Abstract
The molecular genetics of vernalization, defined as the promotion of flowering by cold treatment, is still poorly understood in cereals. To better understand this mechanism, we cloned and characterized a gene that we named TaVRT-1 (wheat [Triticum aestivum] vegetative to reproductive transition-1). Molecular and sequence analyses indicated that this gene encodes a protein homologous to the MADS-box family of transcription factors that comprises certain flowering control proteins in Arabidopsis. Mapping studies have localized this gene to the Vrn-1 regions on the long arms of homeologous group 5 chromosomes, regions that are associated with vernalization and freezing tolerance (FT) in wheat. The level of expression of TaVRT-1 is positively associated with the vernalization response and transition from vegetative to reproductive phase and is negatively associated with the accumulation of COR genes and degree of FT. Comparisons among different wheat genotypes, near-isogenic lines, and cereal species, which differ in their vernalization response and FT, indicated that the gene is inducible only in those species that require vernalization, whereas it is constitutively expressed in spring habit genotypes. In addition, experiments using both the photoperiod-sensitive barley (Hordeum vulgare cv Dicktoo) and short or long day de-acclimated wheat revealed that the expression of TaVRT-1 is also regulated by photoperiod. These expression studies indicate that photoperiod and vernalization may regulate this gene through separate pathways. We suggest that TaVRT-1 is a key developmental gene in the regulatory pathway that controls the transition from the vegetative to reproductive phase in cereals.
Freezing tolerance (FT) in cereals is dependent upon a highly integrated system of structural, regulatory, and developmental genes. The development of maximum low-temperature (LT) tolerance is known to be associated with two important developmentally controlled adaptive features (Mahfoozi et al., 2001a). The first is a vernalization requirement that delays heading by postponing the transition from the vegetative to the reproductive phase. The second is a photoperiod requirement that allows the plant to flower only when exposed to optimal inducing conditions. Time sequence studies have shown that LT-induced gene expression is also developmentally regulated (Fowler et al., 1996a, 1996b). In these studies, transition from the vegetative to the reproductive growth phase can be perceived as a critical switch that initiates the down-regulation of LT-induced genes (Fowler et al., 1996a, 1996b, 2001; Mahfoozi et al., 2001a, 2001b). As a result, full expression of cold hardiness genes only occurs in the vegetative phase, and plants in the reproductive phase have a limited ability to cold acclimate. In addition, plants that are still in the vegetative phase have the ability to re-acclimate following periods of exposure to warm temperatures, whereas plants in the reproductive phase only have a limited ability to do so (Mahfoozi et al., 2001b).
According to our proposed model (Fowler et al., 1999), the developmental genes (vernalization and photoperiod) act as a master switch controlling the duration of expression of LT-induced structural genes (Fowler et al., 1996a, 1996b; Mahfoozi et al., 1998), whereas the level of FT is determined by the length of time and degree that the structural genes are up-regulated. Vernalization requirements allow LT genes to be expressed for a longer period of time at temperatures in the acclimation range (Fowler et al., 1996a, 1996b). Similarly, photoperiod sensitivity allows plants to maintain LT genes in an up-regulated state for a longer period of time under short-day (SD) compared with long-day (LD) environments (Mahfoozi et al., 2000). In both instances, the delay in the transition from the vegetative to the reproductive phase produces increased FT that is sustained for a longer period of time. This observation also explains why a high level of FT has not been observed in spring habit cultivars. Because LT gene expression is only up-regulated when the plant is in the vegetative phase, the genetic potentials of spring habit cultivars are not given an opportunity to be fully expressed, leaving the impression that the spring habit Vrn-A1 allele has a dominant pleiotropic effect for frost susceptibility (Fowler et al., 1999). Thus, vernalization plays an important regulatory role in the growth and development of winter cereals. However, the molecular genetic basis of vernalization is still poorly understood in cereals compared with the model plant Arabidopsis. This is due to the genetic complexity of cereals and the difficulty in obtaining mutants. In Arabidopsis, several vernalization- and flowering-associated genes have been cloned and characterized (Simpson et al., 1999; Blázquez, 2000; Hepworth et al., 2002). These genes encode members of diverse transcription factors such the MADS-box, MYB, Myc and zinc finger families, RNA-binding protein, and other protein families.
Although vernalization has been studied in cereals, few regulatory proteins have been associated with this process in monocots (Jensen et al., 2001). To unravel the molecular processes involved during vernalization and flowering in cereals, it is essential to identify the genes associated with these processes and to functionally characterize them. On the basis of the high number of MADS-box proteins (at least 10) known to be implicated in the vernalization and the flowering pathways in Arabidopsis (Blázquez, 2000), we have begun the identification and characterization of this family in wheat (Triticum aestivum). In this study, we report a detailed molecular characterization of the MADS-box transcription factor TaVRT-1. Expression analysis using different wheat genotypes and near-isogenic lines (NILs) for the Vrn-A1 locus indicated that the accumulation of TaVRT-1 transcript is associated with the vernalization response, the transition from vegetative to reproductive phase, the expression of COR genes, and the degree of FT. We further demonstrate that the level of TaVRT-1 accumulation could be used to determine the capacity of the apical meristem to enter the reproductive phase. Using several wheat deletion lines for chromosome 5A and 5D, we localized copies of the gene to the same region as the major vernalization loci Vrn-A1 and Vrn-D1. The role of this gene in regulating the transition from the vegetative to reproductive phase is discussed.
RESULTS
Molecular Analyses and Mapping of TaVRT-1
Natural variation and mutation analyses as well as molecular characterization have identified the MADS-box family members as important developmental regulators in plants. However, the function of this family in cereals is still poorly understood. In this study, we used two approaches to identify MADS-box genes from wheat. The first consisted of using specific primers to amplify the TaMADS#11 gene (Murai et al., 1998) and then screen a cDNA library with the amplified product. This approach allowed the identification of six homologs of TaMADS#11, and the longest one was completely sequenced (accession no. AY280870). The second used data mining to identify six different MADS-box genes in our EST database. Preliminary expression studies using control and 49-d LT-treated spring and winter wheat cultivars revealed that only the accumulation of the TaMADS#11 homolog was associated with the vegetative to reproductive transition (data not shown). Therefore, we named this gene T. aestivum vegetative to reproductive transition-1 (TaVRT-1). The gene encodes a type II MADS-box protein with the MADS, I, K, and C domains (Fig. 1). Sequence comparison with Arabidopsis MADS-box proteins revealed that TaVRT-1 is highly similar to APETALA 1 (AP1) and could be classified with members of the AP1/SQUA branch of the plant MADS-box family (Gocal et al., 2001; Fig. 1). In monocots, this group includes HvBM5 of barley, OsMADS14/RAP1B of rice, LtMADS1 of L. temulentum (Monn et al., 1999; Kyozuka et al., 2000; Schmitz et al., 2000; Gocal et al., 2001) and a maize EST (BE511439). In dicots, some of the representatives of AP1/SQUA group are AtAP1 and AtFRUITFULL/AGL8 of Arabidopsis, AmDEFH28 of snapdragon, and PhTBP26 of petunia (Mandel et al., 1992; Mandel and Yanofsky, 1995; Immink et al., 1999; Müller et al., 2001). Transcription factors of the AP1/SQUA branch, such as PhTBP26, AtAP1, AtFUL/AGL8, AmDEFH28, and Lt-MADS1, have been associated with the transition of the shoot apex from the vegetative to the reproductive phase. Search for a specific nuclear targeting domain using PSORT (Nakai and Kanehisa, 1992) revealed that the N-terminal end of TaVRT-1 is likely to possess a bipartite nuclear targeting signal (Robbins et al., 1991) that is conserved in all MADS-box transcription factors shown in Figure 1. In addition, the C-terminal region of TaVRT-1 contains a Ser stretch that is predicted to be a conserved phosphorylation site. This putative Ser stretch phosphorylation site is present in all AP1/SQUA MADS-box family members shown in Figure 1 except AP1. On the other hand, AP1 is the only member of the AP1/SQUA family that has a predicted and experimentally verified C-terminal prenylation site (CaaX; Yalovsky et al., 2000). These analyses indicate that TaVRT-1 may not be a functional ortholog of AP1 from Arabidopsis despite the sequence similarity.
