Abstract
Selective serotonin reuptake inhibitors (SSRIs) are widely prescribed to treat depression. Although these drugs presumably have the same mechanism of action, they vary in several clinically important ways, including how long they remain in the body and the extent to which they interfere with the metabolism of other medications. This article reviews the pharmacologic differences among SSRIs and how these differences may affect various aspects of treatment, such as dosing, administration, and discontinuation. Understanding the distinct properties of SSRIs may help primary care physicians to design the most appropriate therapeutic plan for individual patients.
Depression is as common as—and often more debilitating than—chronic medical ailments such as arthritis and diabetes.1,2 When left untreated or when treated improperly, depression can be a life-threatening condition. In addition to increasing the risk of suicide, it may hasten the onset or worsen the course of other serious illnesses. Patients who have suffered a myocardial infarction, for example, tend to die sooner if they also suffer from depression.3 Among older persons, depression appears to be an independent risk factor for both physical decline and premature death.4–6 In light of the significant morbidity and mortality associated with this disorder, vigilant evaluation and appropriate intervention should be health care priorities.
Most patients with symptoms of affective disorders seek treatment in primary care settings7; thus, primary care physicians play an important role in the diagnosis and management of depression. The introduction of selective serotonin reuptake inhibitors (SSRIs) more than a decade ago simplified the treatment of depression in primary care settings. Unlike tricyclic antidepressants (TCAs), the former standard of care, SSRIs have a broad therapeutic range. They also are relatively free of serious side effects, such as urinary retention and slowed cardiac conduction. Consequently, patients who are prescribed SSRIs do not require extensive pharmacologic monitoring (e.g., measurement of antidepressant blood levels, careful dose titration), which can be prohibitive in a highly time-constrained managed care environment.
SSRIs including citalopram, fluoxetine, fluvoxamine (which is approved for obsessive-compulsive disorder but is often used for depression), paroxetine, and sertraline are similarly efficacious8–13 but have distinct pharmacologic profiles. Understanding the differences among the SSRIs may help primary care physicians determine which agent to prescribe and what precautions to take when designing a treatment plan for an individual patient.
DISTINGUISHING FEATURES AMONG THE SSRIs
SSRIs are chemically diverse and thus differ from each other in several clinically important ways, including (1) how effective they are across their recommended dose range, (2) how efficiently they are metabolized across their dose range (kinetics), (3) how quickly they are eliminated from the body (half-life), (4) how patient age affects their elimination, and (5) how they affect the metabolism of other drugs (see Table 1). In addition, they may differ slightly in the way they affect various targets in the body (i.e., neuroreceptors). These individual differences can influence dosing and administration among general and special populations (e.g., the ill and the elderly), side effect profiles, safety during coadministration with other medications, discontinuation effects, and safety when switching from one SSRI to another (or another class of antidepressant).
Table 1.
Clinically Important Differences Between Selective Serotonin Reuptake Inhibitors
DOSING AND ADMINISTRATION
The Dose-Response Relationship
Recent data14 and widespread clinical observation suggest that sertraline, unlike the other SSRIs, is more effective at the higher end of its dose range than at its recommended starting dose of 50 mg/day. Thus, most patients who are prescribed sertraline will probably require upward dose adjustments. This is an important consideration, since administering insufficient doses of an antidepressant can result in treatment failure and unnecessary drug substitutions.
Kinetics
Fluoxetine and paroxetine (and possibly fluvoxamine) inhibit their own metabolism, which can lead to disproportionate increases in plasma levels (nonlinear kinetics) at higher doses.15 Among most healthy patients, the kinetic profile of these drugs does not appear to increase the frequency or severity of adverse events. However, as a precaution, physicians should prescribe reduced doses of fluoxetine, fluvoxamine, and paroxetine to patients whose ability to eliminate drugs is already substantially impaired (e.g., patients with severe liver or kidney disease).16–19 Plasma concentrations of sertraline and citalopram rise in direct proportion to dose; thus, these drugs may be better choices (at the low end of the dose range and with appropriate caution) for patients with significant kidney or liver dysfunction.
