Abstract
Attention-deficit/hyperactivity disorder (ADHD), characterized by developmentally inappropriate inattentiveness, impulsivity, and hyperactivity, is the most common and thoroughly researched neuropsychiatric disorder affecting children and adolescents. The diagnosis of ADHD requires a comprehensive clinical assessment including a detailed patient history, clinical interview and observation, and a thorough physical examination. A variety of other disorders can masquerade as ADHD or coexist with the disorder. The clinician must recognize environmental influences that may affect the severity of symptoms exhibited in the child or adolescent with ADHD. Clinically, treatment with a stimulant can be expected to result in an immediate, often dramatic, improvement in the core symptoms of ADHD. Studies published over the past 20 years indicate that the symptoms of ADHD, which were originally thought to diminish as a child matured, may persist into adolescence and adulthood. This article is a review of the most recent recommendations and clinical data regarding the diagnosis and management of ADHD in children and adolescents to assist with appropriate and prudent clinical decision making.
Attention-deficit/hyperactivity disorder (ADHD) is a neurobehavioral syndrome characterized by developmentally inappropriate degrees of inattentiveness, impulsivity, and hyperactivity. It is the most common and thoroughly researched neuropsychiatric disorder affecting children and adolescents. Individuals with ADHD are a heterogeneous group, displaying high variability in the frequency and pervasiveness of symptoms, as well as the degree of impairment resulting from these symptoms. This disorder is associated with significant functional impairment, including poor academic outcomes, comorbid psychiatric and developmental conditions, and patient and family stress.
An atmosphere of controversy and confusion has arisen during the past decade concerning the apparent rise in prevalence of ADHD and the resultant escalation in psychostimulant prescriptions in the United States. Despite the recognition of the disorder nearly a century ago and the extensive research conducted in the latter half of the 20th century, there are those who continue to believe that ADHD is simply a problem of poor parenting or societal influences. Public and professional concerns include the increasing frequency of diagnosis of ADHD, treatment with stimulant medications, and even the validity of the disorder itself. The purpose of this article is to review the most recent recommendations and clinical data regarding the diagnosis and management of ADHD in children and adolescents to assist with appropriate and prudent clinical decision making.
EPIDEMIOLOGY
The true worldwide prevalence of ADHD is difficult to measure. The reported values in the literature have ranged from 1.7% to as high as 17.8%.1,2 Differences in the prevalence of ADHD may be explained by wide variations in the use of diagnostic and assessment measures, the degree of impairment necessary for diagnosis, cultural differences (e.g., degree of awareness of the disorder as well as variable levels of tolerance of certain behaviors), and the population sampled.3,4
Based on earlier diagnostic criteria, the DSM-IV estimates the prevalence to be 3% to 5% in school-aged children.5 Current estimated prevalence rates in school-aged youth based on DSM-IV criteria are closer to 10%.6 Earlier diagnostic sets had a narrower focus largely based on hyperactivity, whereas current criteria include both hyperactive/impulsive and inattentive subtypes. A recently published analysis of trends regarding diagnosis of ADHD among school-aged children and physician psychostimulant-prescribing practices revealed a 2.3-fold increase in the rate of office-based visits resulting in a diagnosis and a 2.9-fold increase in the rate of ADHD patients prescribed psychostimulant medications.7 The higher rates of diagnosis and treatment may be due to increased recognition of the inattentive subtype, increased awareness and acceptance of the condition, and greater knowledge of the illness course.8 An increase in the use of stimulant medications may also be a reflection of confidence in the efficacy and safety of these medications based on years of experience in clinical practice and numerous controlled clinical trials.
The ratio of male to female patients with ADHD varies depending on factors such as the population studied and the diagnostic criteria utilized. Among patients referred to psychiatrists and psychologists from a clinic setting, the boy-to-girl ratio in school-aged children has been reported as high as 9:1,9,10 whereas in community epidemiologic studies the ratio is closer to 2:1.11,12 The difference in reported sex ratios among school-aged children is most likely due to referral bias in clinical versus population-based studies. The sex ratio among young adults, however, is approximately 1:1.11
ETIOLOGY
Although the exact cause of ADHD has not been fully elucidated, the disorder is most likely caused by a complex interplay of neurologic, biological, and environmental factors. Evidence from neuroimaging studies and family studies implicates neurobiological and genetic factors as the greatest contributors. Several magnetic resonance imaging (MRI) studies have demonstrated abnormalities and/or smaller volumes in specific areas of the brain in patients with ADHD compared with normal subjects. These areas include the caudate nucleus and globus pallidus regions, involved with motor activity; the frontal lobes, associated with attention; and some regions of the corpus callosum.13–16 An analysis of the results from quantitative MRI studies reveals that these regions of the brain are approximately 10% smaller in ADHD patients compared with control patients.17
One of the most well-documented potential etiologies is that of heredity, indicative of a genetic contribution to the pathogenesis of ADHD. Family studies have demonstrated that first-degree relatives of patients with ADHD have a higher risk for the disorder than relatives of controls.18–20 Siblings of children with ADHD have 2 to 3 times the risk of having ADHD compared with siblings of control subjects.21 Concordance is higher in full siblings than in half siblings and in monozygotic twins versus dizygotic twins. Adoptive relatives of children with ADHD are less likely to have the disorder than are biological relatives of these children.22,23 Most studies also demonstrate an increased risk for ADHD in parents of children diagnosed with the disorder.21 Twin studies have indicated that the average heritability of ADHD is approximately 0.80.24 This value means that about 80% of the variance in phenotype can be attributed to genetic rather than environmental factors. Molecular genetic studies have implicated several genes that may be associated with the development of ADHD, including the human thyroid receptor-β gene, the dopamine transporter (DAT1) gene, and the D4 receptor (DRD4) gene.25–29 The genetic mechanisms involved in the heritability of ADHD are likely to be an area of further research.
COURSE OF ILLNESS
Persistence Into Adolescence and Adulthood
Studies published over the past 20 years indicate that the symptoms of ADHD, which were originally thought to diminish as a child matured, may persist into adolescence and adulthood. In a review of follow-up studies, Barkley and Biederman30 estimated that 40% to 80% of patients diagnosed with ADHD in childhood will continue to exhibit symptoms in adolescence and young adulthood. As many as 60% of children with ADHD may continue to display behavioral problems and symptoms of the disorder well into their adult lives.31
Follow-up studies have revealed limited information regarding the timing of remission. ADHD may remit in childhood or adolescence. Early remission (prior to age 12) may be associated with low levels of comorbidity, low familiality, and low psychosocial risk factors.32 Hart et al.33 reported that the persistence of ADHD at a 4-year follow-up was predicted by hyperactive-impulsive symptoms and comorbid conduct disorder. More prospective, longitudinal follow-up studies are necessary to further delineate rates of persistence and the factors affecting remission of ADHD.
