Abstract
Side effects of antidepressants can be predicted by receptor selectivity and site of action. Although the selective serotonin reuptake inhibitors (SSRIs) have better overall safety and tolerability than older antidepressants, broad-based experience with SSRIs has shown the frequency and type of side effects to be increased relative to clinical trial data. The author explores the reasons for the different profiles and discusses adverse effects, especially sexual dysfunction, weight gain, and sleep disturbance, the most troubling adverse events seen during long-term SSRI therapy. The informed management of these side effects by primary care practitioners supports successful treatment of depression.
In the early 1950s, the mood-elevating effects of the monoamine oxidase inhibitors (MAOIs) were discovered serendipitously. Further investigations of these compounds and the tricyclic antidepressants (TCAs) led to early theories relating brain chemistry and mood. These discoveries in the 1950s and 1960s sparked further interest in antidepressant drug therapy and in developing new and better medications for patients suffering from depression.
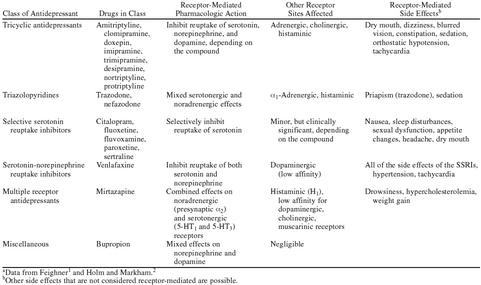
TCAs nonselectively inhibit the reuptake of serotonin, norepinephrine, and dopamine into presynaptic storage vesicles in the brain. Although they are effective in treating depression, their effects on other receptor systems, including histaminic, cholinergic, adrenergic, and postsynaptic serotonin receptors unrelated to depression, led to the development of significant, often intolerable adverse effects that limited their use in clinical practice (Table 1).1,2
Table 1.
Classification of Antidepressants by Receptor Selectivity and Site of Actiona
Despite their efficacy, TCAs have a narrow therapeutic index, and, at high doses, they can cause seizures as well as death due to slowing of intraventricular conduction, leading to complete heart block or ventricular reentry arrhythmias.1 Consequently, research efforts focused on developing drugs with similar efficacy, but with improved safety and tolerability.
In essence, the search for a safer “magic bullet” had begun, with the goal of treating depression with an efficacious agent that had fewer associated side effects.
THE SEARCH FOR A MAGIC BULLET
In the 1970s, second-generation antidepressants were developed with differing receptor-binding activities. They had different side effect profiles, depending on their binding at sites for other classes of receptors (Table 1).1,2 The realization that more highly receptor-selective agents would reduce the number and type of adverse effects but with increased “potency” because of their selectivity spurred the development of the class of selective serotonin reuptake inhibitors (SSRIs).
SSRIs: An Important Step Forward
In 1988, the first SSRI, fluoxetine, was introduced in the United States. The adverse effect profile of fluoxetine was far superior to that of any other available antidepressant because of its selectivity for serotonin receptors. Other SSRIs were soon introduced in the United States and elsewhere (Table 1). Although the efficacy of the SSRIs is comparable to that of the TCAs, the SSRIs have significantly fewer side effects.3 This was confirmed by the finding that fewer patients taking an SSRI discontinued therapy because of adverse effects than did those taking TCAs.4 Unlike TCAs, SSRIs do not cause cardiac conduction abnormalities in overdose and have low propensity to cause seizures.1 Thus, development of the SSRIs was an important milestone in the treatment of depression.
Compared with the TCAs, SSRIs were initially considered almost free of side effects. Unlike the TCAs, they could be used safely in many patient populations, including the elderly and children, both of whom are particularly sensitive to the adverse effects of TCAs. SSRIs also could be prescribed for patients with multiple comorbidities. Because of their overall efficacy, safety, and tolerability, they have become widely prescribed by primary care physicians. Consequently, more patients are now successfully treated for depression than ever before.
Not quite the magic bullet.
