Abstract
Background: This open-label portion of a 2-phase study assessed the effects of the antidepressant bupropion sustained release (SR) on health-related quality of life (QOL) and workplace productivity in patients with major depression.
Method: Patients (N = 816) with DSM-IV major depression were treated with bupropion SR, 300 mg/day, for 8 weeks. The Clinical Global Impressions scale for Improvement of Illness (CGI-I) was completed at weekly clinic visits. At baseline and week 8, QOL and productivity were assessed. QOL was assessed using the Quality of Life in Depression Scale (QLDS).
Results: QOL and productivity were significantly improved from baseline after 8 weeks of treatment with bupropion SR. Mean QLDS scores were 18.98 and 10.36 at baseline and week 8, respectively (mean change = 8.62; p < .001). At week 8 compared with baseline, patients working at a paid job reported missing 1.58 fewer hours of work because of depression during the past 7 days, being 14.6% more effective on the job, working at reduced effectiveness less often, and incurring 6.37 fewer hours of overall lost productivity (p < .001 each variable). Improvements in QOL and productivity were significantly (p < .001) greater in bupropion SR responders (i.e., those with CGI-I scores of “very much improved” or “much improved” during the last 3 weeks of open-label therapy) than in nonresponders.
Conclusion: Effective treatment of major depression with bupropion SR for 8 weeks is associated with improvements in QOL and reductions in lost workplace productivity. Patients who responded clinically to bupropion SR showed significantly greater improvements in these variables than those who did not respond.
Approximately 5% to 10% of patients visiting primary care practitioners meet diagnostic criteria for major depression, and up to 3 times as many suffer less severe depressive disorders.1 Regardless of the severity of depressive symptoms, their impact on the patient extends beyond abnormalities in mood and neurovegetative function to encompass multiple aspects of psychological, social, and physical function.2–6 For example, mental, social, and physical aspects of health-related quality of life as measured by the Medical Outcomes Study Short Form-36 Health Survey and similar instruments are impaired in patients with major depression compared with both the general population and patients suffering from other chronic diseases such as hypertension, diabetes, arthritis, and cardiovascular disease.2–4,6
The functional impairment of depressed patients reduces their ability to perform normal daily activities such as performing at a job. Patients with major depression and mild mood disturbances were 4.8 times and 1.6 times, respectively, more likely than nondepressed individuals to spend all or part of a day in bed or to abstain from usual activities because of illness in one 2980-respondent survey.7 Employed patients with major depression were 3.2 times more likely than asymptomatic individuals to miss work days because of their depression.
These data highlight the importance of supplementing the traditional therapeutic goal of improving core symptoms in depression with recognition of the detrimental impact of depression on well-being and ability to perform normal daily activities. Clinicians choosing appropriate pharmacotherapy for depressed patients require information not only about the efficacy of medications against core depressive symptoms but also about the effects of medications on patients' health-related quality of life and functional ability. In some regards, measures of the impact of medications on health-related quality of life and functional ability are more relevant to patients' global functional status than traditional efficacy measures. The efficacy of an antidepressant at relieving depressive symptoms does not always translate into improvement in well-being and functioning because of the side effects that may negatively impact well-being and function.
The antidepressant bupropion sustained release (SR), a norepinephrine and dopamine reuptake inhibitor (NDRI), is as effective in the treatment of depression as selective serotonin reuptake inhibitors such as sertraline8,9 and paroxetine,10 but it differs from these medications in its lack of association with side effects such as diarrhea, somnolence, and sexual dysfunction. The strong efficacy and superior tolerability of bupropion SR are associated with a positive impact on patients' functional status. The effects of bupropion SR on depressed patients' work and social functioning were assessed in an open-label, 3167-patient study evaluating the safety and efficacy of bupropion SR.11 Prior to treatment with bupropion SR, investigators considered 62% of depressed patients to be markedly or severely impaired in their work or social activities. After 8 weeks of treatment with bupropion SR, investigators considered only 22% of patients to be markedly or severely impaired, and 64% of patients were considered to be less impaired than they were prior to initiation of bupropion SR therapy. Improvements in investigator-rated functional status were strongly correlated with improvements in depressive symptoms.