Clinical Sequelae
By late childhood and early adolescence, many patients with ADHD will display academic, familial, and social dysfunction. The potential consequences of nontreatment of ADHD include low self-esteem, social and academic failure, and possibly an increased risk of later antisocial behavior.34,35 Adolescents with ADHD are up to 3 times as likely as normal controls to have failed 1 or more grades, been suspended, or been expelled in the course of their academic careers.36 A significant percentage of adolescents and adults (25% to 40%) display delinquent behavior or antisocial personality at follow-up, particularly male patients who exhibited conduct problems at an early age.10,37,38 Adolescents with ADHD tend to be involved in auto accidents and receive traffic violations at a greater rate than adolescents without ADHD.39 In addition, these patients are more likely to smoke and experiment with drugs10,37 and, in patients with a comorbid diagnosis of conduct disorder, to develop a substance abuse problem.36
A significant subgroup of children with ADHD will develop serious psychopathology and dysfunction during adolescence and young adulthood, but the predictive factors are unknown.31 Seidman et al.40 reported that adults with ADHD have higher rates of childhood conduct disorder, adult antisocial personality disorder, and mood and anxiety disorders compared with control patients without ADHD matched by age and gender. Adults with persistent symptoms of ADHD have completed less formal education and have lower-status jobs, but not lower rates of employment.10,37,41
DIAGNOSIS
Diagnostic Criteria
There is no single test to diagnose ADHD; rather, ADHD, like other psychiatric and many medical conditions, is a clinical diagnosis based on symptomatology and associated impairments. The diagnostic criteria primarily used in clinical practice in the United States are specified in the DSM-IV (Table 1).5 These criteria are based on field trials that were conducted mainly in children 5 to 12 years of age.42 Diagnostic criteria require that symptoms be pervasive (i.e., appear in a variety of settings), have persisted for at least 6 months to a degree that is maladaptive and inconsistent with developmental level, manifested before the age of 7 years, and are the cause of significant academic or social impairment. The 3 subtypes of ADHD delineated in the DSM-IV include (1) the predominantly inattentive type, (2) the predominantly hyperactive-impulsive type, and (3) the combined type. In addition, the DSM-IV indicates a diagnosis of “ADHD not otherwise specified” for cases in which prominent symptoms are present but full criteria are not met.
Table 1.
Diagnostic Criteria for Attention-Deficit/Hyperactivity Disordera
The clinical features of the disorder vary widely between subtypes and age groups. For instance, the DSM-IV field trials found inattentive subtype patients were more likely to be diagnosed at a later age than combined or hyperactive-impulsive types. The reason for a later diagnosis in this particular subgroup of patients is that they simply do not get “noticed” as early as their hyperactive/impulsive peers. Those with the predominantly inattentive subtype are often described as “daydreamy” and seem unable to listen. Although these patients often fail to finish tasks, require frequent redirection, and fall behind academically, their symptoms are somewhat subtle as opposed to overt symptoms that command attention.
The manifestations of impulsivity and hyperactivity found in the other subtypes are much more likely to be noticed and addressed at an earlier age. Preschoolers with ADHD often display excessive gross motor activity and may be described as “being driven by a motor.” Excessive fidgeting and restlessness will evolve from this gross motor activity as children get older. The physical impulsivity often seen in the child with ADHD will regress over time, and verbal impulsivity may become a more predominant manifestation in adolescence and adulthood. In contrast to the symptoms of hyperactivity, symptoms of inattention frequently do not diminish with age.43
Patient Evaluation
Assessment.
The American Academy of Pediatrics (AAP) has recently released a Clinical Practice Guideline44 recommending an evaluation for ADHD in all children 6 to 12 years of age who present with inattention, hyperactivity, impulsivity, academic underachievement, or behavioral problems. The diagnosis of ADHD requires a comprehensive clinical assessment including a detailed patient history, clinical interview and observation, and a thorough physical examination. Ideally, the process requires several visits if done thoroughly; if limited to one visit, physicians should allot at least 1 hour to perform the initial assessment. Child and adolescent patients may be capable of maintaining behavioral control in the office setting and may lack insight into the impairment resulting from their symptoms. The parent interview, therefore, is the crux of the assessment process. Structured parent interviews and a DSM-IV symptom checklist may be useful tools in this regard. Other environmental or psychiatric causes should be ruled out at this time based on the parent and child interviews.4
It is also necessary to obtain and review report cards and reports from teachers to determine the patient's history of behavior, academic progress, and attendance at school. Parent and teacher rating scales can be very useful in obtaining valuable diagnostic and prognostic information. The most commonly used and the best normed and validated scales include the parent-completed Child Behavior Checklist, the Teacher Report Form (TRF) of the Child Behavior Checklist, the Conners' Parent and Teacher Rating Scales (the long form), the ADD-H Comprehensive Teacher Rating Scale (ACTeRS), and the Barkley Home Situations Questionnaire and School Situations Questionnaire.4 The AAP guideline suggests rating scales as an option, cautioning clinicians not to validate or refute a diagnosis based solely on these instruments.44 ADHD-specific, rather than broadband, scales are more useful in distinguishing between children with and without ADHD.
Physicians should attempt to assess intellectual ability since children with below-average ability may be inattentive or misbehave if class work is too complex or presented too quickly for them. Similarly, pediatricians should be alert to the possibility of a learning disability or auditory processing disorder since these are common coexistent conditions and may be contributing to (or masquerading as) symptoms of ADHD.4 Symptoms suggestive of a learning disability or auditory processing disorder include a history of language delay, confusion with spatial orientation, difficulty following directions even when paying attention, and/or family history of a learning disability.
Medical evaluation.
A comprehensive physical examination should be performed, and height, weight, pulse, and blood pressure should be recorded. A medication history including the use of prescription, over-the-counter, and illicit drugs also should be documented. An evaluation of vision and hearing is required to determine whether any deficits exist that may mimic the symptoms of ADHD. In addition, a neurologic examination should be performed to rule out a central nervous system insult or progressive neurologic condition. The neurologic assessment should include screening of motor coordination, visual-perceptual skills, language skills, and cognitive function. Neurologic soft signs are not diagnostic for ADHD and can be found in children with learning disabilities, psychoses, and autism as well as in children without developmental disorders. No specific laboratory tests are necessary unless specifically indicated by the history or findings on the physical examination. Medical factors possibly predisposing patients to ADHD include prematurity; prenatal exposure to cocaine, alcohol, and possibly cigarettes; and fragile X syndrome.4 Findings from the routine physical examination in assessments of children with ADHD are usually normal.
Differential Diagnoses and Comorbidities
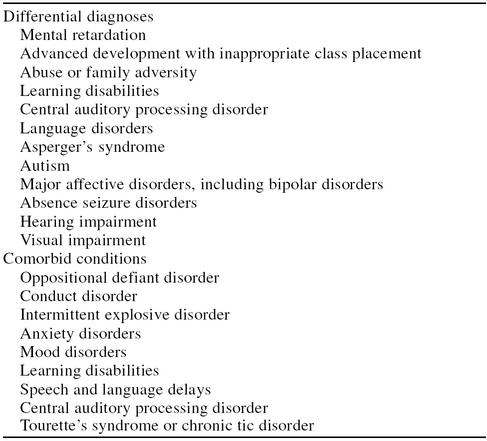
A variety of other disorders can masquerade as ADHD or coexist with the disorder (Table 2).45,46 A comorbid condition exists in up to two thirds of clinically referred children with ADHD, including up to 50% for oppositional defiant disorder, 30% to 50% for conduct disorder, 15% to 20% for mood disorders, and 20% to 25% for anxiety disorders.47,48 Approximately 20% to 30% of children with ADHD have coexistent learning disabilities in the area of reading, spelling, or math.46 The likelihood of a child's having a comorbid condition will be influenced by the age and sex of the child as well as the ADHD subtype. Additionally, there are uncommon conditions that have a high prevalence of comorbid ADHD (i.e., 50% to 60% of patients with Tourette's disorder have ADHD).47
Table 2.
Differential Diagnoses and Comorbidities
The clinician should attempt to identify potential environmental causes of the symptoms suggestive of ADHD. Both abuse (e.g., physical, emotional, sexual) and family dysfunction can produce symptoms similar to those seen in true cases of ADHD. A diagnosis of ADHD should not be given if the patient's symptoms appear to be the result of environmental influences.45 However, the clinician must be cognizant of environmental influences that may affect the severity of symptoms exhibited in the child or adolescent with ADHD.
