Abstract
A critical step in establishing a successful nitrogen-fixing symbiosis between rhizobia and legume plants is the entrapment of the bacteria between root hair cell walls, usually in characteristic 180° to 360° curls, shepherd's crooks, which are formed by the host's root hairs. Purified bacterial signal molecules, the nodulation factors (NFs), which are lipochitooligosaccharides, induce root hair deformation in the appropriate host legume and have been proposed to be a key player in eliciting root hair curling. However, for curling to occur, the presence of intact bacteria is thought to be essential. Here, we show that, when spot applied to one side of the growing Medicago truncatula root hair tip, purified NF alone is sufficient to induce reorientation of the root hair growth direction, or a full curl. Using wild-type M. truncatula containing the pMtENOD11::GUS construct, we demonstrate that MtENOD11::GUS is expressed after spot application. The data have been incorporated into a cell biological model, which explains the formation of shepherd's crook curls around NF-secreting rhizobia by continuous tip growth reorientation.
Bacteria of the genera Rhizobium spp., Bradyrhizobium sp., Azorhizobium sp., Mesorhizobium sp., and Sinorhizobium sp., collectively referred to as rhizobia, can establish a symbiosis with legume plants. The bacteria induce the development of a new plant organ, the root nodule, in which nitrogen fixation takes place. Located in this nodule, the bacteria are provided with photosynthate. In return, the symbiont converts atmospheric nitrogen into ammonia, a form that can be readily assimilated by the host plant. A requirement for successful infection in many legumes is the entrapment of bacteria between root hair cell walls. Usually, this is accomplished by the formation of a tight curl, a shepherd's crook, of the host plant root hairs to which bacteria have become attached (Kijne, 1992; Hadri and Bisseling, 1998). The subsequent formation of the host-produced infection thread (Nutman, 1956) is initiated within this curl, where bacteria have been enclosed between root hair cell walls. In experimental conditions, infection threads can also originate from bacteria that are entrapped between the tips of two uncurled root hairs (Haack, 1964).
Nodulation factors (NFs) are molecules synthesized and excreted by rhizobia in response to plant flavonoids (Fisher and Long, 1992). Application of purified NF to legume roots induces the formation of polarized cytoplasmic bridges (pre-infection threads) in the outer cortical cells (van Brussel et al., 1992) and cell divisions in the inner cortex (van Brussel et al., 1992; for review, see Kijne, 1992). Moreover, various ENOD (early nodulin) genes are expressed in response to NF (Scheres et al., 1990; Pichon et al., 1992; Yang et al., 1993; Pingret et al., 1998; Compaan et al., 2001; Journet et al., 2001).
One of the best characterized biological activities of purified NF is to induce root hair deformation in the appropriate host. Therefore, root hair deformation assays are widely used to evaluate the specificity of NF toward a given legume host (Lerouge et al., 1990; Ardourel et al., 1994; Catoira et al., 2000). In the classical assay, deformation affects growth-terminating root hairs and starts with a swelling of the cell apex from which an outgrowth emerges (Heidstra et al., 1994). This outgrowth exhibits all the characteristics of a growing root hair, with a vesicle-rich area at the extreme tip, followed by a subapical cytoplasmic dense region with the nucleus at its base (Vicia sativa: de Ruijter et al., 1998; Miller et al., 2000; Medicago truncatula: Sieberer and Emons, 2000). Initial swelling of the root hair tip starts within minutes after NF application, whereas outgrowth generally initiates at least 1 h later. Based on such observations, it has been proposed that NF (Emons and Mulder, 2000) might be the inducing principle in bacteria-associated root hair curling (van Batenburg et al., 1986). However, until now, no direct evidence for this hypothesis has been provided. It is generally thought that for root hair curling to occur, the presence of bacteria is essential (Catoira et al., 2001). We now show that NF alone, when spot applied, can induce root hair tip growth reorientation and root hair branching toward NF in M. truncatula.
RESULTS
Spot Application of Host-Specific Nod Factor onto the Tip of Growing Wild-Type Air-Grown Root Hairs Induces Root Hair Growth Axis Reorientation
In all previous reported assays to study the effects of purified NF on legume root hairs (Lerouge et al., 1990; Ardourel et al., 1994; Heidstra et al., 1994; de Ruijter et al., 1998; Catoira et al., 2000; Miller et al., 1999, 2000; Sieberer and Emons, 2000), a liquid medium containing NF is globally applied to the root hairs. In these assays, growth-terminating root hairs respond to the NF application with root hair deformation (Heidstra et al., 1994; de Ruijter et al., 1998; Miller et al., 1999, 2000; Sieberer and Emons, 2000) or root hair branching (Catoira et al., 2000), depending on the assay. However, in nodulation assays in which NF-excreting rhizobia are applied to legume roots (Ardourel et al., 1994), growing root hairs curl around the bacteria (Kijne, 1992). Therefore, we hypothesized that in the case of root hair curling, the local presence of NF, excreted by the bacterial colony, is causing the root hair curling (Emons and Mulder, 2000). To test this hypothesis, we developed the spot application assay, in which a droplet of purified NF is applied to one side of the apical dome of a growing root hair. Because it is technically impossible to locally apply a droplet of NF solution on a root hair growing in liquid or agar-based medium, experiments were carried out on air-growing root hairs from seedlings that were grown along vertical agar plates. These root hairs are further referred to as air-grown root hairs.