However, questions about the safety and tolerability of SSRIs have emerged with their continued use. For example, in the original placebo-controlled clinical trials of fluoxetine in depressed patients, sexual dysfunction was reported in 1.9% of trial participants receiving fluoxetine. However, postmarketing clinical trials have reported rates of sexual dysfunction as high as 75%.5 Although severe SSRI-induced hyponatremia was not reported in the original clinical trials, it is now known to occur in 1 in 200 elderly patients per year receiving treatment with fluoxetine or paroxetine. Hyponatremia (thought to be caused by the syndrome of inappropriate antidiuretic hormone) is less common in patients treated with other SSRIs and venlafaxine.6
Data Capture and Interpretation
Why have the frequency and type of side effects with SSRIs increased with time? Dosages used in early clinical trials may not have been sufficient to allow for a full understanding of the side effect profile of the drugs. Trial design, methods for determining adverse events, and the duration of the studies may have affected the emergence or reporting of side effects. During clinical trials, adverse event data are typically captured through spontaneous reports volunteered by the patient, open-ended questioning by the clinician, and changes in laboratory values and results of physical examinations. Patients may not be comfortable discussing an adverse event such as sexual dysfunction unless the clinician specifically asks about it. Other patients do not attribute adverse events to the use of a drug, and instead think they are getting the “flu” or completely fail to report their symptoms to the doctor. The strict exclusion criteria in clinical trials preclude the participation of patients with significant comorbidities. The terms and coding of the reporting systems used to collect adverse event data during clinical trials can differ among countries, leading to variable statistics. The 6- to 8-week duration of typical antidepressant clinical trials may be insufficient for the capture of adverse events that only become evident with longer term treatment. For example, SSRI-induced hyponatremia may not be noted in short clinical trials because of its time to detection and its nonspecific symptoms (e.g., confusion, weakness, lethargy, drowsiness).7 Rare side effects that occur in less than 5000 patients may not emerge until a drug is marketed and more patients are exposed to the drug. Therefore, the incidence of adverse effects reported in clinical trials does not necessarily represent real-life experience.
Although information about the side effect profile of a given drug is available in both the drug manufacturer's package insert and the Physicians' Desk Reference (PDR),8 this method of obtaining drug information has limitations. Data included in product labeling are derived from the company-sponsored studies that have been reviewed by the U.S. Food and Drug Administration. Uncommon, rare, and unpredictable drug-related adverse effects may not have emerged in clinical trials and may not be included in the PDR until the drug has been available for several years.
Published case reports often contain this type of information; however, most practicing physicians do not have time to read or do not have access to these anecdotal reports. Also, observed side effects reported for a single drug may vary considerably depending on the psychiatric condition studied. These adverse events may, in turn, be summarized without addressing which side effects are condition specific. The calculated rate of occurrence for drug-related side effects could be inappropriate for a specific disorder. For example, headache is reported more often with SSRI therapy in patients receiving treatment for obsessive-compulsive disorder than for depression. However, this must be interpreted with the knowledge that the baseline prevalence of headache is higher in patients with obsessive-compulsive disorder than in those with depression. Therefore, rates of treatment-emergent adverse effects must be compared between groups of patients receiving treatment with active drug or placebo for the same disorder to assess the true incidence of SSRI-induced side effects.
One measure of tolerability of any drug is continued patient use. Consequently, discontinuation rates during clinical trials have been used to measure the tolerability of a given drug.9 Fluvoxamine is associated with the highest discontinuation rates because of adverse events in clinical trials (up to 70% within the first 2 months), followed by fluoxetine (45%) and sertraline (40%).10 Discontinuation rates for any drug after release can be continually redefined using product labeling, published clinical trials, postmarketing surveillance, current literature, case reports, and clinical experience.
