Abstract
Sulfhydryl-endopeptidase (SH-EP) is a papain-type vacuolar proteinase expressed in cotyledons of germinated Vigna mungo seeds, and the enzyme possesses a C-terminal propeptide containing KDEL tail, an endoplasmic reticulum retention signal for soluble proteins. SH-EP is transported to vacuoles via a KDEL vesicle (KV) through a Golgi complex-independent route. To see the function of the KDEL sequence of SH-EP, wild-type SH-EP and its KDEL deletion mutant (SH-EPΔKDEL) were heterologously expressed in Arabidopsis and in cultured tobacco Bright Yellow 2 cells, and their intracellular transport pathways and localizations were analyzed. A combination of the results from analyses for transformed Arabidopsis and tobacco (Nicotiana tabacum) cells indicated that wild-type SH-EP is packed into KV-like vesicles through the KDEL sequence and is transported to vacuoles in the cells of transformants. In contrast, KV was not formed/induced in the cells expressing SH-EPΔKDEL, and the mutant protein was mainly secreted. Therefore, the C-terminal KDEL sequence of the KDEL-tailed cysteine proteinase is thought to be involved in the formation of KV, and in the efficient vacuolar transport of the proteins through KV.
Eukaryotic cells are divided into distinct subcellular compartments or organelles enclosed by one or more membranes. Because protein synthesis occurs mainly in the cytosol, proteins of subcellular compartments have intracellular localization signals that determine their final destinations. A transient signal peptide allows cotranslational entry into the lumen of the endoplasmic reticulum (ER). The ER is the starting compartment for vesicular traffic along the secretory pathway to the Golgi complex, vacuoles, and the cell surface. Because secretion following a route mediated by the Golgi complex is a default destination for proteins introduced into the ER, proteins localizing in the ER, the Golgi complex, or vacuoles must have additional signals. Most soluble ER residents have a permanent C-terminal KDEL or HDEL tetrapeptide sequence, which constitutes an ER retention signal. (Munro and Pelham, 1987; Pelham, 1989). The tetrapeptide is recognized by the ERD2-KDEL receptor on the Golgi complex, resulting in retrieval of H/KDEL proteins from this compartment back into the ER. The H/KDEL system is conserved through mammals, plants, and yeasts (Denecke et al., 1992; Napier et al., 1992; Lee et al., 1993). Beside ER residents found in other eukaryotes, higher plants also have unique papain-type proteinases that possess KDEL tails at the C terminus (Akasofu et al., 1989; Tanaka et al., 1993; Valpuesta et al., 1995; Becker et al., 1997; Lee et al., 1997; Guerrero et al., 1998; Schmid et al., 1998; Cercos et al., 1999). One protein of this family, Vigna mungo KDEL proteinase, designated Sulfhydryl-endopeptidase (SH-EP), has been shown to localize in vacuoles (Okamoto et al., 1994; Toyooka et al., 2000); possibly, other members also have this location. The existence of such KDEL-tailed vacuolar proteinase suggests that plant cells use the C-terminal KDEL sequence for an unidentified vacuolar sorting system in addition to the ER retention of proteins.
In germinating cotyledons of V. mungo seedlings, a KDEL-tailed Cys proteinase, termed SH-EP, is expressed de novo and is involved in degradation of storage proteins accumulated in protein storage vacuoles (Mitsuhashi et al., 1986; Okamoto and Minamikawa, 1998). SH-EP is synthesized in the ER as a proform of 43 kD through cleavage of the signal sequence, and proSH-EP is further processed to the enzymatically active 33-kD mature enzyme via 39- and 36-kD intermediates (Mitsuhashi and Minamikawa, 1989); possibly in vacuoles (Okamoto et al., 1999b, 2001). As for the C-terminal KDEL sequence of SH-EP, analysis of heterologous expression of SH-EP and its KDEL-deleted mutant in insect Sf-9 cells showed that the KDEL-tail of SH-EP promotes the storage of SH-EP in the ER as a transient zymogen (Okamoto et al., 1999a). Recently, immunocytochemical analysis of cotyledon cells of V. mungo seedlings using anti-SH-EP antibody revealed that proSH-EP was accumulated at the edge or middle region of the ER, and that the accumulated proSH-EP molecules were packed in specific vesicles with a diameter of 200 to 500 nm, termed the KDEL vesicle (KV). KVs bud off from the ER and are fused with vacuoles through a Golgi complex-independent pathway (Toyooka et al., 2000).
It has been reported that the addition of KDEL to the C terminus of vacuolar proteins, such as storage proteins, changes the intracellular localization of these fusion proteins to the ER (Herman et al., 1990; Wandelt et al., 1992; Herman and Larkins, 1999). This is consistent with the well-known ER retrieval mechanism of KDEL-tailed proteins using the ERD2-receptor system. In contrast, KDEL-tailed Cys proteinases such as SH-EP are not artificially KDEL-fused proteins, but are instead naturally used as vacuolar hydrolases by plant cells in vivo. Therefore, SH-EP should be a good candidate for studies aimed at additional and/or different role(s) for the KDEL-tail in plant cells, apart from its role in the ER retention system. In the present study, wild-type SH-EP and its KDEL deletion mutant (SH-EPΔKDEL) were heterologously expressed in Arabidopsis and in cultured tobacco (Nicotiana tabacum) Bright Yellow (BY)-2 cells, and the effects of the deletion of the KDEL-tail from the SH-EP polypeptide on intracellular localization of the enzyme were monitored by immunogold electron microscopic observation for transgenic Arabidopsis plants, and by biochemical analyses and subcellular fractionation of the tobacco cells. In addition, green fluorescent protein (GFP)-fused SH-EP or SH-EPΔKDEL was expressed in tobacco BY-2 cells to observe localization of the fusion proteins in the cells. Functions of the KDEL-tail of SH-EP in the formation of KV and its subsequent intracellular localization are discussed.
