Abstract
Sleep disorders can be divided into those producing insomnia, those causing daytime sleepiness, and those disrupting sleep. Transient insomnia is extremely common, afflicting up to 80% of the population. Chronic insomnia affects 15% of the population. Benzodiazepines are frequently used to treat insomnia; however, there may be a withdrawal syndrome with rapid eye movement (REM) rebound. Two newer benzodiazepine-like agents, zolpidem and zaleplon, have fewer side effects, yet good efficacy. Other agents for insomnia include sedating antidepressants and over-the-counter sleep products (sedating antihistamines). Nonpharmacologic behavioral methods may also have therapeutic benefit. An understanding of the electrophysiologic and neurochemical correlates of the stages of sleep is useful in defining and understanding sleep disorders. Excessive daytime sleepiness is often associated with obstructive sleep apnea or depression. Medications, including amphetamines, may be used to induce daytime alertness. Parasomnias include disorders of arousal and of REM sleep. Chronic medical illnesses can become symptomatic during specific sleep stages. Many medications affect sleep stages and can thus cause sleep disorders or exacerbate the effect of chronic illnesses on sleep. Conversely, medications may be used therapeutically for specific sleep disorders. For example, restless legs syndrome and periodic limb movement disorder may be treated with dopamine agonists. An understanding of the disorders of sleep and the effects of medications is required for the appropriate use of medications affecting sleep.
Each of us will spend a third of our lives asleep. Sleep is a complex and pervasive cognitive state affected by medications in many different ways. The field of sleep disorders medicine has become increasingly complex with more than 90 disorders of sleep described, each with clear diagnostic criteria. An even larger group of diseases produces mental or physical discomfort affecting sleep. Sleep disorders can generally be divided into 3 large groups: (1) those producing insomnia (complaints of difficulty falling asleep, staying asleep, or nonrestorative sleep), (2) those with a primary complaint of daytime sleepiness, and (3) those associated with disruptive behaviors during sleep—the disorders of arousal.1,2 There is a full range of medications used to treat these disorders, each with particular benefits as well as potential for harm.
MEDICATIONS FOR THE TREATMENT OF INSOMNIA
Sedatives and Hypnotics
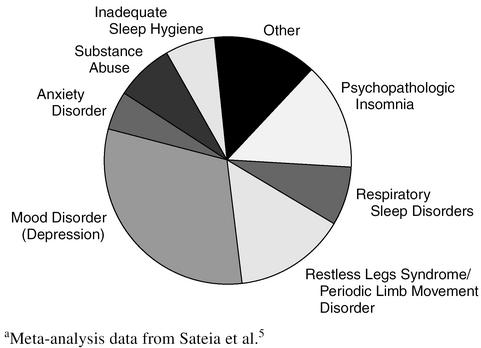
Insomnia is an extremely common complaint. Transient insomnia (< 2 weeks in duration) affects up to 80% of the population on a yearly basis.3 Chronic insomnia affects 15% of the population.1 In the 1990s in the United States, 2.6% of adults were using prescription sedative-hypnotic medications and 3.1%, over-the-counter (OTC) sleep medications (primarily antihistamines).4 The comparative frequency of the more common diagnoses resulting in chronic insomnia is presented graphically in Figure 1.5
Figure 1.