Figure 1.
Structure and sequence alignment of TaVRT-1 with other MADS-box members of the API/SQUA family. Barley (Hordeum vulgare) HvBM5 (Schmitz et al., 2000), Lolium temulentum LtMADS1 (Gocal et al., 2001), indica rice OsMADS14 (Monn et al., 1999), japonica rice OsRAP1B (Kyozuka et al., 2000), maize (Zea mays) ZmMADS from EST BE511439, petunia (Petunia hybrida) PhTBP26 (Immink et al., 1999), Arabidopsis AtFUL/AGL8 (Mandel and Yanofsky, 1995), Snapdragon (Antirrhinum majus) AmDEFH28 (Müller et al., 2001), and Arabidopsis AtAP1 (Mandel et al., 1992). Identical and similar amino acids are shaded in black and gray, respectively. MADS-box domain, DNA-binding domain; I, intervening region; K, keratin-like domain; C, C-terminal region. *, Residues identified as being part of a nuclear targeting signal by PSORT (Nakai and Kanehisa, 1992; http://psort.nibb.ac.jp). Ser stretch, NetPhos 2.0 (Blom et al., 1999) predicts phosphorylation sites in this region (http://www.cbs.dtu.dk/services/NetPhos/).
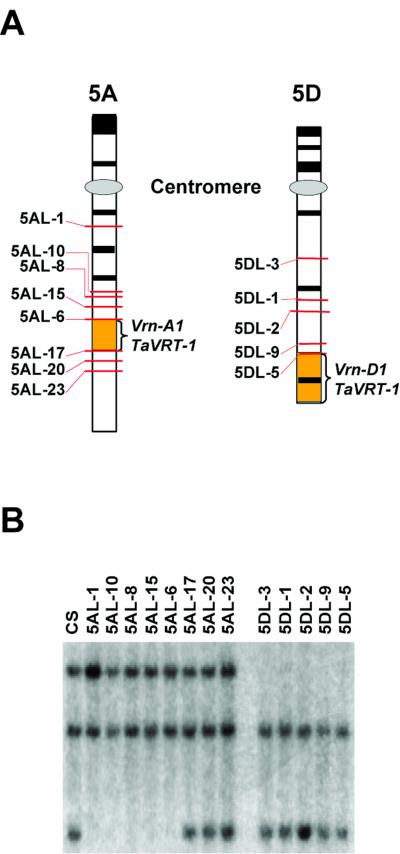
DNA gel-blot analysis of nullisomic-tetrasomic lines with a specific TaVRT-1 probe (lacking the MADS-box domain) showed that there is a copy of TaVRT-1 present on each group 5 chromosome (data not shown). To localize the TaVRT-1 gene more precisely, several deletion lines for homeologous group 5 chromosomes (Endo, 1988; Endo and Gill, 1996) from wheat cv Chinese Spring were analyzed (Fig. 2). The breakpoints of the deletion lines are indicated to the left of the chromosome drawings (Fig. 2A). DNA gel-blot analysis indicates that TaVRT-1 is located on the long arm of chromosome 5A in a region delimited by deletion breakpoints 5AL-6 and 5AL-17 and on the distal end of chromosome 5D (Fig. 2B). These two regions are known to be rich in regulatory genes and carry, more specifically, the Vrn-A1 and Vrn-D1 genes associated with vernalization response and FT (Plaschke et al., 1993; Dubcovsky et al., 1998; Limin and Fowler, 2002).
Figure 2.
Mapping of TaVRT-1. a, Schematic representation of the deletion lines used to map the TaVRT-1 gene on chromosomes 5A and 5D. The numbers to the left indicate the deletion breakpoints of each line, where the distal portion of the chromosome is missing. Black boxes represent telomeric C-band markers. Orange boxes indicate the regions containing Vrn-1 and TaVRT-1. b, DNA gel-blot analysis of genomic DNA from wheat cv Chinese Spring (CS) and chromosome 5A and 5D deletion lines.
TaVRT-1 Expression and the Vernalization Response
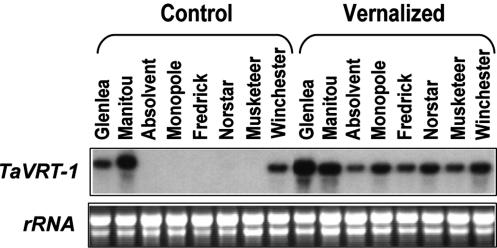
Expression studies using different wheat genotypes and other cereal species indicated that the accumulation of TaVRT-1 mRNA in plants held at 4°C is genotype-dependent (Fig. 3). Spring habit wheat and barley cereals without a vernalization requirement (wheat cvs Glenlea and Manitou and barley cv Winchester) show constitutive expression of the TaVRT-1 gene. In contrast, winter habit wheat and rye (Secale cereale) cereals that require vernalization (wheat cvs Absolvent, Fredrick, Monopole, and Norstar and rye cv Musketeer) expressed the TaVRT-1 gene only upon LT treatment. These observations indicated the possible association between TaVRT-1 expression and the vernalization response in cereals.
Figure 3.
Expression of TaVRT-1 in several wheat cultivars and other cereals. Unvernalized (control) plants were grown for 12 d at 20°C, whereas vernalized plants were grown for 45 d at 4°C after 7 d at 20°C. RNA from two spring wheat (cvs Glenlea and Manitou), one spring barley (cv Winchester), four winter wheat (cvs Absolvent, Monopole, Fredrick, and Norstar), and a winter rye (cv Musketeer) were analyzed. The blot was hybridized with the specific TaVRT-1 probe. rRNA is shown as a load control.