The human aging process is accompanied by reductions in liver and kidney function that can extend the half-life and increase the blood levels of many drugs, including some of the SSRIs. As shown in Table 1, dose adjustments are recommended when prescribing citalopram, paroxetine, and fluvoxamine to elderly patients.
Side Effects
In clinical trials, SSRIs have been well tolerated compared with placebo.44 Their relative lack of anticholinergic effects (e.g., constipation, urinary retention, blurred vision, confusion) and orthostatic effects makes them well suited for the treatment of depression among most adult patients, including the elderly. Their benign cardiovascular profile and broad therapeutic range make them relatively safe in overdose. Common side effects associated with SSRI therapy include nausea and sexual dysfunction.
Although the SSRIs are well tolerated as a class, their distinct secondary effects on the body (i.e., interactions with various neurotransmitter receptors) may produce slightly different side effect profiles.45 Among the SSRIs, paroxetine appears to cause the most sedation,46 fluvoxamine the most gastrointestinal upset,47 and fluoxetine the most short-term weight loss and activation (e.g., anxiety and agitation).45 Some of these side effects can be either advantageous or disadvantageous, depending on the circumstances. For example, significant weight loss may benefit obese patients but may be hazardous to patients who are frail. Likewise, activating effects can be helpful for patients with extreme psychomotor retardation but can lead to added distress and polypharmacy (e.g., combined therapy with benzodiazepines during the early stage of treatment) among patients with anxiety or panic disorder, which often coexist with depression. Although it is impossible to anticipate exactly how a given person will respond to a particular SSRI, consideration of possible differences in secondary effects may help the clinician to make the most favorable match between patient and drug.
ADMINISTERING SSRIs WITH OTHER DRUGS
Depression often requires months or even years of continuous pharmacotherapy. Thus, it is quite likely that many patients will take at least 1 other drug—be it an over-the-counter cough syrup, a nasal decongestant, or an antibiotic—with their SSRI at some time during treatment. For some patients (e.g., those with chronic medical conditions), polypharmacy is a daily necessity.
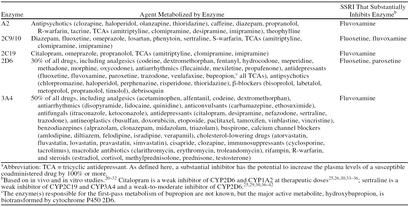
SSRIs are relatively safe when administered alone, but the risk of combining them with other medications varies significantly from agent to agent. Underlying this variability is the cytochrome P450 (CYP) system, a group of enzymes that metabolizes most marketed drugs. All of the SSRIs are extensively biotransformed by the P450 system, but fluoxetine, fluvoxamine, and paroxetine also significantly inhibit 1 or more of the major P450 enzymes.20–32 Therefore, these agents have the potential to impair the metabolism of a wide variety of medications (Table 2). In contrast, citalopram and sertraline do not substantially inhibit P450 enzymes.25,26,29,30,33–42
Table 2.
Cytochrome P450 (CYP) Enzymes That Are Substantially Inhibited by Selective Serotonin Reuptake Inhibitors (SSRIs)a
When initiating therapy with an SSRI, the single most important means of avoiding adverse drug interactions is to make a list of every medication the patient is taking. On the basis of this inventory and what is known about the P450 system, physicians can predict which antidepressants are least likely to conflict with an existing regimen.