TREATMENT
The first component of any treatment plan for ADHD should be the dissemination of educational material to the patients and family. Patients and families should be aware of national organizations such as Children and Adults with Attention-Deficit/Hyperactivity Disorder (CHADD; www.chadd.org) that provide access to accurate medical, legal, and educational information. Parents should be informed that ADHD is a neurobiological disorder without a definitive cause that is often, but not always, inherited. In addition, the family should be reassured that although there is not a cure, effective treatments are available to ameliorate the symptoms associated with the disorder.34
Psychosocial Interventions
Treatment for ADHD may include psychosocial interventions, pharmacotherapy, or a combination of the 2. Results from a comprehensive review of psychosocial treatments for ADHD performed by Pelham and colleagues49 indicate that behavioral parent training (e.g., training parents in contingency management, such as “time out” and point/token economy reward systems) and behavioral interventions in the classroom may be viewed as well-established treatments, but that cognitive interventions, such as self-monitoring, self-reinforcement, and self-evaluation do not meet criteria for empirically supported treatments. The psychosocial interventions have significant limitations, including the fact that they are labor intensive and the effects cannot be generalized to different settings or nontargeted behaviors.3
To investigate the relative merits of these interventions in ADHD treatment, the National Institute of Mental Health and Department of Education sponsored the Multimodal Treatment Study of Children With ADHD (MTA study).50 This multicenter, randomized, controlled trial followed 579 children aged 7 to 9 years for a period of 14 months. Children were randomly assigned to 1 of 4 treatment arms:
medication only, with methylphenidate titrated to “best dose” administered 3 times daily (in cases of inadequate response to methylphenidate, dextroamphetamine was employed as a second choice, followed by pemoline or imipramine)
intensive behavioral treatment only, with parent training, classroom behavioral interventions, and full-time summer treatment program
medication management plus behavioral treatment
community-based care with assessment by investigators and referral to community physicians (roughly two thirds of this group received medication prescribed by their chosen physician)
In terms of improvements in the core symptoms of ADHD, the medication management techniques used in this trial were superior to behavioral treatment as well as to routine community care. Although routine community care included medication, the superior response seen in the carefully titrated medication management group speaks to the importance of after-school dosing for many children, higher dosing, and the need for frequent teacher and parent communication. The combination of medication and intensive behavioral treatment resulted in modest improvements in terms of positive functioning outcomes and non-ADHD symptoms compared with medication alone. However, multimodal therapy did not yield significantly greater benefits than medication alone for the core symptoms of ADHD.50
Results of several short-term studies suggest that the addition of psychosocial interventions to stimulant treatment may allow for the administration of lower, and thus potentially safer, doses of stimulants.49 The efficacy of psychosocial interventions is contingent on the persistence, motivation, and cooperation of teachers and parents, and treatment failure with regard to behavioral interventions is often due to incorrect implementation of these methods.34 Behavioral modification techniques, however, should generally be the initial treatment of choice for preschoolers with ADHD.
Pharmacologic Treatment
A 1996 policy statement by the AAP states that pharmacologic treatment for ADHD is indicated when the child or adolescent displays attentional signs and symptoms and related difficulties to a degree that the problems impair the patient's ability to learn and/or develop interpersonal relationships. This policy statement also contends that drug therapy should not be used as the sole treatment for the disorder, but, rather, should be part of an integrated approach including proper classroom placement, behavior modification, and counseling.51 In mild-to-moderate cases of ADHD, behavior modification at home and school may be attempted prior to initiation of pharmacologic treatment. In severe cases (e.g., if the impulsive behavior places the child at risk), immediate initiation of medical treatment may be warranted.34 The Academy is currently refining detailed Clinical Practice Guidelines for the treatment of ADHD that will soon be published.
Stimulants.
Psychostimulants are the medications most commonly used to treat children and adolescents with ADHD, with U.S. estimates of 2.8% or 1.5 million school-aged children being treated with a stimulant medication annually.7,52 Results from a recently published survey of pediatric prescribing practices indicate that stimulants are the most frequently prescribed psychotropic agents, with nearly 2 million office visits and 6 million drug “mentions” in 1995.53 (A “mention” occurs whenever a particular drug is prescribed, recommended, refilled, or given as a sample.) The efficacy of stimulant medications has been demonstrated in all age groups, ranging from preschool-aged children to adults, although the vast majority of data has been obtained from studies of school-aged youths.54
As previously mentioned, much concern has been raised in recent years regarding the dramatic increase in stimulant prescribing and, specifically, a concern that these medications are being overprescribed or perhaps even abused. A recent analysis, however, conducted by the Council on Scientific Affairs of the American Medical Association concluded that, overall, there is “little evidence of widespread overdiagnosis or misdiagnosis of ADHD or of widespread overprescription of methylphenidate by physicians.”8(p1100) Results from a community-based study conducted as part of the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study indicate that appropriate medication treatment for children with ADHD may be underutilized in some areas of the United States.55
Despite the negative media attention focused on stimulant medications, these agents are the first-line choice for pharmacologic therapy given their established safety and efficacy.4 The volume of literature on the treatment of ADHD with stimulant medications is immense. As a result of the published literature and over 60 years of clinical experience, more is known about the use of stimulants in children than any other class of drug prescribed to the pediatric population.4 The stimulants most widely used in the United States include Adderall (amphetamine mixture containing equal parts d-amphetamine sulfate, d,l-amphetamine sulfate, d,l-amphetamine aspartate, and d-amphetamine saccharate), dextroamphetamine, methylphenidate, and pemoline.
1. Pharmacology. It is believed that stimulants, as a class, affect central norepinephrine and dopamine pathways. By enhancing the functioning of executive control, increases in dopamine concentrations at the synaptic cleft may help to overcome the deficits that children with ADHD have in impulse control and working memory. Stimulants are rapidly absorbed after oral administration, exhibit low plasma protein binding, and undergo rapid and extensive extracellular and hepatic metabolism. The short-acting, immediate-release formulations of stimulants exhibit their pharmacodynamic effects on behavior, such as improved concentration and decreased hyperactivity, within 30 minutes, reach their peak effects within 1 to 3 hours, and have a duration of effect of approximately 3 to 5 hours. This short duration of action often necessitates an in-school dosing regimen, leaving patients at risk for “labeling” and ridicule from their peers. There have also been reports from parents and clinicians of a rebound effect when the effects of the dose begin to dissipate in the late afternoon.
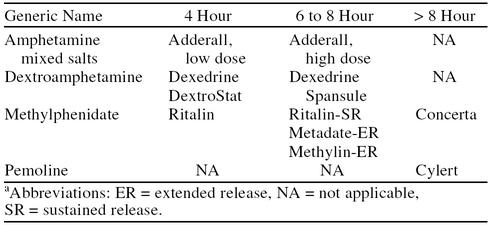
Pemoline is a long-acting stimulant that may be given once-daily for many children, but its use is limited by the potential for hepatotoxicity. Sustained-release preparations of methylphenidate (designed for 8-hour efficacy) are available. Clinical experience with these formulations, however, has found them to be less reliable than multiple doses of the immediate-release formulations for some children. The sustained-release form of dextroamphetamine (Dexedrine Spansule) may produce a more consistent result.4 Some research suggests that the continuous rate of delivery produced by a sustained-release preparation of methylphenidate leads to acute tolerance and, thus, decreased efficacy.56 The need for more reliable, once-daily dosage forms has led to the recent availability of extended-release methylphenidate (Concerta) tablets that utilize an osmotic controlled-release delivery system. These new formulations are designed to be swallowed whole and cannot be chewed or crushed.
