolute neutrophil count below 1500/mm3 would likely require discontinuation of the clozapine until blood cell parameters are restored to normal. It may take as many as 4 weeks until hematologic parameters are restored to normal. In some cases, human granulocyte colony–stimulating factor has been employed in cases of significant agranulocytosis to facilitate restoration of hematologic recovery.110
Cardiorespiratory collapse associated with clozapine has been reported,111,112 but the etiology appears to be unclear. In some cases, it may have been because of the hypotensive effects of clozapine,113 excessively stressing an already compromised cardiovascular system. In other cases, cardiomyopathy may have occurred during the course of (perhaps resulting from) clozapine treatment.111 Clinicians ought to be aware that concomitant use of benzodiazepines or other sedating medications with clozapine may increase the risk of cardiorespiratory compromise.114
Seizures have been associated with clozapine use. While the risk of seizures appears to be increased in those with preexisting seizure disorders or neurologic insults, the likelihood of seizures appears to be dose related and increases with rapid dose escalations.115 Daily doses of clozapine of less than 300 mg/day have a seizure risk comparable with that of traditional antipsychotics, i.e., 1% to 2%. However, doses of 300 to 600 mg/day are associated with an increased seizure risk, approximately 3% to 4%, while doses of 600 to 900 mg/day have a seizure risk of 4% to 6%.116 To minimize the risk of seizures, dose increases should be gradual, the maximum daily dose should be kept below 300 mg/day if at all possible, and electroencephalograph monitoring may be required in cases in which doses exceeding 300 mg/day are required. In those persons who have preexisting seizure disorders, anticonvulsants may also be required. The concomitant use of carbamazepine with clozapine is ill-advised, as there is an increased risk of agranulocytosis. Use of alternate anticonvulsants, such as valproate, is likely suitable.
Fever associated with clozapine use can present a vexing clinical situation. Benign, transient fever can occur early in the course of treatment with clozapine, unrelated to infection and agranulocytosis.20 Nonetheless, the presence of fever would warrant physical examination along with complete blood count, blood cultures, urine cultures, throat cultures, and chest x-ray to establish whether an infection exists. Benign fever associated with clozapine use is often responsive to antipyretic agents. Significant fever, i.e., greater than 102·F, can signal NMS, warranting appropriate physical examination and laboratory investigations, including assessment of stability of autonomic signs, rigidity, delirium, liver function tests, and WBC counts.
The primary care physician treating the patient prescribed with clozapine must be vigilant to a number of potential side effects and toxicities associated with clozapine use. Regular, periodic physical examinations and frequent WBC monitoring are required. Patient education is a must, since patients must understand the importance of notifying the physician of any symptoms suggesting serious untoward effects, e.g., fever, chills, symptoms of infection, seizures, syncope, dyspnea, chest discomfort. Furthermore, caution is advised to avoid the potential of coadministration of medications that may contribute to untoward effects, such as benzodiazepines, carbamazepine, and antihypertensive agents.
Risperidone
Risperidone was the second of the atypical antipsychotics marketed in the United States. Similar to clozapine, it has demonstrated clinical efficacy beyond that of conventional antipsychotics in the treatment of refractory illnesses as well as management of negative symptoms. It affords a major advantage over clozapine: without the risk of agranulocytosis, there is no need for weekly blood monitoring. It is available in liquid as well as tablet formulations from Janssen Pharmaceutica. Dosing is generally begun at 1 mg b.i.d., increased to 2 mg b.i.d. on day 2 then to 3 mg b.i.d. on day 3. While clinical trials assessing the efficacy of risperidone have included doses up to 16 mg/day, the recommended daily dose for most individuals is between 4 and 8 mg/day.29
As compared with conventional antipsychotic use, risperidone use carries with it a decreased risk of EPS.117,118 As noted previously, the propensity toward EPS with risperidone appears to be dose related. At doses exceeding the usual recommended daily dose (16 mg), significantly higher rates of EPS were encountered as compared with placebo.28 The risk of risperidone-associated EPS is reduced if doses are maintained within the recommended dosage range.
The most problematic side effects (see Table 2) include sedation, weight gain, and sexual side effects due to alteration of CNS prolactin release. Sedation appears to be common early in the course of treatment and increases with higher doses. As with each of the atypical antipsychotics, weight gain can occur with risperidone use. Patients may need to be advised about altering dietary habits and incorporating exercise into the usual daily regimen to reduce the potential morbidity and medical complications associated with weight gain. Significant weight increases can adversely effect self-image and lead to noncompliance. The risk of weight gain appears to be less for risperidone than either clozapine or olanzapine.119
Hypothalamic dopamine exerts an inhibitory effect on pituitary prolactin release. As with more conventional antipsychotics, risperidone can lead to hyperprolactinemia through its blockade of dopamine receptors. For women, increases in serum prolactin can result in amenorrhea, galactorrhea, and decreased libido. For men, hyperprolactinemia can result in erectile and ejaculatory dysfunction and decreased libido.120 However, there is no correlation between degree of hyperprolactinemia and side effect rates.51,120 Thus, periodic inquiry into sexual side effects associated with risperidone use is warranted, since they can adversely affect patient compliance. Generally, management of sexual side effects is possible with risperidone dose reductions or the use of alternate antipsychotic agents, such as olanzapine. The addition of prolactin-lowering agents, e.g., bromocriptine, may prove to be hazardous in that these can exacerbate psychosis.
Risperidone has been associated with alteration of cardiac conduction, i.e., prolongation of the corrected QT interval. While this is of minimal clinical significance, primary care physicians ought to be aware of the possibility that the use of risperidone can lead to tachyarrhythmias in patients who have preexisting cardiac conduction disturbances.121
Concomitant administration of other medications can alter risperidone clearance, requiring dosing adjustments. For example, carbamazepine increases risperidone clearance, perhaps requiring higher risperidone doses to effectively mitigate psychotic symptoms, while fluoxetine decreases clearance, perhaps requiring risperidone dose reduction to avoid side effects.