Diagnoses Resulting in Chronic Insomniaa
Historically, sedative/hypnotics have been some of the most commonly prescribed drugs. Chloral hydrate was the original “Mickey Finn” slipped into the drinks of unsuspecting marks for the purposes of criminal activity. Unfortunately, the median lethal dose (LD50) for chloral hydrate is quite close to the therapeutic dose, and murders rather than robberies were often the result. In the years leading up to the 1960s, barbiturates were commonly utilized for their sedative effects. Unfortunately, these medications can be drugs of abuse and have a significant danger of overdose. Marilyn Monroe, Elvis Presley, and Jim Morrison, among others, were celebrities who died during this era from overdoses of sleeping pills. These medications and similar barbiturate-like medications (methaqualone, glutethimide, ethchlorovynol, methyprylon) can still be prescribed, but should be used sparingly because of their potential for abuse and overdose.6
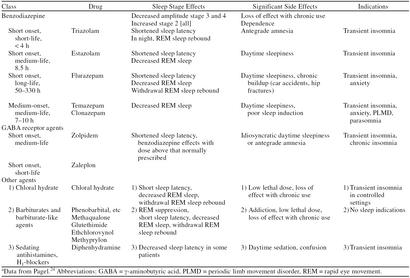
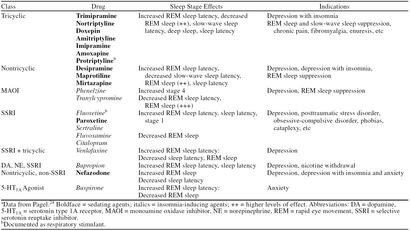
In the 1970s benzodiazepines became available for the treatment of insomnia. These drugs act at γ-aminobutyric acid (GABA) neuroreceptors and have far less overdose danger and abuse potential than previous medications used for sleep. The many drugs in this class are best viewed therapeutically based on their pharmacodynamics (Table 1). Rapid onset of action is characteristic of flurazepam and triazolam, indicating that both of these agents have excellent sleep-inducing effects. Flurazepam, like diazepam and clorazepate, has active breakdown products. This characteristic results in an extraordinarily long active half-life, which can approach 11 days. This prolonged effect in the elderly has been associated with increased auto accidents and falls with hip fractures.7,8 Withdrawal from these long-acting agents can be difficult, causing an initial syndrome of insomnia followed by persistent anxiety that may extend beyond the half-life of the agent.
Table 1.
Sedatives and Hypnoticsa
Benzodiazepines are rapid eye movement (REM) sleep–suppressant medications, and withdrawal often results in episodes of increased REM sleep (REM sleep rebound). REM sleep is known to have a role in learning and memory consolidation. For short-acting agents such as triazolam, this rebound occurs during the same night in which the medication was taken and has been associated with daytime memory impairment, particularly at higher dosages.9,10 Temazepam and estazolam have half-lives compatible with an 8-hour night of sleep. Temazepam, because of its slower onset of action, is less efficacious as a sleep-inducing agent than other drugs used as hypnotics in this class.11 All benzodiazepines can result in respiratory depression in patients with pulmonary disease and may lose sleep-inducing efficacy with prolonged use.10,12
The newer hypnotics zolpidem and zaleplon are benzodiazepine-like agents, exerting effects at the same GABA receptors. Withdrawal from benzodiazepines is not blocked by these agents. Both have excellent efficacy with minimal side effects. Abuse potential for these agents is minimal, although any agent used to induce sleep can result in a dependence on that agent to induce sleep. Idiosyncratic reactions of persistent daytime somnolence and/or memory loss have been reported in some patients. Tachyphylaxis is unusual, and thus they can be used on a long-term basis. Sleep is altered minimally, and REM rebound is not associated with these agents (Table 1).6,9,13 Zolpidem has a 6- to 8-hour half-life and zaleplon is shorter acting (3–4 hours). Clinical comparison of these agents suggests that zolpidem may have greater sleep-inducing efficacy and zaleplon, fewer side effects.
In the last 30 years, although the drugs for treatment of insomnia have become safer, the number of sedatives and hypnotics prescribed in the United States has declined. This decrease most likely reflects the public's and the medical community's increasing understanding of the side effects and limitations of the available hypnotic drugs. Nonpharmacologic behavioral methods, such as sleep hygiene, hypnosis, relaxation training, sleep restriction, and cognitive therapies, have shown therapeutic benefit in the treatment of insomnia.14
The physician treating insomnia should make the appropriate diagnosis before initiating therapy. Insomnia is commonly a symptom of nocturnal discomfort, whether psychological, physical, or environmental. Medications, in general, can be safely utilized on a short-term basis for the treatment of transient insomnia. Chronic hypnotic medication use has been associated with the development of mood disorders (depression) and hypnotic-dependent disorders of sleep.15 Therefore, the underlying reasons and diseases resulting in chronic insomnia should be addressed. Approximately 10% of the cases of chronic insomnia are due to anxiety or panic disorder. For patients in this category and those with idiopathic insomnia (persistent lifelong insomnia without other sleep-associated diagnoses), chronic hypnotic use can be justified and is indicated.5,9,16
Other Sedating Agents
Ethanol is probably the most widely used hypnotic medication. In patients with chronic insomnia, 22% report using ethanol as a hypnotic.5,17 Unfortunately, chronic use to induce sleep can result in tolerance, dependence, and diminished sleep efficiency and quality. When ethanol is used in excess with other sedative/hypnotic agents, overdose can be fatal.
