Abstract
Primary care practitioners are in an ideal position to initiate treatment for patients with behavior, mood, and thought disturbances. It is believed that early identification and treatment of these symptomatic features of primary or secondary central nervous system disorders may significantly reduce morbidity and benefit the patient, his/her family, and involved caregivers, including the primary care physician. A broad list of central nervous system–active medications are utilized by family physicians to treat patients who exhibit symptoms of agitation, altered mood, and disordered thought. Some medications have demonstrated superiority over placebo or active medicines in reported clinical trials. This article is a brief overview of the safety and efficacy from reported studies of the use of medications frequently used to treat symptoms related to behavior, mood, and thought disturbances, with a specific focus on the clinical applicability of olanzapine.
An effective physician-patient relationship is important no matter what illness is being treated. Inherent in the definition of the role of the primary care physician is the ability to successfully engender such a relationship. The primary care physician may be the clinician most available to provide assessment of behavior, mood, and thought-related disorders and therefore can have a paramount role in treatment recommendations. Symptom assessment is a central task of the primary care physician during the physician-patient encounter. At times it may be important for the primary care physician to request a psychiatric consultation to augment the diagnostic evaluation.
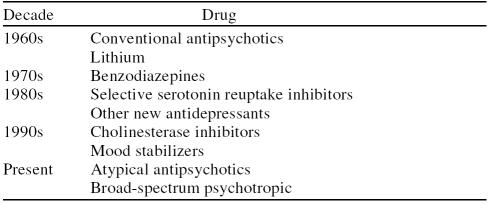
Primary care physicians are required by virtue of their frontline role to assess patients with a myriad of neuropsychiatric symptoms ranging in intensity from subtle to severe. These may include the domains of affect, behavior, cognition, and thought (Table 1). In parallel with developments in the fields of neurology and psychiatry, primary care practitioners have increased their skills over the years in recognizing and appropriately treating central nervous system disturbances. For disturbances in behavior accompanied by changes in mood or thought processes, the primary care physician has learned to prescribe conventional antipsychotics and tricyclic antidepressants (1960s), benzodiazepines (1970s), selective serotonin reuptake inhibitors (SSRIs) (1980s), cholinesterase inhibitors (1990s), and, most recently, atypical antipsychotics and the psychotropic olanzapine (Table 2).
Table 1.
Symptoms of Affective (Mood), Behavioral, and Cognitive/Thought Disturbances
Table 2.
A Chronology of Primary Care Prescribing of Psychotropic Medications
Patients with psychiatric disturbances are frequently reluctant to see a psychiatrist. Those who do agree to see a specialist may have an extended period of delay while waiting for an appointment. Early intervention by the primary care practitioner can help prevent escalation of problems, which may help patients to avoid more complex, expensive, and lengthy treatment. Continuity of care, reduced visits to the emergency room, and/or reduced episodes of hospitalization are further potential advantages of treatment in the primary care setting.1
Early identification and treatment of psychiatric illnesses, often first seen in a primary care setting, may significantly reduce morbidity. A study by the National Institute of Mental Health revealed the startling results that, of completed patient suicides in the elderly, approximately 70% had visited their primary care physician within 1 month prior to their death, and approximately 30% had visited within 1 week prior to their suicide.2
BEHAVIOR, MOOD, AND THOUGHT DISTURBANCES
Behavior
Primary care physicians frequently encounter patients with dementia, primarily Alzheimer's disease, which is by far the most common of all dementia illnesses3 and is accompanied by a myriad of behavioral symptoms. Symptoms include, among others, agitation, aggression, hostility, “sundowning,” and social withdrawal. Such behavioral symptoms have been suggested to be present in at least 50% of outpatients and 75% of nursing home patients with Alzheimer's disease.4,5 However, in the context of early intervention and reducing the burden on the socioeconomic system, it is important to note that some patients who are subsequently diagnosed with Alzheimer's disease have already exhibited numerous behavioral symptoms for up to 3 years prior to diagnosis.6
In the outpatient setting, it is important to recognize and be able to treat difficult-to-manage behaviors such as restlessness, agitation, and aggressiveness. When left untreated, these behaviors can lead to significant emotional distress and caregiver fatigue. Patients with dementia who have significant behavioral symptoms may require placement in a nursing home earlier than would otherwise be necessary.4 Early treatment of symptoms of behavioral disturbances may allow for longer independent living, with less stress on the caregiver. Depression burdens 30% to 50% of caregivers for people with dementia,7 which is a significantly higher number than the general population.8 This prevalence is perhaps related to adaptation to the stress of dealing with noncognitive features associated with the dementia. Depressive disorder is associated with high levels of morbidity and mortality.
Additionally, caregivers frequently rely on over-the-counter sedating medications, which may result in significant side effects. In the absence of empirical studies, it is believed that a well thought out medication regimen for a patient with behavioral problems should reduce agitation but will also lessen the risk of falls and injuries.
Affective (Mood)
In addition to behavioral symptoms, the primary care physician often sees patients who exhibit irritable mood, grandiosity, decreased need for sleep, or somewhat erratic behavior. Sometimes patients are mildly euphoric and somewhat talkative. These symptoms may be caused by mania and may present in a wide spectrum of intensity from mild to severe. It is important to note that behavioral symptoms are not specific to any single disorder and therefore overlap disorders. One example of this is agitation. When choosing the most effective treatment for patients exhibiting manic symptoms, consideration should be given to the numerous symptom domains that are present (Table 3).
Table 3.
Symptoms of Mania
Bipolar mania affects all aspects of the patient's psychological and social functioning and also can adversely affect the patient's physical health. When present in a mild form (i.e., hypomania), the patient may appear to be remarkably productive, but as mania intensifies, it becomes devastating for the patient, the family, the caregivers, and for anyone who comes close to the patient in any capacity. Inadequately treated, the patient may become increasingly exhausted, and the illness may then become life threatening. Bipolar disorder is characterized by a high rate of relapse. Repetitive relapses into mania have been shown to have an accumulating deteriorative effect on the patient's long-term functioning and recovery.9 However, in using the most effective treatment, a patient's function may be maximized, and relapses and associated disruption to the family and caregivers may be minimized.