While these data demonstrate bupropion SR–associated improvements in patients' functional status as assessed by investigators, the effects of bupropion SR on well-being and functional status assessed from the depressed patient's perspective have not been systematically evaluated. The current study, the open-label phase of a clinical study assessing the long-term efficacy of bupropion SR in patients with major depression (Weihs KL, Houser TL, Batey SR, et al., unpublished data, 2000), evaluated as secondary endpoints the effects of bupropion SR on health-related quality of life and workplace productivity measured from the patient's perspective. Furthermore, the relationship between clinical response to bupropion SR and its effects on well-being and functional status was explored by determining whether patients meeting criteria for clinical response to bupropion SR were more likely than nonresponders to manifest improvements in health-related quality of life and productivity.
METHOD
Patients
Men and women aged 18 years and older were eligible for the study if they had been diagnosed with moderate-to-severe recurrent major depression based on DSM-IV criteria12; scored a minimum of 18 on the 21-item Hamilton Rating Scale for Depression13,14 (HAM-D) at screening and baseline; were currently experiencing a recurrent episode of major depression lasting 8 weeks to 24 months; and had experienced at least one other depressive episode within the past 60 months. Patients were excluded from the study if they had a predisposition to seizures or were taking seizure threshold–lowering medications; had a history or current diagnosis of anorexia or bulimia; had a DSM-IV12 Axis II diagnosis suggesting a propensity for noncompliance with or nonresponsiveness to pharmacotherapy for depression; were pregnant or lactating or did not agree to avoid pregnancy during the study; had a past-year history of alcohol or other substance abuse; had used a psychoactive drug within 1 week of initiating bupropion SR treatment (2 weeks for monoamine oxidase inhibitors or protriptyline; 4 weeks for fluoxetine or any investigational drugs); had a past-year history of bupropion treatment or had previously received bupropion in a clinical study; or were actively suicidal. All patients provided written, informed consent to participate in the study.
Procedures
The protocol for this study (Glaxo Wellcome protocol number AK1A4004) was approved by an institutional review board for each of the 22 study sites. During a 1-week screening period, patients who no longer met study criteria were identified and excluded, and patients' prestudy antidepressant medications, if any, were discontinued. Patients continuing to meet selection criteria at the end of screening were dispensed bupropion SR to be taken during an 8-week, open-label evaluation in which the effects of bupropion SR on health-related quality of life and workplace productivity were assessed. Patients were instructed to take bupropion SR 150 mg once daily for the first 3 days and 150 mg twice daily for the remainder of the 8 weeks. At the end of the open-label phase, patients who responded clinically (according to predetermined criteria) to bupropion SR were given the option to enroll in a 44-week, placebo-controlled, double-blind evaluation of the efficacy and tolerability of bupropion SR 150 mg twice daily for the prevention of recurrence/relapse of depression.
The double-blind phase of the study was primarily designed to compare the efficacy and tolerability of bupropion SR versus placebo in the prevention of relapse/recurrence of depression (Weihs KL, Houser TL, Batey SR, et al., unpublished data, 2000). The current article describes the results of health outcomes measures, including health-related quality of life and productivity, obtained during the open-label phase of the study. The open-label phase enrolled patients currently experiencing a major depressive episode; the data from these patients were considered most germane for assessing the influence of therapy on health outcomes in depression. Data from the double-blind, second phase of the study, which enrolled only patients who responded to bupropion SR and discontinued patients who relapsed to depression again, were considered less relevant for assessing outcomes in patients currently experiencing depression.