There is an increasing body of evidence documenting the efficacy and safety of Adderall in the treatment of ADHD.57–62 Adderall has a potential benefit compared with other short-acting preparations due to its longer duration of action. In a 7-week, randomized, double-blind, crossover study, Swanson and colleagues57 found that this medication was as effective as methylphenidate for ADHD and that the duration of action increased with increasing doses. The results of this study demonstrated the safety and efficacy of Adderall in the treatment of children with ADHD and suggest that the differences in time-response patterns among the doses may allow physicians to vary the dose to achieve optimal response as well as a longer duration of action.
Pelham and colleagues,58 during a summer treatment program, performed a 6-week, double-blind, placebo-controlled, crossover study of the efficacy and time course of Adderall (7.5 mg b.i.d. and 12.5 mg b.i.d.) in comparison with Ritalin (10 mg b.i.d. and 17.5 mg b.i.d.) in 25 children with ADHD. Both drugs were superior to placebo and produced improvements in academic productivity, negative behavior, and staff and parent ratings of behavior. Adderall produced significantly more improvement in nearly all counselor-related measures of behavior. The lower dose of Adderall produced effects that were comparable to those of the higher Ritalin dose, indicating a potency ratio of approximately 1 to 2. Additionally, Adderall was favored 3 to 1 over Ritalin for continued medication based on clinical staff recommendations.58
Clinical studies are currently investigating an extended-release formulation of Adderall in an effort to provide a once-daily dosing option to more patients.
2. Therapeutic effects. With appropriate dosing, Adderall, dextroamphetamine, and methylphenidate are efficacious for the treatment of ADHD. It is generally accepted that at least 70% of patients will respond to a trial of one of the major psychostimulants54 in comparison with relatively low rates of response to placebo, ranging from 3% to 30%.63 The response rate to stimulants is even higher if more than one stimulant is tried. In a double-blind, crossover study of 48 male, school-aged patients comparing methylphenidate, dextroamphetamine, and placebo, only 4.2% of the patients failed to respond to at least 1 of the 2 stimulants.64 Similarly, Manos et al.60 reported behavioral benefits from Adderall in 13 of 15 children who previously had been treated unsuccessfully with methylphenidate. Subtle differences among the available psychostimulants may account for variations in efficacy or tolerance among patients. This variability may necessitate a trial of more than one stimulant if response to the first is not satisfactory.65,66 A positive response to stimulant medication, however, is not diagnostic of the presence of ADHD. Patients with other psychiatric disorders and even normal subjects may have a positive response to stimulants.67
Clinically, treatment with a stimulant can be expected to result in an immediate, often dramatic, improvement in the core symptoms of ADHD (inattention, hyperactivity, and impulsivity). In addition to improvements in the core symptoms of ADHD, stimulant treatment may result in diminished physical and verbal aggression; improved social interactions with peers, teachers, and parents; enhanced academic productivity and accuracy; and better compliance. Areas of impairment that have not been shown to improve during treatment with stimulant medications include reading skills, social skills, learning, long-term academic achievement, and antisocial behavior or arrest rate.34
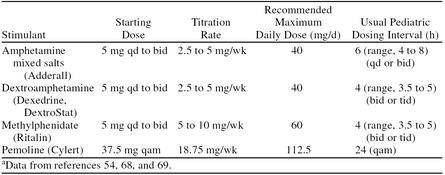
3. Dosing recommendations. The goal of safe and effective pharmacotherapy with stimulant medications is to find the lowest effective dose that allows for maximal therapeutic benefits while minimizing adverse effects.68 Weight-based dosing regimens are often employed in clinical trials; however, response to stimulant medications cannot be reliably predicted by body weight, and current research does not support weight-dependent dosing.66 Pediatric dosing guidelines for immediate-release preparations are presented in Table 3.54,68,69 The clinician treating a patient with ADHD must consider that the optimal dose of any psychostimulant in terms of both efficacy and tolerability varies from person to person and, thus, should be titrated to the needs of the individual patient.70
Table 3.
Pediatric Dosing Recommendations for Immediate-Release Stimulantsa
When initiating therapy with methylphenidate, it is customary to start with a low dose of approximately 5 mg twice daily given in the morning and at noon. The dose is titrated on a weekly basis using increments of 5 or 10 mg until a satisfactory therapeutic benefit is achieved (or significant adverse effects are manifested). Therapeutic benefit is usually seen at doses ranging from 0.3 to 0.8 mg/kg/dose. Due to the short pharmacodynamic duration of action of the immediate-release form of methylphenidate, it is usually necessary to administer the drug 2 or 3 times daily. Sustained-release (8-hour) or extended-release (12-hour) formulations of methylphenidate may be substituted for the immediate-release tablets when the time-release dose corresponds to the total daily dose of immediate-release tablets.
Dextroamphetamine may be administered at one half the dose of methylphenidate because of the approximate 1:2 potency ratio between the 2 agents. For children 3 to 5 years of age, the starting dose is 2.5 mg once daily. The dose is then increased at a rate of 2.5 mg per week until a satisfactory response is exhibited. For children aged 6 years or older, 5 mg once or twice daily is used and titrated to optimal response in 5-mg weekly increments. Sustained-release formulations may be substituted for the immediate-release tablets when the time-release dose corresponds to the total daily dose of immediate-release tablets.
Adderall may be initiated in the same manner as dextroamphetamine. However, research by Swanson and colleagues57 indicates that increasing the dose of Adderall increases the duration of effect. Thus, this medication may be initiated in a single morning dose with dosage increases every 3 to 5 days. The maximum single dose can be determined by efficacy as well as side effects that may emerge. Depending on the duration of effect achieved, a second daily dose may be added. Recent trials indicate that, for some children, a single daily dosing of Adderall appears to be as effective as twice-daily dosing of methylphenidate.59–61
Because of the risk of hepatotoxicity, pemoline, although it has the advantage of being a longer-acting agent, is generally used only in patients who do not respond to a trial of the other stimulants. It is usually initiated at a dose of 37.5 mg administered once daily in the morning and is titrated in aliquots of 18.75 mg every week until a therapeutic benefit is achieved.68,71
The decision of how many times per day and how many days per week to administer medication should be individualized to the specific needs of the patient based on the time course and severity of symptoms.4 Although the duration of effect of stimulant medications varies from patient to patient, approximate times are provided in Table 4. After the initial dose titration phase, some children may be able to achieve adequate benefit from a regimen in which medication is administered only on school days. However, many patients display significant impairments outside the classroom and benefit from medication during weekends and holidays.
Table 4.
Approximate Duration of Effect of Available Stimulantsa
Adverse events. There is essentially no difference among the stimulants in terms of the frequency or severity of adverse events; however, the severity of side effects among the various stimulants may differ in individual patients.4 Thus, as in the case of an unsatisfactory response, patients who are intolerant of one of the stimulants may tolerate another stimulant without any significant problems. All of the available agents share a similar adverse event profile, with the most frequently cited side effects including decreased appetite, headache, irritability, insomnia, and stomachache.69 These effects appear shortly after initiation of the medication trial, are mild in nature, and may be diminished with careful dose titration.63 The frequency of some side effects reported during stimulant treatment such as staring and daydreaming, irritability, anxiety, and nail biting have been shown to decrease with continued treatment.72 Severe adverse events occur only in a minority of patients.