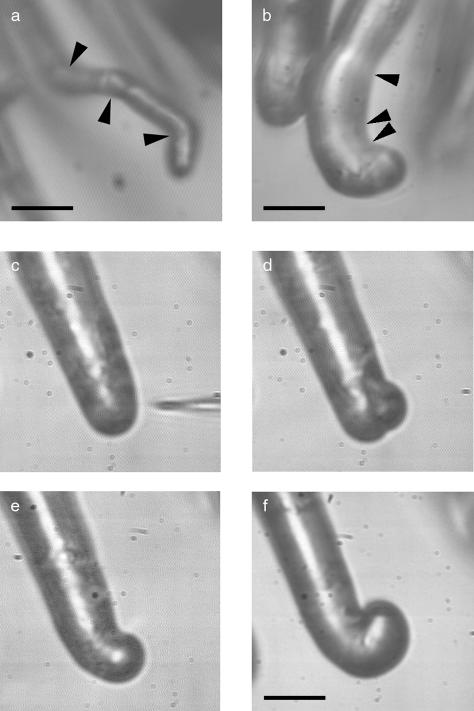
At a concentration of 10–9 m, NF spot application resulted in a reorientation of the growth axis of air-grown root hairs toward the site of application (Fig. 1). Already within minutes after application, a change in cell morphology is visible as the extreme tip of the root hair shifts toward the site of application. Fifteen minutes after application, the reorientation is clearly visible and becomes more pronounced with time (Fig. 1). Provided that the root hair under study maintains growth after the initial reorientation, spot application can be repeated several times, thus giving rise to multiple growth axis reorientations of the same root hair (Fig. 2A). When spot application is performed repeatedly on the same side of the air-grown root hair with short time intervals, partial curls can be obtained (Fig. 2B). Complete curls cannot easily be obtained experimentally because the inner side of the curl becomes less and less accessible for the micropipette with increasing numbers of spot applications. However, single spot applications can sometimes lead to complete curls within 50 min after NF application (n = 6; Fig. 2, c–f).
Figure 1.
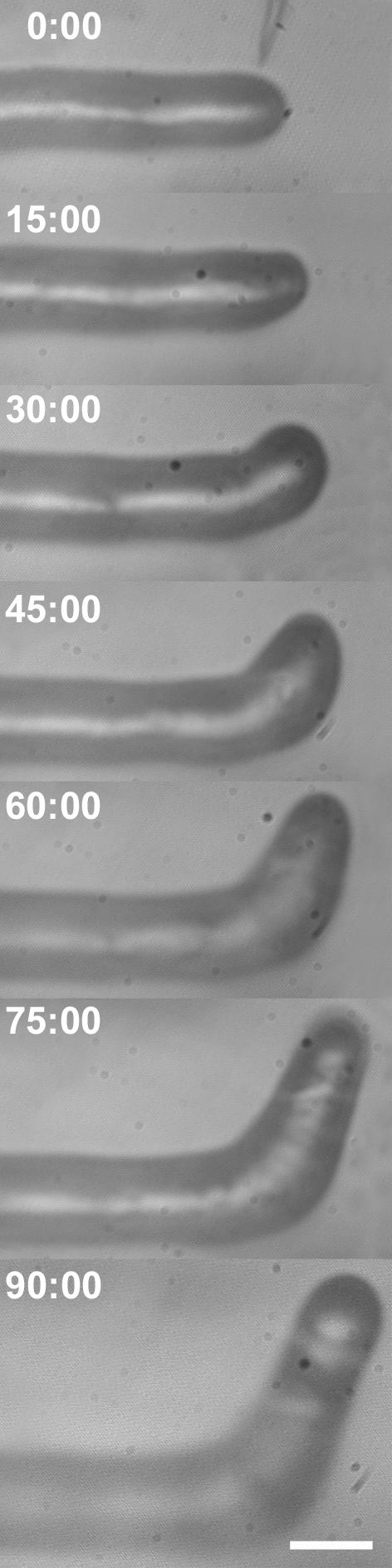
The response of a growing M. truncatula root hair to a single spot application of 10–9 m purified Nod factor (NF). Fifteen minutes after NF application, the reorientation of the root hair growth axis toward the site of application is already visible and becomes more pronounced at 30 min. As can be seen, root hair growth is continuous during and after reorientation, and the root hair diameter does not change. Bar = 15 μm.