Thought
Psychotic symptoms vary in intensity from the subtle to the severely overt and can include positive symptoms (i.e., delusions, hallucinations, disorganized thoughts),10 negative symptoms (i.e., flattened affect, paucity of speech, poor motivation, decreased activity, social withdrawal),10 cognitive deficits (i.e., impairment of attention, memory, executive functions), and comorbid conditions (e.g., mood disturbances, substance abuse, and anxiety and behavioral disturbances). Primary care physicians see patients who may have become increasingly fearful or socially isolated, have difficulty concentrating or making decisions, and may not appear to be thinking clearly. Such patients could have premorbid symptoms of an emerging psychosis. While a definitive disorder-specific diagnosis may not be made at this time, treatment of the symptoms may modify future development of the emerging thought disorder.11
Treatment of psychotic symptoms can improve overall social and occupational functioning. Although patients with psychotic symptoms may have a chronic disorder, medication advances can positively impact their ability to function in their day-to-day lives, including relationships, self-care (diet, exercise, and medical care), and work. Treating these symptoms with newer and safer medications can increase the patient's likelihood of reintegration.12
TREATMENT ISSUES
Selection of a drug class is the first step in treatment of agitation and behavioral symptoms. The U.S. Food and Drug Administration (FDA) has not yet approved the indication for any medication to treat agitation.13 Still, many different medications are used to treat symptoms of agitation and aggression. It is helpful to compare their effectiveness, especially with consideration of the possibility of serious side effects (Table 4). Medications used to treat emergent anxiety and agitation include trazodone and the class of antidepressants known as SSRIs, which have demonstrated some efficacy in treating mild symptoms of agitation but may cause side effects as well. Buspirone may be effective in treating symptoms of anxiety, but its effectiveness in agitation is unproved.14
Table 4.
Safety Issues for Mood Stabilizers and Antipsychotic Drugs
Benzodiazepines are effective in reducing agitation. Their main effects in controlling agitation are sedation, muscle relaxation, and antianxiety action.15,16 Unfortunately, benzodiazepines are also prone to producing many adverse effects, including daytime sedation and dizziness. Benzodiazepines are also associated with falls, which, especially in older adults, can be life threatening.17,18
Mood stabilizers are used for the treatment of agitation due to the parallels in behaviors of agitated patients and bipolar patients, including psychomotor agitation and sleep disturbance.19 Such agents include lithium, carbamazepine, divalproex sodium, and olanzapine. Divalproex sodium has been reported to be useful in the treatment of agitation.20 In spite of this benefit, the FDA's recent addition of a “black box” warning to the package insert for divalproex sodium21 after reports of severe and potentially life-threatening pancreatitis raises concern. This drug also has black box warnings for hepatotoxicity and teratogenicity.
Carbamazepine and lithium are used less frequently than other mood stabilizers due to their side effect potential. Carbamazepine has the potential to induce aplastic anemia or agranulocytosis, and serum monitoring is recommended. Lithium is sometimes used to control extreme agitation but has the potential for cardiotoxicity, nephrotoxicity, teratogenicity, hypothyroidism, and confusion, especially in older adults.
Historically, use of conventional antipsychotic drugs such as haloperidol has sometimes been effective in reducing agitation. These drugs have been studied more intensively than any other drug class used for agitation.13 A review of controlled studies of conventional antipsychotics reported that their effects in treating agitation are modest and that no individual medication is better than any other.22 Treatment with conventional antipsychotics may result in extrapyramidal symptoms (EPS), tardive dyskinesia, hyperprolactinemia, orthostasis, cardiotoxicity, sedation, and acute dystonic reactions. Despite these risks, conventional antipsychotics continue to be among the most widely prescribed medications for patients with agitation and aggression.23
Although use of atypical antipsychotics has resulted in fewer side effects, safety risks persist. Clozapine, the first atypical antipsychotic, initially set the standard for efficacy in the treatment of psychosis and was very effective in the most refractory treatment-resistant patients with schizophrenia. More recently, though, it has been used judiciously due to its black box warnings for agranulocytosis, seizures, and cardiovascular and respiratory problems. It is also a highly anticholinergic drug.24,25
Risperidone, an atypical launched in 1994, is much better tolerated than clozapine,26 though studies have reported an approximately 10% incidence rate of EPS in elderly patients treated with risperidone.26,27 This incidence has been seen even at mean dosages as low as 1.1 mg/day.28 Risperidone also has produced greater changes in serum prolactin levels than other atypical agents.29
The introduction of quetiapine offered physicians another treatment option. It has a beneficial EPS profile and is thought to have a stronger sedative effect than other atypicals.25 However, patients may experience somnolence, dizziness, and lower blood pressure.30 In the package insert, there is a bolded precaution of risk for cataracts and the suggested need to conduct periodic slit lamp examinations.
Ziprasidone, the most recently approved atypical antipsychotic, has just begun to be prescribed for treatment of schizophrenia. Four of 5 registration trials in inpatients (primarily with schizophrenia) distinguished ziprasidone from placebo; the fifth trial showed no significant difference in terms of efficacy.31 No registration trial included outpatients. Ziprasidone has a purported favorable tolerability profile for EPS during treatment, as measured by the Extrapyramidal Symptom Rating Scale (ESRS), and weight change during treatment is comparable to placebo.32 Additionally, it has been indicated for maintenance treatment in schizophrenia. Nonetheless, there is a 10-paragraph bolded warning from the FDA regarding QTc prolongation and the risk of sudden death. Some experts on QTc prolongation have recommended, in accordance with the package insert, not to choose this drug for a patient with preexisting cardiac disease.33 Additionally, ziprasidone has been associated with somnolence.34 When choosing the safest treatment for a patient with psychiatric disturbances, these risks may warrant concern.
Olanzapine, launched in 1996, is the only atypical antipsychotic that is classified as a psychotropic. It is indicated not only for schizophrenia but also for maintenance treatment in schizophrenia and for acute mania in bipolar disorder. In placebo-controlled olanzapine studies,35 there were very few significant differences in the incidence of treatment-emergent adverse events. Somnolence, dizziness, weight gain, and akathisia occurred more frequently during olanzapine treatment. Paranoid reaction, anorexia, flu syndrome, delusions, and weight loss were reported more frequently by placebo-treated patients. Despite these reported events, olanzapine has a broad efficacy and also an overall favorable safety profile as demonstrated in the following clinical studies (Table 5).
Table 5.
Summary of Benefits of Olanzapine Treatment
USE OF OLANZAPINE IN SPECIFIC DISORDERS
Dementia
Street et al.36 conducted a randomized, double-blind, placebo-controlled study of olanzapine treatment with 206 nursing home patients with Alzheimer's disease (mean age = 82.8 years). Baseline Mini-Mental State Examination (MMSE) scores identified 70.9% of this study population as severely cognitively impaired (mean MMSE scores 6.4–7.3). At study entry, 95% of patients had symptoms of agitation/aggression, 56.4% had delusions, 22.8% had hallucinations, and 57.9% had agitation/aggression as well as one or more psychotic symptoms as measured on the Neuropsychiatric Inventory/Nursing Home version (NPI/NH) (Table 6).37
Table 6.
Summary of Neuropsychiatric Inventory/Nursing Home Version (NPI/NH)a
Patients were randomly assigned to treatment with placebo; olanzapine, 5 mg/day; olanzapine, 10 mg/day; or olanzapine, 15 mg/day. In the Street et al.36 study, the NPI/NH,37 which is a well-validated scale for assessing psychiatric symptoms in nursing home settings, was the primary efficacy measurement of psychiatric treatment response of behavioral disturbances (see Table 4). Compared with placebo, olanzapine, 5 mg/day (65.5% vs. 35.6%), and olanzapine, 10 mg/day (57.1% vs 35.6%), groups contained a significantly greater proportion of patients who responded at a ≥ 50% improvement rate at 6 weeks (p < .01 and p < .05, respectively) (Figure 1). The 15-mg/day dose group had no statistically significant differences from placebo.