RESULTS
Formation of KV in Transformed Arabidopsis Expressing Wild-Type SH-EP
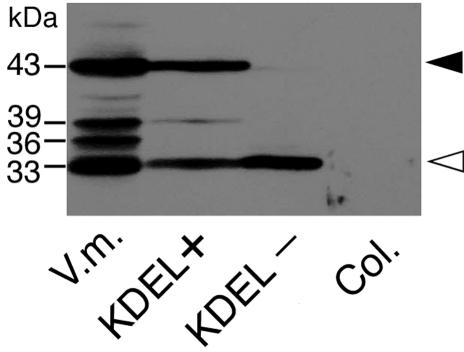
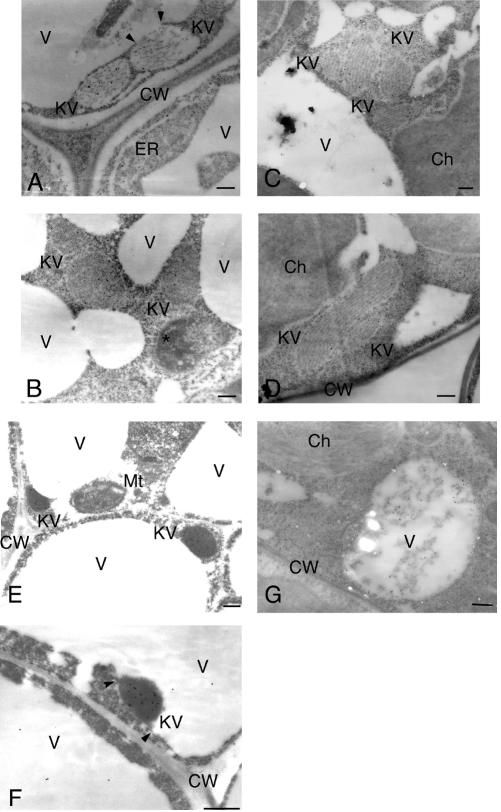
ProSH-EP of 43 kD, SH-EP intermediates of 39- and 36-kD, and mature SH-EP of 33 kD were detected in the extracts from cotyledons of germinated V. mungo seeds (Fig. 1; Mitsuhashi and Minamikawa, 1989). When crude extracts were prepared from rosette leaves of transgenic Arabidopsis expressing wild-type SH-EP and were analyzed by SDS/PAGE-immunoblotting with anti-SH-EP antibody, two major polypeptides of proform and mature SH-EPs were detected (Fig. 1). Detection of the intense signal from proSH-EP in the extracts suggests that wild-type SH-EP accumulated in the ER and packed into KV-like vesicles in cells of the plants, as in cotyledon cells of V. mungo seedlings (Toyooka et al., 2000). Immunocytochemical analyses using anti-SH-EP antibodies were conducted for cells of rosette leaves, stems, sepals, and cotyledons of the plants in which SH-EP accumulated in the vesicles with diameters between 200 and 700 nm (Fig. 2, A–C, and E). These vesicles possibly correspond to KV-like vesicles because the size of the vesicles and the accumulation of KDEL-proteinase are characteristics identical to those of KVs in cotyledons of V. mungo seedlings. In cells from rosette leaves, some KV-like vesicles enlarged and were oblong shaped (Fig. 2D). KV-like vesicles in transgenic plants appeared to fuse with vacuoles (Fig. 2A), and the localization of SH-EP in vacuoles was also observed (Fig. 2, F and G). These observations suggest that SH-EP was transported to vacuoles via a KV-dependent pathway. However, it cannot be ruled out that part of SH-EP remains in KV-like vesicles, and part follows an independent Golgi-mediated route to vacuoles.
Figure 1.
Distributions of SH-EP-related polypeptides in transformed Arabidopsis expressing wild-type SH-EP or SH-EPΔKDEL. Crude extracts (20 μg of protein) were prepared from rosette leaves of the transformants and were analyzed by SDS/PAGE-immunoblotting with anti-SH-EP antibody. Solid and open arrowheads indicate the proform and mature form of SH-EP, respectively. V.m., Crude extract from cotyledons of 3-d dark-grown V. mungo seedlings; KDEL+, Arabidopsis expressing wild-type SH-EP; KDEL–, Arabidopsis expressing SH-EPΔKDEL; Col, nontransformed Arabidopsis.
Figure 2.
Electron micrographs showing development of KV-like vesicles in cells of several tissues from transgenic Arabidopsis expressing wild-type SH-EP. A, SH-EP accumulated in ER and KV-like vesicles in stem cells. A KV-like vesicle fused with a vacuole (arrowheads). B, SH-EP was accumulated in KV-like vesicles in cotyledon cells. C, SH-EP was accumulated in KV-like vesicles in rosette leaf cells. D, Enlarged and oblong-shaped KV-like vesicles were observed in rosette leaf cells. E, SH-EP accumulated in KV-like vesicles in sepal cells. F, A KV-like vesicle was fused with vacuoles in sepal cells. Arrowheads indicate possible fusion sites. G, SH-EP was also localized in vacuoles of cells of rosette leaves. Ch, Chloroplast; CW, cell wall; KV, KV-like vesicle; Mt, Mitochondrion; V, vacuole; An asterisk indicates an unidentified cell compartment. Bars = 200 nm.
Secretion of KDEL-Deleted SH-EP in Transgenic Arabidopsis
KDEL tail was deleted from wild-type SH-EP, and the mutant protein (SH-EPΔKDEL) was expressed in Arabidopsis to investigate the effects of removal of KDEL on localization of SH-EP. In SDS/PAGE-immunoblotting of crude extracts from the rosette leaves of transformed Arabidopsis expressing the mutant SH-EP, only 33-kD SH-EP was detected (Fig. 1). The failure to detect 43-kD proSH-EP in the extracts would have been due to the deletion of the KDEL tail from SH-EP, suggesting the loss of accumulation of proSH-EP in ER and/or KV-like vesicles in cells of the transformants. The localization of mutant SH-EP was observed with immunogold electron microscopy. Gold particles from the anti-SH-EP antibodies existed at the extracellular spaces and at possible air spaces (Fig. 3, A–C). This suggests that SH-EPΔKDEL was mainly secreted from the cells.
Figure 3.
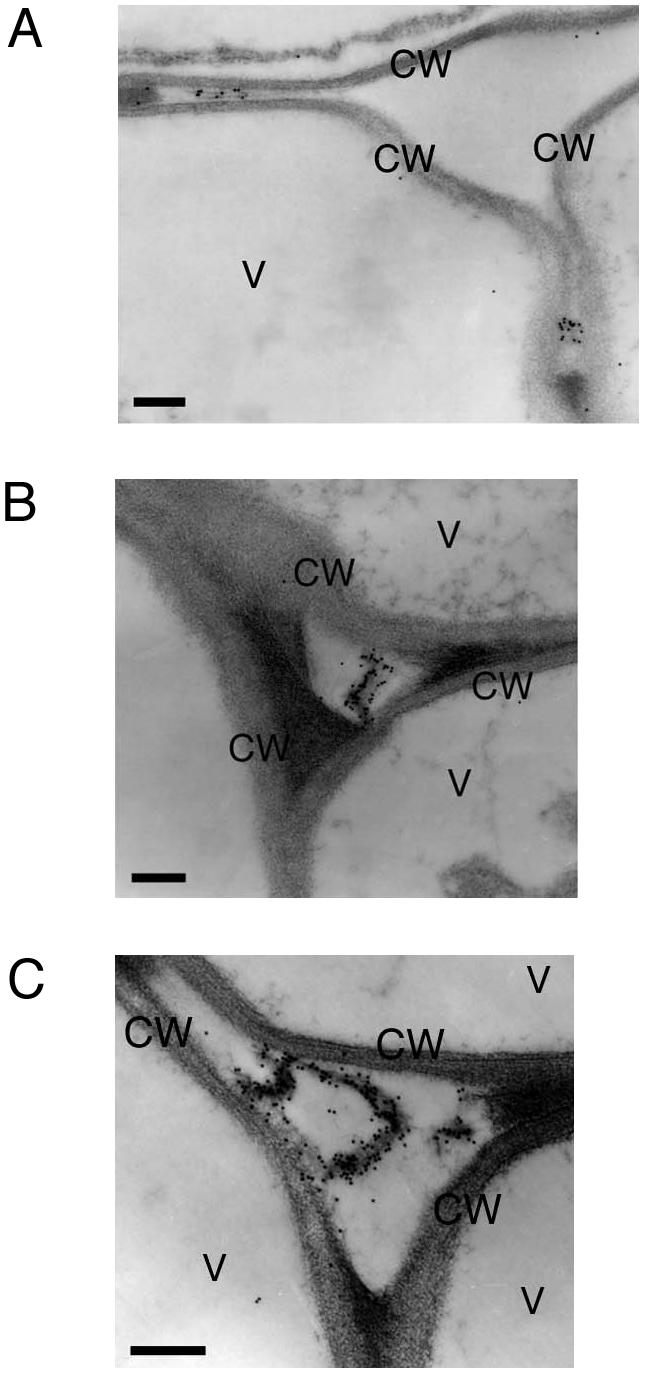
Electron photographs showing secretion of SH-EPΔKDEL from the cells of rosette leaves of transgenic Arabidopsis (A–C). The gold particles from anti-SH-EP antibody were found at extracellular spaces, and possible air spaces. CW, Cell wall; V, vacuole. Bars = 200 nm.