Olanzapine
Olanzapine is dosed between 5 and 20 mg daily. It is provided in unmarked tablet formulations from Eli Lilly; however, several tablet strengths are available for use. Given its half-life of 30 hours, once-daily dosing has been advocated to facilitate treatment compliance. A rapidly dissolving formulation has recently been marketed (Zyprexa Zydis), which dissolves quickly upon contact with saliva within the mouth. Although more costly than the customary tablet formulations, this quick-dissolving formulation is advocated for patients who experience difficulty swallowing pills, for example, because of dysphagia, and those individuals who may attempt to undermine treatment by “cheeking” and spitting out medications administered to them. This tablet is available only in 5- and 10-mg doses. Generally, patients are initiated at 5 mg/day and the dose is gradually increased up to a maximum of 20 mg/day. Common adverse effects associated with olanzapine use include postural hypotension, dizziness, constipation, and weight gain. Of these, weight gain has been the most significant; an average 26-lb weight gain by the end of 1 year of clinical trials has been reported.122 Certainly, weight gain can be a disincentive to comply with treatment and complicates comorbid medical conditions, such as obesity and heart disease. Olanzapine use has also been linked to disturbances in glucose regulation, hyperglycemia, and diabetic ketoacidosis and increases in triglyceride levels.123–127 Consequently, patients with diabetes will need to have more careful monitoring of their blood glucose and hemoglobin A1C levels if administered olanzapine. In nondiabetics, periodic monitoring of triglycerides, serum glucose level, and weight would be indicated. There have been asymptomatic elevations in hepatic enzymes in olanzapine-treated patients, but the significance of such elevations remains uncertain.122 Nonetheless, caution is advised for patients with preexisting hepatic disease including hepatitis, cirrhosis, and significant alcohol abuse histories.
Increased olanzapine clearance has been associated with concomitant administration of omeprazole, rifampin, and carbamazepine. Decreased olanzapine clearance has been noted among patients coadministered fluvoxamine.
Quetiapine
The most recent atypical antipsychotic to be approved by the U.S. Food and Drug Administration (FDA) for treatment of psychotic disorders is quetiapine. Dosing is usually begun at 25 mg b.i.d. or t.i.d. and increased by 25 to 50 mg b.i.d. every 1 to 2 days until a daily dose of 300 mg is achieved by the end of 1 week. Generally, the effective dose ranges between 300 and 500 mg daily in the acutely ill psychiatric patient. Quetiapine is generally well tolerated. The most frequent adverse effects associated with its use include sedation, fatigue, dizziness, and orthostatic hypotension.128 Quetiapine has been noted to have significant blockade of histamine (H1) receptors, which accounts for both its sedating effect as well as its effect on weight gain. Similar to other atypical agents, weight gain with quetiapine use can be quite troublesome to patients and can interfere with medication compliance. Unlike risperidone, quetiapine has little effect on prolactin release.35 Recently, a rare case of delirium attributed to quetiapine use was reported.129
Cataracts developed in canines, but not monkeys, subjected to quetiapine. It is unclear if cataracts develop in humans treated with quetiapine for any extended period. Regular ocular exams are recommended when quetiapine is employed. Patients with preexisting ocular difficulties, such as cataracts and lens deformities, require careful documentation of the extent of visual decrements at treatment outset and more frequent monitoring.130 Because the literature on quetiapine has thus far been limited, details of its side effect profile and untoward effects have as yet to be clarified in more elaborate clinical trials.
Increased quetiapine clearance has been associated with coadministration of phenytoin and thioridazine. Ketoconazole, fluconazole, and erythromycin decrease quetiapine clearance, necessitating quetiapine dose reductions to avoid toxicity.129
Sertindole
Although sertindole has demonstrated efficacy in the management of positive and negative symptoms of psychotic disorders,131 Abbott Laboratories withdrew its licensing application from the FDA for the drug. Sertindole was demonstrated to produce QT interval prolongation among treated patients, although there were no reported cases of torsades de pointes and few cardiac adverse effects associated with its use.132 Nonetheless, extensive investigation to assure safety of sertindole would have been required before licensing would have been authorized; such action appears unlikely given the issues of cardiac safety.
ATYPICAL ANTIPSYCHOTICS AND THE ELDERLY
By virtue of their favorable side effect profiles, the atypical antipsychotics have been safely employed in elderly patients with schizophrenia and other psychotic disorders,133–135 as well as in those manifesting behavioral disturbances associated with dementia.136–138 Elderly patients are more vulnerable to untoward side effects, particularly the extrapyramidal, anticholinergic, and αadrenergic side effects of antipsychotics. The elderly are particularly vulnerable to EPS and tardive dyskinesia associated with conventional antipsychotic use.139 Thus, the atypical antipsychotics may prove to be safer alternatives to conventional agents. Nonetheless, the anticholinergic side effects associated with atypical antipsychotics can produce confusion, impair memory, and exacerbate the cognitive dysfunction of dementia. In addition, highly anticholinergic agents can increase the risk of constipation, ileus, and urinary retention, particularly in men with benign prostatic hypertrophy. α-Adrenergic effects can lead to orthostasis and increase the risk of falls. Atypical antipsychotics are therefore initiated at low doses and increased gradually in this population. The optimal daily doses are generally lower than those previously mentioned. Effective dose ranges suggested for elderly patients include 6.25 to 400 mg/day of clozapine140 (initiated at 6.25 or 12.5 mg/day with increments of 6.25–12.5 mg weekly),141 0.5 to 2 mg/day of risperidone (initiated at 0.25 or 0.5 mg/day and increased by 0.5 mg weekly),142 5 to 10 mg/day of olanzapine,143 and 100 to 300 mg/day of quetiapine (initiated at 25 mg daily or b.i.d. with increments of 25 to 50 mg weekly).144 In clinical settings, a particular patient may require doses lower or higher than those suggested here. Gradual dose increments may safeguard against development of serious side effects and allow the clinician and patient to determine the safest effective dose to be employed.
ATYPICAL AGENTS PENDING FDA APPROVAL
A number of new atypical antipsychotics are currently under investigation, including aripiprazole (Otsuka Pharmaceuticals), amisulpride (available in Europe), remoxipride (Astra Pharmaceuticals), zotepine (available in Japan and the United Kingdom), and ziprasidone (Pfizer Inc). The latter agent appears to have a pharmacologic profile similar to that of risperidone. Efficacy has been demonstrated in clinical trials comparing ziprasidone and haloperidol145 as well as long-term studies demonstrating reduced relapse rates among ziprasidone-treated patients.146 Side effects may include mild sedation, but preliminary evidence suggests that weight gain and hyperprolactinemia may be less of a problem with ziprasidone compared with other atypical antipsychotics.147 One potential advantage offered by ziprasidone, as compared with currently available atypicals, is that it may be available in formulations allowing for intramuscular injection.148 Hence, it may become useful in those individuals for whom oral administration is impossible, e.g., the agitated and aggressive patient who may be unwilling or unable to take the medication by mouth.