Figure 1.
Percentage of Responders With Alzheimer's Disease Showing ≥ 50% Improvement on Neuropsychiatric Inventory/Nursing Home Version (NPI/NH) Core Total (hallucinations, delusions, and agitation/aggression) at 6 Weeksa
Patients who completed the acute phase of this study could continue into an 18-week open-label extension in which they received olanzapine, 5 to 15 mg/day (mean modal dose = 5 mg/day). Of the 137 patients who continued in this phase, 64.7% achieved a protocol-defined clinical response of ≥ 50% on the NPI/NH score; therefore, a majority of patients responded to olanzapine and maintained that response through 18 weeks. Olanzapine, particularly the 5-mg/day dose, was an effective and generally well-tolerated treatment for behavioral disturbances and psychotic symptoms in elderly patients with Alzheimer's disease.
Incidence of treatment-emergent adverse events was similar in the olanzapine groups compared with placebo, with the exception of somnolence (25% of patients on olanzapine, 5 mg, and 26% of patients on olanzapine, 10 mg, p < .05; 35.8% of patients on olanzapine, 15 mg, p < .001; 6.4% of patients on placebo) and abnormal gait (19.6% of patients on olanzapine, 5 mg, p < .01; 17% of patients on olanzapine, 15 mg, p < .05; 2.1% of patients on placebo). Results of a covariance efficacy analysis controlling for somnolence showed that there was not a significant effect of somnolence on the primary efficacy results and that the treatment effects remained statistically significant. Post hoc analysis of the Simpson-Angus gait item revealed no statistically significant differences between any olanzapine group relative to placebo in mean change from baseline to endpoint. This secondary explanatory analysis of the patient group with reported abnormal gait demonstrated that gait improved or did not change in 8 of 9 patients at 5 mg and in 6 of 7 patients at 10 mg, while 4 of 7 patients at 15 mg had a worsening of gait. Only 1 patient taking placebo, 1 patient taking 5 mg of olanzapine, and 1 patient taking 10 mg of olanzapine experienced worsened gait during this study. Of the 11 patients whose gait item score changed during the study, 10 patients already had an abnormal gait assessment (≥ 1) at baseline.
Dementia particularly burdens the elderly population. Whether or not cognitive decline is actually recognized beforehand, these patients do manifest a plethora of abnormal behaviors. Street et al.36 found that olanzapine, particularly the 5-mg/day dose, was an effective and generally well-tolerated treatment for behavioral disturbances and psychotic symptoms in elderly patients with Alzheimer's disease.
Mania and Depression
Many different medications have been traditionally used for bipolar disorder. As previously noted, many of these medications (benzodiazepines, lithium, anticonvulsants, and conventional antipsychotics) carry significant risk of side effects. The newer anticonvulsants, gabapentin, lamotrigine, and topiramate, have not been studied adequately in clinical trials and do not have an indication for the treatment of mania. In an attempt to reduce symptoms in all behavior, mood, and thought domains, the prescribing physician may resort to polypharmacy, resulting in an increase in drug-drug interactions and side effects.
In March 2000, olanzapine received an indication for the short-term treatment of acute manic episodes associated with bipolar I disorder after efficacy was demonstrated in 2 placebo-controlled clinical trials (one 3-week38 and one 4-week39). The first head-to-head comparison of divalproex (N = 125; mean modal daily dose = 1401 mg/day) and olanzapine (N = 123; mean modal dose = 17 mg/day) demonstrated the effectiveness of both drugs.40 However, olanzapine was clinically and statistically superior in the percentage of patients achieving remission (defined as Young Mania Rating Scale score ≤ 12 at endpoint). In fact, during this 3-week study, 47% of olanzapine-treated patients came into the normal or euthymic range, versus only 34% taking divalproex (p = .039). Olanzapine-treated patients experienced significantly greater rates of somnolence (p = .002) and dry mouth (p < .001), significantly increased appetite (p = .003), and greater rates of tremor, speech disorder, neck rigidity, sleep disorder, and tongue edema than patients treated with divalproex. Conversely, the divalproex group demonstrated a significantly higher incidence of nausea (p < .001), perhaps accounting for the lower number of patients with increased appetite. There were no significant differences in study discontinuations due to adverse events.
Treatment-resistant depression, the failure to respond to 2 or more adequate treatment trials with medication from different pharmacologic classes, presents a further treatment challenge. In an 8-week, double-blind study by Shelton et al.,41 patients with treatment-resistant depression without psychotic features were treated with fluoxetine (N = 10; mean modal dose 52.0 mg/day), olanzapine (N = 8; mean modal dose 12.5 mg/day), or a combination of the 2 drugs (N = 10; mean modal dose fluoxetine, 52.0 mg/day, olanzapine, 13.5 mg/day). The olanzapine-fluoxetine combination group demonstrated significant improvement in depressive symptoms as early as week 1 (Figure 2). This robust improvement persisted throughout the 8 weeks of the study with this group of patients. Commonly reported adverse events included somnolence, increased appetite, asthenia, weight gain, headache, dry mouth, and nervousness; increased appetite and weight gain occurred significantly more frequently in the olanzapine monotherapy and combination groups. However, completion rates were high in all groups (fluoxetine, N = 7, 70%; olanzapine, N = 6, 75%; combination, N = 9, 90%).
Figure 2.
Montgomery-Asberg Depression Rating Scale (MADRS) Total Score in the Acute Treatment Phasea
Psychosis
In 4 different analyses of olanzapine clinical trials,42–47 olanzapine proved superior compared with haloperidol in treatment of patients with psychotic symptoms of schizophrenia, as described below. This superiority was evident in 4 symptom domains common in schizophrenia: negative, positive, depressive, and cognitive symptoms.
An international, multicenter, double-blind, 6-week trial comparing olanzapine (N = 1336, mean modal dose 13.2 mg/day) with haloperidol (N = 660, mean modal dose 11.8 mg/day), showed olanzapine's superiority to haloperidol on overall improvement on the Positive and Negative Syndrome Scale (PANSS) negative symptoms subscale.10,42,43 The PANSS was used to measure negative symptoms; its presence implies the absence of appropriate affect and normal emotional responsiveness. These mood disturbances present in primary care as blunted affect, emotional withdrawal, and motor retardation. Study results show that olanzapine significantly outperformed haloperidol in reducing negative signs and symptoms. In addition, patients treated with olanzapine had significantly fewer EPS and a higher rate of completion.