SIDE EFFECTS OF SSRIs
The improved tolerability of the SSRIs is attributable to their selectivity and to their absence of interaction with other receptors, such as histaminic, cholinergic, dopaminergic, and noradrenergic. Serotonin receptors comprise at least 7 classes, which are further divided at the subreceptor level. These receptors mediate a variety of functions unrelated to mood, including sleep, appetite, and sexual function, as well as symptoms such as pain, nausea, depression, and anxiety.11 By increasing the inhibition of serotonin reuptake, more of the neurotransmitter is available to interact with any of these receptors or subtype receptors. Therefore, most SSRI side effects are dose related and can be attributed to serotonergic effects. For example, nausea, a common side effect of SSRI therapy, most likely results from stimulation of 5-HT3 receptors and can usually be alleviated by reducing the dose of the SSRI.10 In contrast, fluoxetine-induced skin reactions are not dose related and apparently are idiosyncratic.
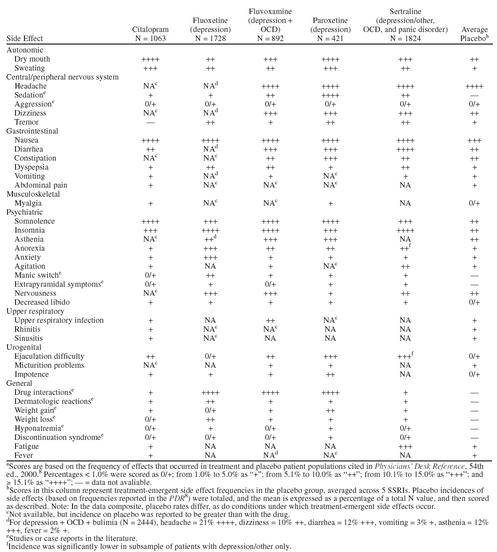
There are some differences in the adverse effect profiles of the available SSRIs (Table 2). Gastrointestinal (GI) disturbances are the most frequently reported side effects.10 Individually, postmarketing surveillance studies suggest that fluvoxamine is associated with the highest frequency of GI disturbances, while anxiety, agitation, and insomnia are most often reported with sertraline and fluoxetine.10,12 Overall, citalopram appears to be the best-tolerated SSRI, followed by fluoxetine, sertraline, paroxetine, and fluvoxamine. The latter 2 drugs are associated with the most side effects and the highest discontinuation rates because of side effects in clinical trials.13 During long-term SSRI therapy, the most troubling adverse effects are sexual dysfunction, weight gain, and sleep disturbance.
Table 2.
Comparison of Frequent Side Effects Associated With Currently Available Selective Serotonin Reuptake Inhibitorsa
Sexual Dysfunction
The interference in sexual functioning caused by SSRIs is quite complicated, possibly involving nitric oxide. The effect appears to be attributable to stimulation of postsynaptic 5-HT2 receptors, possibly in the spinal cord. Clinically, the effect can manifest as decreased libido, male impotence, delayed ejaculation, or anorgasmia. These are common characteristics of depression itself, as well as adverse effects of antidepressant therapy.5 Therefore, effects attributable to antidepressant therapy should be cautiously interpreted and normalized against baseline. According to clinical trials specifically designed to assess sexual function in patients receiving SSRIs, the type and severity of sexual dysfunction vary by gender. Depressed women tend to have greater reduction of sexual desire and increased difficulties with orgasm at baseline compared with men. Women experience some remission of these symptoms with continued SSRI treatment. In contrast, men tend to experience continuing orgasmic inhibition and overall sexual dysfunction as a side effect of SSRI therapy.5 Increased sexual desire and priapism are also possible with SSRI therapy. For both sexes, SSRI-induced orgasmic delay and anorgasmia are more common than decreased libido.5
The method used to obtain information about sexual function directly affects the reporting of sexual side effects with SSRI therapy (Table 3). Only 2% to 7% of patients spontaneously report sexual side effects with SSRI therapy, but when a sexual dysfunction questionnaire is used, the incidence of sexual dysfunction rose to 55% for SSRIs,14 and is as high as 92% for the TCA clomipramine.15 Based on these observations, physicians should obtain thorough sexual function histories before initiating SSRI therapy and, through detailed, frank discussions, compare reported changes during treatment with self-evaluated pretreatment levels of sexual functioning. Ideally, a validated sexual dysfunction questionnaire, such as the Arizona Sexual Experience Scale (ASEX),16 would be used to capture and standardize sexual side effect information during SSRI therapy.