CONCLUSION
In contrast to the conventional antipsychotics, the atypical antipsychotics exert their clinical effects by influencing serotonin and dopamine in the CNS. Most of the atypical antipsychotics are considered the first-line treatment for psychosis associated with schizophrenia on the basis of data on treatment efficacy, better tolerability, and reduced risks of EPS. The exception, clozapine, is reserved as a second-line agent because of the potential for serious, life-threatening complications.
The atypical antipsychotics reduce positive and negative symptoms associated with psychotic disorders and may enhance restoration of functional adaptation and patient rehabilitation. The atypical antipsychotics appear promising for the treatment of psychosis associated with mood disorders, but this use requires further investigation.
The atypical agents can produce undesirable weight gain, most notably encountered among clozapine- and olanzapine-treated patients. Olanzapine can have an impact on patient triglyceride and serum glucose levels. Hence, patients with significant hypertriglyceridemia and diabetes may require more frequent monitoring and laboratory investigations. Among the atypical agents, risperidone appears to be most likely to produce EPS. In addition, secondary symptoms due to hyperprolactinemia appear to be more common among risperidone-treated patients compared with patients treated with other atypical agents. Although adverse effects derived from postmarketing analyses are least known about quetiapine, it appears to be quite sedating and can produce orthostasis. Routine visual monitoring of patients is required during quetiapine treatment, and significant visual disturbances may preclude its use. Although effective, treatment with clozapine is contingent upon compliance with routine WBC testing. The prescription and dispensing of clozapine has largely been handled by psychiatrists because of the requirement that candidates for clozapine treatment have documented refractory response to prior treatment and/or experienced severe consequences from prior antipsychotic exposure. Nonetheless, primary care physicians may be the first to detect and encounter complications associated with clozapine use, such as agranulocytosis, infection, and so on. Hence, close collaboration between psychiatrists and primary care physicians will be required.
Atypical antipsychotics require further investigation to assess long-term utility and safety. EPS, tardive dyskinesia, and NMS appear to be less of a risk with atypical antipsychotics. Furthermore, compliance with atypical agents appears to exceed rates of compliance encountered previously with conventional agents. The atypical agents appear to be safe among elderly patients in low doses, and there appear to be few drug interactions about which to be concerned.
Unfortunately, none of the atypical agents currently in use in the United States are available in injectable forms. Such a formulation would make it possible to administer the antipsychotic to those individuals for whom oral administration is impossible. Similarly, there are no depot injectable forms as there are among conventional antipsychotics (haloperidol, fluphenazine). Several new atypical antipsychotics are currently under development and/or in early stages of clinical trials. These agents may offer a broader range of available formulations and greater flexibility in medication administration than has thus far been possible.
Critics may argue that the cost of the atypical antipsychotics, as compared with conventional agents, is prohibitive. Yet, the data suggest that the cost of a reduced spectrum of psychotic symptoms, reduced suicide rate associated with psychosis, reduced rehospitalization rates, and enhanced quality of life may be well worth the higher prescription cost over the long term.149,150
Drug names: bromocriptine (Parlodel and others), carbamazepine (Tegretol and others), chlorpromazine (Thorazine and others), clonidine (Catapres and others), clozapine (Clozaril and others), erythromycin (Ery-Tab and others), fluconazole (Diflucan), fluoxetine (Prozac), fluvoxamine (Luvox), haloperidol (Haldol and others), ketoconazole (Nizoral and others), olanzapine (Zyprexa), omeprazole (Prilosec), phenytoin (Dilantin and others), pimozide (Orap), quetiapine (Seroquel), rifampin (Rifadin), risperidone (Risperdal), thioridazine (Mellaril and others).
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association. 1994 [Google Scholar]
- Andreasen NC, Olsen S. Negative vs positive schizophrenia definition and validation. Arch Gen Psychiatry. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- Hegarty JD, Baldessarini RJ, Tohen M, et al. One hundred years of schizophrenia: a meta-analysis of the outcome literature. Am J Psychiatry. 1994;151:1409–1416. doi: 10.1176/ajp.151.10.1409. [DOI] [PubMed] [Google Scholar]
- Caldwell CB, Gottesman II. Schizophrenics kill themselves too: a review of risk factors for suicide. Schizophr Bull. 1990;16:571–589. doi: 10.1093/schbul/16.4.571. [DOI] [PubMed] [Google Scholar]
- Drake RE, Gates C, Colton PG, et al. Suicide among schizophrenics: who is at risk? J Nerv Ment Dis. 1984;172:613–617. doi: 10.1097/00005053-198410000-00004. [DOI] [PubMed] [Google Scholar]
- Glazer WM, Dickson RA. Clozapine reduces violence and persistent aggression in schizophrenia. J Clin Psychiatry. 1998;59(suppl 3):8–14. [PubMed] [Google Scholar]
- Meltzer HY. Suicide in schizophrenia: risk factors and clozapine treatment. J Clin Psychiatry. 1998;59(suppl 3):15–20. [PubMed] [Google Scholar]