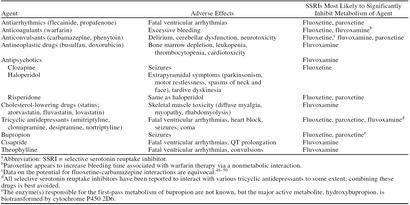
If a patient has already been prescribed an SSRI with a high potential for P450-mediated drug interactions, several steps can be taken to avoid problematic situations when other forms of therapy are initiated. The first step is to become familiar with the drugs that are most likely to interact with the particular SSRI in a clinically meaningful way. These include agents that become toxic with relatively minor elevations above the therapeutic dose (Table 3) or are inactive in their unmetabolized form (e.g., codeine). In either case, the best course is to select an alternative medication, if one is available. For example, ibuprofen or another nonopiate analgesic could be substituted for codeine to treat minor pain. Likewise, an antiarrhythmic drug not in class IC could be prescribed instead of propafenone. If a safer alternative does not exist, agents with a narrow therapeutic range that are quite likely to be affected by an SSRI should be started at a lower-than-usual dose, and the patient should be closely monitored for adverse reactions.
Table 3.
Examples of Medications With Narrow Therapeutic Rangesa
Self-Medication
Self-medication with over-the-counter preparations, leftover or borrowed prescription drugs, and alternative medicines (e.g., herbal remedies) is a common practice. Therefore, educating the patient about the risks of combining SSRIs (either individually or as a class) with other drugs is a critical component of safe and effective therapy. For example, patients taking fluvoxamine (for either depression or obsessive-compulsive disorder) should be cautioned against the use of benzodiazepines outside of a doctor's care, since interactions between fluvoxamine and benzodiazepines can cause oversedation.43 Among the elderly, who are frequently prescribed benzodiazepines, oversedation can lead to falls and fractures.
Two commonly self-administered drugs that have the potential to interact with all SSRIs are dextromethorphan, an ingredient in many cough syrups, and St. John's wort (Hypericum perforatum), an increasingly popular herbal antidepressant. Both agents affect serotonin (in fact, a constituent of St. John's wort appears to be a naturally occurring serotonin uptake inhibitor) and therefore may have additive effects when combined with SSRIs.51–53 In some instances, additive serotonergic effects may result in a toxic reaction (“serotonin syndrome”) marked by confusion, agitation, hyperthermia, myoclonus, and hyperreflexia. (Coprescribing SSRIs with monoamine oxidase inhibitors is contraindicated because of the possibility of such reactions.) To avoid potentially serious clinical situations, physicians should inform patients about both the risks and the warning signs of adverse interactions between SSRIs and other commonly used (and abused) serotonergic drugs, including meperidine and amphetamines.
Augmentation Strategies
Lithium and buspirone are commonly coprescribed with antidepressants to boost efficacy. Although lithium is not susceptible to P450-mediated drug interactions, it appears to have nonspecific serotonergic effects and therefore may interact with SSRIs in the manner described above. Buspirone is metabolized by the CYP3A4 enzyme, which is substantially inhibited by fluvoxamine.
Elderly Patients
The elderly, as a group, tend to take many medications on a daily basis. Because the aging body eliminates drugs less efficiently and is more sensitive to pharmacotherapeutic side effects, adverse reactions resulting from drug-drug interactions are not only more common but also potentially more severe and longer lasting in older patients. Choosing an agent with a low propensity for drug interactions is therefore especially important for the management of late-life depression. All drug combinations should be carefully monitored among elderly patients who are frail or medically ill.
DISCONTINUATION
Withdrawal effects can occur when any antidepressant is abruptly discontinued. For patients taking SSRIs, abrupt withdrawal can cause malaise, light-headedness, restlessness, sleep and sensory disturbances, and headache. Although not life-threatening, such symptoms can be distressing to the patient, since they may easily be mistaken for symptoms of returning depression.
The severity of SSRI withdrawal syndrome appears to vary according to the half-life of the drug. Fluoxetine, which has the longest half-life of the SSRIs (see Table 1), appears to produce the fewest withdrawal symptoms, while paroxetine, which has the shortest half-life, produces the most pronounced discontinuation effects.54 Thus, whereas all SSRIs should be discontinued over a 1-to 2-week period, the smallest stepwise decrements in dose should be used when withdrawing paroxetine. If abrupt discontinuation of any SSRI is medically necessary, patients should be monitored carefully and informed about withdrawal symptoms.