Over-the-counter sleeping pills contain sedating antihistamines, usually diphenhydramine. These agents are varyingly effective, but may result in daytime sleepiness, cognitive impairment, and anticholinergic effects that persist into the day after use, affecting driving performance.18 These agents are not recommended for use in the elderly.19 Seizure thresholds can be lowered by their use in epileptic patients. The side effect profiles of the newer sedatives and hypnotics are generally more benign that those of the sedating antihistamines.13,18
Anidepressants
Sedating antidepressants are often used to treat insomnia. A significant percentage of individuals with chronic insomnia and/or daytime sleepiness also have depressive symptoms. Chronic insomnia itself can lead to depression.17 Depression associated with insomnia is likely a different diagnostic entity than depression without insomnia, and treatment of the former with nonsedating antidepressants may produce no improvement in sleep even when the underlying depression resolves.20 Use of antidepressants is limited by side effects (anticholinergic effects, daytime hangover, etc.) and danger with overdose (particularly the tricyclics).11,21,22 Sedating antidepressants include the tricyclics (amitriptyline, imipramine, nortriptyline, etc.), trazodone, and the newer agents mirtazapine and nefazodone. The selective serotonin reuptake inhibitors (SSRIs) have a tendency to induce insomnia; however, in some patients, paroxetine may induce mild sedation. Depression-related insomnia responds to sedating antidepressants more rapidly and with lower doses compared with other symptoms of depression.23 In patients with insomnia and concomitant depression, antidepressants are often used in combination with sedative/hypnotic medications.14
MEDICATIONS INDUCING INSOMNIA
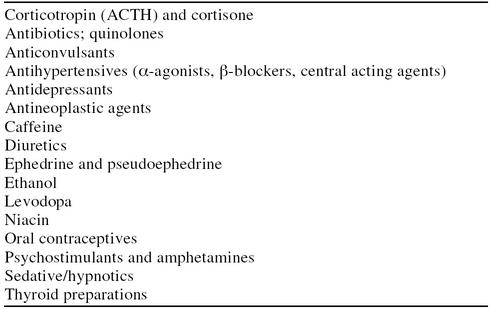
Most medications affecting central nervous system (CNS) functioning can induce insomnia in some patients. A sleep history in a patient with insomnia should include a review of all medications, including OTC products. Common culprits include medications affecting neurotransmitters, such as norepinephrine, serotonin, acetylcholine, or dopamine. Less commonly, agents such as antibiotics, antihypertensives, oral contraceptives, and thyroid replacements can induce insomnia in susceptible individuals (Table 2).11,24 Over-the-counter medications that may induce insomnia include decongestants (including nose sprays), weight loss agents, ginseng preparations, and high-dose vitamin B1. Finally, chronic and long-term sedative/hypnotic use to induce sleep may cause tolerance to the sedative effect and can contribute to chronic insomnia.1,16
Table 2.