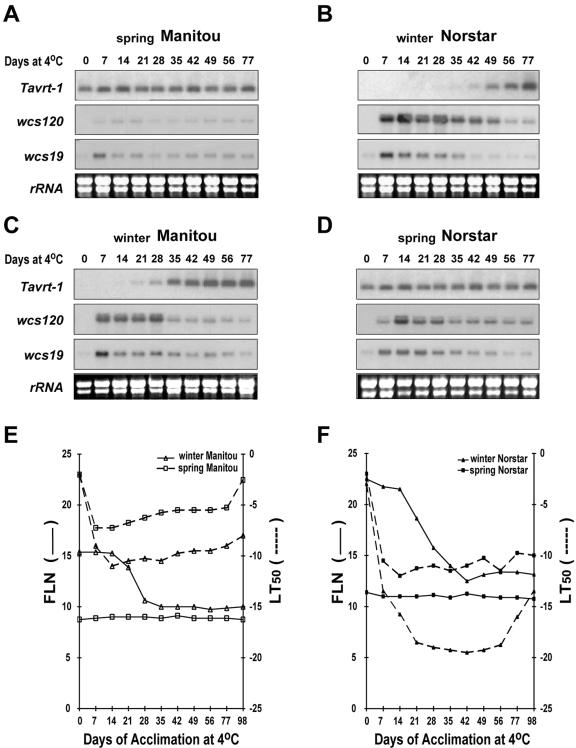
To verify this possibility, the expression of TaVRT-1 during the transition from vegetative to reproductive phase in the winter wheat cv Norstar was compared with identical time points in the reproduction competent spring wheat cv Manitou (Fig. 4). The COR genes WCS120 and WCS19/LEA3-L2 were used for comparative gene expression analyses because the level and duration of their expression have been shown to be affected by the vernalization saturation point (Fowler et al., 1996a). The results indicate a distinct regulation of the vernalization-associated gene TaVRT-1 in spring wheat cv Manitou and winter wheat cv Norstar (Fig. 4A, b). In spring wheat, the constitutive expression of TaVRT-1 was associated with low induction of COR genes and a poor capacity to develop FT (Fig. 4A, E). In contrast, TaVRT-1 transcripts were undetectable in winter wheat cv Norstar at the beginning of the LT treatment (0–35 d), and this period coincides with high induction of COR genes and maximum development of FT (Fig. 4A, F). However, TaVRT-1 transcripts began to accumulate when plants reached their vernalization saturation point, as determined by final leaf number (FLN) measurements (after 42-d LT treatment; Fig. 4B, F). At this point, similarly to spring wheat, TaVRT-1 accumulation was associated with down-regulation of COR genes and the beginning of a decrease in FT. Thus, full expression of COR genes and maximum FT occurred in the vegetative phase before TaVRT-1 transcripts began to accumulate, establishing that TaVRT-1 expression is positively associated with the completion of vernalization requirement in winter habit wheat, concomitantly with the down-regulation of COR genes and the decrease in FT. These results clearly establish that TaVRT-1 expression is associated with the vernalization response in wheat. Whether this holds true for other cereals such as barley and rye will have to await future studies with appropriate cultivars that have well-defined phenotypes for vernalization and FT.
Figure 4.
Expression of TaVRT-1 in relation to vernalization, COR gene expression, FLN, and development of FT. RNA gel-blot analyses showing TaVRT-1 transcript accumulation in wheat parental lines spring wheat cv Manitou (a) and winter wheat cv Norstar (b) and in the wheat near-isogenic lines winter wheat cv Manitou (c) and spring wheat cv Norstar (d). Blots were hybridized sequentially with the specific TaVRT-1 probe and the WCS120 and WCS19/LEA3-L2 probes. rRNA is shown as a load control. FLN (—) and LT50 (....) for wheat cv Manitou (e) and wheat cv Norstar (f), and their NILs are also illustrated.
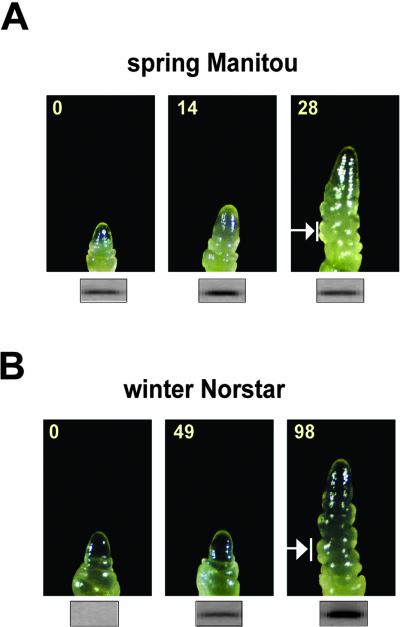
In cereals, the formation of a double-ridge structure has long been considered as an early marker of flowering competence (Kirby and Appleyard, 1987; McMaster, 1997). However, it was recently shown that flowering competence in the meristem may occur well before the visible appearance of the double-ridge formation (Mahfoozi et al., 2001a). Therefore, shoot apices were dissected during the LT treatment to identify the developmental stage of the meristem and to determine whether TaVRT-1 expression was associated with the development of flowering competence or the appearance of double ridges. Results presented in Figure 5 show that TaVRT-1 accumulation preceded the appearance of double ridges, which become visible after LT treatment for 28 d in wheat cv Manitou and 98 d in wheat cv Norstar. These structures were not apparent in 0- and 49-d vernalized wheat cv Norstar and 0- and 14-d LT-treated wheat cv Manitou. On the basis of these results, TaVRT-1 accumulation may represent an early marker of flowering competence.
Figure 5.
Expression of TaVRT-1 during phenological development in wheat. Spring wheat cv Manitou and winter wheat cv Norstar were grown for 14 d at 20°C under LD photoperiod and were then vernalized at 4°C for the times indicated. Shoot apices were dissected and analyzed for the appearance of a double-ridge structure. Arrow indicates the double-ridge formation indicative of transition to the reproductive phase. RNA gel blots indicating TaVRT-1 transcript level are shown for each time point.
The Vrn-A1 Locus and Regulation of TaVRT-1 and COR Genes in Wheat Near Isogenic Lines (NILs)
Vernalization requirement in wheat is a critical determinant between spring and winter habit genotypes. In hexaploid wheat, which has three genomes (AABBDD) with seven chromosome pairs each (2n = 42), three major loci determining vernalization requirement have been identified and mapped: Vrn-A1, Vrn-B1, and Vrn-D1 on the long arm of chromosomes 5A, 5B, and 5D, respectively (Law et al., 1976; Maystrenko, 1980; Galiba et al., 1995; Nelson et al., 1995; Korzun et al., 1997; Snape et al., 1997; McIntosh et al., 1998; Sarma et al., 1998; Kato et al., 1999a). The degree of requirement to complete heading depends on the Vrn genotype. For example, Vrn-A1 does not require vernalization treatment at all, whereas Vrn-B1 and Vrn-D1 require vernalization for 15 to 30 d, and winter habit wheat that is recessive for all of these genes requires 45 to 60 d of vernalization for heading (Kato, 1988; Maystrenko, 1987). The wheat cv Manitou has a single dominant gene for spring growth habit (Vrn-A1/Vrn-A1, vrn-B1/vrn-B1, and vrn-D1/vrn-D1), while wheat cv Norstar (vrn-A1/vrn-A1, vrn-B1/vrn-B1, and vrn-D1/vrn-D1) is recessive for all the Vrn genes (Brule-Babel and Fowler, 1988). Therefore, wheat cvs Manitou/Norstar reciprocal NILs for the chromosomal region containing the Vrn-A1 locus represent a good model system to study the influence of the two alleles on the developmental regulation of genes associated with vernalization and FT.