The possibility of growth suppression in children treated with psychostimulants is an area of concern to both parents and physicians treating children with ADHD. Small, but statistically significant, decreases in growth have been observed in children with ADHD who are treated with stimulants.73,74 This decrease, however, does not appear to affect the final height attained by young adults with ADHD treated with stimulants as children.75 One approach, which has been utilized by some clinicians in an attempt to compensate for the potential for growth suppression during stimulant treatment, has been the use of “drug holidays” (e.g., weekends or summers without treatment). This approach may cause a deterioration in some children's functioning and, therefore, is not an absolute requirement.76 There is also evidence suggesting that the differences in growth observed between children with ADHD and controls may be associated with the disorder itself, rather than a result of treatment with stimulants.77
A potential adverse event that warrants consideration by physicians is the increased risk of development of transient motor or vocal tics during treatment with stimulant medications. The majority of reported cases of tics resulting from stimulant therapy have resolved after discontinuation; however, there are a few cases in which the tics did not diminish once the drug was removed.71 In patients with preexisting tic disorders, treatment with stimulants may occasionally cause exacerbation of the disorder.54 Although most cases are transient and it is relatively rare for a chronic tic disorder to develop (e.g., fewer than 1% of children treated with a stimulant), it is recommended that physicians inquire as to a personal or family history of tics or Tourette's disorder prior to the initiation of a stimulant and proceed with caution in the presence of such a history.71 Physicians are urged to weigh the potential impairment from tics (or Tourette's) with the impairment caused by the ADHD symptoms when making clinical decisions regarding pharmacotherapy in these cases.4
Hepatotoxicity is a rare adverse event associated with long-term pemoline treatment. There have been several cases of elevated liver enzymes reported in the literature, including a handful of cases of fatal hepatic failure.78 As a result of the risk for hepatic injury, pemoline should not be considered as a first-line therapy for children with ADHD. Monitoring of liver enzymes is recommended every 2 weeks; however, the onset of hepatitis is unpredictable. It is imperative, therefore, to educate the patient and family to report if the child experiences nausea, vomiting, or malaise, appears jaundiced, or if abdominal discomfort persists for greater than 2 weeks.4
An understandable concern for parents of children and adolescents treated for ADHD is the potential for abuse or dependence with stimulant medications. Methylphenidate, dextroamphetamine, and Adderall are Schedule II drugs and, thus, are regarded as drugs associated with considerable potential for abuse. Pemoline is a Schedule IV drug and is not associated with as high a potential for abuse; as mentioned above, however, its use is limited by the potential for hepatotoxicity. Prudence necessitates special consideration and careful monitoring in the treatment of adolescents with stimulants. Despite this caution, there are no reports of individual cases of drug addiction or serious dependence with these medications71; nor is there evidence that stimulant use increases the incidence of later substance abuse. On the contrary, there is evidence indicating that treating ADHD with stimulants may prevent later substance abuse.79
Nonstimulant medications.
Although stimulants are effective in the majority of patients, there is a percentage of patients who fail to respond to or are intolerant of these medications. Although none of these nonstimulant medications are approved by the U.S. Food and Drug Administration (FDA) to treat ADHD, the most studied medications in the treatment of ADHD—antidepressants and α2 agonists—are discussed. Other classes of drugs that have been used to treat ADHD include neuroleptics and anticonvulsants.
1. Antidepressants. The most well-established medications for the treatment of children with ADHD in this class are the tricyclic antidepressants (TCAs). These agents have putative inhibitory effects on the reuptake of selective neurotransmitters, specifically norepinephrine and serotonin. The undesirable effects of the TCAs result from their effects on various other neurotransmitter systems such as the histaminic, cholinergic, α-adrenergic, and serotonergic systems.80 TCAs that have been the most extensively studied in the treatment of ADHD include desipramine, imipramine, and nortriptyline.
TCAs are considered second-line agents in the treatment of ADHD, used predominantly for children who do not have a satisfactory response to the stimulant class of medications or for whom stimulants may be contraindicated. The available literature indicates that TCAs are effective in controlling the behavioral symptoms of ADHD, but TCAs do not appear to be as effective as the stimulants in improving attention and concentration in children with ADHD.69 The TCAs may prove to be of benefit, however, in patients with comorbid depression or anxiety or in patients with pre-existing tic or Tourette's disorders.4 Other potential benefits of the TCAs compared with stimulants include a longer half-life, thus reducing the necessity for in-school dosing; fewer disturbances in sleep, appetite, and growth patterns; and a minimal risk of abuse.3,69
The largest concern with the use of TCAs in the pediatric population is that of safety. Sudden, unexplained deaths have been reported following the use of desipramine.81–83 Although the exact mechanism underlying these deaths is not clear, it is recommended that an electrocardiogram be obtained at baseline and at steady-state levels once they are achieved. Routine monitoring of plasma levels is also prudent. The risk of fatality following overdose is another substantial safety concern, with mortality rates up to 1% following desipramine overdose.84 A major drawback with all TCAs is the incidence of adverse events. Anticholinergic side effects (e.g., dry mouth, sedation, weight gain, constipation), cardiotoxicity, and neurologic effects (e.g., lowering of seizure threshold) further limit patient acceptance and tolerance of these agents.
Other antidepressants also have been studied for the treatment of ADHD. Monoamine oxidase inhibitors (MAOIs), specifically tranylcypromine and clorgyline, have been shown to be effective in the treatment of ADHD. Due to the dietary restrictions and risks with these medications, however, MAOIs are rarely used clinically and are generally not recommended for treatment of children with ADHD.84 Selective serotonin reuptake inhibitors (SSRIs) have not been extensively studied in patients with ADHD. An open-label trial with fluoxetine displayed positive results,85 but the lack of controlled data necessitates that the role of SSRIs in treating ADHD be considered preliminary at most.84 SSRIs may be useful as an adjunctive medication for patients with ADHD and comorbid major depressive disorder.86
The most promising data in the management of ADHD with newer antidepressants are with bupropion. There are a number of open and controlled studies documenting its efficacy in both children and adults with ADHD.87–90 Bupropion is usually well tolerated; however, it may lower the seizure threshold at higher dose ranges or in patients with eating disorders.4 There also have been reports of exacerbation of tics with bupropion treatment.91 Generally, however, its side effect profile is better than that of stimulants and TCAs. It may prove to be a useful alternative for the treatment of ADHD, but the reports thus far have involved only a small number of patients. Further research is warranted to determine the appropriate role of bupropion in the treatment of ADHD.92
2.α2 Agonists. Lack of response or intolerance to first- and second-line agents may lead physicians to seek nonstimulant, nonantidepressant alternatives. α2 Agonists such as clonidine and guanfacine have been evaluated in patients with ADHD. Studies with clonidine have described beneficial effects on symptoms of aggression, impulsivity, overarousal, and hyperactivity; fewer benefits are reported for effects on attention and concentration. Common treatment-emergent side effects include drowsiness, dizziness, and sedation. Feelings of dysphoria and irritability may occur with clonidine treatment. Slower dose titration or dose reduction may help to ameliorate some of these effects.93 Although controversial, the combination of clonidine and methylphenidate has been studied in open-label trials and used in clinical practice when only a partial therapeutic response is achieved with stimulants or the stimulant dose is limited by side effects.94 Rare cases of sudden death and cardiac adverse events have been reported with this combination; however, a causal relationship has not been established.
Case reports have described beneficial effects of clonidine in the treatment of sleep disturbances in patients with ADHD, both with and without psychostimulant treatment.93 Results of a retrospective chart review by Prince and colleagues95 suggest clonidine may be an effective treatment for sleep disturbances associated with ADHD.
Guanfacine is another α2 agonist that has been evaluated for potential use in ADHD, although not as extensively as clonidine. Advantages compared with clonidine include a longer duration of action and decreased sedative side effects. Open-label studies are promising96,97; however, currently there are no controlled trials in the literature evaluating its efficacy in ADHD.