Growth Defect of Transgenic Arabidopsis Expressing SH-EPΔKDEL
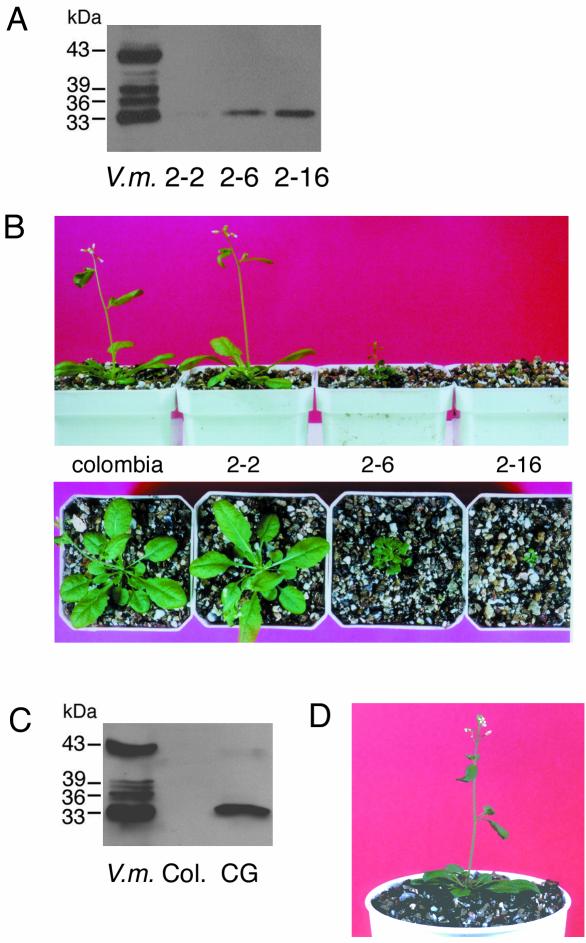
SH-EP belongs to the papain family and has strong proteolytic activity with broad substrate specificities (Okamoto and Minamikawa, 1998). The transformant expressing wild-type SH-EP (Fig. 4A) showed no phenotype and grew normally (Fig. 4B). In contrast, secretion of SH-EPΔKDEL into the extracellular space damaged the growth of transformants. Plants expressing mutant SH-EP at a low level grew normally (Fig. 5, A and B). However, plants with high expressions of the protein died after developing several small rosette leaves. In the case of plants expressing SH-EPΔKDEL at a mid-level, the size of the plant became small. This suggests that secreted SH-EP degrades the cell wall and/or apoplastic proteins, and that the difference of phenotype depends on the expression level of SH-EPΔKDEL. To verify this possibility, the active site Cys of mutant SH-EP was replaced with Gly, and SH-EPΔKDEL(C152G) was expressed in Arabidopsis. The transgenic plants developed normally even if SH-EPΔKDEL(C152G) was expressed at a high level (Fig. 5, C and D). This indicates that the proteolytic activity of secreted SH-EP affects the growth of transformants. Protein components, existing in extracellular spaces, which are essential for cell-cell interaction and/or communication, may be degraded by the secreted SH-EP.
Figure 4.
Transgenic Arabidopsis expressing wild-type SH-EP. A, Crude extracts (20 μg of protein) were prepared from rosette leaves of transformants and nontransformants and were analyzed by SDS/PAGE-immunoblotting with anti-SH-EP antibody. B, The transformants expressing wild-type SH-EP grew normally even when the mutant protein was expressed at a high level as in A. Columbia and SH-EP in B indicates nontransformant and transformant plants, respectively. Col, Nontransformed Arabidopsis; SH, Arabidopsis expressing wild-type SH-EP; V.m., crude extract from cotyledons of 3-d dark-grown V. mungo seedlings.
Figure 5.
Growth defect of transgenic Arabidopsis expressing SH-EPΔKDEL (A and B), and expression of active site-mutated SH-EPΔKDEL (SH-EPΔKDEL, C152G) in Arabidopsis (C and D). A, Crude extracts (20 μg of protein) were prepared from three independent transgenic lines and were analyzed by SDS/PAGE-immunoblotting with anti-SH-EP antibody. 2-2, 2-6, and 2-16 are independent transgenic lines. B, Phenotypes of the same plants used in A. Severe phenotype appeared according to increase of expression level of SH-EPΔKDEL as shown in A. Columbia, nontransformant. C, Crude extracts (20 μg of protein) were prepared from the transgenic Arabidopsis expressing SH-EPΔKDEL(C152G) and were analyzed by SDS/PAGE-immunoblotting with anti-SH-EP antibody. D, The transformants expressing SH-EPΔKDEL(C152G) grow normally even when the mutant protein is expressed at a high level as in A. CG, Arabidopsis expressing SH-EPΔKDEL(C152G); Col, nontransformed Arabidopsis; V.m., crude extract from cotyledons of 3-d dark-grown V. mungo seedlings.