Because TaVRT-1 regulation is closely associated with the vernalization saturation point, we investigated the influence of the two alleles of the Vrn-A1 locus on the developmental regulation of TaVRT-1 and COR genes using the wheat reciprocal NILs. These NILs, which theoretically contain 96.9% of the recurrent parent's genome, have been used to confirm the close association between FT capacity and vernalization saturation point (Limin and Fowler, 2002). Introgression of the recessive vrn-A1 allele from wheat cv Norstar transformed the spring habit wheat cv Manitou into the winter habit wheat cv Manitou. In this NIL, TaVRT-1 is no longer constitutively expressed, but shows an accumulation similar to a genuine winter habit wheat (Fig. 4, compare C with B). The lower TaVRT-1 expression in winter wheat cv Manitou is now associated with higher COR gene expression and FT (Fig. 4C, E); however, several visible differences were noted. The attainment of the vernalization saturation point, the start of TaVRT-1 accumulation, and the loss of COR gene expression occurred after 21 to 28 d of LT treatment in winter wheat cv Manitou compared with approximately 42 d for winter wheat cv Norstar. These differences suggest that the vrn-A1 locus does not act alone in determining the transition from the vegetative to the reproductive phase.
Introgression of the dominant Vrn-A1 allele from wheat cv Manitou transformed the winter habit wheat cv Norstar into the spring habit wheat cv Norstar. In this NIL, TaVRT-1 is now constitutively expressed to a level similar to the spring wheat cv Manitou (Fig. 4, compare D with A). This constitutive expression in spring wheat cv Norstar is associated with a lower induction of COR genes and poorer development of FT (Fig. 4A, F). Spring wheat cv Norstar produced on average 1.1 more leaves than spring wheat cv Manitou as determined by FLN measurements (Fig. 4F), suggesting that it remained in the vegetative phase for a slightly longer period. However, the level of TaVRT-1 expression was similar to a spring habit plant that has committed to the reproductive phase. Overall, these results offer further support to the idea that the Vrn-A1/vrn-A1 locus is the major locus regulating TaVRT-1 expression, COR gene inducibility, and FT and that TaVRT-1 expression closely follows the capacity of the plant to become reproductive.
Influence of Photoperiod on the Developmental Regulation of TaVRT-1 in Winter Cereals
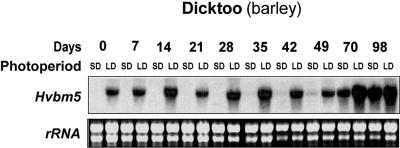
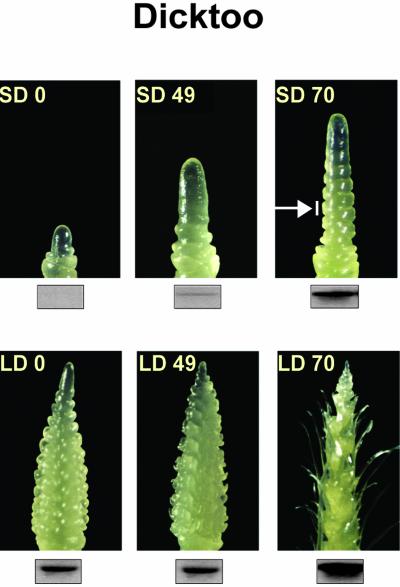
In a recent study, we have shown that the highly SD-sensitive barley cv Dicktoo, which does not have a vernalization requirement, is able to accumulate higher levels of COR proteins and to acquire higher FT when acclimated under SD compared with LD photoperiod (Fowler et al., 2001). Therefore, we grew barley cv Dicktoo under SD and LD photoperiods at 4°C for 98 d to determine the effect of different photoperiod regimes on the expression of the barley TaVRT-1 homolog (HvBM5) relative to flowering capacity and FT. As shown in Figure 6, the barley TaVRT-1 homolog shows the same expression profile in LD-grown barley cv Dicktoo as spring wheat cv Manitou (Fig. 4A). Also as observed in wheat cv Manitou, the constitutive expression of the TaVRT-1 homolog in barley cv Dicktoo is associated with a lower accumulation of COR proteins and lower level of FT (Fowler et al., 2001). Under SD conditions, TaVRT-1 homolog transcripts started accumulating at 49 d and reached a maximum level after 70 d of LT treatment (Fig. 6). This expression profile was similar to the one observed in wheat cv Norstar (Fig. 4B). The main difference between TaVRT-1 induction in wheat cv Norstar and SD-grown barley cv Dicktoo is that its expression in barley cv Dicktoo is not associated with the vernalization saturation point. To better determine the phenological stage of the plants used in this experiment, shoot apices were dissected after the different LT treatments. As shown in Figure 7, the expression of TaVRT-1 homolog in barley cv Dicktoo was clearly associated with the appearance of double ridges. These results show that even though barley cv Dicktoo always has the competence to flower, it requires inductive photoperiod conditions to become reproductive.
Figure 6.
Expression of a barley TaVRT-1 homolog in vernalization-insensitive barley cv Dicktoo during cold acclimation. Barley plants were grown at 4°C for 0 to 98 d under SD and LD. Northern analysis was as described in Figure 3.
Figure 7.
Expression of a barley, TaVRT-1 homolog, during phenological development in barley cv Dicktoo under different photoperiods. Barley plants were grown under SD and LD photoperiods for 70 d of cold acclimation. Apical shoot development in 0-, 49-, and 70-d-old plants is presented. Arrow indicates the double-ridge formation indicative of transition to the reproductive phase. RNA gel blots indicating transcript level are shown for each time point.
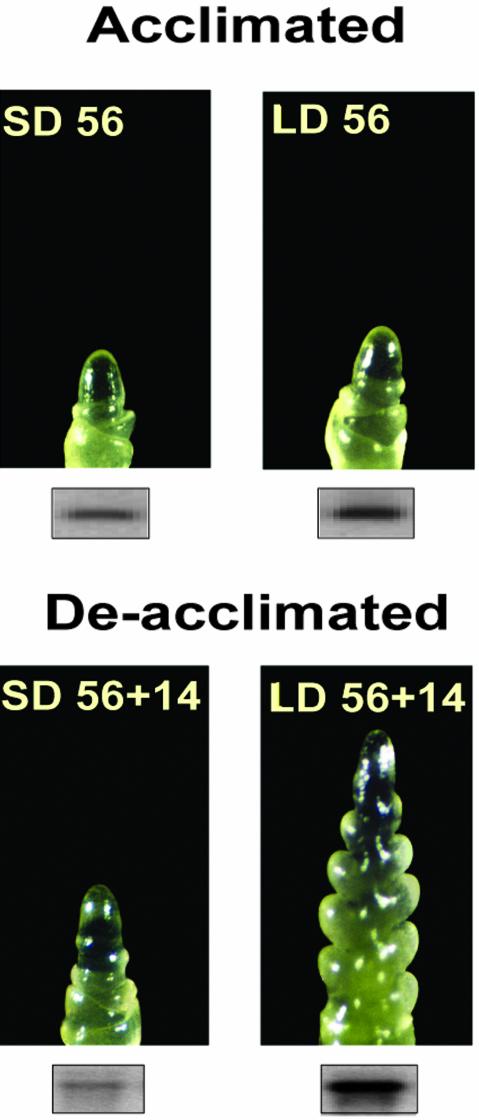
Vernalization and photoperiod are the main environmental cues regulating the flowering developmental switch. They also have a large influence on the capacity of the plant to cold acclimate. In winter wheat cv Norstar, FT and transition to the reproductive phase are dependent upon growth temperature and photoperiod (Mahfoozi et al., 2000; 2001a). In previous experiments, we have shown that after 56 d of vernalization at 4°C under SD, wheat cv Norstar became reproductive only when shifted to warm temperatures and LD photoperiod, with the consequential loss of the capacity to cold acclimate (Mahfoozi et al., 2001b). In contrast, wheat cv Norstar plants maintained under SD photoperiod remained vegetative and retained their ability to cold acclimate for a longer period of time. To determine whether the wheat TaVRT-1 gene is also regulated by photoperiod in wheat cv Norstar, 56-d-acclimated plants that had reached flowering competence but were not yet reproductive were shifted to 20°C under LD and SD photoperiod regimes. The results presented in Figure 8 show that TaVRT-1 expression increased after the transfer to LD-inductive conditions and that this increase is associated with the reproductive transition of the shoot apex and a reduced capacity to reacquire FT (Mahfoozi et al., 2001b). In contrast, transfer to SD conditions resulted in the decrease of TaVRT-1 transcript level that was associated with the absence of double-ridge formation (vegetative/reproductive transition) and a higher capacity to re-acclimate. These results provide evidence that TaVRT-1 expression is also regulated by photoperiod and confirm the link between TaVRT-1 expression, the vegetative/reproductive transition, and the degree of FT.