Figure 2.
Iterative spot application of NF leads to iterative root hair growth axis reorientation, leading to root hair curling. a, Growing root hair after three successive spot applications of NF on different sides, showing that multiple applications lead to multiple growth axis reorientations. Bar = 30 μm. b, Growing root hair after three successive spot applications of NF on one side of the root hair tip, showing multiple reorientations of the root hair growth axis, leading to a partial root hair curl. Arrowheads point to the position of successive NF spot applications. c to f, Time series of root hair curling after single spot application. With a micropipette (c), a microdroplet of 10–9 m Nod factor is applied to one side of the apical dome of a growing root hair (d). After 25 min, a clear reorientation of the root hair growth axis toward the side of application is visible (e), and in 50 min, a partial shepherd's crook is formed (f). Bar in b to f = 18 μm.
Root Hair Reorientation upon NF Spot Application Is NF Type Specific
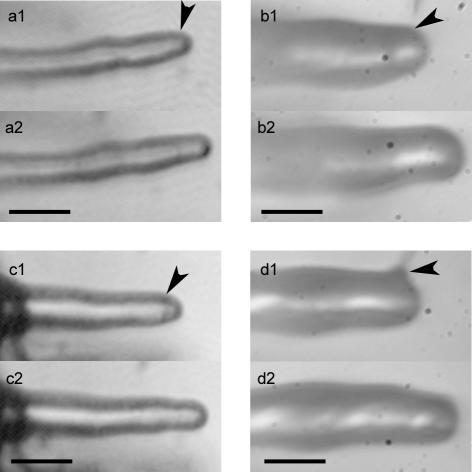
Nod factors are complex molecules, consisting of β-1,4-linked tetramers or pentamers of d-glucosamine, which are mono-N-acylated on the terminal nonreducing residue and N-acetylated on the other residues. Moreover, the chitin oligomer backbone can be decorated with O-acetyl- and O-sulfate groups, which determines the specificity of a bacterium for its host (Lerouge et al., 1990; Roche et al., 1991; Spaink et al., 1991; Truchet et al., 1991; Ardourel et al., 1994). It has been shown that for Medicago sativa, the sulfate decoration is essential to establish a successful infection (Medicago sativa Roche et al., 1991 Ardourel et al., 1994) and that purified non-sulfated NF fails to induce root hair deformation in M. truncatula (Catoira et al., 2000). Therefore, we used spot application of the non-sulfated NF as a control to test the specificity of the root hair reorientation response (Table I). In Figure 3A, we show that upon spot application of 10–9 m non-sulfated NF, root hairs did not respond with root hair reorientation. Spot application of 10–6 M chitotetraose [(GlcNac)4], the β-1,4-linked tetramer backbone of all NFs (Fig. 3B), or 10–9 M sulfated chitotetraose (Fig. 3C), or water (the NF solvent; Fig. 3D) did not result into root hair reorientation (Table I). This indicates that growth axis reorientation of air-grown root hairs upon NF spot application is a NF-specific response and not triggered by mechanical stimuli.
Table I.
Responses of growing and full-grown root hairs to spot application of different NF molecules
| NF application
|
Growing Root Hairs
|
Full-Grown Root Hairs
|
||||
|---|---|---|---|---|---|---|
| N treated | N reoriented | % | N treated | N reoriented | % | |
| Different molecules on the side of the tip | ||||||
| NF NodRm-IV (C16:2,Ac,S) | 34 | 34 | 100 | 16 | 0b | 0 |
| Non-sulfated NF | 16 | 0a | 0 | ndc | ||
| Chitotetraose (GlcNac)4 | 15 | 0a | 0 | nd | ||
| Sulfated chitotetraose | 18 | 0a | 0 | nd | ||
| Water | 7 | 0a | 0 | nd | ||
| N treated | N branched | % | ||||
| NF Nod Rm-IV (C16:2, Ac,S) application on the shank | ||||||
| NF 30 μm below the tip | 8 | 8 | 100 | 4 | 0b | 0 |
| NF 60 μm below the tip | 3 | 3 | 100 | 3 | 0b | 0 |
Hairs were growing straight after application. b No morphological changes were observed. c nd = Not done.
Figure 3.