Table 3.
Incidence (%) of Patients Reporting Sexual Dysfunction With the Selective Serotonin Reuptake Inhibitors in Some Studies, Depending on Methods for Determining Adverse Eventsa
For patients who find sexual dysfunction intolerable as a side effect of SSRI therapy, substituting another SSRI may attenuate these side effects. Citalopram did not seem to cause sexual impairment in patients who had experienced such events with another SSRI.17 Within the class of SSRIs, erectile dysfunction, vaginal lubrication difficulties, and decreased libido in both sexes are most common with paroxetine, particularly in the first month of therapy.5,10 Anorgasmia appears to be the most common dose-dependent side effect of citalopram therapy.18
Table 3 illustrates the differences in published incidence rates for SSRI-induced interference with sexual function, depending on the method used to collect information. Few studies have compared the frequency of sexual dysfunction reported by patients treated with citalopram with that of patients taking other SSRIs. Although the exact mechanism for SSRI-induced sexual dysfunction is unknown, the influence of other receptor systems, including dopaminergic, cholinergic, and serotonergic, and agents such as prolactin and nitric oxide have been proposed.5
Weight Gain
Like sexual dysfunction, weight gain was infrequently reported during premarketing clinical trials of the SSRIs. Because of the weight loss that occurred during the early, short-term clinical trials with fluoxetine, it was investigated as a potential weight loss agent.19 However, weight gain subsequently emerged as a common side effect of long-term SSRI therapy. Although some SSRIs are typically associated with weight loss during initial therapy, weight is often regained after 6 months and can be followed by additional weight gain with long-term use. Uncontrolled studies have reported mean weight gains of 15 lb (6.75 kg) for sertraline, 21 lb (9.45 kg) for fluoxetine, and 24 lb (10.80 kg) for paroxetine after 6 to 12 months of therapy.20,21 Although studies to date suggest that citalopram is less likely to cause weight gain,22 one clinical series of 18 patients reported 8 patients with mixed anxiety and mood disorders who had an average weight gain of 15.7 lb (7.1 kg) after receiving citalopram for 5 weeks.23
Effects on Sleep
In depressed patients, normal sleep patterns are altered, with an increased duration and earlier onset of rapid eye movement (REM) sleep, reduced slow-wave sleep, and more awakenings.10 Additionally, the SSRIs interfere with sleep architecture, potentially complicating the sleep of depressed patients. Fluoxetine, paroxetine, and sertraline delay the onset of REM sleep, and fluoxetine and paroxetine increase awakenings and reduce REM sleep, slow-wave sleep, total sleep time, and sleep efficiency. In contrast, sertraline minimally increases sleep efficiency and reduces nocturnal wakefulness time, which may benefit depressed patients whose sleep disturbance is troubling.24
Other Side Effects
Discontinuation reactions have been reported after withdrawal of prolonged SSRI treatment and constitute a syndrome that is not well characterized. Clinical trials designed to examine this syndrome in terms of receptor physiology have been inconclusive. Presumably, the discontinuation syndrome results from neurophysiologic readjustment in the central nervous system to compensate for the pharmacologic activity of the SSRI. The symptoms include dizziness, nausea, lethargy, headache, anxiety, and agitation. They are generally mild, begin within a week of discontinuing SSRI therapy, and resolve within 3 weeks.25 Some reported problems are more disabling, for example, falls and absence from work. Reinstatement of the SSRI resolves symptoms. The discontinuation syndrome is best avoided by slowly tapering SSRI therapy. As expected, withdrawal side effects are more common with SSRIs that have the shortest half-lives (i.e., paroxetine, fluvoxamine).25