Occasionally, SSRIs may need to be discontinued because of adverse events. In these cases, a long half-life can be problematic. Patients who develop intolerable adverse symptoms while taking fluoxetine, for example, may suffer from these symptoms for several days or weeks while the drug and its metabolites are cleared from the body. In contrast, drug-induced adverse events produced by the other SSRIs most likely will resolve more quickly, since these agents are more rapidly cleared from the body (see Table 1).
SWITCHING ANTIDEPRESSANTS
Several strategies are available for patients who do not respond to the first trial of antidepressant pharmacotherapy, including augmentation (described above) and substitution. Switching from one SSRI to another appears to be effective in most cases,55 probably because of the significant chemical and pharmacologic differences between these agents. Intraclass switching appears to be a safe strategy as well (even when abrupt substitutions are medically necessary), since the SSRIs have a broad therapeutic range.
The risk of switching from an SSRI to an antidepressant from another class depends on the characteristics of both medications. Citalopram and sertraline, which have relatively little effect on the P450 system and wash out of the body relatively quickly, are the least likely of the SSRIs to interact with a replacement drug. Fluvoxamine and paroxetine carry a moderate risk of interaction because they inhibit major P450 enzymes but linger in the body for only a week or so after discontinuation. Fluoxetine, which (along with its active metabolite) potently inhibits relevant P450 enzymes and remains in the system far longer than the other SSRIs, is the agent most likely to interfere with the metabolism of a substitute antidepressant. Drugs to watch when switching from fluoxetine include TCAs, venlafaxine, and bupropion, each of which is metabolized to some extent by CYP2D6 and each of which is associated with potentially serious adverse events (cardiovascular toxicity, hypertension, and seizures, respectively) at elevated blood concentrations of drug.
CONCLUSION
Although the SSRIs are similarly efficacious for the treatment of depression, individual pharmacologic differences may make one SSRI more or less appropriate for a given patient or may dictate which precautions to take in a particular situation. An agent with linear kinetics may be more appropriate for patients with kidney or liver dysfunction, while an agent with a low potential for short-term weight loss may be more appropriate for frail patients. Among patients who take multiple medications, it is preferable to use agents with a low potential for drug interactions. Special attention should be paid to the individual characteristics of SSRIs when discontinuing therapy or when switching to another agent, since the length of the half-life can affect the severity of withdrawal symptoms and the likelihood that a potential inhibitor will affect the metabolism of the replacement drug. It is important to remember that each patient may respond to a given SSRI (or combination of an SSRI and another drug) differently and that it is not always possible to predict which agent will be the most effective for a given patient. However, understanding the individual characteristics of the SSRIs and keeping a watchful eye on a patient's response can help primary care physicians avoid or minimize potentially problematic clinical situations.