Vacuolar Transport and Posttranslational Processing of Wild-Type SH-EP in Tobacco BY-2 Cells
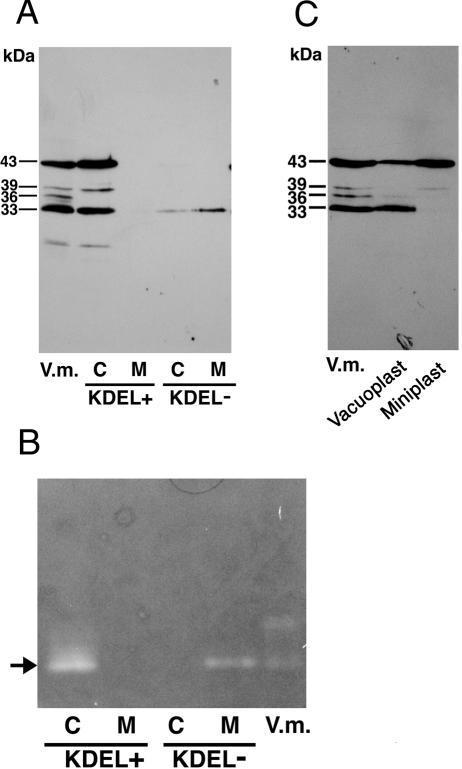
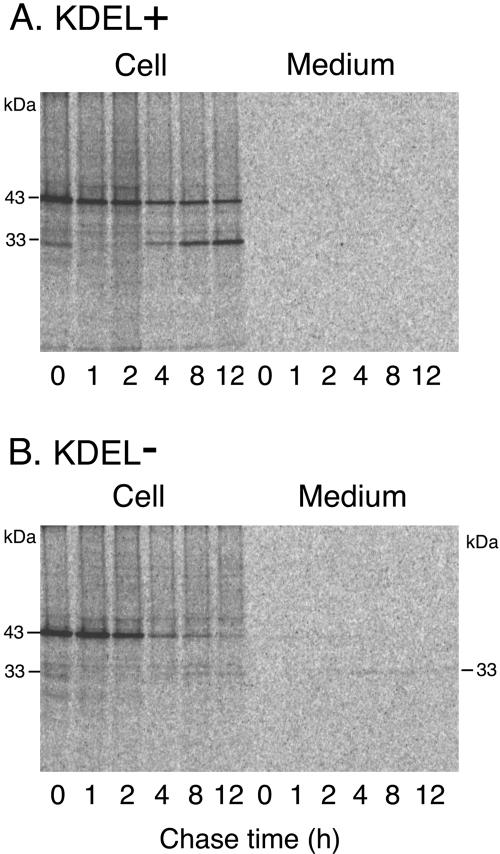
When the cell and medium fractions from transformed tobacco cell cultures expressing wild-type SH-EP were analyzed by SDS/PAGE-immunoblotting with anti-SH-EP antibody, 43-, 39-, and 33-kD SH-EPs were detected in the cell fraction, but no SH-EP-related polypeptide was observed in the medium fraction (Fig. 6A, KDEL+ lanes). No polypeptide immunoreactive with anti-SH-EP antibody was detected in cells or medium fractions prepared from nontransformed tobacco BY2 cell cultures (data not shown). The pattern of the molecular masses of SH-EP-related polypeptides in the transformed tobacco cells was almost the same as that in extracts from cotyledons. The proteinase activity of SH-EP in the cell culture expressing wild-type SH-EP was visualized using a gel-based gelatin plate method (Fig. 6B, KDEL+ lanes). These results suggest that the correct folding and processing of wild-type SH-EP, essential for enzymatic activation, occurred in the tobacco cells.
Figure 6.
Distributions of SH-EP-related polypeptides (A) and proteinase activities (B) in transformed tobacco cell cultures expressing wild-type SH-EP (KDEL+) or SH-EPΔKDEL (KDEL–), and subcellular localization of wild-type SH-EP in tobacco cells (C). A, Cell and medium fractions from tobacco cell cultures expressing wild-type SH-EP or SH-EPΔKDEL were prepared as described in “Materials and Methods” and were analyzed by SDS/PAGE-immunoblotting with anti-SH-EP antibody. B, Cell and medium fractions from tobacco cell cultures expressing wild-type SH-EP or SH-EPΔKDEL were prepared as described in “Materials and Methods” except for extraction buffer (50 mm sodium acetate, pH 5.4, and 10 mm 2-mercaptoethanol). Both fractions were separated by 12.5% (w/v) nondenaturing PAGE, and proteinase activities in the gel were visualized with a gel-based gelatin plate method (Mitsuhashi and Minamikawa 1989). Arrow indicates proteinase activity derived from SH-EP (Mitsuhashi and Minamikawa 1989). C, Vacuoplasts and miniplasts were prepared from the transformed tobacco cells expressing wild-type SH-EP as described in “Materials and Methods.” Both fractions were analyzed by SDS/PAGE-immunoblotting with anti-SH-EP antibody. V.m., Crude extracts from cotyledons of 3-d dark-grown V. mungo seedlings; C, cell fraction; M, medium fraction.
To observe the subcellular localization of 43- or 33-kD SH-EP in tobacco cells, cells expressing wild-type SH-EP were separated into vacuoplasts and miniplasts. A vacuoplast is mostly composed of a vacuole that is surrounded with a plasma membrane, and a miniplast is composed of intracellular compartments other than vacuoles (Sonobe, 1990). That is, vacuoles and the other organelle, including ER and KV-like vesicles, are separated into vacuoplast and miniplast fractions, respectively. Both fractions were analyzed by SDS/PAGE-immunoblotting with anti-SH-EP antibody (Fig. 6C). In the vacuoplast fraction, 33-kD mature SH-EP and 43-kD proSH-EP were observed, but only 43-kD SH-EP was detected in the miniplast fraction. The band of 33-kD SH-EP was detected only in the vacuoplast fraction, indicating that 33-kD SH-EP exists in vacuoles and that conversion of proSH-EP to the mature form accompanies the transport of proSH-EP to the vacuoles. In addition, 43-kD SH-EP was enriched in the miniplast fraction, possibly suggesting that wild-type SH-EP was transiently accumulated in the ER and/or KV-like vesicles, as has been shown in cotyledon cells (Toyooka et al., 2000) and insect Sf-9 cells expressing wild-type SH-EP (Okamoto et al., 1999a). These suggest that the intracellular transport and posttranslational processing of wild-type SH-EP progressed in the tobacco cells in a manner similar to that in the cotyledon cells.
In vivo labeling of the transformed cells expressing wild-type SH-EP with 35S-labeled amino acids and the subsequent immunoprecipitation with anti-SH-EP antibody was carried out to monitor the traffic of SH-EP in tobacco cells. Cell and medium fractions were prepared from the in vivo-labeled cells at the indicated chase times to follow the changes with time in the molecular masses of the SH-EP-related polypeptides. The 43-kD SH-EP band was detected in cells at the end of the pulse (Fig. 7A). The band around 30 kD detected at 0-h chase is a nonspecific signal. After a 4-h chase, the 33-kD SH-EP band appeared, and its intensity gradually increased during a 12-h chase. As shown by the subcellular fractionation of the cells and the subsequent analysis with SDS/PAGE-immunoblotting (Fig. 6C), in vivo conversion of 43-kD SH-EP into 33-kD SH-EP (Fig. 7A) is due to the transport of the SH-EP to the vacuoles. Detection of the 33-kD SH-EP after a 4-h chase suggests that vacuolar sorting of wild-type SH-EP from the ER takes at least 4 h. In the medium fraction of the cell culture, no radioactive SH-EP-related polypeptide was observed (Fig. 7A). This is consistent with the finding that there was no polypeptide immunoreactive with anti-SH-EP antibody in the medium fraction of the tobacco cell cultures (Fig. 6A, KDEL+ lanes).
Figure 7.
Pulse-chase analysis of SH-EP in transformed tobacco cells expressing wild-type SH-EP (A) or SH-EPΔKDEL (B). Transformed cell cultures were pulse-labeled with 35S-amino acids for 15 min and were chased with unlabeled Met and Cys for the indicated periods of time. SH-EP-related polypeptides in the cell or medium fraction from the cell cultures were immunoprecipitated with anti-SH-EP antibody. The immunoprecipitated samples were separated by SDS/PAGE and were detected by an imaging analyzer (BAS 2000; Fuji Film, Tokyo).