Measures
The Clinical Global Impressions scale for Improvement of Illness15 (CGI-I) as well as other psychiatric evaluations such as the HAM-D and the Hamilton Rating Scale for Anxiety16 (HAM-A) were completed at weekly clinic visits during the open-label phase. The CGI-I comprises a 7-point scale on which the clinician rates the global improvement of patients (1 = very much improved to 7 = very much worse). Health-related quality of life and productivity were assessed at baseline and week 8 of treatment (or when patients discontinued from the study). For the quality-of-life assessments, patients completed the 34-item Quality of Life in Depression Scale (QLDS), previously demonstrated to be valid and reliable.17,18 QLDS scores range from 0 (corresponding to favorable health-related quality of life) to 34 (corresponding to poor health-related quality of life). For the productivity assessments, study coordinators interviewed part- and full-time employed patients during the baseline and week 8 (or discontinuation) study visits to determine for the 7 days prior to the interview the number of hours the patient had planned or been scheduled to work at a paid job; the number of hours missed from a paid job because of depression; an estimate of the percentage of effectiveness at the job (100% = usual effectiveness); and a patient rating of the frequency with which depression reduced effectiveness at the job (0 = never; 1 = rarely; 2 = sometimes; 3 = usually; 4 = always).
Data Analysis
CGI-I.
Data from all patients taking at least 1 bupropion SR tablet and having at least 1 posttreatment assessment were included in the analyses of CGI-I data, which were summarized using last-observation-carried-forward imputation of missing values for raw scores. Patients with a CGI-I score of 1 (very much improved) or 2 (much improved) during each of the last 3 weeks of open-label treatment were classified as responders to bupropion SR; all other patients were classified as nonresponders.
Quality of life.
Baseline and week 8 (or discontinuation) quality-of-life data for patients taking at least 1 bupropion SR tablet and having at least 1 posttreatment assessment were scored as recommended by the developers of the QLDS19 and summarized using means and standard deviations. Missing quality-of-life data were imputed using the last-observation-carried-forward method. T tests were used to determine whether QLDS scores at week 8 were significantly different from baseline. In addition, differences between CGI-I responders (those with a CGI-I rating of very much improved or much improved during each of the last 3 weeks of open-label treatment) and nonresponders in week 8 quality-of-life scores were tested using analysis of covariance controlled for age, gender, and study site.
Productivity.
Patients' answers to each of the productivity questions were summarized using means and standard deviations. Productivity data were analyzed for the subset of patients who indicated at baseline that they worked part- or full-time at a paid job and recorded the number of hours they had planned or been scheduled to work during the past 7 days. Only patients who provided productivity data at both baseline and week 8 (or when patients discontinued from the study) were included in the analyses.
In addition to responses to the 4 productivity-related questions, overall productivity loss for the past 7 days (in hours equivalent) was computed as the sum of productivity loss attributed to absenteeism and productivity loss while present at work. Absenteeism was measured as the hours missed from work, whereas productivity loss while present at work was calculated as (hours scheduled to work – hours missed from work) × (1 – percentage effectiveness at work). This overall measure accounts for productivity loss attributable both to time working at reduced effectiveness and to time missed from work. Only patients who reported to work at a full- or part-time paid job and provided productivity data at both baseline and week 8 (or at discontinuation from the study) were included in the analysis.
T tests were used to determine whether productivity scores at week 8 were significantly different from baseline. In addition, differences between CGI-I responders and nonresponders in week 8 productivity measures were tested using analysis of covariance controlled for age, gender, and study site.
RESULTS
Patients
The number of patients enrolling in the open-label phase was 828, of whom 816 took at least one bupropion SR tablet and had at least 1 posttreatment assessment. The number of patients indicating that they worked part- or full-time at both baseline and week 8 was 466.