Drug names: alfentanil (Alfenta and others), alprazolam (Xanax and others), amitriptyline (Elavil and others), amlodipine (Lotrel, Norvasc), atorvastatin (Lipitor), bisoprolol (Zebeta), bupropion (Wellbutrin), buspirone (BuSpar), busulfan (Myleran), carbamazepine (Tegretol and others), chlorpromazine (Thorazine and others), cisapride (Propulsid), citalopram (Celexa), clarithromycin (Biaxin), clomipramine (Anafranil and others), clonazepam (Klonopin and others), clozapine (Clozaril and others), cyclosporine (Neoral, SangCya), desipramine (Norpramin and others), diazepam (Valium and others), diltiazem (Cardizem and others), disopyramide (Norpace), doxorubicin (Adriamycin, Doxil, and others), erythromycin (Ery-Tab and others), ethosuximide (Zarontin), etoposide (VePesid), felodipine (Lexxel), fentanyl (Actiq and others), flecainide (Tambocor), fluoxetine (Prozac), fluvastatin (Lescol), fluvoxamine (Luvox), haloperidol (Haldol and others), ibuprofen (Motrin and others), itraconazole (Sporanox), ketoconazole (Nizoral and others), labetalol (Normodyne, Trandate), lidocaine (Xylocaine and others), lithium (Eskalith and others), losartan (Cozaar), lovastatin (Mevacor), meperidine (Demerol and others), methylprednisolone (Medrol and others), metoprolol (Lopressor and others), mexiletine (Mexitil and others), midazolam (Versed), nefazodone (Serzone), nortriptyline (Pamelor and others), olanzapine (Zyprexa), omeprazole (Prilosec), oxycodone (OxyContin, Percocet, and others), paclitaxel (Taxol), paroxetine (Paxil), perphenazine (Trilafon and others), phenytoin (Dilantin and others), pravastatin (Pravachol), propafenone (Rythmol), propranolol (Inderal and others), quinidine (Quinaglute), rifampin (Rifadin and others), risperidone (Risperdal), sertraline (Zoloft), simvastatin (Zocor), tacrine (Cognex), tacrolimus (Prograf), tamoxifen (Nolvadex), testosterone (Androderm, Testoderm), theophylline (Aerolate, Marax, and others), timolol (Blocadren and others), triazolam (Halcion), troleandomycin (Tao), venlafaxine (Effexor), verapamil (Calan and others), vinblastine (Velban), vincristine (Oncovin), warfarin (Coumadin).
Acknowledgments
Support for this article was provided by Forest Laboratories, Inc.
REFERENCES
- Hays RD, Wells KB, Sherbourne CD, et al. Functioning and well-being outcomes of patients with depression compared with chronic general medical illnesses. Arch Gen Psychiatry. 1995;52:11–19. doi: 10.1001/archpsyc.1995.03950130011002. [DOI] [PubMed] [Google Scholar]
- Wells KB, Sherbourne CD. Functioning and utility for current health of patients with depression or chronic medical conditions in managed, primary care practices. Arch Gen Psychiatry. 1999;56:897–904. doi: 10.1001/archpsyc.56.10.897. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F, Juneau M, et al. Gender, depression, and one-year prognosis after myocardial infarction. Psychosom Med. 1999;61:26–37. doi: 10.1097/00006842-199901000-00006. [DOI] [PubMed] [Google Scholar]
- Rovner BW. Depression and increased risk of mortality in the nursing home patient. Am J Med. 1993;94:19S–22S. [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Ferrucci L, et al. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279:1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Geerlings SW, Deeg DJ, et al. Minor and major depression and the risk of death in older persons. Arch Gen Psychiatry. 1999;56:889–895. doi: 10.1001/archpsyc.56.10.889. [DOI] [PubMed] [Google Scholar]