Secretion and Vacuolar Transport of SH-EPΔKDEL in Tobacco BY-2 Cells
When the cell and medium fractions from the transformed tobacco cell cultures expressing SH-EPΔKDEL were analyzed by SDS/PAGE-immunoblotting with anti-SH-EP antibody, the 33-kD SH-EP band was detected in the medium fraction, along with another weak signal in the cell fraction (Fig. 6A, KDEL–lanes). These results indicate that deletion of the KDEL tail from SH-EP resulted in secretion of the enzyme and in a marked decrease of the efficiency of the vacuolar transport of SH-EP. Next, pulse-chase experiments of the transformed tobacco cell cultures were conducted. In the cell fraction, most of the 43-kD SH-EP detected at 0-h chase disappeared during the 12-h chase, and a weak signal from mature SH-EP was detected after 8-h of chase (Fig. 7B). In the medium fraction, the 43-kD SH-EP band, with a weak signal, was detected after a 1-h chase, and thereafter, secreted proSH-EP was converted to mature 33-kD SH-EP (Fig. 7B). This indicates that SH-EPΔKDEL was secreted as proenzyme, and that the proSH-EPΔKDEL was processed to a mature form in the medium. Because our earlier study of in vitro processing of the recombinant proSH-EP revealed that the proenzyme has the potential to be activated in an autocatalytic fashion at an acidic pH (pH 5.4–5.8; Okamoto et al., 1999b), which corresponds to the pH of the culture medium of tobacco cells, proSH-EP in the medium presumably was autocatalytically activated. However, it cannot be ruled out that maturation of SH-EPΔKDEL occurs en route to secretion rather than after secretion, because mature protein was detected in cell fractions (Fig. 7B). The mature SH-EP in the medium fraction from the cell culture expressing SH-EPΔKDEL showed proteinase activity (Fig. 6B, KDEL–lanes), indicating that the KDEL tail of SH-EP is not needed for the correct folding of the enzyme. This is consistent with the results from detection of enzymatically active SH-EPΔKDEL that was heterologously expressed in insect Sf9 cells (Okamoto et al., 1999a). In contrast to the detection of enzymatic activity of 33-kD SH-EPΔKDEL in the medium fraction, that of mature SH-EPΔKDEL in the cell fraction was not detected (Fig. 6B, KDEL–lanes). Although we have not addressed the reason, SH-EPΔKDEL may be unstable in vacuoles or secretion route, and therefore, the level of accumulation of the protein might remain low.
Intracellular Localization of GFP-Fused SH-EP or SH-EPΔKDEL in Tobacco BY-2 Cells
Signal peptide (SP)-GFP-SHEP or SP-GFP-SHEP ΔKDEL was expressed in tobacco BY-2 cells to visualize their localizations in the cells. When SP-GFP-SHEP was expressed, strong fluorescence was detected in small vesicles (Fig. 8, A and B). The diameter of the GFP-labeled small vesicles appeared to be similar to that of KV-like vesicles, which were detected under immunogold microscopic observation of cells of transformed Arabidopsis expressing wild-type SH-EP (Fig. 2, A–E). These results suggest that the GFP-labeled small vesicles correspond to KV-like vesicles. In case of expression of SP-GFP-SHEPΔKDEL in tobacco cells, such small vesicles were not detected (Fig. 8C). This suggests that KV-like vesicles are not induced in the transformed cells expressing SP-GFP-SHEPΔKDEL, but in cells expressing SP-GFP-SHEP.
Figure 8.
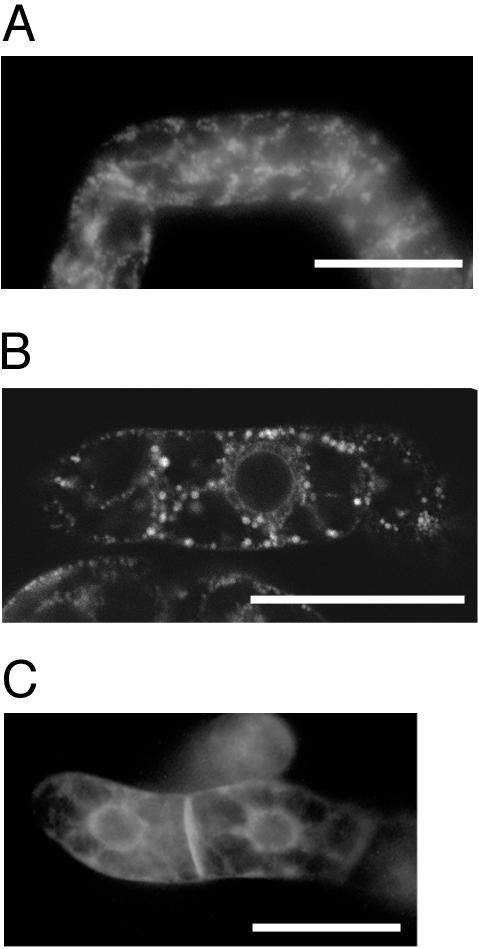
Intracellular localization of SP-GFP-SHEP or SP-GFP-SHEPΔKDEL in tobacco BY-2 cells. Transformed tobacco cells expressing SP-GFP-SHEP were observed with fluorescent (A) or confocal laser scanning microscope (B). Transformed tobacco cells expressing SP-GFP-SHEPΔKDEL were observed with fluorescent microscope (C). Bars = 50 μm.
DISCUSSION
The present study of the heterologous expression of wild-type SH-EP in Arabidopsis and in tobacco BY2 cells showed that protein accumulated in KV-like vesicles and was transported to vacuoles of cells. With expression of KDEL-truncated SH-EP in Arabidopsis, an intense signal from the anti-SH-EP antibody was detected at apoplastic spaces by immunocytochemical analyses (Fig. 3), strongly suggesting that SH-EPΔKDEL was secreted. However, the possibility that some portion of SH-EPΔKDEL is transported to vacuoles cannot be excluded because the volume of apoplast is much smaller than that of vacuoles, and immunocytochemistry has a better chance of detecting signals in apoplasts than in vacuoles, in which protein concentrations including SH-EPΔKDEL will be at low levels. In fact, immunoblot and pulse-chase analyses with tobacco cell culture expressing SH-EPΔKDEL revealed that mature SH-EPΔKDEL (possible vacuolar form of SH-EP) existed in cell fractions in addition to medium fractions (Figs. 6A and 7B), suggesting that the mutant SH-EP appeared to be transported to vacuoles as well as secreted. Alternatively, the possibility that maturation of the mutant protein occurs en route to secretion cannot be excluded.
Putative vacuolar sorting receptor, termed VmVSR, has been isolated from microsomes of cotyledons of V. mungo seedlings, and the receptor was revealed to bind to the N-terminal propeptide of SH-EP (Tsuru-Furuno et al., 2001). This suggests that the N-terminal propeptide of SH-EP may possesses a vacuolar targeting signal for SH-EP, and that SH-EPΔKDEL can be sorted to vacuoles via the unidentified signal on the N-terminal prosequence. However, deletion of KDEL from SH-EP resulted in the loss of formation/development of KVs (Fig. 8), secretion of the mutant protein, and in accumulation of SH-EPΔKDEL in cells at a low level. This suggests that the putative vacuolar sorting signal on the N-terminal propeptide of SH-EP is not enough for the formation of KVs, which effectively transport wild-type SH-EP to vacuoles. The KDEL sequence of SH-EP will be needed for formation of KVs and the subsequent mass transport of SH-EP to vacuoles via KVs. Secretion of SH-EP resulted in growth defects of transformed Arabidopsis through possible degradation of apoplastic proteins (Fig. 5, A and B), indicating that the efficient and mass transport of KDEL-proteinase via KV will be a biologically important transport system in cells in which KDEL-proteinase is highly expressed.