In a post hoc analysis by Kinon et al.43 of a 6-week study, patients with predominantly positive psychotic symptoms at baseline (N = 388; score ≥ 4 on 3 or more PANSS positive subscale items and a score ≥ 4 on no more than 2 PANSS negative subscale items) showed significantly greater improvement in the Brief Psychiatric Rating Scale (BPRS) positive symptom subscore during olanzapine treatment than during haloperidol treatment (p = .013, last observation carried forward). Olanzapine improvement was significantly greater than haloperidol at weeks 4, 5, and 6. The improvement of behavioral agitation and prominent positive psychotic symptoms supports the beneficial effects of olanzapine in controlling these acute symptoms. The comparative data support the effectiveness of olanzapine in treating patients experiencing an acute psychotic episode.
Tollefson et al.44 reported that more than 50% of patients with schizophrenia who were enrolled in a comparative trial with olanzapine and haloperidol suffered from clinically significant depressive signs and symptoms as indicated by a score of ≥ 16 on the Montgomery-Åsberg Depression Rating Scale total score, secondary to their diagnosis of schizophrenia. This comorbidity of a mood disturbance with a thought disturbance can occur in a primary care setting as well. The ideal treatment option would treat both types of symptoms.
Purdon et al.45 analyzed data from stable outpatients with schizophrenia or schizophreniform disorder (N = 65) from multiple centers across Canada who were within 5 years of their first neuroleptic exposure. These patients were randomly assigned to olanzapine (N = 21; mean dose = 11.7 mg/day), risperidone (N = 21; mean dose = 6.1 mg/day), or haloperidol (N = 23; mean dose = 10.2 mg/day) treatment groups, and a comprehensive neuropsychological test battery was administered. These 17 individual test scores were grouped into 6 different cognitive domains: motor skills, attention span, verbal fluency and reasoning, nonverbal fluency and construction, executive function, and immediate recall. Results showed that olanzapine was superior to risperidone and haloperidol in improving the general cognitive index, motor, and nonverbal functions. Olanzapine also had a robust positive effect on attention, motor, executive, spatial, and immediate recall.
A head-to-head study46 comparing olanzapine with risperidone showed a significant number of olanzapine-treated patients achieving a higher improvement level than those treated with risperidone, as measured by the PANSS total score. Of the olanzapine-treated patients (N = 166, 16.7 mg/day), 36.8% achieved ≥ 40% improvement of the PANSS total score from baseline to endpoint, compared with 26.7% of patients treated with risperidone (N = 165, 6.8 mg/day) (p = .049). Similarly, 21.7% of patients in the olanzapine group achieved ≥ 50% improvement, versus 12.1% of risperidone-treated patients (p = .02). Treatment-emergent adverse events, which were reported in at least 10% of patients in either treatment group, were somnolence, anxiety, weight gain, headache, insomnia, rhinitis, depression, and nausea. Significantly fewer olanzapine-treated patients than patients in the risperidone group reported adverse events overall, and the only event reported significantly more frequently by olanzapine-treated patients was weight gain. Nine events were reported significantly more often in risperidone-treated patients (nausea, amblyopia, EPS, increased salivation, suicide attempt, abnormal ejaculation, back pain, creatine phosphokinase increases, and urinary tract infection). This study also reflected the superiority of olanzapine over risperidone in maintaining response (p = .001), based on the PANSS, a widely used scale that assesses positive and negative symptoms of schizophrenia, and the Clinical Global Impressions-Severity of Illness scale, a broad 3-item assessment of overall symptom severity, at endpoint (week 28).46 An additional analysis pooling data from comparative studies of olanzapine and haloperidol showed olanzapine to be superior in maintenance of treatment response (p = .034), defined as BPRS total score ≤ 18 or decreased ≤ 40% from baseline.47
SAFETY ISSUES
Atypical antipsychotics share, in general, a more diverse receptor activity level than conventional antipsychotics, affording them an improved safety profile with respect to depressive signs and symptoms and tardive dyskinesia. Olanzapine in particular has a broader receptor-binding profile than several other atypical antipsychotics, which may correlate clinically with its previously described efficacy in mania and (based on data from schizophrenia trials) in psychoses, depressive symptoms, negative symptoms, and improvement in cognition. Olanzapine has a favorable safety profile regarding EPS and tardive dyskinesia. Inferentially, based on clinical trials in humans and animal receptor data, it is believed that broad efficacy and safety are correlated with receptor agonist activity.
Some antipsychotics carry a substantial list of limitations both in terms of side effect profile as well as the potential for poor compliance and inadequate response. Even though conventional antipsychotic drug treatment frequently may result in troublesome side effects such as EPS, tardive dyskinesia, and cardiovascular toxicity associated with clinically significant QT prolongation, IMS Health reports that conventional antipsychotics account for more than half of all antipsychotics currently prescribed by primary care physicians (IMS Health, National Disease and Therapeutic Index, Plymouth Meeting, Pa., 2001).
Tardive dyskinesia is more of a consideration in longer-term treatment, but risk seems lower during treatment with olanzapine than with conventional antipsychotic drugs. A study evaluating patients for up to 2.6 years48 suggested rates during olanzapine treatment less than 1/11 of those during haloperidol treatment. Given that there is a baseline rate of tardive dyskinesia in patients with mental illness, the 0.5% incidence during treatment with olanzapine may be approaching that of the baseline incidence.
The annual risk of treatment-emergent tardive dyskinesia for patients treated with olanzapine is low. In a population of patients with bipolar mania, which has a heightened risk for the development of dyskinetic movement disorders, no patients were reported to have treatment-emergent tardive dyskinesia at the end of the extension phase after a total treatment time of 52 weeks.49
Older patients are particularly vulnerable to tardive dyskinesia secondary to medications such as conventional antipsychotics.50–53 The literature suggests that the rate of treatment-emergent tardive dyskinesia in middle-aged and elderly patients treated with neuroleptic (conventional) antipsychotic medicines is about 6 times greater than is expected in younger patients.54 The expected rate of emergent tardive dyskinesia in the younger patient treated with neuroleptic antipsychotic medicines is about 5% per year.55 The risk of tardive dyskinesia is higher in the case of older patients treated with even low doses of neuroleptics; thus, the newer agents with a more favorable EPS profile are rapidly becoming the standard of care.
Another concern when prescribing antipsychotic medication is cardiac safety. Many medications, including psychotropics, can prolong the QT interval. Recently, both thioridazine and mesoridazine received black box warnings for QT prolongation. Ziprasidone was recently approved with a bold warning regarding QT prolongation and risk of sudden death. As a result, it is recommended that, in many cases, other antipsychotics should be tried first. Prolongation of the QT interval by medications has been linked to symptoms such as dizziness, light-headedness, palpitations/racing heart, and syncope and, unfortunately, in some cases has been associated with ventricular tachyarrhythmias, some more serious than others, and sudden death. In registration trials, olanzapine-treated patients were not significantly different from placebo in the occurrence of potentially clinically significant electrocardiogram abnormalities, including QT prolongation.