- Preskorn SH. Outpatient Management of Depression: A Guide for the Primary-Care Practitioner. Caddo, Okla: Professional Communications. 1999 [Google Scholar]
- Ekselius L, von Knorring L, Eberhard G. A double-blind multicenter trial comparing sertraline and citalopram in patients with major depression treated in general practice. Int Clin Psychopharmacol. 1997;12:323–331. doi: 10.1097/00004850-199711000-00005. [DOI] [PubMed] [Google Scholar]
- Fava M, Amsterdam JD, Deltito JA, et al. A double-blind study of paroxetine, fluoxetine, and placebo in outpatients with major depression. Ann Clin Psychiatry. 1998;10:145–150. doi: 10.1023/a:1022337927842. [DOI] [PubMed] [Google Scholar]
- Aguglia E, Casacchia M, Cassano GB, et al. Double-blind study of the efficacy and safety of sertraline versus fluoxetine in major depression. Int Clin Psychopharmacol. 1993;8:197–202. doi: 10.1097/00004850-199300830-00010. [DOI] [PubMed] [Google Scholar]
- Patris M, Bouchard JM, Bougerol T, et al. Citalopram versus fluoxetine: a double-blind, controlled, multicentre, phase 3 trial in patients with unipolar major depression treated in general practice. Int Clin Psychopharmacol. 1996;11:129–136. [PubMed] [Google Scholar]
- De Wilde J, Spiers R, Mertens C, et al. A double-blind, comparative, multicentre study comparing paroxetine with fluoxetine in depressed patients. Acta Psychiatr Scand. 1993;87:141–145. doi: 10.1111/j.1600-0447.1993.tb03345.x. [DOI] [PubMed] [Google Scholar]
- Kiev A, Feiger A. A double-blind comparison of fluvoxamine and paroxetine in the treatment of depressed outpatients. J Clin Psychiatry. 1997;58:146–152. doi: 10.4088/jcp.v58n0402. [DOI] [PubMed] [Google Scholar]
- Cantrell R, Gillespie W, Altshuler L. Fluoxetine and sertraline dosages in major depression. Depress Anxiety. 1999;9:78–82. [PubMed] [Google Scholar]
- Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78:203–208. doi: 10.1111/j.1600-0773.1996.tb00206.x. [DOI] [PubMed] [Google Scholar]
- Dalhoff K, Almdal TP, Bjerrum K, et al. Pharmacokinetics of paroxetine in patients with cirrhosis. Eur J Clin Pharmacol. 1991;41:351–354. doi: 10.1007/BF00314966. [DOI] [PubMed] [Google Scholar]
- Prozac (fluoxetine). Physicians' Desk Reference. Montvale, NJ: Medical Economics. 1999 924–928. [Google Scholar]
- Luvox (fluvoxamine). Physicians' Desk Reference. Montvale, NJ: Medical Economics. 1999 3121–3124. [Google Scholar]
- Paxil (paroxetine). Physicians' Desk Reference. Montvale, NJ: Medical Economics. 1999 3078–3083. [Google Scholar]
- Iribarne C, Picart D, Dreano Y, et al. In vitro interactions between fluoxetine or fluvoxamine and methadone or buprenorphine. Fundam Clin Pharmacol. 1998;12:194–199. doi: 10.1111/j.1472-8206.1998.tb00941.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Nielsen TL, Brosen K. Fluvoxamine is a potent inhibitor of the metabolism of caffeine in vitro. Pharmacol Toxicol. 1998;83:240–245. doi: 10.1111/j.1600-0773.1998.tb01476.x. [DOI] [PubMed] [Google Scholar]
- von Moltke LL, Greenblatt DJ, Court MH, et al. Inhibition of alprazolam and desipramine hydroxylation in vitro by paroxetine and fluvoxamine: comparison with other selective serotonin reuptake inhibitor antidepressants. J Clin Psychopharmacol. 1995;15:125–131. doi: 10.1097/00004714-199504000-00008. [DOI] [PubMed] [Google Scholar]
- Jeppesen U, Rasmussen BB, Brosen K. Fluvoxamine inhibits the CYP2C19-catalytic bioactivation of chloroguanide. Clin Pharmacol Ther. 1997;62:279–286. doi: 10.1016/S0009-9236(97)90030-8. [DOI] [PubMed] [Google Scholar]