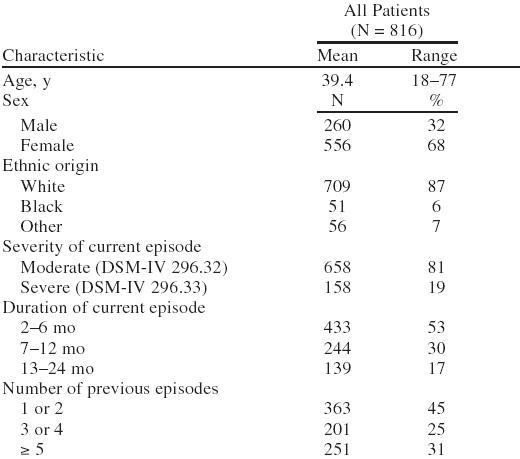
Most patients were female (68%) and white (87%). Patients' mean age was 39.4 years (Table 1). The current depressive episode was moderate in 81% of patients and severe in 19% (Table 1). Approximately half of the patients had been depressed for 2 to 6 months and had experienced 1 or 2 previous depressive episodes (Table 1).
Table 1.
Demographic and Patient Characteristics in Patients Taking Bupropion SR During the Open-Label Phase
CGI-I
Approximately 55% (448/816) of patients had a CGI-I score of 1 or 2 during the last 3 weeks of open-label treatment and were therefore categorized as bupropion SR responders; the remaining 368 patients were categorized as nonresponders for the purposes of this analysis. For the last week of open-label treatment, 69% of patients had a CGI-I score of 1 or 2.
Quality of Life
Health-related quality of life after 8 weeks of treatment of depression with bupropion SR was significantly improved relative to baseline. Mean ± SD QLDS scores were 18.98 ± 7.78 and 10.36 ± 9.68 at baseline and week 8, respectively (mean change = 8.62 ± 9.49; p < .001).
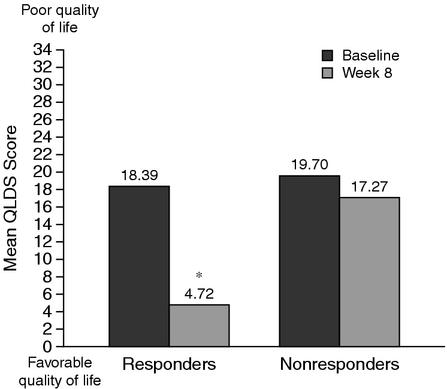
Improvement in health-related quality of life after 8 weeks of treatment with bupropion SR was significantly greater in responders to bupropion SR than in nonresponders. While mean QLDS scores were similar at baseline between responders (18.39) and nonresponders (19.70), week 8 scores reflected significantly more favorable health-related quality of life in responders (4.72 ± 5.41) compared with nonresponders (17.27 ± 9.28; p < .001; Figure 1).
Figure 1.
Mean Quality of Life in Depression Scale (QLDS) Scores at Baseline (before initiation of bupropion SR) and at Week 8 of Therapy in Bupropion SR Responders and Nonresponders
Productivity
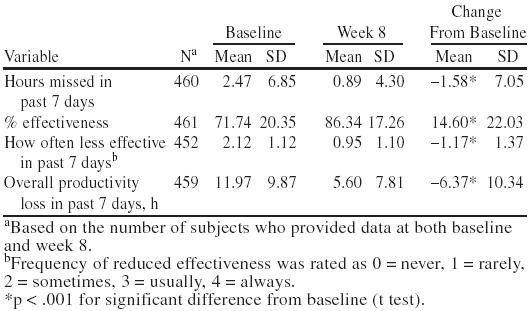
Lost workplace productivity after 8 weeks of treatment with bupropion SR was significantly reduced relative to baseline. Patients working part- or full-time at a paid job planned or were scheduled to work in the past 7 days a mean of 37.84 ± 11.60 hours at baseline and a mean of 38.23 ± 12.16 hours at week 8. Patients reported missing 1.58 fewer hours of work because of depression during the past 7 days; being 14.6% more effective on the job; working at reduced effectiveness less often; and incurring a mean of 6.37 fewer hours of overall lost productivity at week 8 compared with baseline (p < .001 for each variable; Table 2).
Table 2.