Drugs Known to Cause Insomnia
Daytime Sleepiness
Surprisingly, despite insomnia being such a common complaint, many patients presenting with symptoms of a sleep disorder are not complaining of insomnia. Excessive daytime sleepiness is present in 5% to 15% of the population.1,3,17 Many patients with excessive daytime sleepiness, particularly those who also complain of snoring, will require overnight sleep evaluation (polysomnography) because of the potential diagnosis of obstructive sleep apnea. Obstructive sleep apnea is usually treated with continuous positive airway pressure (CPAP), a system that utilizes positive nasal pressure to maintain airway patency during sleep. Other treatment approaches for obstructive sleep apnea include ear-nose-throat surgery and dental mouthpieces. Symptoms of a mood disorder (depression), which is also a common cause of daytime sleepiness, can be difficult to distinguish from the symptoms of obstructive sleep apnea.17 Chronic sleep deprivation as a basis for daytime sleepiness is particularly common in the adolescent and young adult population.3 Less common causes of excessive daytime sleepiness are neurologic diseases that induce sleepiness: narcolepsy and idiopathic hypersomnolence. A major concern in such sleepy patients is the potential danger to self and others while working and/or driving motor vehicles.25
Altering Medications
Medications that are used in somnolent patients to induce alertness include the amphetamines (dextroamphetamine and methylphenidate) and pemoline. Pemoline can cause hepatic toxicity in susceptible patients. The amphetamines are considered to have high abuse potential and are Schedule II prescription drugs. The newer alerting agent modafinil is pharmacologically distinct and has less potential for abuse (Schedule IV). Side effects of these drugs include personality changes, tremor, hypertension (dextroamphetamine and methylphenidate), headaches, and gastrointestinal reflux.4,25,26
MEDICATION-INDUCED ALTERATIONS IN SLEEP STAGES AND SLEEP EEG
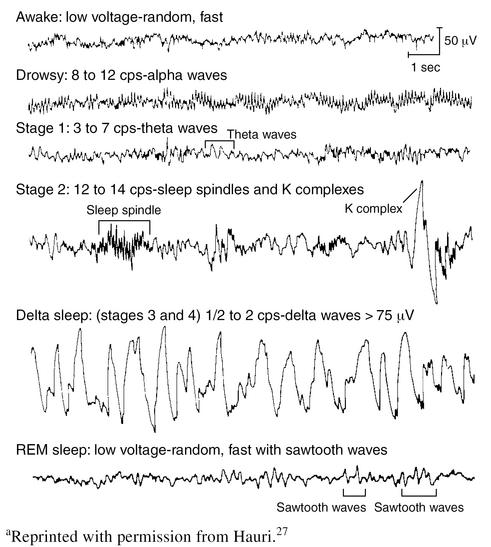
Sleep stages were first defined in the mid-1960s, after telemetric techniques developed for monitoring the physiologic functions of astronauts were adapted for sleep monitoring (Figure 2). Polysomnographic recordings using electrooculogram (EOG), electromyogram (EMG), and electroencephalogram (EEG) can be used to divide sleep into stages. In some ways, sleep staging is an artificial construct designed for analysis of sleep based on our available monitoring techniques. However, research has revealed that these sleep stages have physiologic and behavioral correlates that are clinically important. REM sleep occurs about every 90 minutes and is sometimes followed by short periods of waking. During REM sleep, low voltage, fast EEG activity is associated with rapid movements of the eyes and low EMG tone in most antigravity muscles. Non-REM (NREM) sleep is divided into stages 1 to 4. Stage 1 sleep is the transition from drowsy wake to sleep and is characterized by slow rolling eye movements and the disappearance of the EEG alpha rhythm. Stage 2, often the stage dominating much of the night, is light sleep, defined by the presence of sleep spindles and K complexes on the EEG. Stages 3 and 4, also known as deep sleep, include large amounts of the slow (1 Hz) delta rhythm on the EEG. Sleep stages occur in cycles throughout the night.3,21
Figure 2.
Characteristic Electroencephalographic Patterns of Human Sleep Stagesa
Sleep-state alteration is frequently seen with psychoactive medication use. CNS active medications often alter the occurrence, latency, and EEG characteristics of specific sleep/dream states, either with therapeutic intent or as side effects. Even some nonpharmacologic therapies, such as oxygen, CPAP, and electroconvulsive therapy, can alter REM sleep and deep sleep.28
Medications that produce psychoactive effects alter the EEG. Psychoactive medication effects can vary with the alterations that the drugs produce in the EEG. Typically, psychoactive medications alter background EEG frequencies and the occurrence, frequency, and latency of the various sleep stages (Tables 1 and 3).24,29–31 In general, drug-induced EEG changes are associated with characteristic behavioral effects. This relationship has been utilized to suggest therapeutic possibilities for medications that produce characteristic EEG effects.29–31
Table 3.