Other Treatments
There are a variety of alternative management strategies that have been touted by their proponents as effective for the treatment of ADHD without adequate evidence to support such claims. Treatments for ADHD including megavitamin therapy, dietary restrictions, herbal remedies, mineral or amino acid supplementation, and chelating agents have proven ineffective in controlled clinical trials.98 Electroencephalogram (EEG) biofeedback training remains quite controversial; although several poorly controlled studies have demonstrated benefits of EEG biofeedback,99,100 there are many reasons to question its efficacy. An extensive review of alternative treatments for ADHD has been published elsewhere.98 Several psychosocial interventions also have not been proved efficacious in ADHD, including cognitive treatments (e.g., problem-solving strategies and self-monitoring training), modeling, individual counseling, and play therapy.3,4,45
CONCLUSION
ADHD is a chronic, neurodevelopmental disorder affecting children, adolescents, and—it is becoming increasingly evident—adults. Although not conclusively determined, it appears to be caused by a combination of neurobiological and genetic factors. The diagnosis is made on the basis of a detailed patient history, with reports from both parents and teachers a necessity for a comprehensive evaluation. A variety of other disorders can coexist or masquerade as ADHD; physicians must consider the numerous potential differential diagnoses and comorbidities when performing the initial assessment of a patient with symptoms of ADHD. Stimulant medications (methylphenidate and amphetamine preparations), despite controversy in recent years, remain first-line therapy in the treatment of ADHD. Overdiagnosis of ADHD and inappropriate prescription of stimulants continue to be a concern; however, nontreatment of ADHD may be associated with low self-esteem, social and academic failure, as well as an increased risk of later antisocial behavior and drug abuse. Accurate diagnosis and optimal management of this disorder may be the keys to improving long-term outcomes of youth with this disorder.
Drug names: bupropion (Wellbutrin and others), clonidine (Catapres and others), desipramine (Norpramin and others), dextroamphetamine (Dexedrine and others), fluoxetine (Prozac), guanfacine (Tenex and others), methylphenidate (Ritalin, Concerta, and others), nortriptyline (Pamelor and others), pemoline (Cylert), tranylcypromine (Parnate).
REFERENCES
- Esser G, Schmidt MH, Woerner W. Epidemiology and course of psychiatric disorders in school-age children: results of a longitudinal study. J Child Psychol Psychiatry. 1990;31:243–263. doi: 10.1111/j.1469-7610.1990.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Baumgaertel A, Wolraich ML, Dietrich M. Comparison of diagnostic criteria for attention deficit disorders in a German elementary school sample. J Am Acad Child Adolesc Psychiatry. 1995;34:629–638. doi: 10.1097/00004583-199505000-00015. [DOI] [PubMed] [Google Scholar]
- Elia J, Ambrosini PJ, Rapoport JL. Treatment of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340:780–788. doi: 10.1056/NEJM199903113401007. [DOI] [PubMed] [Google Scholar]
- American Academy of Child and Adolescent Psychiatry. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(suppl 10):85S–121S. doi: 10.1097/00004583-199710001-00007. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Attention-deficit and disruptive behavior disorders. In: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association. 1994 78–85. [Google Scholar]
- Wolraich ML, Hannah JN, Pinnock TY, et al. Comparison of diagnostic criteria for attention-deficit hyperactivity disorder in a county-wide sample. J Am Acad Child Adolesc Psychiatry. 1996;35:319–324. doi: 10.1097/00004583-199603000-00013. [DOI] [PubMed] [Google Scholar]
- Robison LM, Sclar DA, Skaer TL, et al. National trends in the prevalence of attention-deficit/hyperactivity disorder and the prescribing of methylphenidate among school-age children: 1990–1995. Clin Pediatrics. 1999;38:209–217. doi: 10.1177/000992289903800402. [DOI] [PubMed] [Google Scholar]
- Goldman LS, Genel M, Bezman RJ, et al. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. JAMA. 1998;279:1100–1107. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- Weiss G, Hechtman L, Milroy T, et al. Psychiatric status of hyperactives as adults: a controlled prospective 15-year follow-up of 63 hyperactive children. J Am Acad Child Adolesc Psychiatry. 1985;24:211–220. doi: 10.1016/s0002-7138(09)60450-7. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Edelbrock CS, et al. The adolescent outcome of hyperactive children diagnosed by research criteria, 1: an 8-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990;29:546–557. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Kasen S, et al. An epidemiological study of disorders in late childhood and adolescence, 1: age- and gender-specific prevalence. J Child Psychol Psychiatry. 1993;34:851–867. doi: 10.1111/j.1469-7610.1993.tb01094.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Prevalence and comorbidity of DSM-III-R diagnoses in a birth cohort of 15 year olds. J Am Acad Child Adolesc Psychiatry. 1993;32:1127–1134. doi: 10.1097/00004583-199311000-00004. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Semrud-Clikeman M, Steingard RJ, et al. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Reiss AL, Reader MJ, et al. Basal ganglia volumes in children with attention-deficit hyperactivity disorder. J Child Neurol. 1996;11:112–115. doi: 10.1177/088307389601100210. [DOI] [PubMed] [Google Scholar]
- Hynd GW, Hern KL, Novey ES, et al. Attention deficit-hyperactivity disorder and asymmetry of the caudate nucleus. J Child Neurol. 1993;8:339–347. doi: 10.1177/088307389300800409. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Sergeant JA, Taylor E, et al. Attention-deficit hyperactivity disorder and hyperkinetic disorder. Lancet. 1998;351:429–433. [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Keenan K, et al. Family-genetic and psychosocial risk factors in DSM-III attention deficit disorder. J Am Acad Child Adolesc Psychiatry. 1990;29:526–533. doi: 10.1097/00004583-199007000-00004. [DOI] [PubMed] [Google Scholar]
- Morrison JR, Stewart MA. A family study of the hyperactive child syndrome. Biol Psychiatry. 1971;3:189–195. [PubMed] [Google Scholar]
- Cantwell DP. Psychiatric illness in the families of hyperactive children. Arch Gen Psychiatry. 1972;27:414–417. doi: 10.1001/archpsyc.1972.01750270114018. [DOI] [PubMed] [Google Scholar]
- Faraone S, Biederman J. Genetics of attention-deficit hyperactivity disorder. Child Adolesc Psychiatr Clin North Am. 1994;3:285–302. doi: 10.1016/j.chc.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Alberts-Corush J, Firestone P, Goodman JT. Attention and impulsivity characteristics of the biological and adoptive parents of hyperactive and normal control children. Am J Orthopsychiatry. 1986;56:413–423. doi: 10.1111/j.1939-0025.1986.tb03473.x. [DOI] [PubMed] [Google Scholar]
- Morrison JR, Stewart MA. The psychiatric status of the legal families of adopted hyperactive children. Arch Gen Psychiatry. 1973;28:888–891. doi: 10.1001/archpsyc.1973.01750360098015. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J. Neurobiology of attention-deficit hyperactivity disorder. Biol Psychiatry. 1998;44:951–958. doi: 10.1016/s0006-3223(98)00240-6. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Sunohara GA, Kennedy JL, et al. Association of the dopamine receptor D4 (DRD4) gene with a refined phenotype of attention deficit hyperactivity disorder (ADHD): a family-based approach. Mol Psychiatry. 1998;3:38–41. doi: 10.1038/sj.mp.4000354. [DOI] [PubMed] [Google Scholar]