Recently, Frigerio et al. (2001) reported a KDEL-dependent vacuolar transport using transgenic tobacco expressing KDEL-tagged phaseolin, a French bean (Phaseolus vulgaris) storage protein. Interestingly, the phaseolin-KDEL was transported to vacuoles in a Golgi complex-independent manner, as judged from the lack of modifications in the sugar chain of the protein. This indicates that the Golgi complex-independent vacuolar sorting is not specific to the KDEL-tailed Cys proteinases, but is an alternative transport mechanism for KDEL-tailed proteins. Other than Cys proteinases, three RNases are known to have a putative ER retention sequence, RDEL or HDEF, at the C terminus; these enzymes are expressed during xylogenesis (Ye and Droste, 1996) or stress treatment (Kaletta et al., 1998). These enzymes may be sorted to vacuoles via KV-like vesicles, because an efficient transport system via KV-like vesicles permits cells to deliver mass proteins to vacuoles, while cells should also be able to quickly differentiate or respond to the environment.
In cotyledon cells of transgenic Arabidopsis expressing signal peptide (SP)-GFP-HDEL, spindle-shaped structures have been observed (Haseloff et al., 1997; Gunning, 1998; Kohler, 1998). Hayashi et al. (2001) indicated that the spindle-shaped structures, termed ER bodies, fuse with vacuoles when the cells are stressed with a 0.1 m salt solution. It was also revealed that two stress-inducible vacuolar protease, non-KDEL-tailed proteases, are sequestered into ER bodies, suggesting that ER bodies are sorting vesicles/organelles for stress-inducible vacuolar proteins to vacuoles. In addition, it has recently been indicated that ER bodies are induced in rosette leaves of Arabidopsis by the application of methyl jasmonate, a plant hormone involved in the defense against wounding and chewing by insects (Matsushima et al., 2002). ER bodies have some different characteristics from KVs, and are specifically observed in epidermal cells of Arabidopsis seedlings even if the SP-GFP-HDEL was driven under cauliflower mosaic virus 35S promoter. In contrast, KVs were formed/induced in cells of most tissues by expression of SH-EP under the same promoter in Arabidopsis (Fig. 2). The shape and size of KVs are also different from ER bodies. In the case of KVs, it has been reported that approximately 90% of luminal proteins of ricinosome, a KV-like vesicle detected in endosperm cells of castor bean (Ricinus communis) seedlings, are occupied with the proform of a KDEL-tailed Cys proteinase (pro-Cys-EP), suggesting that the KV is a specific vesicle for the enzyme (Schmid et al., 2001). However, KV and ER bodies may be formed at the ER and fuse with vacuoles using similar or identical molecular machineries because both vesicles emerge from ER and directly fuse with vacuoles.
KDEL-tailed Cys proteinases such as SH-EP are present exclusively in the plant kingdom, and are expressed in senescing organs in which rapid and massive protein mobilization occurs (Tanaka et al., 1993; Yamauchi et al., 1992; Valpuesta et al., 1995; Becker et al., 1997; Guerrero et al., 1998; Cercos et al., 1999). It seems likely that higher plants use the KDEL tail as an enhancer for vacuolar transport of KDEL-proteinases to massively and rapidly degrade proteins in senescing organs. The ER of plant cells has enormous plasticity (for review, see Chrispeels and Herman, 2000). In fact, KV-like vesicles were induced/developed in cells of most transgenic plant tissues by heterologous expression of wild-type SH-EP, providing an example of the plasticity.
MATERIALS AND METHODS
Antibody, SDS/PAGE, Immunoblotting, and Gel-Based Proteinase Assay
Antibody against SH-EP was purified from anti-SH-EP antiserum by a recombinant SH-EP-conjugated affinity column as described by Toyooka et al. (2000). SDS/PAGE was conducted on 12.5% (w/v) gels, and immunoblotting and gel-based proteinase assays were performed as described elsewhere (Mitsuhashi et al., 1986; Mitsuhashi and Minamikawa, 1989).
Preparations of Transgenic Arabidopsis and Tobacco (Nicotiana tabacum) BY-2 Cells Expressing Wild-Type SH-EP or Its KDEL Deletion Mutant (SH-EPΔKDEL)
pUC119 vector harboring full-length SH-EP cDNA was digested with XbaI and SacI to excise SH-EP cDNA, and the cDNA was subcloned into the binary vector pBI121 cut with the same enzymes. cDNA encoding KDEL-deleted mutant SH-EP, in which Lys codon (AAA) in C-terminal KDEL sequence was changed to a stop codon (TAA) (Okamoto et al., 1999a), was cut out of pVL1393 vector (BD PharMingen, San Diego) containing the cDNA using XbaI and PstI. The cDNA was blunted using a DNA blunting kit (Takara, Otsu, Japan) and was subsequently subcloned into pUC119 vector cleaved with SmaI. The resultant vector harboring KDEL-deleted SH-EP cDNA was cleaved by XbaI and SacI to excise the cDNA insert, and the insert was then subcloned into a pBI121 vector cut with the same enzymes. pBI121 vector harboring SH-EP or KDEL-deleted SH-EP cDNA at downstream of the cauliflower mosaic virus 35S promoter was used for Agrobacterium tumefaciens-mediated transformation of suspension-cultured BY-2 tobacco cells and BY-2 cells were cultured according to Matsuoka and Nakamura (1991). Arabidopsis (ecotype Columbia) was transformed by the in planta method (Bechtold and Pelletier, 1998) with the transformed A. tumefaciens as described above. Arabidopsis plants were grown at 22°C under a 16-h light/8-h dark cycle.
Preparations and Microscopic Observations of Transgenic Tobacco BY-2 Cells Expressing SP-GFP-SHEP or Its KDEL Deletion Mutant (SP-GFP-SHEPΔKDEL)
SP of SH-EP was amplified by PCR using TCTATGAAGAAGCTCTTGTGGGT and CCATGGCTTGGCCACTCCAAGAAC as primers and SH-EP cDNA as template. The amplified fragment was subcloned to pGEM-T Easy vector (Promega, Madison, WI), and the vector was cut by XbaI and NcoI. The insert was subcloned into XbaI-NcoI site of 35S promoter-GFP(S65T) plasmid (Chiu et al., 1996) to produce pSP-GFP. The chimeric gene encodes 26-amino acid signal peptide followed by GFP. For chimeric gene encoding SP-GFP-SHEP, the DNA region encoding proSH-EP was amplified by PCR with using TGTACAAGTTTGATTTTCATGAGAAGGA and GAGCTCTCAAAGTTCATCTTTGGGAG as primers and SH-EP cDNA as template. After subcloning the amplified fragment to pGEM-T Easy vector, the vector was cut by BsrGI and NotI, and the excised fragment encoding proSH-EP was inserted into BsrGI-NotI site of pSP-SHEP to produce pSP-GFP-SHEP. pSP-GFP-SHEPΔKDEL was produced with the same procedures as those for pSP-GFP-SHEP except for using cDNA encoding KDEL-deleted SH-EP as PCR template. pSP-GFP-SHEP or pSP-GFP-SHEPΔKDEL was digested with XbaI and SacI to excise DNA region for SP-GFP-SHEP or SP-GFP-SHEPΔKDEL, and the insert was subcloned into the binary vector pBI121 cut with the same enzymes. A. tumefaciens-mediated transformation of tobacco BY-2 cells was conducted as above. DNA sequences of all PCR products were verified by DNA sequencing.