Spot application controls showing that root hair reorientation is an NF-specific response. a, Spot application of 10–9 m non-sulfated NF (from the Sinorhizobium meliloti NodH mutant) does not lead to growth reorientation. Bar = 30 μm. a1, At the moment of application; a2, 25 min after application. b, Growing M. truncatula wild-type root hair after spot application with 10–6 m chitotetraose showing no growth axis reorientation. Bar = 18 μm. b1, At the moment of application; b2, 45 min after application. c, Spot application of 10–9 m sulfated chitotetraose does not lead to growth reorientation. Bar = 30 μm. c1, Before application; c2, 30 min after application. d, Growing M. truncatula wild-type root hair after spot application of Millipore water (Millipore, Bedford, MA) showing no growth axis reorientation. Bar = 18 μm. d1, At the moment of application; d2, 55 min after application. Arrowheads point to the site of application.
Expression of the NF-Induced Early Nodulin Gene MtENOD11 Is Maintained after NF Spot Application
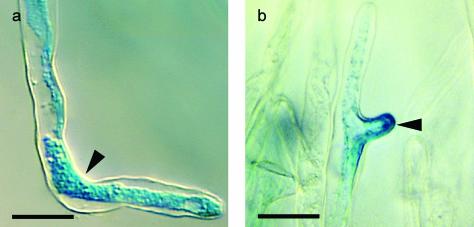
We now demonstrated that root hair reorientation after NF spot application is a NF specific response. In addition, we wanted to test if ENOD expression is induced by NF spot application. Therefore, we performed single spot applications of 10–9 m NF on the side of the tip of growing root hairs of transgenic M. truncatula plants. These transgenics are transformed with the pMtENOD11::GUS reporter construct. MtENOD11::GUS is not expressed during normal root hair development but is strongly expressed after global NF application (Vernoud et al., 1999; Journet et al., 2001). As can be seen in Figure 4A, NF spot application on these transgenic root hairs resulted in root hair reorientation and MtENOD11::GUS expression, indicating that this NF-specific response is induced by NF spot application. Neither spot application of the non-sulfated NF, nor the chitotetraose backbone or the sulfated chitotetraose resulted in MtENOD11::GUS expression (data not shown).
Figure 4.
pMtENOD11::GUS expression is maintained in reorienting and branching wild-type M. truncatula root hairs after NF spot application. a, Transgenic M. truncatula wild-type root hair carrying the pMtENOD11::GUS fusion construct stained for β-glucuronidase (GUS) expression 120 min after spot application of 10–9 m NF showing positive GUS staining of the cytoplasm. Bar = 30 μm. b, Transgenic M. truncatula wild-type root hair carrying the pMtENOD11::GUS fusion construct stained for GUS expression 75 min after spot application of 10–9 m NF 60 μm below the growing tip, showing root hair branching at the site of application and GUS staining of the cytoplasm. Bar: 30 μm.
Root Hair Branching after Nod Factor Spot Application at 30 or 60 μM below the Tip of a Growing Root Hair
A bacteria-entrapping curl does not necessarily have to develop at the root hair tip. In the presence of bacteria, lateral root hair branches can develop which subsequently curl around the bacteria (Dart, 1974). Moreover, depending on the assay, root hairs can branch after global NF application (Catoira et al., 2000). Because we now show that the local presence of NF at the side of the dome of the growing root hair tip is sufficient to induce reorientation of the root hair growth axis, we hypothesized that local presence of NF on the shank of a growing root hair would result in a new growth axis at the site of application, i.e. root hair branching. When NF was applied 30 (n = 8) to 60 (n = 3) μm distally from the growing tip, the existing tip stopped growing, and within 20 min, a branch started to grow at the site of NF spot application. Moreover, branching hairs express MtENOD11::GUS after NF spot application (Fig. 4B), indicating that this branching response is a physiological response.
DISCUSSION
Root Hair Curling Is Continuous Tip Growth Reorientation
Root hair curling is a critical step in the establishment of a successful invasion of the root hair by rhizobia, but the mechanisms underlying this curling process remain largely unknown. In this work, we show that spot application of purified NF is sufficient to induce (partial) root hair curling and, therefore, that the presence of bacteria is not required, which was thought before (Catoira et al., 2001). These results strongly support the model of van Batenburg et al. (1986) that root hair curling is continuous reorientation of tip growth. In this model, it was proposed that root hair curling can only occur when at least the following conditions are fulfilled (van Batenburg et al., 1986): (a) the attachment of one inducing principle (e.g. the NF droplet), (b) within the growth area of the root hair; (c) translocation of the inductor along the growing root hair tip (e.g. iterative spot application); and (d) redirection of the original plant-driven tip growth. An alternative hypothesis for root hair curling could be that reorientation of the root hair growth axis is achieved by differential stimulation of wall expansion on the opposing site of NF presence. However, this is inconsistent with: (a) the observation that spot application of NF on the shank of a growing root hair results in a growing branch at the site of application, and (b) the computer simulations by van Batenburg et al. (1986).