- Xu ZH, Xie HG, Zhou HH. In vivo inhibition of CYP2C19 but not CYP2D6 by fluvoxamine. Br J Clin Pharmacol. 1996;42:518–521. doi: 10.1046/j.1365-2125.1996.45319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosen K, Skjelbo E, Rasmussen BB, et al. Fluvoxamine is a potent inhibitor of cytochrome P4501A2. Biochem Pharmacol. 1993;45:1211–1214. doi: 10.1016/0006-2952(93)90272-x. [DOI] [PubMed] [Google Scholar]
- Skjelbo E, Brosen K. Inhibitors of imipramine metabolism by human liver microsomes. Br J Clin Pharmacol. 1992;34:256–261. doi: 10.1111/j.1365-2125.1992.tb04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandel S, Bertschy G, Baumann P, et al. Fluvoxamine and fluoxetine: interaction studies with amitriptyline, clomipramine and neuroleptics in phenotyped patients. Pharmacol Res. 1995;31:347–353. doi: 10.1016/1043-6618(95)80088-3. [DOI] [PubMed] [Google Scholar]
- Kashuba AD, Nafziger AN, Kearns GL, et al. Effect of fluvoxamine therapy on the activities of CYP1A2, CYP2D6, and CYP3A as determined by phenotyping. Clin Pharmacol Ther. 1998;64:257–268. doi: 10.1016/S0009-9236(98)90174-6. [DOI] [PubMed] [Google Scholar]
- Schmider J, Greenblatt DJ, von Moltke LL, et al. Inhibition of CYP2C9 by selective serotonin reuptake inhibitors in vitro: studies of phenytoin p-hydroxylation. Br J Clin Pharmacol. 1997;44:495–498. doi: 10.1046/j.1365-2125.1997.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belpaire FM, Wijnant P, Temmerman A, et al. The oxidative metabolism of metoprolol in human liver microsomes: inhibition by the selective serotonin reuptake inhibitors. Eur J Clin Pharmacol. 1998;54:261–264. doi: 10.1007/s002280050456. [DOI] [PubMed] [Google Scholar]
- Avenoso A, Spina E, Campo G, et al. Interaction between fluoxetine and haloperidol: pharmacokinetic and clinical implications. Pharmacol Res. 1997;35:335–339. [PubMed] [Google Scholar]
- Bergstrom RF, Peyton AL, Lemberger L. Quantification and mechanism of the fluoxetine and tricyclic antidepressant interaction. Clin Pharmacol Ther. 1992;51:239–248. doi: 10.1038/clpt.1992.18. [DOI] [PubMed] [Google Scholar]
- Gram LF, Hansen MG, Sindrup SH, et al. Citalopram: interaction studies with levomepromazine, imipramine, and lithium. Ther Drug Monit. 1993;15:18–24. [PubMed] [Google Scholar]
- Jeppesen U, Gram LF, Vistisen K, et al. Dose-dependent inhibition of CYP1A2, CYP2C19 and CYP2D6 by citalopram, fluoxetine, fluvoxamine and paroxetine. Eur J Clin Pharmacol. 1996;51:73–78. doi: 10.1007/s002280050163. [DOI] [PubMed] [Google Scholar]
- Priskorn M, Sidhu JS, Larsen F, et al. Investigation of multiple dose citalopram on the pharmacokinetics and pharmacodynamics of racemic warfarin. Br J Clin Pharmacol. 1997;44:199–202. doi: 10.1046/j.1365-2125.1997.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Yamamoto T, Chiba K, et al. The effects of selective serotonin reuptake inhibitors and their metabolites on S-mephenytoin 4′-hydroxylase activity in human liver microsomes. Br J Clin Pharmacol. 1995;40:481–485. doi: 10.1111/j.1365-2125.1995.tb05793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir V, Naranjo CA, Herrmann N, et al. The extent and determinants of changes in CYP2D6 and CYP1A2 activities with therapeutic doses of sertraline. J Clin Psychopharmacol. 1998;18:55–61. doi: 10.1097/00004714-199802000-00009. [DOI] [PubMed] [Google Scholar]