Antidepressantsa
Sleep State–Specific Diagnoses and Symptoms
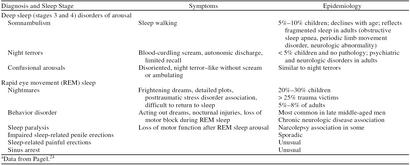
Parasomnias are sleep disorders that occur during arousal, partial arousal, or sleep state transition.1 The arousal disorders are associated with arousals from deep sleep, usually during the first deep-sleep episode of the night (typically 1:00–3:00 a.m.). Arousal disorders include sleep terrors, somnambulism (sleep walking), and confusional arousals. These conditions are most common in children, with occurrence declining markedly after the onset of adolescence (Table 4).
Table 4.
Parasomniasa
REM sleep parasomnias include sleep paralysis, sleep-related painful erections, REM sleep–related sinus arrest, nightmare syndrome, and REM behavior disorder. REM sleep alters many physiologic processes, and therefore it is not surprising that a variety of physical illnesses become symptomatic during REM sleep. Respiratory muscle atonia associated with REM sleep can result in increased sleep apnea, particularly in patients with chronic obstructive pulmonary disease (COPD). Lower esophageal pressure, also characteristic of REM sleep, can result in symptomatic gastrointestinal reflux. Chronic diseases manifesting symptoms during REM sleep include angina, migraines, and cluster headaches.1 REM sleep latency (the length of time from sleep onset to the first REM sleep period of the night) is often shorter in actively depressed patients.32 An increase in REM sleep latency has been correlated with improvements in psychometric depression scales and can be a marker for the efficacy of antidepressant medication.22,32,33
Nocturnal seizures, asthma, and panic attacks are more likely to occur in the NREM stages of sleep. The sleep manifestations of posttraumatic stress disorder include stereotypic frightening dreams that occur either at sleep onset or during REM sleep. Such disordered dreaming can result in both sleep onset and sleep maintenance insomnia.34,35
Clinical Use of Sleep Stage and EEG Effects
Medication-induced changes in sleep stages can lead to an increase in symptoms occurring during those specific sleep/dream states. For example, insomnia and nightmares are associated with the REM sleep rebound that occurs after discontinuation of REM suppressive drugs (i.e., ethanol, barbiturates, benzodiazepines). Medications such as lithium that can increase deep sleep can induce the occurrence of arousal disorders such as somnambulism.2,24
The influence of psychoactive medications on sleep states has a positive side as well. For example, REM sleep suppressive medications can be useful adjuncts in the treatment of REM sleep parasomnias and symptoms. Both benzodiazepines and antidepressants can be used to decrease REM sleep. Similarly, the arousal disorders can be treated with medications affecting deep sleep (benzodiazepines and others) (see Tables 1 and 3).24,36,37
SPECIFIC SLEEP-RELATED MEDICATION EFFECTS
Respiratory Effects
Certain medications are known to affect respiratory drive. Benzodiazepines, barbiturates, and narcotics can exacerbate respiratory failure in patients with COPD, central sleep apnea, and restrictive lung disease. These medications can also negatively affect obstructive sleep apnea. The newer hypnotics (zolpidem and zaleplon) have less respiratory suppressant effects. Medroxyprogesterone, protriptyline, and fluoxetine have been documented to have respiratory stimulant effects that may be clinically useful in some patients.12
Enuresis
Enuresis, defined as persistent bed-wetting more than twice a month past the age of 5 years, is present in 15% of 5-year-olds. Medication has been shown to be symptomatically useful. Tricyclic antidepressants have been used for decades in this disorder, but there has been concern about long-term safety in children. The current treatment of choice is desmopressin nasal spray, which corrects the lack of cyclic antidiuretic hormone increase during sleep, typically seen in these patients. Symptoms can be controlled until neurophysiologic maturity occurs, bringing a resolution of nocturnal enuresis.38