The Cell Biology of Root Hair Curling. A Hypothesis
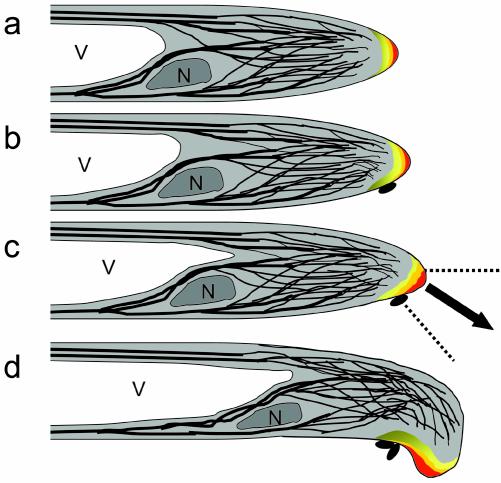
Based on what we know about root hair tip growth and how root hairs react to global NF application at the cell biological level, we would like to propose a hypothesis to explain the formation of a tight curl, the shepherd's crook, around a colony of bacteria. In a growing root hair (Fig. 5A), the dense subapical fine bundles of actin filaments deliver Golgi-derived vesicles to the root hair tip (Miller et al., 1999, 2000). Aided by a tip-localized cytosolic calcium gradient (Wymer et al., 1997; de Ruijter et al., 1998; Cárdenas et al., 1999), the membrane of these vesicles inserts into the plasma membrane, thus delivering the vesicle content into the existing cell wall via exocytosis (Battey and Blackbourn, 1993; Battey et al., 1996). Because the newly inserted cell wall is flexible (Cosgrove, 1993; Roberts, 1994) and the cell is under turgor pressure (Passioura and Fry, 1992), the tip expands. When a host-specific bacterium attaches to the root hair dome, the bacterium locally excretes NF, which is then immobilized within the cell wall (Goedhart et al., 2000). Because NF increases the cytosolic calcium ion concentration, [Ca2+]c (Felle et al., 1998) at the plasma membrane (Cárdenas et al., 1999), the local presence of bound NF in the cell wall induces a local [Ca2+]c increase. Therefore, the region of high [Ca2+]c at the plasma membrane, i.e. the region where exocytosis occurs (Miller et al., 1997), will gradually shift from the tip toward the cell wall area with bound NF (Fig. 5B). Furthermore, the density of subapical fine bundles of actin filaments, which deliver the Golgi-derived vesicles to the tip area, increases upon global NF application (de Ruijter et al., 1999). Because NF is immobilized locally within the cell wall (Goedhart et al., 2000), NF spot application will only locally increase the subapical fine bundles of actin filaments. This results in a shift in the region where vesicles are being delivered and inserted into the plasma membrane from the existing vesicle-rich area to the side of the hair that has become the new center of activity under the influence of NF. Thus, a new growth axis that is the resultant of the original root hair growth axis and the NF-induced growth direction is initiated toward the attachment side, as previously proposed by Emons and Mulder (2000; Fig. 5C). The attached bacterial colony creates a new center of growth activity, thus redirecting tip growth in the direction of the colony. The multiplication of the present bacteria enlarges the area of surface contact between the two organisms. Thus, the new cell tip contacts new bacteria, which also excrete NF, and the process described above is repeated (Fig. 5D). The continuous reorientation of tip growth results in a continuous rotation of the tip in a single direction and can give rise to the tight curl (van Batenburg et al., 1986; Emons and Mulder, 2000) within which the bacteria are entrapped, the shepherd's crook.
Figure 5.
Cartoon of the putative mechanism involved in root hair growth axis reorientation during curl formation around rhizobia. a, Growing root hair with a tip-focused calcium gradient in an area devoid of detectable actin filaments, a subapical fine F actin area (black lines), the nucleus (N) positioned at the base of the subapical fine F-actin area, and the vacuole (V). b, Bacterium attaches to the root hair tip and locally excretes NF, which induces a local calcium influx, leading to a gradual increase in [Ca2+]c. c, High [Ca2+]c; thus, the growth area, shifts toward the attached bacteria, redirecting the growth. Note that the new growth direction is the resultant of the NF-induced direction and the original growth axis. d, The enlarging bacterial colony also produces NF, thus shifting again the growth area toward itself. In the end, these continuously repeated growth axis reorientations give rise to a tight curl, entrapping the bacteria.
MtENOD11::GUS expression after NF spot application will be a valuable tool for deciphering NF-induced signaling pathways. Spot application of NF in combination with pharmacological agents on these transgenics and early symbiosis mutants of M. truncatula, will give new insights in the role of certain proteins in NF-induced signaling.