An additional advantage of SSRIs over TCAs is a reduced risk of drug-drug interactions. However, the administration of 2 or more serotonergic drugs or an overdose of 1 agent can cause the serotonin syndrome, a potentially life-threatening disorder characterized by myoclonus, hyperreflexia, sweating, shivering, incoordination, and mental status changes.11 The serotonin syndrome can be distinguished from other SSRI-induced side effects by the clustering of clinical features, their severity, and duration.10 The coadministration of serotonergic drugs (e.g., 2 SSRIs or an SSRI plus an MAOI) should be avoided. Additionally, when substituting one SSRI for another in a given patient, a suitable washout period should be ensured that reflects the half-life of the drug being replaced.9
CONCLUSION
Based on their tolerability profile, the SSRIs are a significant advancement over the TCAs for the treatment of depression. Although some SSRI-associated adverse effects can be intolerable or troubling, except for the serotonin syndrome, they are not life-threatening. As with other classes of antidepressants, SSRIs induce side effects that can be predicted by receptor physiology. Through the broad-based experience with the SSRIs, the frequency of side effects such as sexual dysfunction and sleep disturbance has increased. Therefore, selectivity for serotonergic receptors does not ensure freedom from adverse effects. The shift of treatment of depression to primary care practitioners, who manage heavy patient schedules across all therapeutic areas, has created the need to enhance the successful treatment of depression. The wealth of experience with SSRIs has set the stage for a next generation of antidepressants that are at least as effective, but better tolerated and safer than their predecessors. The search for the magic bullet continues.
Drug names: amitriptyline (Elavil and others), bupropion (Wellbutrin), citalopram (Celexa), clomipramine (Anafranil and others), desipramine (Norpramin and others), doxepin (Sinequan and others), fluoxetine (Prozac), fluvoxamine (Luvox), mirtazapine (Remeron), nefazodone (Serzone), nortriptyline (Pamelor and others), paroxetine (Paxil), protriptyline (Vivactil), sertraline (Zoloft), trimipramine (Surmontil), venlafaxine (Effexor).
Footnotes
Partially funded by an unrestricted educational grant from Merck and Company.
REFERENCES
- Feighner JP. Mechanism of action of antidepressant medications. J Clin Psychiatry. 1999;60(suppl 4):4–11. [PubMed] [Google Scholar]
- Holm KJ, Markham A. Mirtazapine: a review of its use in major depression. Drugs. 1999;57:607–631. doi: 10.2165/00003495-199957040-00010. [DOI] [PubMed] [Google Scholar]
- Anderson IM. SSRIs versus tricyclic antidepressants in depressed patients: a meta-analysis of efficacy and tolerability. Depress Anxiety. 1998;7(suppl 1):11–17. [PubMed] [Google Scholar]
- Edwards JG, Anderson I. Systemic review and guide to selection of selective serotonin reuptake inhibitors. Drugs. 1999;57:507–533. doi: 10.2165/00003495-199957040-00005. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Lane RG, Menza M. Effects of SSRIs on sexual function: a critical review. J Clin Psychopharmacol. 1999;19:67–85. doi: 10.1097/00004714-199902000-00013. [DOI] [PubMed] [Google Scholar]
- Wilkinson TJ, Begg EJ, Winter AC, et al. Incidence and risk factors for hyponatraemia following treatment with fluoxetine or paroxetine in elderly people. Br J Clin Pharmacol. 1999;47:211–217. doi: 10.1046/j.1365-2125.1999.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner V, Silver LE, Kelly CA. Selective serotonin reuptake inhibitors and hyponatremia: review and proposed mechanisms in the elderly. J Psychopharmacol. 1998;12:396–400. doi: 10.1177/026988119801200411. [DOI] [PubMed] [Google Scholar]