Restless Legs Syndrome and Periodic Limb Movement Disorder
Symptoms of restless legs syndrome include uncomfortable limb sensations at sleep onset and motor restlessness exacerbated by relaxation. Periodic limb movement disorder is characterized by repetitive, stereotypic limb movements occurring in 15- to 40-second cycles in NREM sleep and often leading to recurrent arousals from sleep.39 These disorders are quite common, occurring in up to 15% of the population and increasing in frequency with age.40
Historically, both periodic limb movement disorder and restless legs syndrome have been treated with benzodiazepines, particularly clonazepam.37,41 Low dosages of dopamine precursors and dopamine receptor agonists at bedtime have been demonstrated to be efficacious in these disorders. Possible side effects from these medications, which include carbidopa/levodopa, pergolide, pramipexole, selegiline, and ropinirole, are nausea, headache, and occasional augmentation of symptoms.42,43
Circadian Rhythm Disturbance
A number of sleep disorders are linked to abnormally timed sleep-wake cycles. These include delayed and advanced sleep phase syndromes in which the sleep period is markedly later or earlier than what is socially accepted, jet lag, shift work, and certain sleep abnormalities associated with aging. Melatonin is the photoneuroendocrine transducer that conveys information controlling sleep-wake cycles and circadian rhythms in the CNS. Low doses may be useful in treating these disorders.44 Because melatonin is marketed as a dietary supplement, there are minimal data on safety, side effects, and drug interactions for this compound.45 Jet lag and shift work disorders can also be effectively treated with short-term sedatives and hypnotics.46
CONCLUSION
A philosophy that remains cogent in regard to the CNS is that new research discoveries almost always show this system to be more complex than previously thought. Only a few years ago, if patients complained of difficulty sleeping, medications that were often dangerous and addictive were prescribed to induce sleep, while the basis of the patient's complaint was not addressed. Now sleeping pills are safer, and our understanding of the sleep state has increased exponentially. Insomnia is no longer a diagnosis, it is a complaint to be addressed—a symptom of a sleep disorder for which specific and appropriate treatment exists.
Drug names: amitriptyline (Elavil and others), amoxapine (Asendin and others), bupropion (Wellbutrin), buspirone (BuSpar), carbidopa-levodopa (Sinemet and others), citalopram (Celexa), clonazepam (Klonopin and others), clorazepate (Tranxene and others), desipramine (Norpramin and others), desmopressin (DDAVP and others), dextroamphetamine (Dexedrine and others), diazepam (Valium and others), doxepin (Sinequan and others), estazolam (ProSom and others), fluoxetine (Prozac), flurazepam (Dalmane and others), fluvoxamine (Luvox), medroxyprogesterone (Provera and others), methylphenidate (Ritalin and others), mirtazapine (Remeron), modafinil (Provigil), nefazodone (Serzone), nortriptyline (Pamelor and others), paroxetine (Paxil), pemoline (Cylert), pergolide (Permax), phenelzine (Nardil), phenobarbital (Donnatal and others), pramipexole (Mirapex), protriptyline (Vivactil), ropinirole (Requip), selegiline (Eldepryl), sertraline (Zoloft), temazepam (Restoril and others), tranylcypromine (Parnate), trazodone (Desyrel and others), triazolam (Halcion and others), trimipramine (Surmontil), venlafaxine (Effexor), zaleplon (Sonata), zolpidem (Ambien).
Footnotes
Financial disclosure: Dr. Pagel has received grant/research support from Sepacor and has served on the speakers/advisory board for Cephalon, Wyeth-Ayerst, and Searle.