- Volavka J, Krakowski M. Schizophrenia and violence. Psychol Med. 1989;19:559–562. doi: 10.1017/s0033291700024144. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Rupp A, Keith S. The costs of schizophrenia. Psychiatr Clin North Am. 1993;16:413–423. [PubMed] [Google Scholar]
- Wyatt RJ, Henter ID. The effects of early and sustained intervention on the long-term morbidity of schizophrenia. J Psychiatr Res. 1998;32:169–177. doi: 10.1016/s0022-3956(97)00014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt S, Andreasen NC, Flaum M, et al. A longitudinal study of symptom dimensions in schizophrenia: predictions and patterns of change. Arch Gen Psychiatry. 1995;52:352–360. doi: 10.1001/archpsyc.1995.03950170026004. [DOI] [PubMed] [Google Scholar]
- Richelson E. Receptor pharmacology of neuroleptics: relation to clinical effects. J Clin Psychiatry. 1999;60(suppl 10):5–14. [PubMed] [Google Scholar]
- Expert Consensus Guideline Series: Treatment of Schizophrenia. J Clin Psychiatry. 1996;57(suppl 12B):1–58. [PubMed] [Google Scholar]
- Mason P, Harrison G, Glazebrook C, et al. The course of schizophrenia over 13 years: a report from the International Study of Schizophrenia (ISoS) coordinated by the World Health Organization. Br J Psychiatry. 1996;169:580–586. doi: 10.1192/bjp.169.5.580. [DOI] [PubMed] [Google Scholar]
- Kane JM. Treatment-resistant schizophrenic patients. J Clin Psychiatry. 1996;57(suppl 9):35–40. [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Kirkpatrick B, et al. Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151:20–26. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Breier A, Kirkpatrick B, et al. Positive and negative symptom response to clozapine in schizophrenic patients with and without deficit syndrome. Am J Psychiatry. 1998;155:751–760. doi: 10.1176/ajp.155.6.751. [DOI] [PubMed] [Google Scholar]
- Hong CJ, Chen JY, Chiu HJ, et al. A double-blind comparative study of clozapine versus chlorpromazine on Chinese patients with treatment-refractory schizophrenia. Int Clin Psychopharmacol. 1997;12:123–130. doi: 10.1097/00004850-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Kane JM, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine/benztropine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- Kumra S, Frazier JA, Jacobsen LK, et al. Childhood-onset schizophrenia: a double-blind clozapine-haloperidol comparison. Arch Gen Psychiatry. 1996;53:1090–1097. doi: 10.1001/archpsyc.1996.01830120020005. [DOI] [PubMed] [Google Scholar]
- Pickar D, Owen RR, Litman RE, et al. Clinical and biological response to clozapine in patients with schizophrenia. Arch Gen Psychiatry. 1992;49:345–353. doi: 10.1001/archpsyc.1992.01820050009001. [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Cramer J, Xu W, et al. A comparison of clozapine and haloperidol in hospitalized patients with refractory schizophrenia. N Engl J Med. 1997;337:809–815. doi: 10.1056/NEJM199709183371202. [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Dunn L, Peszke M, et al. Impact of clozapine on negative symptoms and on the deficit syndrome in refractory schizophrenia. Am J Psychiatry. 1999;156:88–93. doi: 10.1176/ajp.156.1.88. [DOI] [PubMed] [Google Scholar]
- Ceskova E, Svestka J. Double-blind comparison of risperidone and haloperidol in schizophrenic and schizoaffective psychoses. Pharmacopsychiatry. 1993;26:121–124. [PubMed] [Google Scholar]
- Chouinard G, Jones B, Remington G, et al. A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. J Clin Psychopharmacol. 1993;13:25–40. [PubMed] [Google Scholar]
- Claus A, Bollen J, De Cuyper H, et al. Risperidone versus haloperidol in the treatment of chronic schizophrenic patients: a multicenter double-blind comparative study. Acta Psychiatr Scand. 1992;85:295–305. doi: 10.1111/j.1600-0447.1992.tb01473.x. [DOI] [PubMed] [Google Scholar]
- Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151:825–835. doi: 10.1176/ajp.151.6.825. [DOI] [PubMed] [Google Scholar]
- Peuskens J. for the Risperidone Study Group. Risperidone in the treatment of patients with chronic schizophrenia: a multi-national, multi-centre, double-blind, parallel-group study versus haloperidol. Br J Psychiatry. 1995 166:712–726. [DOI] [PubMed] [Google Scholar]
- Wirshing DA, Marshall BD Jr, Green MF, et al. Risperidone in treatment-refractory schizophrenia. Am J Psychiatry. 1999;156:1374–1379. doi: 10.1176/ajp.156.9.1374. [DOI] [PubMed] [Google Scholar]
- Beasley CM Jr, Hamilton SH, Crawford AM, et al. Olanzapine versus haloperidol: acute phase results of the international double-blind olanzapine trial. Eur Neuropsychopharmacol. 1997;7:125–137. doi: 10.1016/s0924-977x(96)00392-6. [DOI] [PubMed] [Google Scholar]
- Beasley CM Jr, Tollefson G, Tran P, et al. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14:111–123. doi: 10.1016/0893-133X(95)00069-P. [DOI] [PubMed] [Google Scholar]
- Conley RR, Tamminga CA, Bartko JJ, et al. Olanzapine compared with chlorpromazine for treatment-resistant schizophrenia. Am J Psychiatry. 1998;155:914–920. doi: 10.1176/ajp.155.7.914. [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Beasley CM, Tran PV, et al. Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry. 1997;154:457–465. doi: 10.1176/ajp.154.4.457. [DOI] [PubMed] [Google Scholar]
- Arvanitis LA, Miller BG. for the Seroquel Trial 13 Study Group. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. Biol Psychiatry. 1997 42:233–246. [DOI] [PubMed] [Google Scholar]
