Abstract
The E2F family plays a pivotal role in cell cycle control and is conserved among plants and animals, but not in fungi. This provides for the possibility that the E2F family was integrated during the development of higher organisms, but little is known about this. We examined the effect of E2F ectopically expressed in transgenic tobacco (Nicotiana tabacum) plants on growth and development using E2Fa (AtE2F3) and DPa from Arabidopsis. E2Fa-DPa double transgenic lines exhibited altered phenotypes with curled leaves, round shaped petals, and shortened pistils. In mature but not immature leaves of the double transgenic lines, there were enlarged nuclei with increasing ploidy levels accompanied by the ectopic expression of S phase- but not M phase-specific genes. This indicates that a high expression of E2F promotes endoreduplication by accelerating S phase entry in terminally differentiated cells with limited mitotic activity. Furthermore, mature leaves of the transgenic plants contained increased numbers of small cells, especially on the palisade (adaxial) side of the outer region toward the edge, and the leaf strips exhibited hormone-independent callus formation when cultured in vitro. These observations suggest that an enhanced E2F activity modulates cell cycle in a cell type-specific manner and affects plant morphology depending on a balance between activities for committing to S phase and M phase, which likely differ between organs or tissues.
Transition from G1(G0) to S phase is a critical step in the control of the cell cycle, as well as a mitotic step. The control of this process closely involves E2F transcription factors in animals. E2F proteins form heterodimers with DP proteins and regulate promoters of genes involved in deoxynucleotide biosynthesis, DNA replication, and cell cycle control, mainly those required for entering S phase, but also apparently unrelated genes such as the myc and myb families with different expression profiles (Lavia and Jansen-Dürr, 1999; Müller and Helin, 2000). Recent studies using DNA microarray analysis suggest that E2F regulates also the expression of genes involved in DNA repair, differentiation, apoptosis, and mitosis (Ishida et al., 2001; Müller et al., 2001; Ren et al., 2002; Weinmann et al., 2002).
In humans, six E2F and two DP proteins have been identified. These E2F members, except for E2F-6, which lacks a transactivation domain, have not only a transcriptional activating function, but also repressive activity mediated by the retinoblastoma protein (pRb) or related pocket proteins (p107 and p130). The E2F/pocket protein complex blocks transcription by masking the transactivation domain of E2F and by directly binding target promoters to recruit chromatin-remodeling complexes (Dyson, 1998; Müller and Helin, 2000; DeGregori, 2002). Despite the similar biochemical properties of these five proteins, recent genetic and biochemical analyses revealed that a subgroup of members, E2F-1, E2F-2, and E2F-3, is involved in cell cycle progression to enter S phase, whereas E2F-4 and E2F-5 mainly function as a repressor of interaction with a pocket protein, p130, thereby mediating G1 (G0) arrest (Lindeman et al., 1998; Gaubatz et al., 2000; Paramio et al., 2000; Wang et al., 2000; Wu et al., 2001). Additionally, it has been shown that E2F-6 also plays an important role in G0 arrest by forming a complex containing p130, polycomb group protein, and mga/max on E2F-regulated promoters (Trimarchi et al., 2001; Ogawa et al., 2002).