Figure 8.
TaVRT-1 accumulation and phenological development in wheat during de-acclimation under different photoperiods. Wheat cv Norstar plants were acclimated for 56 d at 4°C under SD and LD photoperiods and then de-acclimated at 20°C for 14 d (56 + 14) under the same photoperiod. In the LD 56+14 treatment, the shoot apex has advanced development beyond the double-ridge phase. RNA gel blots indicating TaVRT-1 transcript level are shown for each time point.
DISCUSSION
Molecular and physiological studies aimed at understanding the mechanisms regulating the cold acclimation process revealed that it was closely associated with vernalization and photoperiodic responses (Fowler et al., 1996a, 2001). Identification of the molecular components involved in the vernalization process is usually carried out with the selection of mutants that show altered phenotypes and subsequent cloning and characterization of the corresponding genes. However, this is not feasible in cereal species such as hexaploid wheat that contain three large genomes and long generation times. To circumvent this problem, we used expression profiling to screen several wheat MADS-box genes. The gene that showed an association with vernalization response was selected for further analysis. Detailed expression studies were carried out using cereal cultivars with well-defined phenotypes that have been fully characterized for vernalization response. In addition, NILs for the vernalization locus Vrn-A1 or vrn-A1 were used to further delineate the effect of each allele within two reciprocal genetic backgrounds (Limin and Fowler, 2002).
Results presented in this study provide evidence that the expression of TaVRT-1, which maps to the Vrn-1 genomic regions, is closely and in dose-dependent fashion associated with the capacity of the shoot apex meristem to enter into the reproductive phase. The gene exhibits sequence homology to members of the AP1/SQUA branch of the plant MADS-box family. Three members of this group in Arabidopsis, AP1, FRUITFULL, and CAULIFLOWER were identified by positional cloning of mutations affecting the development of flowers (Mandel et al., 1992; Kempin et al., 1995; Mandel and Yanofsky, 1995). It was recently shown that apart from their specific role in the flower formation, they also act redundantly in the regulation of the meristem identity (Ferràndiz et al., 2000). The mutations caused primordia to develop shoot characteristics instead of flowers. However, members of the AP1/SQUA branch in Arabidopsis have not yet been associated with the vernalization process. In fact, these proteins are considered downstream effectors in flower development and are controlled by upstream signaling events (Simpson et al., 1999). Similarly, TaVRT-1 may function in a manner identical to that of its close Arabidopsis homolog AP1, but its expression could be controlled directly or indirectly by the Vrn-1 regulon.
The use of the wheat NILs in this study provides evidence that the constitutive expression of TaVRT-1 is highly dependent on the presence of the dominant Vrn-A1 allele. These experiments also highlighted the existence of a complex interplay that regulates the vernalization process where it can be hypothesized that additional genes or loci promote flowering in the wheat cv Manitou background and/or delay flowering in the wheat cv Norstar background. These conclusions are based on the observations that the dominant Vrn-A1 allele in spring wheat cv Norstar did not by itself decrease the FLN to the level found in spring wheat cv Manitou and that the recessive allele vrn-A1 in winter wheat cv Manitou did not by itself delay the attainment of the vernalization saturation point to the one found in winter wheat cv Norstar. This is not surprising because, although the Vrn-1 loci were found to be the major determinants of vernalization requirement and FT, 15 chromosomes or loci in total were found in genetic experiments to have an effect on FT, vernalization response, and flowering time in wheat (Pan et al., 1994; Law and Worland, 1997; Limin et al., 1997; Limin and Fowler, 2002). The existence of additional genetic pathways regulating these processes has a direct effect on the level of COR gene expression and FT development. In the NIL winter wheat cv Manitou, the lower level and duration of COR gene expression leads to a lower FT compared with winter wheat cv Norstar, whereas in the NIL spring wheat cv Norstar, COR gene expression is not completely down-regulated and leads to a higher FT than in spring wheat cv Manitou. Overall, the results give further support to the idea that the Vrn-1 locus is the major locus regulating TaVRT-1 expression, COR gene inducibility, and FT.
The function of the Vrn genes with respect to heading has not yet been clarified. Shindo and Sasakuma (2002) raised the question of how vernalization treatments promote the heading of winter wheat without the dominant Vrn genes. One of the difficulties in answering such a question is that the Vrn/vrn genes have not been isolated for analyses at the functional genomics level. In rice, a cereal species that does not require vernalization to flower, two genes associated with heading time have been identified by positional cloning of quantitative trait loci (Yano et al., 2000; Takahashi et al., 2001). The first locus identified (Hd1) is located on chromosome 6 and encodes a transcriptional regulator similar to CONSTANS from Arabidopsis (Yano et al., 2000). The second (Hd6), located on chromosome 3, encodes a homolog of Casein kinase II (Takahashi et al., 2001). Because this last locus is syntenic to the wheat Vrn-1 region (Kato et al., 1999b), fine mapping studies were undertaken and have shown that Casein kinase II is located 1.1 centiMorgan from the Vrn-A1 locus in wheat (Kato et al., 2002). Because the nature of the Vrn-A1 gene remains unknown, it would be interesting to clarify the exact genetic linkage of TaVRT-1 in relation to the Vrn-A1 locus.
An interesting finding in this study was the positive effect of LD photoperiod on TaVRT-1 expression and flower induction. Overall, these results suggest that photoperiod is a second developmental regulator of TaVRT-1 expression. The question that arises from these observations is what is the role of photoperiod in flower induction? The promotive effect of vernalization can be viewed as the first step in floral induction. It is hypothesized that, at this developmental stage, cells in the apices become competent to form the flower primordia. The second step is the activation of these cells to divide, elongate, and initiate flower primordia under inductive conditions such as LD photoperiod. Within this context, plants may require a minimum photoperiod length to keep the expression of flowering control genes (example TaVRT-1) sufficiently up-regulated and/or their transcripts stabilized to initiate flowering. Alternatively, it may be that SD photoperiod acts by repressing flowering control genes and/or by accelerating the degradation of their transcripts. These hypotheses may in part explain the higher level of TaVRT-1 expression in LD-grown barley cv Dicktoo and deacclimated wheat (Figs. 7 and 8). Although the exact nature of events regulating TaVRT-1 sensitivity to photoperiod is unknown, genetic studies in wheat have shown that the Ppd loci located on the group 2 chromosomes are largely responsible for the photoperiodic sensitivity of flowering induction (Law and Worland, 1997). These genes have not yet been isolated, but it would be interesting to study their genetic affects on the accumulation pattern of TaVRT-1 during and after the vernalization saturation point (Mahfoozi et al., 2000; Fig. 8).