Transformed tobacco cells were cultured as described (Matsuoka and Nakamura, 1991). At 3 d after inoculation of the cells, cells were observed with a fluorescent microscope (IX70; Olympus, Melville, NY) with U-MNIB filter set, or with a confocal laser scanning microscopy (LSM 410; Zeiss, Jena, Germany) with 488 nm excitation and 510 to 520 nm emission wavelengths.
Immunogold Electron Microscopy
Stems, rosette leaves, and flowers were harvested from fully grown transgenic Arabidopsis. Cotyledons were collected from 3-d-old dark-grown Arabidopsis seedlings. Each tissue was fixed with 4% (w/v) paraformaldehyde and 2% (w/v) glutaraldehyde in 0.1 m potassium phosphate buffer (pH 7.2) for 4 h at 4°C. After fixation of the tissues, they were dehydrated in a graded methanol series and the dehydrated pieces were embedded in a hard formulation of LR White resin. Ultrathin sections mounted on nickel grids (600 mesh; Electron Microscopy Sciences, Fort Washington, PA) were blocked with 10% (w/v) fetal bovine serum in Tris-buffered saline (TBS; 25 mm Tris-Cl, pH 7.4, and 150 mm NaCl) for 20 min at room temperature. The sections were then labeled with affinity-purified antibody to SH-EP (1:10) in TBS. After being washed with TBS, the sections were indirectly labeled with colloidal gold particles coupled to goat anti-rabbit immunoglobulin G. The gold-labeled sections were then washed with TBS, rinsed in water, and stained with 4% (w/v) aqueous uranyl acetate. The grids were examined and photographed with a transmission electron microscope (model 1010EX; JEOL, Tokyo, Japan) at 80 kV.
Fractionation of Tobacco Cell Cultures
Cell, medium, vacuoplast, and miniplast fractions were prepared from 5-d-old cultures. A 1-mL aliquot of the cell culture was centrifuged at 2,000g for 3 min, and the precipitate and supernatant were used for further preparations of the cell and medium fractions, respectively. The supernatant was again centrifuged at 5,000g for 10 min, and the supernatant used as the medium fraction. The cells precipitated by centrifugation at 2,000g were resuspended in fresh BY-2 culture medium and then centrifuged again at 2,000g for 3 min. This procedure was carried out again and the precipitated cells were resuspended in 0.5 mL of TBS (20 mm Tris-Cl, pH 7.5, and 150 mm NaCl) containing 0.05% (w/v) SDS, 0.05% (w/v) Triton X-100, 1 mm EDTA, 1 mm phenylmethane sulfonyl fluoride, and 1 mm N-ethylmaleimide. The suspension was sonicated (3 × 1 min, 30W, UR-20P; Tomy Seiko, Tokyo), and the sample was centrifuged at 15,000g for 10 min. The volume of the supernatant was then adjusted to that of the medium fraction and was used as the cell fraction.
Protoplasts were prepared from tobacco-cultured cells as described (Matsuoka and Nakamura, 1991), and miniplasts and vacuoplasts were isolated from the protoplasts according to Sonobe (1990). In brief, protoplasts prepared from 2 mL of tobacco cells were resuspended in 30 mL of 0.7 m mannitol, pH 7.0, containing 30% (v/v) Percoll (Pharmacia, Piscataway, NJ) and 20 mm MgCl2, and the resuspension was centrifuged at 10,000g for 60 min. Two layers were generated by the centrifugation of the protoplasts. The upper and lower layers in the centrifuge tube were isolated and used as vacuoplasts and miniplast fractions, respectively. Both fractions were checked by observation with light microscopy.
Pulse-Chase Experiments
Pulse-chase experiments with the transformed cells were conducted essentially as described (Matsuoka et al., 1990; Matsuoka and Nakamura, 1991). Cells from 5-d-old cultures were centrifuged at 2,000g for 3 min, and the precipitated cells were resuspended in an equal volume of fresh BY-2 medium. A 0.5-mL aliquot of the resuspended cells was incubated with 2.1 MBq of Tran35S-label (ICN Pharmaceuticals, Cost Mesa, CA) at 28°C for 15 min, and then 50 μL of 50 mm Met-Cys was added. The cell and medium fractions were prepared from the chased cells as described above, and SH-EP-related polypeptides were immunoprecipitated with anti-SH-EP antibody. The immunoprecipitated proteins were separated by SDS/PAGE and were detected by photophorimaging with an image analyzer (BAS 2000; Fuji Film).
Acknowledgments
We thank Dr. Yasuo Niwa (University of Shizuoka, Shizuoka) for providing the 35S-GFP(S65T) plasmid, and Dr. Masaki Ito (University of Tokyo, Tokyo) for providing tobacco BY-2 cells.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.021147.
This work was supported in part by the Ministry of Education, Science and Culture of Japan (Grant-in-Aid for Scientific Research no. 12740441) and by the Japan Science Society (Sasakawa Scientific Research grant no. 12–246).