REFERENCES
- Reite ML, Nagel KE, and Ruddy JR. The Evaluation and Management of Sleep Disorders. Washington, DC: American Psychiatric Press. 1990 [Google Scholar]
- American Sleep Disorders Association. Thorpy MJ, ed. The International Classification of Sleep Disorders: Diagnosis and Coding Manual. Lawrence, Kansas: Allen Press. 1990 [Google Scholar]
- Kryger MH, Roth T, and Dement WC. Principles and Practice of Sleep Medicine. 2nd ed. Philadelphia, Pa: WB Saunders. 1994 [Google Scholar]
- Wysowski DK, Baum C. Outpatient use of prescription sedative-hypnotic drugs in the United States, 1970 thought 1989. Arch Intern Med. 1991;151:1779–1783. [PubMed] [Google Scholar]
- Sateia MJ, Doghramji K, Hauri PJ, et al. Evaluation of chronic insomnia. Sleep. 2000;23:243–314. [PubMed] [Google Scholar]
- Mitler MM. Nonselective and selective benzodiazepine receptor agonists: where are we today? Sleep. 2000;23(suppl 1):S39–S47. [PubMed] [Google Scholar]
- Aldrich MS. Automobile accidents in patients with sleep disorders. Sleep. 1989;12:487–494. doi: 10.1093/sleep/12.6.487. [DOI] [PubMed] [Google Scholar]
- Ray WA, Griffen MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA. 1989;262:3303–3307. [PubMed] [Google Scholar]
- Doghramji K. The need for flexibility in dosing hypnotic agents. Sleep. 2000;23(suppl 1):S16–S20. [PubMed] [Google Scholar]
- Greenblatt DJ. Benzodiazepine hypnotics: sorting the pharmacodynamic facts. J Clin Psychiatry. 1991;52(9, suppl):4–10. [PubMed] [Google Scholar]
- Pagel JF. The treatment of insomnia. Am Fam Phys. 1994;49:1417–1422. [PubMed] [Google Scholar]
- George CFP. Perspectives in the management of insomnia in patients with chronic respiratory disorders. Sleep. 2000;23(suppl 1):S31–S35. [PubMed] [Google Scholar]
- Vermeeren A, Danjou PE, O'Hanlon JF. Residual effects of evening and middle-of-the-night administration of zaleplon 10 and 20 mg on memory and actual driving performance. Hum Psychopharmacol Clin Exp. 1998;13:S98–S107. [Google Scholar]
- Pagel JF, Zafralotfi S, Zammit G. How to prescribe a good nights sleep. Patient Care. 1997 Feb;31(4):87–94. [Google Scholar]
- Kessler RC, McGonagle KC, Zhao S. Epidemiology of psychiatric disorders. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Richardson GS. Managing insomnia in the primary care setting: raising the issues. Sleep. 2000;23(suppl 1):S9–S12. [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorder: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Weiler JM, Bloomfield JR, Woodworth GG, et al. Effects of fexofenadine, diphenhydramine, and alcohol on driving performance: a randomized, placebo controlled trial in the Iowa driving simulator. Ann Intern Med. 2000;132:354–363. doi: 10.7326/0003-4819-132-5-200003070-00004. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S. Insomnia in the elderly: a review for the primary care practitioner. Sleep. 2000;23(suppl 1):S23–S30. [PubMed] [Google Scholar]
- Ware JC, Brown FW, Moorad PJ, et al. Effects on sleep: a double blind study comparing trimipramine to imipramine in depressed insomniac patients. Sleep. 1989;12:537–549. doi: 10.1093/sleep/12.6.537. [DOI] [PubMed] [Google Scholar]
- Pagel JF. Disease, psychoactive medication, and sleep states. Primary Psychiatry. 1996;3:47–51. [Google Scholar]
- Settle EC Jr. Antidepressant drugs: disturbing and potentially dangerous adverse effects. J Clin Psychiatry. 1998;59(suppl 16):25–30. [PubMed] [Google Scholar]
- Rickles K, Schweizer E, Clary C, et al. Nefazodone and imipramine in major depression: a placebo controlled trial. Br J Psychiatry. 1994;164:802–805. doi: 10.1192/bjp.164.6.802. [DOI] [PubMed] [Google Scholar]
- Pagel JF. Pharmacologic alterations of sleep and dream: a clinical framework for utilizing the electrophysiological and sleep stage effects of psychoactive medications. Hum Psychopharmacol. 1996;11:217–223. [Google Scholar]