Productivity Data at Baseline and Week 8 for All Patients Employed Part- or Full-Time at Baseline and Returning Data at Baseline and Week 8
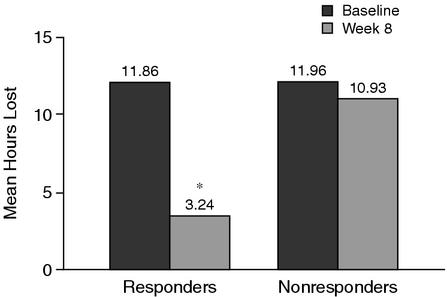
Reductions in lost workplace productivity after 8 weeks of treatment with bupropion SR were significantly greater in responders to bupropion SR than in nonresponders. While the mean numbers of hours of planned or scheduled work in the past 7 days were similar between responders and nonresponders at baseline (38.23 hours for responders and 36.95 hours for nonresponders) and week 8 (38.19 hours for responders and 38.32 hours for nonresponders), week 8 results reflected significant (p < .001) reductions in lost workplace productivity in responders compared with nonresponders for the mean number of hours missed because of depression; mean percent effectiveness on the job; and mean hours of overall lost productivity (Table 3; Figure 2). The mean ratings of how often effectiveness on the job was reduced were also significantly (p < .001) lower for responders compared with nonresponders (Table 3).
Table 3.
Productivity Data at Week 8 for Bupropion SR Responders and Nonresponders Employed Part- or Full-Time at Baseline and Returning Data on Baseline and Week 8
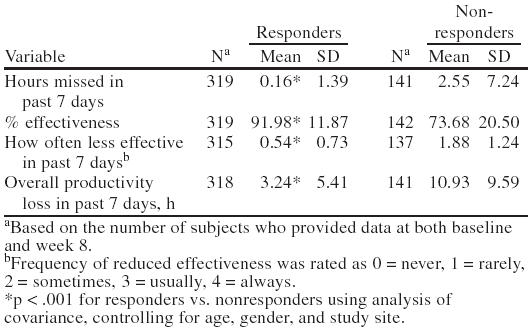
Figure 2.
Overall Lost Workplace Productivity (mean hours) in the Last 7 Days at Baseline (before initiation of bupropion SR) and at Week 8 of Therapy in Bupropion SR Responders and Nonresponders
DISCUSSION
Researchers studying depression in the primary care setting contend that “when assessing the impact of depression … an evaluation of symptoms alone is insufficient, so that an assessment of disability becomes a valuable addition. Furthermore … there is increasing concern about the differing perceptions between what the health professional and the patient considers [sic] an appropriate level of care. Therefore, focusing on specific deficits of functioning and their alteration as reported by the patient … may well improve the therapeutic alliance between patient and doctor. … ”4(pp97–98) The current study, which measured the effects of pharmacotherapy on well-being and functional status from the patient's perspective, provides information useful for improving this patient-doctor therapeutic alliance. The results of this study demonstrate that treatment with bupropion SR for 8 weeks is associated with improvements in health-related quality of life and reductions in lost workplace productivity in patients with major depression. Patients who responded clinically to bupropion SR showed significantly greater improvements in well-being and functional status than those who did not respond.
These improvements in well-being and functional status were accompanied by improvements in standard clinical efficacy measures. The clinical data from the open-label portion of the current study show that 66% of patients achieved a 50% reduction in HAM-D scores and 69% of patients achieved a CGI-I rating of 1 or 2 by the end of the 8-week treatment period (Weihs KL, Houser TL, Batey SR, et al., unpublished data, 2000). Similar improvements in depressive symptoms have been observed in other studies with bupropion SR.8,9,20,21
Bupropion SR–associated improvements in well-being and functional status were observed by the first scheduled measurement 8 weeks after initiation of therapy in this study. A similar effect of bupropion SR was observed in a separate study11 in which investigators rated patients' functional status using the Work and Social Disability Scale after 8 weeks of treatment. Whereas 62% of patients were considered markedly or severely impaired in work or social functioning at baseline, only 22% were markedly or severely impaired after 8 weeks of therapy. Bupropion SR–associated improvements possibly occurred earlier than the eighth week of treatment in both the study employing investigator ratings of functional status11 and the current one employing patient ratings; however, neither study evaluated patients' functional ability earlier than 8 weeks after treatment initiation.