- Borison RL, Arvanitis LA, and Miller BG. for the US Seroquel Study Group. ICI 204,6336, an atypical anti-psychotic: efficacy and safety in a multicenter, placebo-controlled trial in patients with schizophrenia. J Clin Psychopharmacol. 1996 16:158–169. [DOI] [PubMed] [Google Scholar]
- Peuskens J, Link CGG. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Psychiatr Scand. 1997;96:265–273. doi: 10.1111/j.1600-0447.1997.tb10162.x. [DOI] [PubMed] [Google Scholar]
- Small JG, Hirsch SR, Arvanitis LA, et al. Quetiapine in patients with schizophrenia: a high- and low-dose double-blind study comparison with placebo. Arch Gen Psychiatry. 1997;54:549–557. doi: 10.1001/archpsyc.1997.01830180067009. [DOI] [PubMed] [Google Scholar]
- Emsley RA, McCreadie RG, Livingston M, et al. Risperidone in the treatment of first-episode patients with schizophreniform disorder: a double-blind multicenter study [abstract] Eur Neuropsychopharmacol. 1995;5:350. [Google Scholar]
- Robinson DG, Lieberman JA, Sheitman B, et al. Pilot study of atypical antipsychotic agents in first episode schizophrenia [abstract] Schizophr Res. 1997;24:196. [Google Scholar]
- Sanger TM, Lieberman JA, Tohen M, et al. Olanzapine versus haloperidol treatment in first-episode psychosis. Am J Psychiatry. 1999;156:79–87. doi: 10.1176/ajp.156.1.79. [DOI] [PubMed] [Google Scholar]
- Bradford DW, Chakos MH, Sheitman BB, et al. Atypical antipsychotic drugs in treatment-refractory schizophrenia. Psychiatr Ann. 1998;28:618–626. [Google Scholar]
- Tran PV, Tollefson GD, Sanger TM, et al. Olanzapine versus haloperidol in the treatment of schizoaffective disorder. Br J Psychiatry. 1999;174:15–22. doi: 10.1192/bjp.174.1.15. [DOI] [PubMed] [Google Scholar]
- Chengappa KNR, Sheth S, Brar JS, et al. Risperidone use at a state hospital: a clinical audit 2 years after the first wave of risperidone prescriptions. J Clin Psychiatry. 1999;60:373–378. [PubMed] [Google Scholar]
- Conley RR, Love RC, Kelly DL, et al. Rehospitalization rates of patients recently discharged on a regimen of risperidone or clozapine. Am J Psychiatry. 1999;156:863–868. doi: 10.1176/ajp.156.6.863. [DOI] [PubMed] [Google Scholar]
- Csernansky J, Okamoto A, and Brecher M. Risperidone vs haloperidol for prevention of relapse in schizophrenia and schizoaffective disorders: a long-term double-blind comparison. Presented at the 54th annual meeting of the Society of Biological Psychiatry; May 13–15, 1999; Washington, DC. [Google Scholar]
- Moore DB, Kelly DL, Sherr JD, et al. Rehospitalization rates for depot antipsychotics and pharmacoeconomic implications: comparison with risperidone. Am J Health Syst Pharm. 1998;55(24, suppl 4):S17–S19. doi: 10.1093/ajhp/55.suppl_4.S17. [DOI] [PubMed] [Google Scholar]
- Bondolfi G, Dufour H, Patris M, et al. Risperidone versus clozapine in treatment-resistant chronic schizophrenia: a randomized double-blind study. Am J Psychiatry. 1998;155:499–504. doi: 10.1176/ajp.155.4.499. [DOI] [PubMed] [Google Scholar]
- Conley RR, Brecher M. for the Risperidone/Olanzapine Study Group. Risperidone versus olanzapine in patients with schizophrenia or schizoaffective disorder. Presented at the 11th annual congress of the European College of Neuropsychopharmacology; Oct 31–Nov 4, 1998; Paris, France. [Google Scholar]
- Klieser E, Lehmann E, Kinzler E, et al. Randomized, double-blind, controlled trial of risperidone versus clozapine in patients with chronic schizophrenia. J Clin Psychopharmacol. 1995;15(suppl 1):45–51. doi: 10.1097/00004714-199502001-00008. [DOI] [PubMed] [Google Scholar]
- Tran PV, Hamilton SH, Kuntz AJ, et al. Double blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol. 1997;17:407–418. doi: 10.1097/00004714-199710000-00010. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Selecting an atypical antipsychotic by combining clinical experience with guidelines from clinical trials. J Clin Psychiatry. 1999;60(suppl 10):31–41. [PubMed] [Google Scholar]
- Guille C, Sachs GS, Ghaemi SN. A naturalistic comparison of clozapine, risperidone, and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2000;61:638–642. doi: 10.4088/jcp.v61n0907. [DOI] [PubMed] [Google Scholar]
- Kimmel SE, Calabrese JR, Woyshville MJ, et al. Clozapine in treatment-refractory mood disorders. J Clin Psychiatry. 1994;55(9, suppl B):91–93. [PubMed] [Google Scholar]
- Rothschild AJ, Bates KS, Boehringer KL, et al. Olanzapine response in psychotic depression. J Clin Psychiatry. 1999;60:116–118. doi: 10.4088/jcp.v60n0208. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Narendran R, Tohen M, et al. Clinical predictors of acute response with olanzapine in psychotic mood disorders. J Clin Psychiatry. 1998;59:24–28. doi: 10.4088/jcp.v59n0106. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Tohen M, Baldessarini RJ. Clozapine in severe mood disorders. J Clin Psychiatry. 1995;56:411–417. [PubMed] [Google Scholar]
- Tollefson GD, Sanger TM, Lu Y, et al. Depressive signs and symptoms in schizophrenia: a prospective blinded trial of olanzapine and haloperidol. Arch Gen Psychiatry. 1998;55:250–258. doi: 10.1001/archpsyc.55.3.250. [DOI] [PubMed] [Google Scholar]
- Segal J, Berk M, Brook S. Risperidone compared with both lithium and haloperidol in mania: a double-blind randomized controlled trial. Clin Neuropharmacol. 1998;21:176–180. [PubMed] [Google Scholar]
- Tohen M, Sanger TM, McElroy SL, et al. Olanzapine versus placebo in the treatment of acute mania. Am J Psychiatry. 1999;156:702–709. doi: 10.1176/ajp.156.5.702. [DOI] [PubMed] [Google Scholar]
- Berk M, Ichim L, Brook S. Olanzapine compared to lithium in mania: a double-blind randomized controlled trial. Int Clin Psychopharmacol. 1999;14:339–343. doi: 10.1097/00004850-199911000-00003. [DOI] [PubMed] [Google Scholar]