References
- Akasofu H, Yamauchi D, Mitsuhashi W, Minamikawa T (1989) Nucleotide sequence of cDNA for sulfhydryl-endopeptidase (SH-EP) from cotyledons of germinating Vigna mungo seeds. Nucleic Acids Res 17: 6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82: 259–266 [DOI] [PubMed] [Google Scholar]
- Becker C, Senyuk VI, Shutov AD, Nong VH, Fischer J, Horstmann C, Muntz K (1997) Proteinase A, a storage-globulin-degrading endopeptidase of vetch (Vicia sativa L.) seeds, is not involved in early steps of storage-protein mobilization. Eur J Biochem 248: 304–312 [DOI] [PubMed] [Google Scholar]
- Cercos M, Santamaria S, Carbonell J (1999) Cloning and characterization of TPE4A, a thiol-protease gene induced during ovary senescing and seed germination in pea. Plant Physiol 119: 1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W-I, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6: 325–330 [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ, Herman EM (2000) ER-derived compartments function in storage and as mediators of vacuolar remodeling via a new type of organelle, precursor protease vesicle (PPV). Plant Physiol 123: 1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke J, De Rycke R, Botterman J (1992) Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J 11: 2345–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, Pastres A, Prada A, Vitale A (2001) Influence of KDEL on the fate of trimeric or assembly-defective phaseolin: selective use of an alternative route to vacuoles. Plant Cell 13: 1109–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero C, Calle M, Reid MS, Valpuesta V (1998) Analysis of the expression of two thiolprotease genes from daylily (Hemerocallis spp.) during flower senescence. Plant Mol Biol 36: 565–571 [DOI] [PubMed] [Google Scholar]
- Gunning BES (1998) The mystery organelles in Arabidopsis expressing GFP. Trends Plant Sci 3: 417. [DOI] [PubMed] [Google Scholar]
- Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94: 2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Yamada K, Shimada T, Matsushima R, Nishizawa NK, Nishimura M, Hara-Nishimura I (2001) A proteinase-sorting body that prepares for cell death or stress in the epidermal cells of Arabidopsis. Plant Cel Physiol 42: 894–899 [DOI] [PubMed] [Google Scholar]
- Herman EM, Larkins BA (1999) Protein storage vacuoles. Plant Cell 11: 601–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman EM, Tague BW, Hoffman LM, Kjemtrup SE, Chrispeels MJ (1990) Retention of phytohaemagglutinin with carboxy terminal tetrapeptide KDEL in the nuclear envelope and the endoplasmic reticulum. Planta 182: 305–312 [DOI] [PubMed] [Google Scholar]
- Kaletta K, Kunze I, Kunze G, Kock M (1998) The peptide HDEF as a new retention signal is necessary and sufficient to direct proteins to the endoplasmic reticulum. FEBS Lett 434: 377–381 [DOI] [PubMed] [Google Scholar]
- Kohler RH (1998) GFP for in vivo imaging of subcellular structures in plant cells. Trends Plant Sci 3: 317–320 [Google Scholar]
- Lee HI, Gal S, Newman TC, Raikhel NV (1993) The Arabidopsis endoplasmic reticulum retention receptor functions in yeast. Proc Natl Acad Sci USA 90: 11433–11437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Liu ZW, Tan-Wilson AL, Wilson WA (1997) Transcript levels for a mung bean cysteine protease during early seedling growth. Seed Sci Res 7: 359–372 [Google Scholar]
- Matsuoka K, Matsumoto S, Hattori T, Nakamura K (1990) Vacuolar targeting and posttranslational processing of the precursor to the sweet potato tuberous root storage protein in heterologous plant cells. J Biol Chem 265: 19750–19757 [PubMed] [Google Scholar]
- Matsuoka K, Nakamura K (1991) Propeptide of a precursor to a plant vacuolar protein required for vacuolar targeting. Proc Natl Acad Sci USA 88: 834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima R, Hayashi Y, Kondo M, Shimada T, Nishimura M, Hara-Nishimura I (2002) An endoplasmic reticulum-derived structure that is induced under stress conditions in Arabidopsis. Plant Physiol 130: 1807–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi W, Koshiba T, Minamikawa T (1986) Separation and characterization of two endopeptidases from cotyledons of germinating Vigna mungo seeds. Plant Physiol 80: 628–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi W, Minamikawa T (1989) Synthesis and posttranslational activation of sulfhydryl-endopeptidase in cotyledons of germinating Vigna mungo seeds. Plant Physiol 89: 274–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Pelham HBR (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell 48: 899–907 [DOI] [PubMed] [Google Scholar]
- Napier RM, Fowke LC, Hawes CR, Lewis M, Pelham HBR (1992) Immunological evidence that plants use both HDEL and KDEL for targeting proteins to the endoplasmic reticulum. J Cell Sci 102: 261–271 [DOI] [PubMed] [Google Scholar]
- Okamoto T, Minamikawa T (1998) A vacuolar cysteine endopeptidase (SH-EP) that digests seed storage globulin: characterization, regulation of gene expression, and posttranslational processing. J Plant Physiol 152: 675–682 [Google Scholar]
- Okamoto T, Minamikawa T, Edward C, Vakharia V, Herman E (1999a) Posttranslational removal of the carboxy-terminal KDEL of the cysteine protease SH-EP occurs prior to maturation of the enzyme. J Biol Chem 274: 11390–11398 [DOI] [PubMed] [Google Scholar]
- Okamoto T, Nakayama H, Seta K, Isobe T, Minamikawa T (1994) Posttranslational processing of a carboxy-terminal propeptide containing a KDEL sequence of plant vacuolar cysteine endopeptidase (SH-EP). FEBS Lett 351: 31–34 [DOI] [PubMed] [Google Scholar]
- Okamoto T, Toyooka K, Minamikawa T (2001) Identification of a membrane-associated cysteine protease (MCP) with possible dual roles in the endoplasmic reticulum and protein storage vacuole. J Biol Chem 276: 742–751 [DOI] [PubMed] [Google Scholar]
- Okamoto T, Yuki A, Mitsuhashi N, Minamikawa T (1999b) Asparaginyl endopeptidase (VmPE-1) and autocatalytic processing synergistically activate the vacuolar cysteine proteinase (SH-EP). Eur J Biochem 264: 223–232 [DOI] [PubMed] [Google Scholar]
- Pelham HBR (1989) Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol 5: 1–23 [DOI] [PubMed] [Google Scholar]
- Schmid M, Simpson D, Kalousek F, Gietl C (1998) A cysteine endopeptidase with a C-terminal KDEL motif isolated from castor bean endosperm is a marker enzyme for the ricinosome, a putative lytic compartment. Planta 206: 466–475 [DOI] [PubMed] [Google Scholar]
- Schmid M, Simpson DJ, Sarioglu H, Lottspeich F, Gietl C (2001) The ricinosomes of senescing plant tissue bud from the endoplasmic reticulum. Proc Natl Acad Sci USA 98: 5353–8535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonobe S (1990) Cytocharasin B enhances cytokinetic cleavage in miniplasts isolated from cultured tobacco cells. Protoplasma 155: 239–242 [Google Scholar]
- Tanaka T, Minamikawa T, Yamauchi D, Ogushi Y (1993) Expression of an endopeptidase (EP-C1) in Phaseolus vulgaris plants. Plant Physiol 101: 421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K, Okamoto T, Minamikawa T (2000) Mass transport of a proform of a KDEL-tailed cysteine proteinase (SH-EP) to protein storage vacuoles by endoplasmic reticulum-derived vesicle is involved in protein mobilization in germinating seeds. J Cell Biol 148: 453–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuru-Furuno A, Okamoto T, Minamikawa T (2001) Isolation of a putative receptor for KDEL-tailed cysteine proteinase (SH-EP) from cotyledons of Vigna mungo seedlings. Plant Cell Physiol 42: 1062–1070 [DOI] [PubMed] [Google Scholar]
- Valpuesta V, Lange NE, Guerrero C, Reid MS (1995) Up-regulation of a cysteine protease accompanies the ethylene-insensitive senescence of daylily (Hemerocallis spp.) flowers. Plant Mol Biol 28: 575–582 [DOI] [PubMed] [Google Scholar]
- Wandelt CI, Khan MRI, Craig S, Schroeder HE, Spencer D, Higgins TJV (1992) Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants. Plant J 2: 181–192 [DOI] [PubMed] [Google Scholar]
- Yamauchi D, Akasofu H, Minamikawa T (1992) Cysteine endopeptidase from Vigna mungo: gene structure and expression. Plant Cell Physiol 33: 789–797 [DOI] [PubMed] [Google Scholar]
- Ye Z-H, Droste DL (1996) Isolation and characterization of cDNAs encoding xylogenesis-associated and wounding-induced ribonucleases in Zinnia elegance. Plant Mol Biol 30: 697–709 [DOI] [PubMed] [Google Scholar]