- Gill M, Daly G, Heron S, et al. Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Mol Psychiatry. 1997;2:311–313. doi: 10.1038/sj.mp.4000290. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, et al. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Cook EH Jr, Stein MA, Krasowski MD, et al. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet. 1995;56:993–998. [PMC free article] [PubMed] [Google Scholar]
- Hauser P, Zametkin AJ, Martinez P, et al. Attention deficit-hyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med. 1993;328:997–1001. doi: 10.1056/NEJM199304083281403. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Biederman J. Toward a broader definition of the age-of-onset criterion for attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:1204–1210. doi: 10.1097/00004583-199709000-00012. [DOI] [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity disorder: a life-span perspective. J Clin Psychiatry. 1998;59(suppl 7):4–16. [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Milberger S, et al. Predictors of persistence and remission of ADHD: results from a four year prospective follow-up study of ADHD children. J Am Acad Child Adolesc Psychiatry. 1996;35:343–351. doi: 10.1097/00004583-199603000-00016. [DOI] [PubMed] [Google Scholar]
- Hart E, Lahey B, Loeber R, et al. Developmental change in attention-deficit hyperactivity disorder in boys: a four-year longitudinal study. J Abnorm Child Psychol. 1995;23:729–749. doi: 10.1007/BF01447474. [DOI] [PubMed] [Google Scholar]
- Zametkin AJ, Ernst M. Problems in the management of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340:40–46. doi: 10.1056/NEJM199901073400107. [DOI] [PubMed] [Google Scholar]
- Satterfield JH, Schell A. A prospective study of hyperactive boys with conduct problems and normal boys: adolescent and adult criminality. J Am Acad Child Adolesc Psychiatry. 1997;12:1726–1735. doi: 10.1097/00004583-199712000-00021. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Developmental course, adult outcome, and clinic-referred ADHD adults. In: Barkley RA, ed. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. 2nd ed. New York, NY: Guilford Press. 1998 186–224. [Google Scholar]
- Mannuzza S, Klein RG, Bonagura N, et al. Hyperactive boys almost grown up, 5: replication of psychiatric status. Arch Gen Psychiatry. 1991;48:77–83. doi: 10.1001/archpsyc.1991.01810250079012. [DOI] [PubMed] [Google Scholar]
- Gittelman R, Mannuzza S, Shenker R, et al. Hyperactive boys almost grown up, 1: psychiatric status. Arch Gen Psychiatry. 1985;42:937–947. doi: 10.1001/archpsyc.1985.01790330017002. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Guevremont DC, Anastopoulos AD, et al. Driving-related risks and outcomes of attention deficit hyperactivity disorder in adolescents and young adults: a 3- to 5-year follow-up survey. Pediatrics. 1993;92:212–218. [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Weber W, et al. Neuropsychological function in adults with attention-deficit hyperactivity disorder. Biol Psychiatry. 1998;44:260–268. doi: 10.1016/s0006-3223(97)00392-2. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Bessler A, et al. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998;155:493–498. doi: 10.1176/ajp.155.4.493. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, McBurnett K, et al. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. Am J Psychiatry. 1994;151:1673–1685. doi: 10.1176/ajp.151.11.1673. [DOI] [PubMed] [Google Scholar]
- Castellanos FX. Toward a pathophysiology of attention-deficit/hyperactivity disorder. Clin Pediatr. 1997;36:381–393. doi: 10.1177/000992289703600702. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Quality Improvement, Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics. 2000;105:1158–1170. doi: 10.1542/peds.105.5.1158. [DOI] [PubMed] [Google Scholar]
- Baumgaertel A, Wolraich ML. Practice guideline for the diagnosis and management of attention deficit hyperactivity disorder. Ambulatory Child Health. 1998;4:45–58. [Google Scholar]
- Pliszka SR. Comorbidity of attention-deficit/hyperactivity disorder with psychiatric disorder: an overview. J Clin Psychiatry. 1998;59(suppl 7):50–58. [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S, et al. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry. 1991;148:564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Halperin JM. Comorbidity among disruptive behavior disorders: impact on severity, impairment, and response to treatment. Child Adolesc Psychiatr Clin North Am. 1994;3:227–252. [Google Scholar]
- Pelham WE, Wheeler T, Chronis A. Empirically supported psychosocial treatments for attention-deficit/hyperactivity disorder. J Clin Child Psychol. 1998;27:190–205. doi: 10.1207/s15374424jccp2702_6. [DOI] [PubMed] [Google Scholar]
- The MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999 56:1073–1086. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Children with Disabilities, Committee on Drugs. Medication for children with attentional disorders. Pediatrics. 1996 98:301–304. [PubMed] [Google Scholar]
- Safer DJ, Zito JM, and Fine EM. Increased methylphenidate usage in the 1990s. Pediatrics. 1996 98(6, pt 1). 1084–1088. [PubMed] [Google Scholar]
- Jensen PS, Bhatara VS, Vitiello B, et al. Psychoactive medication prescribing practices for US children: gaps between research and clinical practice. J Am Acad Child Adolesc Psychiatry. 1999;38:557–565. doi: 10.1097/00004583-199905000-00017. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35:409–432. doi: 10.1097/00004583-199604000-00008. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Kettle L, Roper MT, et al. Are stimulants overprescribed? treatment of ADHD in four US communities. J Am Acad Child Adolesc Psychiatry. 1999;38:797–804. doi: 10.1097/00004583-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Swanson J, Gupta S, Guinta D, et al. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66:295–305. doi: 10.1016/S0009-9236(99)70038-X. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Wigal S, Greenhill LL, et al. Analog classroom assessment of Adderall in children with ADHD. J Am Acad Child Adolesc Psychiatry. 1998;37:519–526. [PubMed] [Google Scholar]
- Pelham WE, Aronoff HR, Midlam JK, et al. A comparison of Ritalin and Adderall: efficacy and time-course in children with attention-deficit/hyperactivity disorder. Pediatrics. 1999;103:e43. doi: 10.1542/peds.103.4.e43. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Chronis AM. A comparison of morning-only and morning/late afternoon Adderall to morning-only, twice-daily, and three times-daily methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 1999;104:1300–1311. doi: 10.1542/peds.104.6.1300. [DOI] [PubMed] [Google Scholar]
- Manos MJ, Short EJ, Findling RL. Differential effectiveness of methylphenidate and Adderall in school-age youths with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1999;38:813–819. doi: 10.1097/00004583-199907000-00010. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Browne RG, Olvera RL, et al. A double-blind, placebo-controlled study of Adderall and methylphenidate in the treatment of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:619–626. doi: 10.1097/00004583-200005000-00016. [DOI] [PubMed] [Google Scholar]
- Ahmann PA, Theye FW, Berg R, et al. Placebo-controlled evaluation of amphetamine mixture–dextroamphetamine salts and amphetamine salts (Adderall): efficacy rate and side effects. Pediatrics. 2001;107:e10. doi: 10.1542/peds.107.1.e10. [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Halperin JM, Abikoff H. Stimulant medications. J Am Acad Child Adolesc Psychiatry. 1999;38:503–512. doi: 10.1097/00004583-199905000-00011. [DOI] [PubMed] [Google Scholar]