- Aubry JM, Simon AE, Bertschy G. Possible induction of mania and hypomania by olanzapine or risperidone: a critical review of reported cases. J Clin Psychiatry. 2000;61:649–655. doi: 10.4088/jcp.v61n0910. [DOI] [PubMed] [Google Scholar]
- Galdi J. The causality of depression in schizophrenia. Br J Psychiatry. 1983;142:621–625. doi: 10.1192/bjp.142.6.621. [DOI] [PubMed] [Google Scholar]
- Glazer WM. Extrapyramidal side effects, tardive dyskinesia, and the concept of atypicality. J Clin Psychiatry. 2000;61(suppl 3):16–21. [PubMed] [Google Scholar]
- Egan MF, Apud J, Wyatt RJ. Treatment of tardive dyskinesia. Schizophr Bull. 1997;23:583–609. doi: 10.1093/schbul/23.4.583. [DOI] [PubMed] [Google Scholar]
- Kane JM, Woerner M, Borenstein M, et al. Integrating incident and prevalence of tardive-dyskinesia. Psychopharmacol Bull. 1986;22:254–258. [PubMed] [Google Scholar]
- Kane JM, Woerner M, Lieberman JA. Tardive dyskinesia: prevalence, incidence and risk factors. J Clin Psychopharmacol. 1988;8(suppl 4):52–56. [PubMed] [Google Scholar]
- Simpson GM, Lindenmayer JP. Extrapyramidal symptoms in patients treated with risperidone. J Clin Psychopharmacol. 1997;17:194–201. doi: 10.1097/00004714-199706000-00010. [DOI] [PubMed] [Google Scholar]
- Beasley CM Jr, Tollefson GD, Tran PV. Safety of olanzapine. J Clin Psychiatry. 1997;58(suppl 10):13–17. [PubMed] [Google Scholar]
- Ho B-C, Miller D, Nopoulos P, et al. A comparative effectiveness study of risperidone and olanzapine in the treatment of schizophrenia. J Clin Psychiatry. 1999;60:658–663. doi: 10.4088/jcp.v60n1003. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G, Zipursky RB, et al. The D2 dopamine receptor occupancy of risperidone and its relationship to extrapyramidal symptoms: a PET study. Life Sci. 1995;57:103–107. doi: 10.1016/0024-3205(95)02037-j. [DOI] [PubMed] [Google Scholar]
- Farde L, Nordstrom AL, Wiesel FA, et al. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine: relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5HT2 and D2 receptor occupancy of clozapine, risperidone and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky RB, Remington G, et al. 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry. 1998;155:921–928. doi: 10.1176/ajp.155.7.921. [DOI] [PubMed] [Google Scholar]
- Remington G, Kapur S. D2 and 5-HT2 receptor effects of antipsychotics: bridging basic and clinical findings using PET. J Clin Psychiatry. 1999;60(suppl 10):15–19. [PubMed] [Google Scholar]
- Beasley CM, Dellva MA, Tamura RN, et al. Randomised double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. Br J Psychiatry. 1999;174:23–30. doi: 10.1192/bjp.174.1.23. [DOI] [PubMed] [Google Scholar]
- Kane JM, Woerner MG, Pollack S, et al. Does clozapine cause tardive dyskinesia? J Clin Psychiatry. 1993;54:327–330. [PubMed] [Google Scholar]
- Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503–510. doi: 10.1192/bjp.158.4.503. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Luchins DJ. Effect of clozapine in severe tardive dyskinesia: a case report. J Clin Psychopharmacol. 1984;4:286–287. [PubMed] [Google Scholar]
- Small JG, Milstein V, Marhenke JD, et al. Treatment outcome with clozapine in tardive dyskinesia, neuroleptic sensitivity, and treatment-resistant psychosis. J Clin Psychiatry. 1987;48:263–267. [PubMed] [Google Scholar]
- Spivak B, Mester R, Abesgaus J, et al. Clozapine treatment for neuroleptic-induced tardive dyskinesia, parkinsonism, and chronic akathisia in schizophrenic patients. J Clin Psychiatry. 1997;58:318–322. doi: 10.4088/jcp.v58n0706. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Thaker GK, Moran M, et al. Clozapine in tardive dyskinesia: observations from human and animal model studies. J Clin Psychiatry. 1994;55(9, suppl 9B):102–106. [PubMed] [Google Scholar]
- Gilbert PL, Harris MJ, McAdams LA, et al. Neuroleptic withdrawal in schizophrenic patients. Arch Gen Psychiatry. 1995;52:173–188. doi: 10.1001/archpsyc.1995.03950150005001. [DOI] [PubMed] [Google Scholar]
- Kane JM. Treatment of schizophrenia. Schizophr Bull. 1987;13:133–156. doi: 10.1093/schbul/13.1.133. [DOI] [PubMed] [Google Scholar]
- Corrigan PW, Liberman RP, Engel JD. From noncompliance to collaboration in the treatment of schizophrenia. Hosp Community Psychiatry. 1990;41:1203–1211. doi: 10.1176/ps.41.11.1203. [DOI] [PubMed] [Google Scholar]
- Van Putten T. Why do schizophrenic patients refuse to take their drugs? Arch Gen Psychiatry. 1974;31:67–72. doi: 10.1001/archpsyc.1974.01760130049008. [DOI] [PubMed] [Google Scholar]
- Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatr Ann. 2000;30:314–321. [Google Scholar]
- Karagianis JL, Phillips LC, Hogan KP, et al. Clozapine-associated neuroleptic malignant syndrome: two new cases and a review of the literature. Ann Pharmacother. 1999;33:623–630. doi: 10.1345/aph.18286. [DOI] [PubMed] [Google Scholar]
- Auzou P, Ozsancak C, Hannequin D, et al. Clozapine for the treatment of psychosis in Parkinson's disease: a review. Acta Neurol Scand. 1996;94:329–336. doi: 10.1111/j.1600-0404.1996.tb07075.x. [DOI] [PubMed] [Google Scholar]
- Friedman JH, Lannon MC, Stewart RL, et al. Low dose clozapine for the treatment of drug-induced psychosis (DIP) in idiopathic Parkinson's disease (PD): results of the double-blind, placebo-controlled PSYCLOPS trial [abstract] Neurology. 1998;50(suppl 4):A70. [Google Scholar]
- Wolters EC, Hurwitz TA, Mak E, et al. Clozapine in the treatment of parkinsonian patients with dopaminomimetic psychosis. Neurology. 1990;40:832–834. doi: 10.1212/wnl.40.5.832. [DOI] [PubMed] [Google Scholar]