The data reported in this study indicate that both vernalization (Vrn-A1/vrn-A1) and photoperiod influence the accumulation of TaVRT-1. Moreover, the expression of the gene was closely associated with phenological development and the appearance of flowering competence in cereals, suggesting that TaVRT-1 represents an early marker of this process. In wheat and barley, the accumulation of TaVRT-1 was shown to be associated with the progressive down-regulation of COR genes and decrease in FT. From these results, it appears that TaVRT-1 may act as a negative regulator of COR gene expression and FT development. Confirmation of these relationships will have to await future studies using transgenic plants. Overall, these observations suggest that separate genetic pathways converge to control the transition from the vegetative to the reproductive phases in which TaVRT-1 appears to play a central role. However, TaVRT-1 expression may be regulated by higher order developmental cues such as vernalization requirement, photoperiod sensitivity, and other factors not analyzed in this study such as the biological clock and the GA3-dependent growth pathway (Simpson et al., 1999).
MATERIALS AND METHODS
Plant Materials
Two spring wheat (Triticum aestivum L. cvs Glenlea and Manitou), four winter wheat (cvs Absolvent, Fredrick, Monopole, and Norstar), a winter rye (Secale cereale L. cv Musketeer), and a spring barley (Hordeum vulgare L. cv Winchester) were used in the initial gene expression studies. For the detailed analyses of gene expression, we used the photoperiod-sensitive spring habit barley cv Dicktoo, the non-hardy spring habit wheat cv Manitou, the very cold-hardy winter habit wheat cv Norstar, and two wheat reciprocal near-isogenic lines (NILs) that differ in vernalization requirement.
Reciprocal NILs were generated using the wheat cvs Manitou (dominant Vrn-A1 allele) and Norstar (recessive vrn-A1 allele; Limin and Fowler, 2002). In brief, the parents were crossed to produce an initial hybrid that was then backcrossed to each parent. In subsequent generations, progeny heterozygous for the Vrn-A1 locus were identified and crossed with each parent. When wheat cv Norstar was the recurrent parent, heterozygosity (Vrn-A1/vrn-A1) was based on spring habit due to the dominance of the Vrn-A1 locus. When wheat cv Manitou was the recurrent parent, heterozygous genotypes (Vrn-A1/vrn-A1) could be selected because they flowered approximately 2 weeks later than homozygous (Vrn-A1/Vrn-A1) genotypes. Selection based on phenotype ensured that the donor allele was incorporated into the genetic background of the recurrent parent. After four back-crosses, heterozygous plants were selected and selfed. Homozygous lines (vrn-A1/vrn-A1 wheat cv Manitou and Vrn-A1/Vrn-A1 wheat cv Norstar) with theoretically 96.9% of the recurrent parent DNA were recovered. This procedure produced a spring habit wheat cv Norstar with the Vrn-A1 allele of wheat cv Manitou and a winter habit wheat cv Manitou with the vrn-A1 allele of wheat cv Norstar.
Growth Conditions
The experimental design for these studies was a 4 (genotypes) × 11 (acclimation periods) factorial in a two replicate randomized complete block design. All NILs and parental material were evaluated for 11 LT periods (0, 7, 14, 21, 28, 35, 42, 49, 56, 77, and 98 d). Imbibed seeds were kept for 3 d at 5°C and were then transferred to 22°C for 2 d. The seedlings were grown for 14 d in hydroponics at 20°C with a 20-h d and light intensity of 320 μmol m–2s–1, at which stage they had developed three to four leaves. They were then transferred to 4°C with a 20-h photoperiod and a light intensity of 220 μmol m–2s–1 and were sampled at regular intervals. For FLN measurements, germinated seeds were grown at 20°C for 14 d in pots (2 plants pot–1), exposed to 4°C, and transferred weekly to 20°C chambers for floral induction conditions.
For photoperiod studies, imbibed seeds of barley cv Dicktoo and winter wheat cv Norstar were grown for 14 d at 20°C under either a LD (20-h) or a SD (8-h) photoperiod, transferred to 4°C under identical photoperiods, and then sampled at regular intervals. In addition, 56-d LT-treated wheat cv Norstar plants were de-acclimated at 20°C for 14 d under identical photoperiod conditions used for LT treatments.
Phenological Development, LT50, and Vernalization Saturation Point Determination
Two methods were used to determine the stage of phenological development: double-ridge formation (Kirby and Appleyard, 1987) and FLN measurements (Wang et al., 1995). The double-ridge stage was identified by dissecting the crown to reveal the shoot apex development. For FLN measurements, leaves were numbered and the plants were grown until the flag leaf emerged and the FLN on the main shoot could be determined. For each genotype, vernalization saturation was considered complete once the LT treatment no longer reduced its FLN. To determine the LT50 (temperature at which 50% of the plants are killed), the procedure outlined by Limin and Fowler (1988) was used. Analyses of variance were conducted to determine the level of significance of differences due to genotypes and acclimation periods and the genotype by acclimation period interaction in each experiment as described by Limin and Fowler (2002).
Cloning Strategy of TaVRT-1
A PCR approach was used to clone the wheat MADS-box gene. Poly(A+) RNA was isolated from cold-acclimated winter wheat cv Fredrick (Danyluk and Sarhan, 1990) and reverse-transcribed with a first-strand cDNA synthesis kit (Roche Diagnostics, Mannheim, Germany). PCR was performed using the TaMADS#11 (Murai et al., 1998)-specific primers 5′-gcagctgaagcggatcgagaacaaga-3′ and 5′-ggagggaaactggggtggacaaagtg-3′, Taq DNA polymerase (Amersham Biosciences, Uppsala), and 5% (v/v) dimethyl sulfoxide. The correct 900-bp fragment was amplified and subcloned in pSTblue1 plasmid (Novagen, Madison, WI). The clone was used to screen a cold-acclimated wheat cv Norstar cDNA library prepared in the pCMV.SPORT6 vector (Invitrogen, Carlsbad, CA) to identify the full-length cDNA and other MADS-box family members.
Southern and Northern Blots
Genomic DNA was extracted by the cetyl-trimethyl-ammonium bromide method (Limin et al., 1997) from several wheat stocks (cv Chinese Spring): nullisomic-tetrasomic lines (a pair of chromosomes is removed and replaced by another pair of homeologous chromosomes) and deletion lines for homeologous group 5AL and 5DL chromosomes (Endo, 1988; Endo and Gill, 1996) generated using the gametocidal chromosome of Aegilops cylindrical. DNA (1.8 μg) was digested with BamHI and was separated using a FIGE mapper (Bio-Rad Laboratories, Hercules, CA). For northern-blot analyses, total RNA was extracted from wheat leaves as described (Frenette-Charron et al., 2002), and equal amounts (5 μg) were electrophoresed on formaldehyde gels. Transfers and hybridizations were performed as previously described (Houde et al., 1992). For hybridizations, a specific TaVRT-1 probe designed outside of the MADS-box domain was PCR-amplified using the following primers: 5′-aaggatccgttctccaccgagtcatgtat-3′ and 5′-gtgaattcccttcagccgttgatgtggct-3′. All filters were washed at high stringency and exposed to x-ray films (BioMax-MS, Eastman Kodak, Rochester, NY).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material.