Positive effects of antidepressant pharmacotherapy on functional status have also been observed in other studies employing measures of psychosocial function.22–24 For example, chronically depressed patients' scores on self- and interviewer-rated psychosocial measures improved by the fourth week of treatment with antidepressant therapy in one open-label study.22 Stewart and colleagues23 found in another study that improvements in patient-reported social functioning were significantly greater after 6 weeks of treatment with phenelzine or imipramine compared with placebo. Patients whose clinical symptoms responded to treatment demonstrated greater improvements in social functioning than did nonresponders.
Similarly, in the current study, patients whose clinical symptoms responded to bupropion SR demonstrated greater improvements in health-related quality of life and productivity than did nonresponders. This finding suggests that effective treatment of depressive symptoms with bupropion SR may be responsible for the improvements in well-being and functional status. The favorable tolerability of bupropion SR8–10 also may contribute to these improvements.
Unlike the placebo-controlled design of Stewart et al.,23 the open-label design of the current study does not allow definitive conclusions to be drawn about effects of study medication. The effects of factors such as patient expectations and regression toward the mean on functional status cannot be separated from those of bupropion SR. These considerations notwithstanding, the consistency of the bupropion SR–associated improvements in functional status across 2 studies (reference 11 and the current study) supports the contention that the improvements may be attributed to the drug. Further research with a placebo-controlled design to compare the effects of bupropion SR on quality of life and productivity with the effects of other antidepressants is warranted.
A second limitation of the current study is the lack of information about the clinical relevance of changes in QLDS scores. Clinically meaningful differences on this quality-of-life scale have not been defined; in this study, improvement in QLDS scores after 8 weeks of bupropion SR treatment was evaluated based on statistically significant differences. Nevertheless, the greater improvements in QLDS scores among responders relative to nonresponders support clinical validity of the QLDS measure. The consistency of the favorable changes in QLDS scores with other measures of efficacy and functioning is consonant with the probability that the quality-of-life changes reflected in the QLDS were clinically significant.
The baseline productivity data reflect significant functional impairment among these depressed patients. At baseline (before they had received bupropion SR), patients reported 12 hours, or 1.5 working days, of productivity loss in a 7-day period. Patients estimated that when they worked with depressive symptoms, they were only three quarters as effective as normal. These data are based on patients' estimates rather than their actual behavior. Time-and-motion studies, in which observers time or record counts of activities performed by subjects, are used to assess patients' actual behavior.25 It is difficult and impractical to perform a time-and-motion study in a multicenter clinical trial due to the amount of resources required. Although it may be feasible to conduct time-and-motion studies in a single employment setting, the results may lack generalizability. In addition, because patients may behave differently when they are observed than when they are not under observation, results obtained from time-and-motion studies may be confounded by strategic bias.
Another factor to consider in evaluating the productivity data is that only patients who worked part- or full-time and provided productivity data at both baseline and week 8 (or at discontinuation from the study) were included in the productivity analyses. This method of analysis was adopted to assess the benefits of bupropion SR on workplace productivity loss among depressed patients who were employed. While it is possible to use the last-observation-carried-forward method to impute missing productivity data for the follow-up visit using baseline data, this method assumes that patients' employment and productivity status at the follow-up visit were the same as at baseline, which could possibly lead to bias of study results.
A study of the 18,000-employee First Chicago Corporation evaluated the economic ramifications of depression in the workplace.26 Over a 4-year period, the average length of a disability leave was greater for depression (40 days) than for low back pain (37 days), heart disease (37 days), high blood pressure (27 days), or diabetes mellitus (26 days). Likewise, 1-year recidivism rates for short-term disability leave (i.e., work absenteeism for more than 5 consecutive days because of illness) were higher for depressive disorders (26%) than for high blood pressure (11%), low back pain (10%), or heart disease (8%). Depression, which accounted for 52% of employee and dependent mental health medical plan claims, was associated with higher medical plan costs than any other mental illness. In another analysis of data from 2 United States surveys, depressed workers compared with nondepressed workers incurred up to 3.2 more work-disability days in a 30-day period for a productivity loss of up to $395.00 per worker.27 Authors of both studies conclude that employer implementation of appropriate treatment strategies including pharmacotherapy would significantly reduce depression-associated disability and be cost-effective to employers.