- Elia J, Borcherding BG, Rapoport JL, et al. Methylphenidate and dextroamphetamine treatments of hyperactivity: are there true non-responders? Psychiatry Res. 1991;36:141–155. doi: 10.1016/0165-1781(91)90126-a. [DOI] [PubMed] [Google Scholar]
- Elia J, Rapoport JL. Ritalin versus dextroamphetamine in ADHD: both should be tried. In: Greenhill LL, Osman BB, eds. Ritalin: Theory and Patient Management. New York, NY: Mary Ann Liebert. 1991 69–74. [Google Scholar]
- Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry. 1996;35:1304–1313. doi: 10.1097/00004583-199610000-00017. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Buchsbaum MS, Weingartner H, et al. Dextroamphetamine: its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch Gen Psychiatry. 1980;37:933–943. doi: 10.1001/archpsyc.1980.01780210091010. [DOI] [PubMed] [Google Scholar]
- Findling RL, Dogin JW. Psychopharmacology of ADHD: children and adolescents. J Clin Psychiatry. 1998;59(suppl 7):42–49. [PubMed] [Google Scholar]
- Cyr M, Brown CS. Current drug therapy recommendations for the treatment of attention deficit hyperactivity disorder. Drugs. 1998;56:215–223. doi: 10.2165/00003495-199856020-00005. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Denney C. Titrating methylphenidate in children with attention-deficit/hyperactivity disorder: is body mass predictive of clinical response? J Am Acad Child Adolesc Psychiatry. 1997;36:523–530. doi: 10.1097/00004583-199704000-00015. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Barkley RA, and Connor DF. Stimulants. In: Barkley RA, ed. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. 2nd ed. New York, NY: Guilford Press. 1998 510–551. [Google Scholar]
- Ahmann PA, Waltonen SJ, Olson KA, et al. Placebo-controlled evaluation of Ritalin side effects. Pediatrics. 1993;91:1101–1106. [PubMed] [Google Scholar]
- Safer D, Allen R, Barr E. Depression of growth in hyperactive children on stimulant drugs. N Engl J Med. 1972;287:217–220. doi: 10.1056/NEJM197208032870503. [DOI] [PubMed] [Google Scholar]
- Klein RG, Landa B, Mattes JA, et al. Methylphenidate and growth in hyperactive children: a controlled withdrawal study. Arch Gen Psychiatry. 1988;45:1127–113075. doi: 10.1001/archpsyc.1988.01800360075011. [DOI] [PubMed] [Google Scholar]
- Klein RG, Mannuzza S. Hyperactive boys almost grown up, 3: methylphenidate effects on ultimate height. Arch Gen Psychiatry. 1988;45:1131–1134. doi: 10.1001/archpsyc.1988.01800360079012. [DOI] [PubMed] [Google Scholar]
- Pliszka SR. The use of psychostimulants in the pediatric patient. Pediatr Clin North Am. 1998;45:1085–1098. doi: 10.1016/s0031-3955(05)70063-8. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T. Growth deficits in children with attention deficit hyperactivity disorder. Pediatrics. 1998;102:501–506. [PubMed] [Google Scholar]
- Shevell M, Schreiber R. Pemoline-associated hepatic failure: a critical analysis of the literature. Pediatr Neurol. 1997;16:14–16. doi: 10.1016/s0887-8994(96)00266-4. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, and Wilens T. Pharmacotherapy of ADHD with antidepressants. In: Barkley RA, ed. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. 2nd ed. New York, NY: Guilford Press. 1998 552–563. [Google Scholar]
- Riddle MA, Nelson JC, Kleinman CS, et al. Sudden death in children receiving Norpramin: a review of three reported cases and commentary. J Am Acad Child Adolesc Psychiatry. 1991;30:104–108. doi: 10.1097/00004583-199101000-00016. [DOI] [PubMed] [Google Scholar]
- Riddle MA, Geller B, Ryan N. Another sudden death in a child treated with desipramine. J Am Acad Child Adolesc Psychiatry. 1993;32:792–797. doi: 10.1097/00004583-199307000-00013. [DOI] [PubMed] [Google Scholar]
- Varley CK, McClellan J. Case study: two additional sudden deaths with tricyclic antidepressants. J Am Acad Child Adolesc Psychiatry. 1997;36:390–394. doi: 10.1097/00004583-199703000-00018. [DOI] [PubMed] [Google Scholar]
- Popper CW. Antidepressants in the treatment of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 1997;58(suppl 14):14–29. [PubMed] [Google Scholar]
- Barrickman L, Noyes R, Kuperman S, et al. Treatment of ADHD with fluoxetine: a preliminary trial. J Am Acad Child Adolesc Psychiatry. 1991;30:762–767. [PubMed] [Google Scholar]
- Pliszka SR, Greenhill LL, Lynn CM, et al. The Texas Children's Medication Algorithm Project: report of the Texas consensus conference panel on medication treatment of childhood attention-deficit/hyperactivity disorder, pt 1. J Am Acad Child Adolesc Psychiatry. 2000;39:908–919. doi: 10.1097/00004583-200007000-00021. [DOI] [PubMed] [Google Scholar]
- Simeon JG, Ferguson HB, Van Wyck Fleet J. Bupropion effects in attention deficit and conduct disorders. Can J Psychiatry. 1986;31:581–585. doi: 10.1177/070674378603100617. [DOI] [PubMed] [Google Scholar]
- Wender PH, Reimherr FW. Bupropion treatment of attention-deficit hyperactivity disorder in adults. Am J Psychiatry. 1990;147:1018–1020. doi: 10.1176/ajp.147.8.1018. [DOI] [PubMed] [Google Scholar]
- Conners CK, Casat CD, Gualtieri CT, et al. Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1996;35:1314–1321. doi: 10.1097/00004583-199610000-00018. [DOI] [PubMed] [Google Scholar]
- Riggs PD, Leon SL, Mikulich SK, et al. An open trial of bupropion for ADHD in adolescents with substance use disorders and conduct disorder. J Am Acad Child Adolesc Psychiatry. 1998;37:1271–1278. doi: 10.1097/00004583-199812000-00010. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Steingard R, et al. Bupropion exacerbates tics in children with attention-deficit hyperactivity disorder and Tourette's syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32:211–214. doi: 10.1097/00004583-199301000-00030. [DOI] [PubMed] [Google Scholar]
- Cantwell DP. ADHD through the life span: the role of bupropion in treatment. J Clin Psychiatry. 1998;59(suppl 4):92–94. [PubMed] [Google Scholar]
- Connor DF. Other medications in the treatment of child and adolescent ADHD. In: Barkley RA, ed. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. 2nd ed. New York, NY: Guilford Press. 1998 564–581. [Google Scholar]
- Wilens TE, Spencer TJ. Combining methylphenidate and clonidine: a clinically sound medication option. J Am Acad Child Adolesc Psychiatry. 1999;38:614–616. doi: 10.1097/00004583-199905000-00025. [DOI] [PubMed] [Google Scholar]
- Prince JB, Wilens TE, Biederman J, et al. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry. 1996;35:599–605. doi: 10.1097/00004583-199605000-00014. [DOI] [PubMed] [Google Scholar]
- Hunt RD, Arnsten AF, Asbell MD. An open trial of guanfacine in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34:50–54. doi: 10.1097/00004583-199501000-00013. [DOI] [PubMed] [Google Scholar]
- Horrigan JP, Barnhill LJ. Guanfacine treatment of attention-deficit hyperactivity disorder in boys. J Child Adolesc Psychopharmacol. 1995;5:215–223. [Google Scholar]
- Arnold LE. Treatment alternatives for attention-deficit/hyperactivity disorder (ADHD) J Attention Disord. 1999;3:30–48. [Google Scholar]
- Lubar JF, Swartwood MO, Swartwood JN, and et al. Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in T.O.V.A. scores, behavioral ratings, and WISC-R performance. Biofeedback Self Regul. 1995 20:83–99. [DOI] [PubMed] [Google Scholar]
- Linden M, Habib T, Radojevic V. A controlled study of the effects of EEG biofeedback on cognition and behavior of children with attention deficit disorder and learning disabilities. Biofeedback Self Regul. 1996;21:35–49. doi: 10.1007/BF02214148. [DOI] [PubMed] [Google Scholar]