- Physicians' Desk Reference. Montvale, NJ: Medical Economics. 2000 [Google Scholar]
- Skerritt U, Evans R, Montgomery SA. Selective serotonin reuptake inhibitors in older patients: a tolerability perspective. Drugs Aging. 1997;10:209–218. doi: 10.2165/00002512-199710030-00005. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Goodnick PJ. Selective serotonin reuptake inhibitors in the treatment of affective disorders, 3: tolerability, safety, and pharmacoeconomics. J Psychopharmacol. 1998;12(suppl B):S55–S87. doi: 10.1177/0269881198012003041. [DOI] [PubMed] [Google Scholar]
- Nelson JC. Safety and tolerability of the new antidepressants. J Clin Psychiatry. 1997;58(suppl 6):26–31. [PubMed] [Google Scholar]
- Spigset O. Adverse reactions of selective serotonin reuptake inhibitors: reports from a spontaneous reporting system. Drug Saf. 1999;20:277–287. doi: 10.2165/00002018-199920030-00007. [DOI] [PubMed] [Google Scholar]
- Dewan MJ, Anand VS. Evaluating the tolerability of the newer antidepressants. J Nerv Ment Dis. 1999;187:96–101. doi: 10.1097/00005053-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Montejo AL, Llorca G, and Izquierdo JA. Sexual dysfunction with SSRIs: a comparative analysis. In: New Research Program and Abstracts of the 149th Annual Meeting of the American Psychiatric Association; May 9, 1996; New York, NY. Abstract. NR717. 266. [Google Scholar]
- Monteiro WO, Noshirvani HF, Marks IM, et al. Anorgasmia from clomipramine in obsessive-compulsive disorder: a controlled trial. Br J Psychiatry. 1987;151:107–112. doi: 10.1192/bjp.151.1.107. [DOI] [PubMed] [Google Scholar]
- McGahuey CA, Gelenberg AJ, Laukes CA, and et al. The Arizona Sexual Experience Scale: validity and reliability. In: New Research Program and Abstracts of the 150th Annual Meeting of the American Psychiatric Association; May 19, 1997; San Diego, Calif. Abstract. NR184. 116–117. [Google Scholar]
- Pallanti S, Koran LM. Citalopram and sexual side effects of selective serotonin reuptake inhibitors [letter] Am J Psychiatry. 1999;156:796. doi: 10.1176/ajp.156.5.796. [DOI] [PubMed] [Google Scholar]
- Noble S, Benfield P. Citalopram: a review of its pharmacology, clinical efficacy and tolerability in the treatment of depression. CNS Drugs. 1997;8:410–431. [Google Scholar]
- Ferguson JM, Feighner JP. Fluoxetine-induced weight loss in overweight non-depressed humans. Int J Obes. 1987;11(suppl 3):163–170. [PubMed] [Google Scholar]
- Sussman N, Ginsberg D. Rethinking side effects of the selective serotonin reuptake inhibitors: sexual dysfunction and weight gain. Psychiatr Ann. 1998;28:89–97. [Google Scholar]
- De Wilde J, Spiers R, Mertens C, et al. A double-blind, comparative, multicentre study comparing paroxetine with fluoxetine in depressed patients. Acta Psychiatr Scand. 1993;87:141–145. doi: 10.1111/j.1600-0447.1993.tb03345.x. [DOI] [PubMed] [Google Scholar]
- Mackle M, Kocsis J. Effects on body weight of the SSRI citalopram. Presented at the 37th annual meeting of the American College of Neuropsychopharmacology; Dec 14–18, 1998; Las Croabas, Puerto Rico. [Google Scholar]
- Bouwer CD, Harvey BH. Phasic craving for carbohydrate observed with citalopram. Int Clin Psychopharmacol. 1996;11:273–278. doi: 10.1097/00004850-199612000-00009. [DOI] [PubMed] [Google Scholar]
- Winokur A, Lexon N, Allen K, and et al. Sertraline administered for 8 weeks to depressed patients did not alter sleep architecture: a preliminary report. In: New Research Program and Abstracts of the 147th Annual Meeting of the American Psychiatric Association; May 24, 1994; Philadelphia, Pa. Abstract. NR212. 110. [Google Scholar]
- Haddad P. The SSRI discontinuation syndrome. J Psychopharmacol. 1998;12:305–313. doi: 10.1177/026988119801200311. [DOI] [PubMed] [Google Scholar]