- Fry JM. Current issues in the diagnosis and management of narcolepsy. Neurology. 1998;50(2, suppl 1):S1–S48. doi: 10.1212/wnl.50.2_suppl_1.s43. [DOI] [PubMed] [Google Scholar]
- McClellan KJ, Spencer CM. Modafinil: a review of its pharmacology and clinical efficacy in the management of narcolepsy. CNS Drugs. 1998;9:311–324. doi: 10.2165/00023210-199809040-00006. [DOI] [PubMed] [Google Scholar]
- Hauri PJ. Current Concepts: The Sleep Disorders. Kalamazoo, Mich: The Upjohn Company. 1992 [Google Scholar]
- Hart LL, Middleton RK, Schott WJ. Drug treatment for sleep apnea. DICP Ann Pharmacother. 1989;23:308–315. [Google Scholar]
- Hermann WM. Development and critical evaluation of an objective procedure for the electroencephalographic classification of psychotropic drugs. In: Hermann WM, ed. Electroencephalography in Drug Research. Stuttgart, Germany: Gustav Fisher. 1982 385–445. [Google Scholar]
- Itil TM. The discovery of psychotrophic drugs by computer-analyzed cerebral bioelectrical potentials (CEEG) Drug Dev Res. 1981;1:373–407. [Google Scholar]
- Mamdema JW, Danhof M. Electroencephalogram effect measures and relationships between pharmacokinetics and pharmacodynamics of centrally acting drugs. Clin Pharmacokinet. 1992;23:191–215. doi: 10.2165/00003088-199223030-00003. [DOI] [PubMed] [Google Scholar]
- Armitage R, Rochlen A, Fitch T, et al. Dream recall and major depression: a preliminary report. Dreaming. 1995;5:189–198. [Google Scholar]
- Kupfer DJ, Ehlers CL, Frean E, et al. Resistant effects of antidepressants: EEG sleep studies in depressed patients during maintenance treatment. Biol Psychol. 1994;35:781–793. doi: 10.1016/0006-3223(94)91140-1. [DOI] [PubMed] [Google Scholar]
- Krakow B, Tandberg D, Barey M, et al. Nightmares and sleep disturbance in sexually assaulted women. Dreaming. 1995;5:199–206. [Google Scholar]
- Pagel JF. Nightmares and disorders of dreaming. Am Fam Phys. 2000;61:2037–2044. [PubMed] [Google Scholar]
- Pagel JF. Modeling drug actions on electrophysiologic effects produced by EEG modulated potentials. Hum Psychopharmacol. 1993;8:211–216. [Google Scholar]
- Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333–337. doi: 10.1016/S0002-9343(97)89493-4. [DOI] [PubMed] [Google Scholar]
- Klauber GT. Clinical efficacy and safety of desmopressin in the treatment of nocturnal enuresis. J Pediatr. 1989 114(4, pt 2). 719–722. [DOI] [PubMed] [Google Scholar]
- Walters AS. for the International Restless Legs Syndrome Study Group. Toward a better definition of the restless leg syndrome. Mov Disord. 1995 10:634–642. [DOI] [PubMed] [Google Scholar]
- Lavigne GJ, Montplaisir JY. Restless leg syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994;17:739–743. [PubMed] [Google Scholar]
- Boghen D, Lamothe L, Elie R, et al. The treatment of restless leg syndrome with clonazepam: a prospective controlled study. Can J Neurol Sci. 1986;13:245–247. doi: 10.1017/s0317167100036350. [DOI] [PubMed] [Google Scholar]
- Early CJ, Allen RP. Pergolide and carbidopa/levodopa treatment of the restless leg syndrome and periodic leg movements in sleep in a consecutive series of patients. Sleep. 1996;19:801–810. doi: 10.1093/sleep/19.10.801. [DOI] [PubMed] [Google Scholar]
- Walker SL, Fine A, Kryger MH. l-DOPA/carbidopa for nocturnal movement disorders in uremia. Sleep. 1996;19:214–218. [PubMed] [Google Scholar]
- Stone BM, Turner C, Mills SL, et al. Hypnotic activity of melatonin. Sleep. 2000;23:663–670. [PubMed] [Google Scholar]
- Arendt J, Middleton B, Stone B, et al. Complex effects of melatonin: evidence for photoperiodic responses in humans? Sleep. 1999;2:625–636. doi: 10.1093/sleep/22.5.625. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Copinschi G, Van Onderbergen A, et al. A benzodiazepine hypnotic facilitates adaptation of circadian rhythms and sleep-wake homeostasis to an eight hour delay shift simulating westward jet lag. Sleep. 2000;23:915–928. [PubMed] [Google Scholar]