Although the aforementioned risks are low, some patients treated with olanzapine have been reported to gain weight during therapy. In general, there is a higher prevalence of obesity in persons with schizophrenia than in the general population.56 This, in addition to the greatly increasing prevalence of obesity in the general population worldwide,57 suggests the necessity of weight management interventions, not limited to patients taking antipsychotic drugs. Weight change during antipsychotic drug treatment is not a new finding; in fact, it has been a reported side effect for over 30 years,58 occurring during treatment with both conventional and novel antipsychotic drugs.59 Still, antipsychotic agents are at times considered the best treatment option for patients suffering from schizophrenia, bipolar disorder, and related mental illnesses, despite the possibility of weight gain.
Weight change during antipsychotic drug treatment appears to plateau over time.60,61 A recent retrospective analysis by Kinon et al.62 concluded that mean weight change during olanzapine treatment trended to a plateau after the initial 39 weeks of treatment, and patients (N = 573; mean dose = 15.1 mg/day) did not exhibit a significant increase in weight from that point on for up to 3 years. Patients with higher baseline body mass index (BBMI) gained less weight than those patients with a low BBMI. Dose was not a significant predictor of weight change, nor was there a statistically significant association between weight change and glucose levels.
Different weight interventions have been utilized to manage weight gain in patients taking olanzapine. These interventions include counseling and assessment, behavior interventions, and different pharmacologic interventions.63 Breier et al.63 performed a study with 132 patients with schizophrenia, who were not previously on antipsychotic treatment or participating in a formal weight-loss program, to assess the effectiveness of nizatidine treatment for the prevention of weight gain in olanzapine-treated patients. Results showed that patients treated with a combination of olanzapine (mean modal dose = 12.8 mg/day) and nizatidine, 300 mg b.i.d., gained significantly less weight than patients treated with olanzapine (mean modal dose = 10.4 mg/day) alone. (This dose of nizatidine exceeds the usual recommended daily maximum dose of 300 mg/day.64) These significant results appeared as early as week 3, and effects were maintained to the 16-week endpoint. In fact, weight gain appeared to plateau by week 8 of the study. No significant adverse events were associated with this combination treatment, despite the higher nizatidine dose. Study conclusions suggest that combination therapy with nizatidine, 300 mg b.i.d., may help diminish weight gain during olanzapine treatment.
Perhaps aligned with the issue of weight gain, the argument has arisen that the incidence of new-onset diabetes in patients undergoing antipsychotic drug treatment may be partially or entirely a result of pharmacotherapy, with published cases attributed to clozapine, olanzapine, and quetiapine.65,66 Although the causes of antipsychotic-induced diabetes mellitus are not clear, possible causes include dopamine antagonism (leading to decrease in blood glucose concentrations), nonspecific serotonin receptor antagonism (leading to increased blood glucose concentrations), primary damage to the pancreatic islet cells, insulin resistance, and weight gain.66 Notably, most reports in the literature of antipsychotic-induced diabetes have consisted of prevalence studies with relatively small patient samples.66–69 It is also important to note that diabetes mellitus has always been particularly problematic in the population with schizophrenia, even before the introduction of novel antipsychotic drugs.70,71 The incidence of diabetes mellitus is, in fact, 2 to 4 times higher in the population with severe mental illness than in the general population.72 Only 11 new-onset cases of diabetic ketoacidosis or diabetes mellitus during olanzapine treatment have been reported in the adult population (age range, 31–56 years), varying from acute diabetic ketoacidosis to symptoms of polyuria and polydipsia.65,73–76 Premarketing trials showed the incidence of diabetes mellitus in olanzapine-treated patients as 0.6%.75,77,78
Impaired glucose tolerance also appears to have a higher incidence rate in the population with psychotic illness. In a pooled analysis of 78 clinical trials, Beasley et al.79 found that estimated rates of treatment-emergent impaired glucose tolerance or diabetes were higher than anticipated, both with active therapy and with placebo (12.2% over 1.5 years). Olanzapine was not significantly different from placebo, haloperidol, or risperidone.
When treating a patient with atypical antipsychotic drugs, the practitioner should consider the overall benefit associated with the treatment. Weight change is manageable. Patients, especially those with a personal or family history of the disease, can be monitored for development of diabetes; monitoring is an inexpensive and highly effective preventive measure. Ultimately, the patient's overall health and quality of life should be the primary concerns.
OLANZAPINE TREATMENT OPTIONS
Olanzapine is generally easy to use due to its once-a-day dosing without regard to meals, no required blood monitoring, and dosing flexibility. Oral doses are available in 2.5-, 5-, 7.5-, 10-, 15-, and 20-mg tablets; the approved dose range is 5 to 20 mg/day. Titration is not mandatory but may be desirable in special populations, such as the elderly or medically ill. Higher starting doses (10–15 mg/day) are recommended for most patients with acute mania associated with bipolar disorder. A bedtime or early evening dose is recommended in the event of treatment-emergent sedation.
As with any drug treatment, some patients may have difficulty with normal dosing. Sometimes a patient will have difficulty swallowing a regular tablet or perhaps will refuse to take the medication. For patients resistant to traditional oral medication, Zyprexa Zydis was formulated. A rapidly dissolving tablet, it is effective in patients who have trouble swallowing or who have a tendency to “cheek” their medications.
Studies have shown that the rapidly dissolving tablet formulation is well tolerated by patients. In acutely ill patients with schizophrenia, this formulation is comparable in efficacy to the approved dose of olanzapine. In a study of patients (N = 85; 10–20 mg/day) treated with this formulation for up to 6 weeks, significant results were seen as early as the first week of treatment; patients became more compliant, improved their attitude toward taking medication, and accepted this type of antipsychotic treatment.80 These improvements have led directly to a reduction in caregiver burden. Interestingly, most patients who were treated with the rapidly dissolving tablet opted to continue with this form of olanzapine even when offered the option to switch to regular olanzapine tablets.
Olanzapine received an approvable letter from the FDA in April 2001 for an intramuscular formulation, although it is not yet available to clinicians. In a study by Wright et al.,81 intramuscular olanzapine (10 mg/injection) and intramuscular haloperidol (7.5 mg/injection) were superior to intramuscular placebo (p < .05) in reducing agitation in acutely agitated patients with schizophrenia (N = 311) at 2 hours after the first intramuscular injection. However, intramuscular olanzapine also showed superior efficacy over intramuscular haloperidol at the earliest timepoint (15 minutes) and at 30 and 45 minutes after the first intramuscular injection (p < .05). Intramuscular olanzapine was superior to intramuscular placebo at all timepoints (p < .001).
Another study of intramuscular olanzapine (5–10 mg) in acutely agitated patients with bipolar mania demonstrated its superior efficacy (p < .01) over intramuscular placebo and intramuscular lorazepam (1–2 mg) at the earliest timepoint (30 minutes) and at 60, 90, and 120 minutes after the first intramuscular injection.82 These results demonstrate the rapid onset of action of intramuscular olanzapine, which may offer a significant clinical advantage for the treatment of acute agitation.