- Ford B, Lynch T, Greene P. Risperidone in Parkinson's disease. Lancet. 1994;344:681. doi: 10.1016/s0140-6736(94)92114-8. [DOI] [PubMed] [Google Scholar]
- Rich SS, Friedman JH, Ott BR. Risperidone versus clozapine in the treatment of psychosis in six patients with Parkinson's disease and other akinetic-rigid syndromes. J Clin Psychiatry. 1995;56:556–559. [PubMed] [Google Scholar]
- Aarsland D, Larsen JP, Lim NG, et al. Olanzapine for psychosis in patients with Parkinson's disease with and without dementia. J Neuropsychiatry Clin Neurosci. 1999;11:392–394. doi: 10.1176/jnp.11.3.392. [DOI] [PubMed] [Google Scholar]
- Wolters EC, Jansen EN, Tuynman-Qua HG, et al. Olanzapine in the treatment of dopaminomimetic psychosis in patients with Parkinson's disease. Neurology. 1996;47:1085–1087. doi: 10.1212/wnl.47.4.1085. [DOI] [PubMed] [Google Scholar]
- Fernandez HH, Friedman JH, Jacques C, et al. Quetiapine for the treatment of drug-induced psychosis in Parkinson's disease. Mov Disord. 1999;14:484–487. doi: 10.1002/1531-8257(199905)14:3<484::aid-mds1016>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Dallocchio C, Tineeli C, Mazzerello P. Effectiveness of risperidone in Huntington chorea patients. J Clin Psychopharmacol. 1999;19:101–103. doi: 10.1097/00004714-199902000-00020. [DOI] [PubMed] [Google Scholar]
- Madhusoodanan S, Brenner R. Use of risperidone in psychosis associated with Huntington's disease. Am J Geriatr Psychiatry. 1998;6:347–349. [PubMed] [Google Scholar]
- Madhusoodanan S, Brenner R, Moise D, et al. Psychiatric and neuropsychological abnormalities in Huntington's disease: a case study. Ann Clin Psychiatry. 1998;10:117–120. doi: 10.1023/a:1022302305262. [DOI] [PubMed] [Google Scholar]
- Parsa MA, Szigethy E, Voci JM, et al. Risperidone in treatment of choreoathetosis of Huntington's disease. J Clin Psychopharmacol. 1997;17:134–135. doi: 10.1097/00004714-199704000-00023. [DOI] [PubMed] [Google Scholar]
- VanderZwaag C, McGee M, McEvoy JP, et al. Response of patients with treatment-refractory schizophrenia to clozapine within three serum level ranges. Am J Psychiatry. 1996;153:1579–1584. doi: 10.1176/ajp.153.12.1579. [DOI] [PubMed] [Google Scholar]
- Marinkovic D, Timotijevic I, Babinski T, et al. The side effects of clozapine: a four year follow-up study. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:537–544. doi: 10.1016/0278-5846(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Lieberman JA. Maximizing clozapine therapy: managing side effects. J Clin Psychiatry. 1998;59(suppl 3):38–43. [PubMed] [Google Scholar]
- Cohen S, Chiles J, MacNaughton A. Weight gain associated with clozapine. Am J Psychiatry. 1990;147:503–504. doi: 10.1176/ajp.147.4.503. [DOI] [PubMed] [Google Scholar]
- Leadbetter R, Shutty M, Pavalonis D, et al. Clozapine-induced constipation [letter] Am J Psychiatry. 1992;149:68–72. doi: 10.1176/ajp.149.1.68. [DOI] [PubMed] [Google Scholar]
- Honigfeld G. The Clozapine National Registry System: forty years of risk management. J Clin Psychiatry Monograph. 1996;14(2):29–32. [Google Scholar]
- Alvir JMJ, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. N Engl J Med. 1993;329:162–167. doi: 10.1056/NEJM199307153290303. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M, Park K. Mechanism of clozapine-induced agranulocytosis: current status of research and implications for drug development. CNS Drugs. 1997;7:139–158. doi: 10.2165/00023210-199707020-00005. [DOI] [PubMed] [Google Scholar]
- Clozaril (clozapine). Physicians' Desk Reference. 54th ed. Montvale, NJ: Medical Economics. 2000 2008–2012. [Google Scholar]
- Barnas C, Zwierzina H, Hummer M, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) treatment of clozapine-induced agranulocytosis: a case report. J Clin Psychiatry. 1992;53:245–247. [PubMed] [Google Scholar]
- Leo RJ, Kreeger JL, Kim KY. Cardiomyopathy associated with clozapine. Ann Pharmacother. 1996;30:603–605. doi: 10.1177/106002809603000606. [DOI] [PubMed] [Google Scholar]
- Povlsen UJ, Noring U, Fog R, et al. Tolerability and therapeutic effect of clozapine: a retrospective investigation of 216 patients treated with clozapine for up to 12 years. Acta Psychiatr Scand. 1985;71:176–185. doi: 10.1111/j.1600-0447.1985.tb01268.x. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Safferman AZ. Clinical profile of clozapine: adverse reactions and agranulocytosis. Psychiatr Q. 1992;63:51–70. doi: 10.1007/BF01064682. [DOI] [PubMed] [Google Scholar]
- Sassim N, Grohmann R. Adverse drug reactions with clozapine and simultaneous application of benzodiazepines. Pharmacopsychiatry. 1988;21:306–307. doi: 10.1055/s-2007-1016987. [DOI] [PubMed] [Google Scholar]
- Toth P, Frankenberg FR. Clozapine and seizures: a review. Can J Psychiatry. 1994;39:236–238. doi: 10.1177/070674379403900409. [DOI] [PubMed] [Google Scholar]
- Miller DD. Review and management of clozapine side effects. J Clin Psychiatry. 2000;61(suppl 8):14–17. [PubMed] [Google Scholar]
- Guitierrez-Esteinou R, Grebb JA. Risperidone: an analysis of the first three years in general use. Int Clin Psychopharmacol. 1997;12(suppl 4):3–10. [PubMed] [Google Scholar]
- Lemmens P, Brecher M, and Van Baelen B. Extrapyramidal symptoms in patients treated with risperidone. Presented at the 35th annual meeting of the American College of Neuropsychopharmacology; Dec 9–13, 1996; San Juan, Puerto Rico. [Google Scholar]
- Wirshing DA, Wirshing WC, Kysar L, et al. Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry. 1999;60:358–363. [PubMed] [Google Scholar]