The data from the current study, which demonstrate that lost workplace productivity is reduced by half in responders to bupropion SR pharmacotherapy compared with nonresponders, are consonant with this conclusion. Patients using bupropion SR reported a 6.37-hour reduction of lost workplace productivity in the past 7 days (for 25.48 hours in a month) at the end of the 8-week treatment period. Assuming an average hourly wage of $15.09,28 the cost savings to employers of this reduction in lost workplace productivity are estimated at $384.49 per depressed patient per month. This figure conservatively estimates the benefit of bupropion SR because it does not encompass direct medical costs of depression. Even so, the cost savings to employers of the reduction in lost workplace productivity exceed the average wholesale price of $91.6429 for a month's supply of bupropion SR.
CONCLUSION
Bupropion SR is an effective and well-tolerated antidepressant with a low incidence of sexual dysfunction, sedation, and weight gain. Treatment with bupropion SR may improve health-related quality of life and reduce productivity loss. With this profile, bupropion SR enhances clinicians' ability to optimize patient care and improve clinical outcomes for patients. Considered together, these data suggest that bupropion SR should be a treatment of choice for depression.
Drug names: bupropion (Wellbutrin), fluoxetine (Prozac), paroxetine (Paxil), phenelzine (Nardil), protriptyline (Vivactil), sertraline (Zoloft).
Acknowledgments
The authors acknowledge Jane Saiers, Ph.D., of The WriteMedicine, Inc., for assistance with writing the manuscript.
Footnotes
Glaxo Wellcome Inc. funded the research described in this manuscript.
REFERENCES
- Katon W, Schulberg H. Epidemiology of depression in primary care. Gen Hosp Psychiatry. 1992;14:237–247. doi: 10.1016/0163-8343(92)90094-q. [DOI] [PubMed] [Google Scholar]
- Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients: results from the Medical Outcomes Study. JAMA. 1989;262:914–919. [PubMed] [Google Scholar]
- Hays RD, Wells KB, Sherbourne CD, et al. Functioning and well-being outcomes of patients with depression compared with chronic general medical illnesses. Arch Gen Psychiatry. 1995;52:11–19. doi: 10.1001/archpsyc.1995.03950130011002. [DOI] [PubMed] [Google Scholar]
- The Counseling Versus Antidepressants in Primary Care Study Group. How disabling is depression? evidence from a primary care sample. Br J Gen Pract. 1999;49:95–98. [PMC free article] [PubMed] [Google Scholar]
- Coulehan JL, Schulberg HC, Block MR, et al. Treating depressed primary care patients improves their physical, mental, and social functioning. Arch Intern Med. 1997;157:1113–1120. [PubMed] [Google Scholar]
- Williams JW Jr, Kerber CA, Mulrow CD, et al. Depressive disorders in primary care: prevalence, functional disability, and identification. J Gen Intern Med. 1995;10:7–12. doi: 10.1007/BF02599568. [DOI] [PubMed] [Google Scholar]
- Broadhead WE, Blazer DG, George LK, et al. Depression, disability days, and days lost from work in a prospective epidemiologic survey. JAMA. 1990;264:2524–2528. [PubMed] [Google Scholar]
- Croft H, Settle E Jr, Houser T, et al. A placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline. Clin Ther. 1999;21:643–658. doi: 10.1016/S0149-2918(00)88317-4. [DOI] [PubMed] [Google Scholar]
- Coleman CC, Cunningham LA, Foster VJ, et al. Sexual dysfunction associated with the treatment of depression: a placebo-controlled comparison of bupropion sustained release and sertraline treatment. Ann Clin Psychiatry. 1999;11:205–215. doi: 10.1023/a:1022309428886. [DOI] [PubMed] [Google Scholar]