Switching medications sometimes leads to withdrawal effects. For patients switched to olanzapine from prior therapy with conventional antipsychotic drugs, one study83 found that an immediate initial full dose of olanzapine (10 mg/day) with gradual tapering of previous treatment (reduced to 50% by the end of week 1 and completely discontinued by the end of week 2) showed the greatest efficacy and tolerability, which was evident as early as the first week of olanzapine treatment. Similar strategy is recommended for integrating olanzapine into existing therapy. In a clinical trial of 209 outpatients with schizophrenia,83 none of 4 switching paradigms were associated with overall clinical worsening, and a majority of clinically stable patients experienced no relapse or clinically significant withdrawal symptoms.
CONCLUSION
The primary care physician is in a key position to assess and treat a patient with psychiatric symptoms, which may range in intensity from subtle to severe. Early assessment and treatment of psychiatric illnesses with psychiatric consultation as needed is valuable in preventing further progression of the illness and functional decline. It may ultimately help delay or prevent the need for hospitalization and/or institutionalization. Further, total cost to patient, family, and society may be reduced.
Primary care physicians have been prescribing psychiatric medications for years and are familiar with a variety of medication classes. Current studies suggest that atypical antipsychotics provide better efficacy and a more favorable safety profile than conventional treatments. Of the atypical antipsychotics, each has its own advantages and disadvantages. Olanzapine is considered a broad-spectrum psychotropic due to its breadth of efficacy and multiple psychiatric uses. Olanzapine also has a favorable safety profile, resulting in key advantages over many therapeutic alternatives.
Drug names: carbamazepine (Tegretol and others), clozapine (Clozaril and others), divalproex sodium (Depakote), fluoxetine (Prozac and others), gabapentin (Neurontin), haloperidol (Haldol and others), lamotrigine (Lamictal), lorazepam (Ativan and others), mesoridazine (Serentil), nizatidine (Axid), olanzapine (Zyprexa), quetiapine (Seroquel), risperidone (Risperdal), topiramate (Topamax), trazodone (Desyrel and others), ziprasidone (Geodon).
Footnotes
Sponsored by Eli Lilly and Company.
REFERENCES
- Olafsdottir M, Marcusson J. Diagnosis of dementia at the primary care level. Acta Neurol Scand Suppl. 1996;93:58–62. doi: 10.1111/j.1600-0404.1996.tb05873.x. [DOI] [PubMed] [Google Scholar]
- Conwell Y. Suicide in elderly patients. In: Schneider LS, Reynolds CFI, Lebowitz BD, et al, eds. Diagnosis and Treatment of Depression in Late Life. 1st ed. Washington, DC: American Psychiatric Press. 1994 397–418. [Google Scholar]
- Morris JC. Alzheimer's disease: a review of clinical assessment and management issues. Geriatrics. 1997;52:S22–S25. [PubMed] [Google Scholar]
- Rovner BW, Kafonek S, Filipp L, et al. Prevalence of mental illness in a community nursing home. Am J Psychiatry. 1986;143:1446–1449. doi: 10.1176/ajp.143.11.1446. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Borenstein J, Salob SP, et al. Behavioral symptoms in Alzheimer's disease: phenomenology and treatment. J Clin Psychiatry. 1987;48(5, suppl):9–15. [PubMed] [Google Scholar]
- Jost BC, Grossberg GT. The evolution of psychiatric symptoms in Alzheimer's disease: a natural history study. J Am Geriatr Soc. 1996;44:1078–1081. doi: 10.1111/j.1532-5415.1996.tb02942.x. [DOI] [PubMed] [Google Scholar]
- Coope B, Ballard C, Saad K, et al. The prevalence of depression in the carers of dementia sufferers. Int J Geriatr Psychiatry. 1995;10:477–485. [Google Scholar]
- Wright LK, Hickey JV, Buckwalter KC, et al. Emotional and physical health of spouse caregivers of persons with Alzheimer's disease and stroke. J Adv Nurs. 1999;30:552–563. doi: 10.1046/j.1365-2648.1999.01124.x. [DOI] [PubMed] [Google Scholar]
- Gelenberg AJ, Hopkins HS. Antipsychotics in bipolar disorder. J Clin Psychiatry. 1996;57(suppl 9):49–52. [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- DeQuardo JR. Pharmacologic treatment of first-episode schizophrenia: early intervention is key to outcome. J Clin Psychiatry. 1998;59(suppl 19):9–17. [PubMed] [Google Scholar]
- Larsen TK, Johannessen JO, Opjordsmoen S. First-episode schizophrenia with long duration of untreated psychosis: pathways to care. Br J Psychiatry. 1998;172:45–52. [PubMed] [Google Scholar]
- Tariot PN. Treatment of agitation in dementia. J Clin Psychiatry. 1999;60(suppl 8):11–20. [PubMed] [Google Scholar]
- Franson K, Chelsey D. Beta-blockers, benzodiazepines, and other miscellaneous agents. In: Hay DP, Klein D, Hay L, et al, eds. A Practical Guide to the Diagnosis and Management of Agitation in Patients With Dementia. In press. [Google Scholar]
- Ancill R, Carlyle R, Liang R, et al. Agitation in the demented elderly: a role for benzodiazepines? Int Clin Psychopharmacol. 1991;6:141–146. doi: 10.1097/00004850-199100630-00002. [DOI] [PubMed] [Google Scholar]
- Coccaro E, Kramer E, Zemishlany Z, et al. Pharmacologic treatment of non-cognitive behavioral disturbances in elderly demented patients. Am J Psychiatry. 1990;147:1640–1645. doi: 10.1176/ajp.147.12.1640. [DOI] [PubMed] [Google Scholar]
- Cummings R, Miller P, Kelsey J, et al. Medications and multiple falls in elderly people: the St. Louis OASIS study. Age Ageing. 1991;20:455–461. doi: 10.1093/ageing/20.6.455. [DOI] [PubMed] [Google Scholar]
- Goldney R. Paradoxical reaction to new minor tranquilizers. Med J Aust. 1977;1:139–140. [PubMed] [Google Scholar]
- Janicak PG. The relevance of clinical pharmacokinetics and therapeutic drug monitoring: anticonvulsant mood stabilizers and antipsychotics. J Clin Psychiatry. 1993;54(9, suppl):35–41. [PubMed] [Google Scholar]
- Porsteinsson AP, Tariot PN, Erb R, et al. Placebo-controlled study of divalproex sodium for agitation in dementia. Am J Geriatr Psychiatry. 2001;9:58–66. [PubMed] [Google Scholar]