MATERIALS AND METHODS
Medicago truncatula Seed Preparation and Seedling Growth
Seeds of M. truncatula jemalong A17 and M. truncatula jemalong carrying a pMtENOD11::GUS fusion construct (Journet et al., 2001) were scarified in concentrated sulfuric acid for 10 min and thoroughly washed with running demineralized water. Seeds were then sterilized in a mixture of 30% (w/v) hydrogen peroxide and 96% (w/v) ethanol (1:1 [w/v]) for 2 min and extensively rinsed with sterile demineralized water. Seeds were subsequently imbibed overnight in sterile demiwater at 4°C. To synchronize germination, the imbibed seeds were allowed to vernalize in the refrigerator for 4 d at 4°C on plates containing 0.8% (w/v) agar in sterile demiwater wrapped in aluminum foil. For germination, the agar plates containing the vernalized seeds were transferred to the plant growth room at 25°C.
After germination, 24 h later, about 1- to 1.5-cm-long seedlings were transferred to fresh agar plates and allowed to grow for 8 d at 25°C, with a 16-h-light/8-h-dark rhythm in a slightly oblique position from the vertical. In such growth conditions, the root hairs that develop in air above the agar are suitable for spot application.
Spot Application Assay
A water pressure microinjection device (water pressure device: Gilmont, Barrington, IL; needle holder: Eppendorf, Merck Eurolab BV, Amsterdam) was used to apply microdroplets (0.2 pL) of purified NF [NodRm-IV(C16:2, Ac, S)] diluted with Millipore water to a final NF concentration of 10–9 m, 10–9 m non-sulfated NF, 10–6 m chitotetraose [(GlcNac)4], 10–9 m sulfated chitotetraose backbone, or Millipore water to one side of growing root hair tips. Subsequent growth axis reorientations were recorded every 15 min with a video camera linked to an inverted Diaphot microscope (Nikon, Tokyo).
pMtENOD11::GUS Expression
pMtENOD11::GUS expression was assessed by incubating the seedlings for 24 h in the GUS substrate X-Gluc {2 mm 5-bromo-4-chloro-3-indolylglucuronide, 1% [w/v] dimethylformamide, 0.1 mm K3[Fe(CN)6], 0.1 mm, K4[Fe(CN6)] · 3H2O, 1 mm EDTA, and 50 mm KH2PO4 [pH 7.0]} at 37°C (Journet et al., 1994). Images were recorded with a CCD camera (Sony, Tokyo) linked to a Nikon Optiphot upright DIC microscope.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owner of all parts of the material. Obtaining any permission will be the responsibility of the requestor.
Acknowledgments
We thank Dr. David Barker (Laboratory of Plant-Microbe Interactions, Institut National de la Recherche Agronomique-Centre National de la Recherche Scientifique, BP27, 31326 Castanet-Tolosan Cedex, France) for providing M. truncatula seeds carrying the pMtENOD11::GUS construct and for useful discussions on the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.021634.
This work was supported by the Dutch Organization for Scientific Research, Division of Earth and Life Sciences (Nederlandse organisatie voor Wetenschappelijk Onderzoek-Aard-en Levens Wetenschappen, ALW no. 805–33–342 to J.J.E.), by the European Community Training and Mobility of Researchers Program (grant no. FMRX CT 98 0239 to F.G.P.L.), and by the Region Haute-Normandie (postdoctoral fellowship to F.G.P.L.).