- Weihs KL, Settle EC Jr, Batey SR, et al. Bupropion sustained release versus paroxetine for the treatment of depression in the elderly. J Clin Psychiatry. 2000;61:196–202. doi: 10.4088/jcp.v61n0309. [DOI] [PubMed] [Google Scholar]
- Mauskopf JA, Simeon GP, Miles MA, et al. Functional status in depressed patients: the relationship to disease severity and disease resolution. J Clin Psychiatry. 1996;57:588–592. doi: 10.4088/jcp.v57n1207. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Mood disorders. In: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association. 1994 317–345. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychiatry. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare publication (ADM) 76-338. Rockville, Md: National Institute of Mental Health. 1976 218–222. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hunt SM, McKenna SP. The QLDS, a scale for the measurement of quality of life in depression. Health Pol. 1992;22:307–319. doi: 10.1016/0168-8510(92)90004-u. [DOI] [PubMed] [Google Scholar]
- McKenna SP, Hunt SM. A new measure of quality of life in depression: testing the reliability and construct validity of the QLDS. Health Policy. 1992;22:321–330. doi: 10.1016/0168-8510(92)90005-v. [DOI] [PubMed] [Google Scholar]
- The QLDS: A Scale for the Measurement of Quality of Life in Depression. User Manual. Manchester, United Kingdom: Galen Research & Consultancy. 1994 [DOI] [PubMed] [Google Scholar]
- Kavoussi RJ, Segraves RT, Hughes AR, et al. Double-blind comparison of bupropion sustained release and sertraline in depressed outpatients. J Clin Psychiatry. 1997;58:532–537. doi: 10.4088/jcp.v58n1204. [DOI] [PubMed] [Google Scholar]
- Reimherr FW, Cunningham LA, Batey SR, et al. A multicenter evaluation of the efficacy and safety of 150 and 300 mg/d sustained-release bupropion tablets versus placebo in depressed outpatients. Clin Ther. 1998;20:505–516. doi: 10.1016/s0149-2918(98)80060-x. [DOI] [PubMed] [Google Scholar]
- Miller IW, Keitner GI, Schatzberg AF, et al. Treatment of chronic depression, pt 3: psychosocial functioning before and after treatment with sertraline or imipramine. J Clin Psychiatry. 1998;59:608–619. doi: 10.4088/jcp.v59n1108. [DOI] [PubMed] [Google Scholar]
- Stewart JW, Quitkin FM, McGrath PJ, et al. Social functioning in chronic depression: effect of 6 weeks of antidepressant treatment. Psychiatry Res. 1988;25:213–222. doi: 10.1016/0165-1781(88)90053-4. [DOI] [PubMed] [Google Scholar]
- Agnosti V, Stewart JW, Quitkin FM. Life satisfaction and psychosocial functioning in chronic depression: effect of acute treatment with antidepressants. J Affect Disord. 1991;23:35–41. doi: 10.1016/0165-0327(91)90033-o. [DOI] [PubMed] [Google Scholar]
- Finkler SA, Knickman JR, Hendrickson G, et al. A comparison of work-sampling and time-and-motion techniques for studies in health services research. Health Serv Res. 1993;28:577–597. [PMC free article] [PubMed] [Google Scholar]
- Conti DJ, Burton WN. The economic impact of depression in a workplace. J Occup Med. 1994;36:983–988. [PubMed] [Google Scholar]
- Kessler RC, Barber C, Birnbaum HG, and et al. Depression in the workplace: effects on short-term disability. Health Aff. 1999 September/October. 163–171. [DOI] [PubMed] [Google Scholar]
- US Dept of Labor. Bureau of Labor Statistics. National Compensation Survey: Occupational Wages in the United States: 1997. Washington, DC: US Bureau of Labor Statistics; 1999. Bulletin 2519. [Google Scholar]
- Drug Topics Red Book. Montvale, NJ: Medical Economics. 2000. [Google Scholar]