- Depakote (divalproex sodium). Physicians' Desk Reference. Montvale, NJ: Medical Economics. 2001 427–437. [Google Scholar]
- Wragg RE, Jeste DV. Overview of depression and psychosis in Alzheimer's disease. Am J Psychiatry. 1989;146:577–587. doi: 10.1176/ajp.146.5.577. [DOI] [PubMed] [Google Scholar]
- Currier GW. Atypical antipsychotic medications in the psychiatric emergency service. J Clin Psychiatry. 2000;61(suppl 14):21–26. [PubMed] [Google Scholar]
- Chengappa KN, Pollock BG, Parepally H, et al. Anticholinergic differences among patients receiving standard clinical doses of olanzapine or clozapine. J Clin Psychopharmacol. 2000;20:311–316. doi: 10.1097/00004714-200006000-00004. [DOI] [PubMed] [Google Scholar]
- Stanniland C, Taylor D. Tolerability of atypical antipsychotics. Drug Saf. 2000;22:195–214. doi: 10.2165/00002018-200022030-00004. [DOI] [PubMed] [Google Scholar]
- Goldberg RJ, Goldberg J. Risperidone for dementia-related disturbed behavior in nursing home residents: a clinical experience. Int Psychogeriatr. 1997;9:65–68. doi: 10.1017/s1041610297004213. [DOI] [PubMed] [Google Scholar]
- Borison RL. Clinical efficacy of serotonin-dopamine antagonists relative to classic neuroleptics. J Clin Psychopharmacol. 1995;15:24S–29S. doi: 10.1097/00004714-199502001-00005. [DOI] [PubMed] [Google Scholar]
- DeDeyn PP, Rabheru K, Rasmussen A, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology. 1999;53:946–955. doi: 10.1212/wnl.53.5.946. [DOI] [PubMed] [Google Scholar]
- Worrel JA, Marken PA, Beckman SE, et al. Atypical antipsychotic agents: a critical review. Am J Health Syst Pharm. 2000;57:238–258. doi: 10.1093/ajhp/57.3.238. [DOI] [PubMed] [Google Scholar]
- Folks DG. Neuroleptics in the treatment of agitation in dementia. In: Hay DP, Klein D, Hay L, et al, eds. A Practical Guide to the Diagnosis and Management of Agitation in Patients with Dementia. In press. [Google Scholar]
- Geodon [package insert]. New York, NY: Pfizer Inc. 2001. [Google Scholar]
- Simpson G, Romano SJ, Horne RL, et al. Ziprasidone vs olanzapine in schizophrenia: results of a double-blind trial (abstract) Schizophr Res. 2001;49(suppl 1–2):241. [Google Scholar]
- Fayek M, Kingsbury SJ, Zada J, et al. Psychopharmacology: cardiac effects of antipsychotic medications. Psychiatr Serv. 2001;52:607–609. doi: 10.1176/appi.ps.52.5.607. [DOI] [PubMed] [Google Scholar]
- Tandon R, Harrigan E, Zorn SH. Ziprasidone: a novel antipsychotic with unique pharmacology and therapeutic potential. J Serotonin Res. 1997;4:159–177. [Google Scholar]
- Beasley CM, Grundy SL, Gannon KS, and et al. Overview of the safety of olanzapine. In: Tran PV, Bymaster FP, Tye N, et al, eds. Olanzapine (Zyprexa): A Novel Antipsychotic. Philadelphia, Pa: Lippincott Williams & Wilkins. 2000 280–299. [Google Scholar]
- Street JS, Clark WS, Gannon KS, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities. Arch Gen Psychiatry. 2000;57:968–976. doi: 10.1001/archpsyc.57.10.968. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Ray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Tohen M, Sanger TM, McElroy SL, et al. Olanzapine versus placebo in the treatment of acute mania. Am J Psychiatry. 1999;156:702–709. doi: 10.1176/ajp.156.5.702. [DOI] [PubMed] [Google Scholar]
- Tohen M, Jacobs TG, Grundy SL, et al. Efficacy of olanzapine in acute bipolar mania: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2000;57:841–849. doi: 10.1001/archpsyc.57.9.841. [DOI] [PubMed] [Google Scholar]
- Baker RW, Tohen M, Milton D, et al. Olanzapine versus divalproex for the treatment of acute mania [abstract] Schizophr Res. 2001;49(suppl 1–2):220. [Google Scholar]
- Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry. 2001;158:131–134. doi: 10.1176/appi.ajp.158.1.131. [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Beasley CM Jr, Tran PV, et al. Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry. 1997;154:457–465. doi: 10.1176/ajp.154.4.457. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Roychowdhury SM, Milton DR, et al. Effective resolution of acute presentation of behavioral agitation and positive psychotic symptoms in schizophrenia. J Clin Psychiatry. 2001;62(suppl 2):17–21. [PubMed] [Google Scholar]
- Tollefson GD, Sanger TM, Lu Y, et al. Depressive signs and symptoms in schizophrenia. Arch Gen Psychiatry. 1998;55:250–258. doi: 10.1001/archpsyc.55.3.250. [DOI] [PubMed] [Google Scholar]
- Purdon SE, Jones BDW, Stip E, et al. Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. Arch Gen Psychiatry. 2000;57:249–258. doi: 10.1001/archpsyc.57.3.249. [DOI] [PubMed] [Google Scholar]
- Tran PV, Hamilton SH, Kuntz AJ, et al. Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol. 1997;17:407–418. doi: 10.1097/00004714-199710000-00010. [DOI] [PubMed] [Google Scholar]
- Tran PV, Dellva MA, Tollefson GD, et al. Oral olanzapine versus oral haloperidol in the maintenance treatment of schizophrenia and related psychoses. Br J Psychiatry. 1998;172:499–505. doi: 10.1192/bjp.172.6.499. [DOI] [PubMed] [Google Scholar]
- Beasley CM Jr, Dellva MA, Tamura RN, et al. Randomised double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. Br J Psychiatry. 1999;174:23–30. doi: 10.1192/bjp.174.1.23. [DOI] [PubMed] [Google Scholar]
- Sanger TM, Grundy SL, Gibson PJ, et al. Long-term olanzapine therapy in the treatment of bipolar I disorder: an open-label continuation phase study. J Clin Psychiatry. 2001;62:273–281. doi: 10.4088/jcp.v62n0410. [DOI] [PubMed] [Google Scholar]
- Jeste DV. Tardive dyskinesia in older patients. J Clin Psychiatry. 2000;61(suppl 4):27–32. [PubMed] [Google Scholar]
- Caligiuri MR, Jeste DV, Lacro JP. Antipsychotic-induced movement disorders in the elderly: epidemiology and treatment recommendations. Drugs Aging. 2000;17:363–384. doi: 10.2165/00002512-200017050-00004. [DOI] [PubMed] [Google Scholar]
- Sajatovic M, Perez D, Brescan D, et al. Olanzapine therapy in elderly patients with schizophrenia. Psychopharmacol Bull. 1998;34:819–823. [PubMed] [Google Scholar]