Acknowledgments
The excellent technical assistance of Garcia Schellhorn (Crop Development Centre, University of Saskatchewan) in conducting the FT evaluations and phenological development studies and of France Allard (Université du Québec à Montréal) for the RNA extraction of the wheat samples is greatly appreciated.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.023523.
Note Added in Proof
After acceptance of this report, TaVRT-1 was mapped to a 0.03-centimorgan interval that was completely linked to the VRN1 locus in Triticum monococcum [Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRNI. PNAS 100: 6263–6268]. In this study, three independent deletions were found in its promoter region in spring genotypes, suggesting that it may be the VRN1 gene.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (research grants to F.S. and D.B.F.), by Genome Canada, by Génome Québec, and by Genome Prairie.
References
- Blázquez MA (2000) Flower development pathways. J Cell Sci 113: 3547–3548 [DOI] [PubMed] [Google Scholar]
- Blom N, Gammeltoft S, Brunak S (1999) Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294: 1351–1362 [DOI] [PubMed] [Google Scholar]
- Brule-Babel AL, Fowler DB (1988) Genetic control of cold hardiness and vernalization requirement in winter wheat. Crop Sci 28: 879–884 [Google Scholar]
- Danyluk J, Sarhan F (1990) Differential mRNA transcription during the induction of freezing tolerance in spring and winter wheat. Plant Cell Physiol 31: 609–619 [Google Scholar]
- Dubcovsky J, Lijavetzky D, Appendino L, Tranquilli G (1998) Comparative RFLP mapping of Triticum monococcum genes controlling vernalization requirement. Theor Appl Genet 97: 968–975 [Google Scholar]
- Endo TR (1988) Induction of chromosomal structural changes by a chromosome of Aegilops cylindrica L in common wheat. J Hered 79: 366–370 [DOI] [PubMed] [Google Scholar]
- Endo TR, Gill BS (1996) The deletion stocks of common wheat. J Hered 87: 295–307 [Google Scholar]
- Ferràndiz C, Gu Q, Martienssen R, Yanofsky MF (2000) Redundant regulation of meristem identity and plant architecture by FRUITFULL APETALA1 and CAULIFLOWER. Development 127: 725–734 [DOI] [PubMed] [Google Scholar]
- Fowler DB, Breton G, Limin AE, Mahfoozi S, Sarhan F (2001) Photoperiod and temperature interactions regulate low-temperature-induced gene expression in barley. Plant Physiol 127: 1676–1681 [PMC free article] [PubMed] [Google Scholar]
- Fowler DB, Chauvin LP, Limin AE, Sarhan F (1996a) The regulatory role of vernalization in the expression of low-temperature-induced genes in wheat and rye. Theor Appl Genet 93: 554–559 [DOI] [PubMed] [Google Scholar]
- Fowler DB, Limin AE, Ritchie JT (1999) Low-temperature tolerance in cereals: model and genetic interpretation. Crop Sci 39: 626–633 [Google Scholar]
- Fowler DB, Limin AE, Wang S-Y, Ward RW (1996b) Relationship between low-temperature tolerance and vernalization response in wheat and rye. Can J Plant Sci 76: 37–42 [Google Scholar]
- Frenette-Charron JB, Breton G, Badawi M, Sarhan F (2002) Molecular and structural analyses of a novel temperature stress-induced lipocalin from wheat and Arabidopsis. FEBS Lett 517: 129–132 [DOI] [PubMed] [Google Scholar]
- Galiba G, Quarrie SA, Sutka J, Morgounov A (1995) RFLP mapping of the vernalization (Vrn1) and frost resistance (Fr1) genes on chromosome 5A of wheat. Theor Appl Genet 90: 1174–1179 [DOI] [PubMed] [Google Scholar]
- Gocal GFW, King RW, Blundell CA, Schwartz OM, Andersen CH, Weigel D (2001) Evolution of floral meristem identity genes analysis of Lolium temulentum genes related to APETALA1 and LEAFY of Arabidopsis. Plant Physiol 125: 1788–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth SR, Valverde F, Ravenscroft D, Mouradov A, Coupland G (2002) Antagonist regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J 21: 4327–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde M, Danyluk J, Sarhan F (1992) A molecular marker to select for freezing tolerance in gramineae. Mol Gen Genet 234: 43–48 [DOI] [PubMed] [Google Scholar]
- Immink RG, Hannapel DJ, Ferrario S, Busscher M, Franken J, Lookeren Campagne MM, Angenent GC (1999) A petunia MADS-box gene involved in the transition from vegetative to reproductive development. Development 126: 5117–5126 [DOI] [PubMed] [Google Scholar]
- Jensen CS, Salchert K, Nielsen KK (2001) A TERMINAL FLOWER1-like gene from perennial ryegrass involved in floral transition and axillary meristem identity. Plant Physiol 125: 1517–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K (1988) Method for evaluation of chilling requirement and narrow-sense earliness of wheat cultivars. Jpn J Breed 38: 172–186 [Google Scholar]
- Kato K, Kidou S, Miura H, Sawada S (2002) Molecular cloning of the wheat CK2α gene and detection of its linkage with Vrn-A1 on chromosome 5A. Theor Appl Genet 104: 1071–1077 [DOI] [PubMed] [Google Scholar]
- Kato K, Miura H, Sawada S (1999a) QTL mapping of genes controlling ear emergence time and plant height on chromosome 5A of wheat. Theor Appl Genet 98: 472–477 [Google Scholar]
- Kato K, Miura H, Sawada S (1999b) Comparative mapping of the wheat Vrn-A1 region and the rice Hd-6 region. Genome 42: 204–209 [Google Scholar]
- Kempin SA, Savidge B, Yanofsky MF (1995) Molecular basis of the cauliflower phenotype in Arabidopsis. Science 267: 522–525 [DOI] [PubMed] [Google Scholar]
- Kirby EJM, Appleyard M (1987) Cereal development guide. In Russell GE, ed, Stoneleigh Kenilworth, Ed 2. Arable Unit National Agricultural Centre, Warwickshire, UK
- Korzun V, Roder M, Worland AJ, Borner A (1997) Interchromosomal mapping of genes for dwarfing (Rht 12) and vernalization response (Vrn1) in wheat by using RFLP and microsatellite markers. Plant Breed 116: 227–232 [Google Scholar]
- Kyozuka J, Kobayashi T, Morita M, Shimamoto K (2000) Spatially and temporally regulated expression of rice MADS-box genes with similarity to Arabidopsis class A, B and C genes. Plant Cell Physiol 41: 710–718 [DOI] [PubMed] [Google Scholar]
- Law CN, Worland AJ (1997) Genetic analysis of some flowering time and adaptive traits in wheat. New Phytol 137: 19–28 [Google Scholar]
- Law CN, Worland AJ, Giorgi B (1976) The genetic control of ear-emergence time by chromosomes 5A and 5D of wheat. Heredity 36: 49–58 [Google Scholar]