References
- Ardourel M, Demont N, Debellé F, Maillet F, De Billy F, Promé J-C, Dénarié J, Truchet G (1994) Rhizobium meliloti lipooligosaccharide nodulation factors: different structural requirements for bacterial entry into target root hair cells and induction of plant symbiotic developmental responses. Plant Cell 6: 1357–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey NH, Blackbourn HD (1993) The control of exocytosis in plant cells. New Phytol 125: 307–338 [DOI] [PubMed] [Google Scholar]
- Battey N, Carroll A, van Kesteren P, Taylor A, Brownlee C (1996) The measurement of exocytosis in plant cells. J Exp Bot 47: 717–728 [Google Scholar]
- Cárdenas L, Feijó JA, Kunkel JG, Sánchez F, Holdaway-Clarke T, Hepler PK, Quinto C (1999) Rhizobium Nod factors induce increases in intracellular free calcium influxes in bean root hairs. Plant J 19: 347–352 [DOI] [PubMed] [Google Scholar]
- Catoira R, Galera C, de Billy F, Penmetsa RV, Journet EP, Maillet F, Rosenberg C, Cook D, Gough C, Dénarié J (2000) Four genes of Medicago truncatula controlling components of a Nod factor transduction pathway. Plant Cell 12: 1647–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoira R, Timmers ACJ, Maillet F, Galera C, Penmetsa RV, Cook D, Dénarié J, Gough C (2001) The HCL gene of Medicago truncatula controls Rhizobium-induced root hair curling. Development 128: 1507–1518 [DOI] [PubMed] [Google Scholar]
- Compaan B, Yang WC, Bisseling T, Franssen H (2001) ENOD40 expression in the pericycle precedes cortical cell division in Rhizobium-legume interaction and the highly conserved internal region of the gene does not encode a peptide. Plant Soil 230: 1–8 [Google Scholar]
- Cosgrove DJ (1993) Water uptake by growing cells: an assessment of the controlling roles of wall relaxation, solute uptake and hydraulic conductance. Int J Plant Sci 154: 10–21 [DOI] [PubMed] [Google Scholar]
- Dart PJ (1974) The infection process. In A Quispel, ed, The Biology of Nitrogen Fixation. North Holland, Amsterdam, pp 381–429
- de Ruijter NCA, Bisseling T, Emons AMC (1999) Rhizobium Nod factors induce an increase in subapical fine bundles of actin filaments in Vicia sativa root hairs within minutes. Mol Plant-Microbe Interact 12: 829–832 [Google Scholar]
- de Ruijter NCA, Rook MB, Bisseling T, Emons AMC (1998) Lipochitooligosaccharides reinitiate root hair tip growth in Vicia sativa with high [Ca2+]c and spectrin-like antigen at the tip. Plant J 13: 341–350 [Google Scholar]
- Emons AMC, Mulder BM (2000) Nodulation factors trigger an increase of fine bundles of subapical actin filaments in Vicia root hairs: implications for root hair curling around bacteria. In PJGM De Wit, T Bisseling, W Stiekema, eds Biology of Plant-Microbe Interaction, Vol 2. International Society for Molecular Plant-Microbe Interactions, St. Paul, pp 44–49 [Google Scholar]
- Felle HH, Kondorosi E, Kondorosi A, Schultze M (1998) The role of ion fluxes in Nod factor signalling in Medicago sativa. Plant J 13: 455–465 [Google Scholar]
- Fisher RF, Long SR (1992) Rhizobium-plant signal exchange. Nature 357: 655–660 [DOI] [PubMed] [Google Scholar]
- Goedhart J, Hink MA, Visser AJWG, Bisseling T, Gadella TWJ Jr (2000) In vivo fluorescence correlation microscopy (FCM) reveals accumulation and immobilization of Nod factors in root hair cell walls. Plant J 21: 109–119 [DOI] [PubMed] [Google Scholar]
- Haack A (1964) Über den Einfluβ der Knöllchenbakterien auf die Wurzelhaare von Leguminosen und Nichtleguminosen. Zentralblatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten; Zweite Abteilung, Band 117, Heft 4; Jena. Verlag von Gustar Fisher, Germany, pp 343–366
- Hadri A-E, Bisseling T (1998) Responses of the plant to Nod factors. In HP Spaink, A Kondorosi, PJJ Hooykaas, eds, The Rhizobiaceae: Molecular Biology of Model Plant-Associated Bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 403–416
- Heidstra R, Geurts R, Franssen H, Spaink HP, van Kammen A, Bisseling T (1994) Root hair deformation activity of nodulation factors and their fate on Vicia sativa. Plant Physiol 105: 787–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet EP, Pichon M, Dedieu A, de Billy F, Truchet G, Barker DG (1994) Rhizobium meliloti Nod factors elicit cell-specific transcription of the ENOD12 gene in transgenic alfalfa. Plant J 6: 241–249 [DOI] [PubMed] [Google Scholar]
- Journet EP, El-Gachtouli N, Vernoud V, De Billy F, Pichon M, Dedieu A, Arnould C, Morandi D, Barker DG, Gianinazzi-Pearson V (2001) Medicago truncatula ENOD11: a novel RPRP-encoding early nodulin gene expressed during mycorrhization in arbuscule-containing cells. Mol Plant-Microbe Interact 14: 737–748 [DOI] [PubMed] [Google Scholar]