- Eastham JH, Jeste DV. Treatment of schizophrenia and delusional disorder in the elderly. Eur Arch Psychiatry Clin Neurosci. 1997;247:209–218. doi: 10.1007/BF02900217. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Rockwell E, Harris MJ, et al. Conventional vs newer antipsychotics in elderly patients. Am J Geriatr Psychiatry. 1999;7:70–76. [PubMed] [Google Scholar]
- Kane JM, Woerner M, Lieberman J. Tardive dyskinesia: prevalence, incidence, and risk factors. J Clin Psychopharmacol. 1988;8:52S–56S. [PubMed] [Google Scholar]
- Allison DB, Fontaine KR, Heo M, et al. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60:215–220. doi: 10.4088/jcp.v60n0402. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kuczmarski RJ, et al. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- Stanton JM. Weight gain associated with neuroleptic medication: a review. Schizophr Bull. 1995;21:463–472. doi: 10.1093/schbul/21.3.463. [DOI] [PubMed] [Google Scholar]
- Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- Lamberti JS, Bellnier T, Schwarzkopf SB. Weight gain among schizophrenic patients treated with clozapine. Am J Psychiatry. 1992;149:689–690. doi: 10.1176/ajp.149.5.689. [DOI] [PubMed] [Google Scholar]
- Leadbetter R, Shutty M, Pavalonis D, et al. Clozapine-induced weight gain: prevalence and clinical relevance. Am J Psychiatry. 1992;149:68–72. doi: 10.1176/ajp.149.1.68. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Basson BR, Gilmore JA, et al. Long-term olanzapine treatment: weight change and weight-related health factors in schizophrenia. J Clin Psychiatry. 2001;62:92–100. [PubMed] [Google Scholar]
- Breier A, Tanaka Y, Roychowdhury S, and et al. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders: a randomized controlled double blind study. Presented at a meeting of the College of Psychiatric and Neurologic Pharmacists (CPNP); March 25, 2001; San Antonio, Tex. [Google Scholar]
- Axid (nizatidine). Physicians' Desk Reference. Montvale, NJ: Medical Economics. 2001 1704–1706. [Google Scholar]
- Wirshing DA, Spellberg BJ, Erhart SM, et al. Novel antipsychotics and new onset diabetes. Biol Psychiatry. 1998;44:778–783. doi: 10.1016/s0006-3223(98)00100-0. [DOI] [PubMed] [Google Scholar]
- Liebzeit KA, Markowitz JS, Caley CF. New onset diabetes and atypical antipsychotics. Eur Neuropsychopharmacol. 2001;11:25–32. doi: 10.1016/s0924-977x(00)00127-9. [DOI] [PubMed] [Google Scholar]
- Meyer JM. One-year comparison of lipids, glucose, and weight with olanzapine or risperidone. In: New Research Abstracts of the 154th Annual Meeting of the American Psychiatric Association; May 9, 2001; New Orleans, La. Abstract NR490:133. [Google Scholar]
- Casey DE, Danielson EM, and Fishman NB. Prevalence of diabetes in schizophrenia patients treated with antipsychotics. In: New Research Abstracts of the 154th Annual Meeting of the American Psychiatric Association; May 8, 2001; New Orleans, La. Abstract NR315:85–86. [Google Scholar]
- Sernyak MJ, Rosenheck RA, and Leslie D. Association of diabetes mellitus with atypical neuroleptics. In: New Research Abstracts of the 154th Annual Meeting of the American Psychiatric Association; May 9, 2001; New Orleans, La. Abstract NR506:137–138. [Google Scholar]
- Mukherjee S. High prevalence of type II diabetes in schizophrenic patients. Schizophr Res. 1995;37:68–73. [Google Scholar]
- Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull. 2000;26:903–912. doi: 10.1093/oxfordjournals.schbul.a033504. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Decina P, Bocola V, et al. Diabetes mellitus in schizophrenic patients. Compr Psychiatry. 1996;37:68–73. doi: 10.1016/s0010-440x(96)90054-1. [DOI] [PubMed] [Google Scholar]
- Gatta B, Rigalleau V, Gin H. Diabetic ketoacidosis with olanzapine treatment. Diabetes Care. 1999;22:1002–1003. doi: 10.2337/diacare.22.6.1002. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Sporn J, Brown S, et al. New-onset diabetes mellitus and diabetic ketoacidosis associated with olanzapine treatment. Psychosomatics. 1999;40:438–443. doi: 10.1016/S0033-3182(99)71210-7. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Patel R. Olanzapine induced ketoacidosis with diabetes mellitus [letter] Am J Psychiatry. 1999;9:1471. doi: 10.1176/ajp.156.9.1471. [DOI] [PubMed] [Google Scholar]
- Selva KA, Scott SM. Diabetic ketoacidosis associated with olanzapine in an adolescent patient. J Pediatr. 2001;138:936–938. doi: 10.1067/mpd.2001.114016. [DOI] [PubMed] [Google Scholar]
- Keskiner A, el-Toumi A, Bousquet T. Psychotropic drugs, diabetes and chronic mental patients. Psychosomatics. 1973;14:176–181. doi: 10.1016/s0033-3182(73)71353-0. [DOI] [PubMed] [Google Scholar]
- McKee HA, D'Arcy PF, Wilson PJ. Diabetes and schizophrenia: a preliminary study. J Clin Hosp Pharmacy. 1986;11:297–299. doi: 10.1111/j.1365-2710.1986.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Beasley CM Jr, Kwong K, Berg PH, and et al. Incidence and rate of treatment-emergent potential impaired glucose tolerance and potential diabetes with olanzapine compared to other antipsychotic agents and placebo. Presented at the 30th annual meeting of the American College of Neuropsychopharmacology; Dec 12, 2000; San Juan, Puerto Rico. [Google Scholar]
- Kinon BJ, Milton DR, Hill AL, and et al. Efficacy of Zyprexa Zydis in the treatment of acutely ill, noncompliant schizophrenic patients. In: New Research Abstracts of the 154th Annual Meeting of the American Psychiatric Association; May 8, 2001; New Orleans, La. Abstract NR281:76. [Google Scholar]
- Wright P, Birkett M, Ferchland I, et al. A double-blind study of intramuscular olanzapine, haloperidol and placebo in acutely agitated schizophrenic patients. Eur Neuropsychopharmacol. 2000;10:S304. [Google Scholar]
- Meehan K, Breier A, David SR, et al. Efficacy and safety of intramuscular (IM) olanzapine versus IM lorazepam and IM placebo in acutely agitated patients diagnosed with mania associated with bipolar disorder [abstract] Schizophr Res. 2001;49:239. [Google Scholar]
- Kinon BJ, Basson BR, Gilmore JA, et al. Strategies for switching from conventional antipsychotic drugs or risperidone to olanzapine. J Clin Psychiatry. 2000;61:833–840. doi: 10.4088/jcp.v61n1105. [DOI] [PubMed] [Google Scholar]