- Limin AE, Danyluk J, Chauvin LP, Fowler DB, Sarhan F (1997) Chromosome mapping of low-temperature induced WCS120 family genes and regulation of cold-tolerance expression in wheat. Mol Gen Genet 253: 720–727 [DOI] [PubMed] [Google Scholar]
- Limin AE, Fowler DB (1988) Cold hardiness expression in interspecific hybrids and amphiploids of Triticeae. Genome 30: 361–365 [Google Scholar]
- Limin AE, Fowler DB (2002) Developmental traits affecting low-temperature tolerance response in near-isogenic lines for the vernalization locus Vrn-A1 in wheat (Triticum aestivum L em Thell). Ann Bot 89: 579–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfoozi S, Limin AE, Fowler DB (2001a) Influence of vernalization and photoperiod response on the expression of cold hardiness in winter cereals. Crop Sci 41: 1006–1011 [Google Scholar]
- Mahfoozi S, Limin AE, Fowler DB (2001b) Developmental regulation of low-temperature tolerance in winter wheat. Ann Bot 87: 751–757 [Google Scholar]
- Mahfoozi S, Limin AE, Hayes PM, Hucl P, Fowler DB (1998) Developmental control of low temperature tolerance in cereals. In Slinkard AE, ed, Proceedings of the Ninth International Wheat Genetic Symposium, Vol 4. University Extension Press, University of Saskatchewan, Saskatoon, Canada, pp 54–56 [Google Scholar]
- Mahfoozi S, Limin AE, Hayes PM, Hucl P, Fowler DB (2000) Influence of photoperiod response on the expression of cold hardiness in wheat and barley. Can J Plant Sci 80: 721–724 [Google Scholar]
- Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF (1992) Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360: 273–277 [DOI] [PubMed] [Google Scholar]
- Mandel MA, Yanofsky MF (1995) The Arabidopsis AGL8 MADS-box gene is expressed in inflorescence meristems and is negatively regulated by APETALA1. Plant Cell 7: 1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maystrenko OI (1980) Cytogenetic study of the growth habit and ear-emergence time in wheat (Triticum aestivum L). In Well-Being in Mankind and Genetics. Proceedings of the Fourteenth International Congress of Genetics, Moscow, Vol 1. MIR Publishers, Moscow, Russia, pp 267–282 [Google Scholar]
- Maystrenko OI (1987) Discovery of allelism in the Vrn2 locus of common wheat its development type and its chromosome reports. Third All-Union Conference, Kishmev, Shtintsa, Moscow, Russia, pp 148–149
- McIntosh RA, Hart GE, Gale MD (1998) Catalogue of gene symbols for wheat. In ZS Li, ZY Xin, eds, Proceedings of the Eighth International Wheat Genetic Symposium on Agricultural. Scientech Press, Beijing, pp 1333–1500
- McMaster GS (1997) Phenology development and growth of the wheat (Triticum aestivum L) shoot apex: a review. Adv Agron 59: 63–118 [Google Scholar]
- Monn YH, Kang HG, Jung JH, Jeon JS, Sung SK, An G (1999) Determination of the motif responsible for interaction between the rice APETALA/AGAMOUS-LIKE9 family proteins using a yeast two-hybrid system. Plant Physiol 120: 1193–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller BM, Saedler H, Zachgo S (2001) The MADS-box gene DEF28 from Anthirrhinum is involved in the regulation of floral meristem identity and fruit development. Plant J 28: 169–179 [DOI] [PubMed] [Google Scholar]
- Murai K, Murai R, Takumi S, Ogihara Y (1998) cDNA cloning of three MADS-box genes in wheat (accession nos. AB007504, AB007505, and AB007506) (PGR98–159). Plant Physiol 118: 330 [Google Scholar]
- Nakai K, Kanehisa M (1992) A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics 14: 897–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JC, Sorrells ME, Van Deyne AE, Lu YH, Atkinson M, Bernard M, Leroy P, Faris JD, Anderson JA (1995) Molecular mapping of wheat: major genes and rearrangements in homoeologous groups 4, 5 and 7. Genetics 141: 721–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Hayes PM, Chen F, Chen THH, Blake T, Wright S, Karsai I, Bedo Z (1994) Genetic analysis of the components of winterhardiness in barley (Hordeum vulgare L). Theor Appl Genet 89: 900–910 [DOI] [PubMed] [Google Scholar]
- Plaschke J, Borner A, Xie DX, Koebner RMD, Schlegel R, Gale MD (1993) RFLP mapping of genes affecting plant height and growth habit in rye. Theor Appl Genet 85: 1049–1054 [DOI] [PubMed] [Google Scholar]
- Robbins J, Caulfield MP, Higashida H, Brown DA (1991) Genotypic m3-muscarinic receptors preferentially inhibit M-currents in DNA-transfected NG108-15 neuroblastoma × glioma hybrid cells. Eur J Neurosci 3: 820–824 [DOI] [PubMed] [Google Scholar]
- Sarma RN, Gill BS, Sasaki T, Galiba G, Sutka J, Laurie DA, Snape JW (1998) Comparative mapping of the wheat chromosome 5A Vrn-A1 region with rice and its relationship to QTL for flowering time. Theor Appl Genet 97: 103–109 [Google Scholar]
- Schmitz J, Franzen R, Ngyuen TH, Garcia-Maroto F, Pozzi C, Salamini F, Rohde W (2000) Cloning mapping and expression analysis of barley MADS-box genes. Plant Mol Biol 42: 899–913 [DOI] [PubMed] [Google Scholar]
- Shindo C, Sasakuma T (2002) Genes responding to vernalization in hexaploid wheat. Theor Appl Genet 104: 1003–1010 [DOI] [PubMed] [Google Scholar]
- Simpson GG, Gendall AR, Dean C (1999) When to switch to flowering. Annu Rev Cell Dev Biol 99: 519–550 [DOI] [PubMed] [Google Scholar]
- Snape JW, Semikhodskii A, Fish L, Sarma RN, Quarrie SA, Galiba G, Sutka J (1997) Mapping frost tolerance loci in wheat and comparative mapping with other cereals. Acta Agron Hung 45: 265–270. [Google Scholar]
- Takahashi Y, Shomura A, Sasaki T, Yano M (2001) Hd6 a rice quantitative trait locus involved in photoperiod sensitivity encodes the alpha subunit of protein kinase CK2. Proc Natl Acad Sci USA 98: 7922–7927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-Y, Ward RW, Ritchie JT, Schultless U (1995) Vernalization in wheat I A model based on the interchangeability of plant age and vernalization duration. Field Crops Res 41: 91–100 [Google Scholar]
- Yalovsky S, Rodriguez-Concepcion M, Bracha K, Toledo-Ortiz G, Gruissem W (2000) Prenylation of the floral transcription factor APETALA1 modulates its function. Plant Cell 12: 1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J (2003) Positional cloning of wheat vernalization gene VRN1. Proc Natl Acad Sci U S A 100: 6263–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y et al. (2000) Hd1 a major photoperiod sensitivity quantitative trait locus in rice is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12: 2299–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]