- Kleinberg DL, Davis JM, de Coster R, et al. Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol. 1999;19:57–61. doi: 10.1097/00004714-199902000-00011. [DOI] [PubMed] [Google Scholar]
- Brecher MB, Lemmens P, and van Baelen B. Tolerability and cardiovascular safety of risperidone. In: New Research Program and Abstracts of the 150th Annual Meeting of the American Psychiatric Association; May 21, 1997; San Diego, Calif. Abstract NR612:233. [Google Scholar]
- Beasley CM. Safety of olanzapine. J Clin Psychiatry Monograph. 1997;15(2):19–21. [PubMed] [Google Scholar]
- Fertig MK, Brooks VG, Shelton PS, et al. Hyperglycemia associated with olanzapine [letter] J Clin Psychiatry. 1998;59:687–689. doi: 10.4088/jcp.v59n1208c. [DOI] [PubMed] [Google Scholar]
- Gatta B, Rigalleau V, Gin H. Diabetic ketoacidosis with olanzapine treatment [letter] Diabetes Care. 1999;22:1002–1003. doi: 10.2337/diacare.22.6.1002. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Sporn J, Brown S, et al. New-onset diabetes mellitus and diabetic ketoacidosis associated with olanzapine treatment. Psychosomatics. 1999;40:438–443. doi: 10.1016/S0033-3182(99)71210-7. [DOI] [PubMed] [Google Scholar]
- Lindenmayer J-P, Patel R. Olanzapine-induced ketoacidosis with diabetes mellitus [letter] Am J Psychiatry. 1999;156:1471. doi: 10.1176/ajp.156.9.1471. [DOI] [PubMed] [Google Scholar]
- Ober SK, Hudak R, Rusterholtz A. Hyperglycemia and olanzapine [letter] Am J Psychiatry. 1999;156:970. doi: 10.1176/ajp.156.6.970. [DOI] [PubMed] [Google Scholar]
- Garver DL. Review of quetiapine side effects. J Clin Psychiatry. 2000;61(suppl 8):31–33. [PubMed] [Google Scholar]
- Ike N. Delirium induced by quetiapine: a rare event? Psychiatr Ann. 2000;29:616–618. [Google Scholar]
- Seroquel (quetiapine). Physicians' Desk Reference. 54th ed. Montvale, NJ: Medical Economics. 2000 562–566. [Google Scholar]
- Van Kammen DP, McEvoy JP, Targum SD, and et al. A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia. Presented at the 32nd annual meeting of the American College of Neuropsychopharmacology; Dec 13–17, 1993; Honolulu, Hawaii. [Google Scholar]
- Daniel D, Swann A, Silber C, and et al. Long-term cardiovascular safety of sertindole. Presented at the 35th annual meeting of the American College of Neuropsychopharmacology; Dec 9–13, 1996; San Juan, Puerto Rico. [Google Scholar]
- Howanitz E, Pardo M, Smelson DA, and et al. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia. J Clin Psychiatry. 1999 60:41–44.Correction. 1999 60: 341. [DOI] [PubMed] [Google Scholar]
- Madhusoodanan S, Brecher M, Brenner R, et al. Risperidone in the treatment of elderly patients with psychotic disorder. Am J Geriatr Psychiatry. 1999;7:132–138. [PubMed] [Google Scholar]
- Reams SG, Sanger TM, Beasley CM Jr. Olanzapine in the treatment of elderly patients with schizophrenia and related disorders. Schizophr Res. 1998;29:151–152. [Google Scholar]
- Katz IR, Jeste DV, Mintzer JE, et al. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psychiatry. 1999;60:107–115. doi: 10.4088/jcp.v60n0207. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Yeung PP, Sweitzer DE, et al. Quetiapine may reduce hostility in patients with psychosis related to Alzheimer's disease. Schizophr Res. 1999;36:295–296. [Google Scholar]
- Street JS, Clark WS, Gannon KS, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing facilities: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2000;57:968–976. doi: 10.1001/archpsyc.57.10.968. [DOI] [PubMed] [Google Scholar]
- Masand PS. Side effects of antipsychotics in the elderly. J Clin Psychiatry. 2000;61(suppl 8):43–49. [PubMed] [Google Scholar]
- Sajatovic M. Clozapine for elderly patients. Psychiatr Ann. 2000;30:170–174. [Google Scholar]
- Lacro JP, Jeste DV. Geriatric psychosis. Psychiatric Q. 1997;68:247–260. doi: 10.1023/a:1025436207315. [DOI] [PubMed] [Google Scholar]
- Madhusoodanan S, Brenner R, Cohen CI. Risperidone for elderly patients with schizophrenia or schizoaffective disorder. Psychiatr Ann. 2000;30:175–180. [Google Scholar]
- Street JS, Tollefson GD, Tohen M, et al. Olanzapine for psychotic conditions in the elderly. Psychiatr Ann. 2000;30:191–196. [Google Scholar]
- Yeung PP, Tariot PN, Scheider LS, et al. Quetiapine for elderly patients with psychotic disorders. Psychiatr Ann. 2000;30:197–201. [Google Scholar]
- Goff DC, Posever T, Herz L, et al. An exploratory haloperidol-controlled dose-finding study of ziprasidone in hospitalized patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 1998;18:296–304. doi: 10.1097/00004714-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Arato M, O'Connor R, Bradbury JE, and et al. Long-term ziprasidone in schizophrenia. In: New Research Program and Abstracts of the 151st Annual Meeting of the American Psychiatric Association; June 3, 1998; Toronto, Ontario, Canada. Abstract NR464:193. [Google Scholar]
- Bender KJ. Antipsychotic evolution from A to Z: examining ziprasidone. Psychiatric Times Monograph (December). 1998 7–10. [Google Scholar]
- Brook S, Krams M, and Gunn KP. Intramuscular ziprasidone versus intramuscular haloperidol. In: New Research Program and Abstracts of the 151st Annual Meeting of the American Psychiatric Association; June 3, 1998; Toronto, Ontario, Canada. Abstract NR506:204. [Google Scholar]
- Meltzer HY. Outcome in schizophrenia: beyond symptom reduction. J Clin Psychiatry. 1999;60(suppl 3):3–7. [PubMed] [Google Scholar]
- Meltzer HY, Cola P, Way L, et al. Cost effectiveness of clozapine in neuroleptic-resistant schizophrenia. Am J Psychiatry. 1993;150:1630–1638. doi: 10.1176/ajp.150.11.1630. [DOI] [PubMed] [Google Scholar]