- Kijne JW (1992) The Rhizobium infection process. In G Stacey, R Burris, H Evans, eds, Biological Nitrogen Fixation. Chapman and Hall, New York, pp 349–398
- Lerouge P, Roche P, Faucher C, Maillet F, Truchet G, Promé J-C, Dénarié J (1990) Symbiotic host-specificity of Rhizobium meliloti is determined by a sulfated and acetylated glucosamine oligosaccharide signal. Nature 344: 781–784 [DOI] [PubMed] [Google Scholar]
- Miller DD, de Ruijter NCA, Bisseling T, Emons AMC (1999) The role of actin in root hair morphogenesis: studies with lipochito-oligosaccharides as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J 17: 141–154 [Google Scholar]
- Miller DD, de Ruijter NCA, Emons AMC (1997) From signal to form: aspects of the cytoskeleton-plasma membrane-cell wall continuum in root hair tips. J Exp Bot 48: 1881–1896 [Google Scholar]
- Miller DD, Leferink-ten Klooster HB, Emons AMC (2000) Lipochitooligosaccharide nodulation factors stimulate cytoplasmic polarity with longitudinal endoplasmatic reticulum and vesicles at the tip in vetch root hairs. Mol Plant-Microbe Interact 13: 1385–1390 [DOI] [PubMed] [Google Scholar]
- Nutman PS (1956) The influence of the legume in root-nodule symbiosis: a comparative study of host determinants and function. In H Munro Fox, ed, Biological Reviews of the Cambridge Philosophical Society, Vol 31. Cambridge University Press, Cambridge, UK, pp 109–151 [Google Scholar]
- Passioura JB, Fry SC (1992) Turgor and cell expansion: beyond the Lockhart equation. Aust J Plant Physiol 19: 565–576 [Google Scholar]
- Pichon M, Journet EP, Dedieu A, De Billy F, Truchet G, Barker DG (1992) Rhizobium meliloti elicits transient expression of the early nodulin gene ENOD12 in the differentiating root epidermis of transgenic alfalfa. Plant Cell 4: 1199–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingret JL, Journet EP, Barker DG (1998) Rhizobium Nod factor signaling: evidence for a G protein-mediated transduction mechanism. Plant Cell 10: 659–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K (1994) The plant extracellular matrix: in a new expansive mood. Curr Opin Cell Biol 6: 688–694 [DOI] [PubMed] [Google Scholar]
- Roche P, Debelle F, Maillet F, Lerouge P, Faucher C, Truchet G, Dénarié J, Promé J-C (1991) Molecular basis of symbiotic host specificity in Rhizobium meliloti: nodH and nodPQ genes encode the sulfation of lipooligosaccharide signals. Cell 67: 1131–1143 [DOI] [PubMed] [Google Scholar]
- Scheres B, van de Wiel C, Zalensky A, Horvath B, Spaink H, van Eck H, Zwartkruis F, Wolters AM, Gloudemans T, van Kammen A et al. (1990) The ENOD12 gene product is involved in the infection process during the pea-Rhizobium interaction. Cell 60: 281–294 [DOI] [PubMed] [Google Scholar]
- Sieberer B, Emons AMC (2000) Cytoarchitecture and pattern of cytoplasmic streaming in root hairs of Medicago truncatula during development and deformation by nodulation factors. Protoplasma 214: 118–127 [Google Scholar]
- Spaink HP, Sheeley DM, van Brussel AAN, Glushka J, York WS, Tak T, Geiger O, Kennedy EP, Reinhold VN, Lugtenberg BJJ (1991) A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature 354: 125–130 [DOI] [PubMed] [Google Scholar]
- Truchet G, Roche P, Lerouge P, Vasse J, Camut S, de Billy F, Promé J-C, Dénarié J (1991) Sulphated lipo-oligosaccharide signals of Rhizobium meliloti elicit root nodule organogenesis in alfalfa. Nature 351: 670–673 [Google Scholar]
- van Batenburg FHD, Jonker R, Kijne JW (1986) Rhizobium induces marked root hair curling by redirection of tip growth: a computer simulation. Physiol Plant 66: 476–480 [Google Scholar]
- van Brussel AAN, Bakhuizen R, Van Spronsen PC, Spaink HP, Tak T, Lugtenberg BJJ, Kijne JW (1992) Induction of pre-infection thread structures in the leguminous host plant by mitogenic lipo-oligosaccharides of Rhizobium. Science 257: 70–72 [DOI] [PubMed] [Google Scholar]
- Vernoud V, Pingret J-L, Chabaud M, Dedieu A, de Carvalho Niebel F, Journet EP, Barker DG (1999) Nod factor signal transduction in the Medicago truncatula Nod–/Myc– mutants TR25/26. In PJGM de Wit, T Bisseling, WJ Stiekema, eds, Biology of Plant-Microbe Interactions, Vol 2. International Society for Molecular Plant-Microbe Interactions, St. Paul, pp 114–119 [Google Scholar]
- Wymer CL, Bibikova TN, Gilroy S (1997) Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J 12: 427–439 [DOI] [PubMed] [Google Scholar]
- Yang W-C, Katinakis P, Hendriks P, Smolders A, De Vries F, Spee J, Van Kammen A, Bisseling T, Franssen H (1993) Characterization of Gm-ENOD40, a gene showing novel patterns of cell-specific expression during soybean nodule development. Plant J 3: 573–585 [DOI] [PubMed] [Google Scholar]