- Bhatara VS, Bandettini FC. Possible interaction between sertraline and tranylcypromine. Clin Pharm. 1993;12:222–225. [PubMed] [Google Scholar]
- Tremaine LM, Wilner KD, Preskorn SH. A study of the potential effect of sertraline on the pharmacokinetics and protein binding of tolbutamide. Clin Pharmacokinet. 1997;32:31–36. doi: 10.2165/00003088-199700321-00005. [DOI] [PubMed] [Google Scholar]
- Kurtz DL, Bergstrom RF, Goldberg MJ, et al. The effect of sertraline on the pharmacokinetics of desipramine and imipramine. Clin Pharmacol Ther. 1997;62:145–156. doi: 10.1016/S0009-9236(97)90062-X. [DOI] [PubMed] [Google Scholar]
- Solai LK, Mulsant BH, Pollock BG, et al. Effect of sertraline on plasma nortriptyline level in depressed elderly. J Clin Psychiatry. 1997;58:440–443. doi: 10.4088/jcp.v58n1006. [DOI] [PubMed] [Google Scholar]
- Alderman J, Preskorn SH, Greenblatt DJ, et al. Desipramine pharmacokinetics when coadministered with paroxetine or sertraline in extensive metabolizers. J Clin Psychopharmacol. 1997;17:284–291. doi: 10.1097/00004714-199708000-00008. [DOI] [PubMed] [Google Scholar]
- Sproule BA, Naranjo CA, Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions: a critical review of the evidence. Clin Pharmacokinet. 1997;33:454–471. doi: 10.2165/00003088-199733060-00004. [DOI] [PubMed] [Google Scholar]
- Masand PS, Gupta S. Selective serotonin-reuptake inhibitors: an update. Harv Rev Psychiatry. 1999;7:69–84. [PubMed] [Google Scholar]
- Stahl SM. Essential Psychopharmacology. 2nd ed. New York, NY: Cambridge University Press. 1999 [Google Scholar]
- Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996 16:1S–7S.discussion 7S–9S. [DOI] [PubMed] [Google Scholar]
- Haffmans PM, Timmerman L, and Hoogduin CA. for the LUCIFER Group. Efficacy and tolerability of citalopram in comparison with fluvoxamine in depressed outpatients: a double-blind, multicentre study. Int Clin Psychopharmacol. 1996 11:157–164. [DOI] [PubMed] [Google Scholar]
- Grimsley SR, Jann MW, Carter JG, et al. Increased carbamazepine plasma concentrations after fluoxetine coadministration. Clin Pharmacol Ther. 1991;50:10–15. doi: 10.1038/clpt.1991.98. [DOI] [PubMed] [Google Scholar]
- Pearson HJ. Interaction of fluoxetine with carbamazepine [letter] J Clin Psychiatry. 1990;148:1604–1605. [PubMed] [Google Scholar]
- Gidal BE, Anderson GD, Seaton TL, et al. Evaluation of the effect of fluoxetine on the formation of carabamazepine epoxide. Ther Drug Monit. 1993;15:405–409. doi: 10.1097/00007691-199310000-00008. [DOI] [PubMed] [Google Scholar]
- Gordon JB. SSRIs and St. John's wort: possible toxicity? [letter] Am Fam Physician. 1998;57:950, 953. [PubMed] [Google Scholar]
- Skop BP, Finkelstein JA, Mareth TR, et al. The serotonin syndrome associated with paroxetine, an over-the-counter cold remedy, and vascular disease. Am J Emerg Med. 1994;12:642–644. doi: 10.1016/0735-6757(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Lantz MS, Buchalter E, Giambanco V. St. John's wort and antidepressant drug interactions in the elderly. J Geriatr Psychiatry Neurol. 1999;12:7–10. doi: 10.1177/089198879901200103. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44:77–87. doi: 10.1016/s0006-3223(98)00126-7. [DOI] [PubMed] [Google Scholar]
- Nurnberg HG, Thompson PM, Hensley PL. Antidepressant medication change in a clinical treatment setting: a comparison of the effectiveness of selective serotonin reuptake inhibitors. J Clin Psychiatry. 1999;60:574–579. doi: 10.4088/jcp.v60n0902. [DOI] [PubMed] [Google Scholar]