Plants have a conserved regulatory pathway of G1(G0)-S phase transition mediated by E2F transcription factors. Promoters or the 5′-untranslated region of proliferating cell nuclear antigen (PCNA), ribonucleotide reductase (RNR), and MCM3 genes from tobacco (Nicotiana tabacum), rice (Oryza sativa), and Arabidopsis contain E2F sites that are responsible for the S phase or meristematic tissue-specific expression and that bind E2F/DP complexes from plants (Chaboute et al., 2000, 2002; Egelkrout et al., 2001, 2002; Kosugi and Ohashi, 2002a; Stevens et al., 2002). It has been also found that two different sequences for E2F binding are conserved in predicted 5′-upstream regions of a large number of replication- and mismatch repair-associated genes in Arabidopsis (Kosugi and Ohashi, 2002a). Many E2F and DP homologs have been isolated from plants (Ramírez-Parra et al., 1999; Sekine et al., 1999; Albani et al., 2000; Ramírez-Parra and Gutierrez, 2000). In structure and biochemical properties, they are very similar to known animal E2Fs and DPs, and their expression is restricted to meristematic regions in Arabidopsis and an early S phase in synchronized tobacco cells (De Veylder et al., 2002). Arabidopsis expresses six E2F: E2Fa-c and E2L1-3 (also referred to as AtE2F1-3 or DEL1-3; de Jager et al., 2001; Kosugi and Ohashi, 2002b; Mariconti et al., 2002; Vandepoele et al., 2002), and two DP: DPa and DPb (Magyar et al., 2000). Arabidopsis E2Fs exhibit an overall similarity to animal E2Fs and interact with DPa and DPb to stimulate their DNA-binding activity. E2Fa (AtE2F3) and E2Fb (AtE2F1) are strong transcriptional activators only when interacting with DPa, whereas E2Fc (AtE2F2) has no transactivational activity even in a complex with either DP protein (Kosugi and Ohashi, 2002c), and its degradation is stimulated in response to light (Del Pozo et al., 2002). The DPa-specific transactivation of AtE2F1 and AtE2F3 is conferred by the interaction-dependent nuclear import of these proteins through nuclear import- and nuclear export-like sequences conserved in E2Fs (Kosugi and Ohashi, 2002c). It has been shown that transient expression of E2Fa and/or DPa can stimulate S phase entry in Arabidopsis protoplasts (Rossignol et al., 2002). On the other hand, the other three E2F-like proteins, E2L1-3, have a limited similarity only to the DNA-binding domain of E2F proteins and can bind E2F sites as a monomer (Kosugi and Ohashi, 2002b; Mariconti et al., 2002). E2Ls repress E2F-regulated reporter genes, indicating that they function as repressors to antagonize E2F/DP-mediated transactivation.
It is quite important to understand how the control of G1(G0)-S transition by the E2F family affects plant growth, proliferation, and development. Although the functions of these plant E2Fs, as well as animal E2Fs, have been well studied in cultured cells, little is known about the role of E2F in whole organisms. In mammalian cells and imaginal discs of transgenic flies, overexpression of E2F induces S-phase entry followed by apoptosis. In many cases, the E2F-induced apoptosis abolishes the next round of cell cycle progression (Qin et al., 1994; Shan and Lee, 1994; Asano et al., 1996; Du et al., 1996), which hinders observations on developmental effects of overexpressed E2F in animals. A recent study using Arabidopsis plants overexpressing E2Fa and DPa cDNAs demonstrated that E2Fa and DPa synergistically induced ectopic cell division, enhanced endoreduplication, and caused developmental arrest at an early stage (De Veylder et al., 2002), indicating that the ectopic expression of E2Fa/DPa affects plant development.
We analyzed the effect of E2Fa (AtE2F3) and DPa on the regulation of the cell cycle and development using transgenic tobacco plants overexpressing these proteins. In contrast to the transgenic Arabidopsis plants, E2Fa-DPa double transgenic tobacco plants grew until a late stage and exhibited morphological abnormalities specific to certain organs. The ectopic expression of E2Fa and DPa induced not only endoreduplication and/or cell division, which were specifically enhanced in specific tissues of mature leaves, but also hormone-independent formation of callus in leaf strips cultured in vitro. These observations suggest that the activity of E2F can induce S phase entry and continuous cell cycle progression or cell cycle arrest, thereby modulating tissue and organ growth in the presence or absence of other cell cycle-associated factors such as mitotic factors.
RESULTS
Phenotype of Tobacco Plants Overexpressing E2Fa and DPa
We have previously demonstrated that E2Fa (AtE2F3) exerts its transactivation function when DPa is coexpressed in cultured tobacco cells (Kosugi and Ohashi, 2002c). To examine the role of E2Fa and DPa in planta, we produced transgenic tobacco plants carrying the E2Fa and DPa cDNA under the constitutive control of the cauliflower mosaic virus (CaMV) 35S promoter. Eight and nine plants overexpressing E2Fa and DPa cDNAs with variable levels of expression were obtained by a screening with northern analysis. Although none of the primary transgenic plants exhibited phenotypic alterations, plants of the next generation with high levels of the E2Fa transcript exhibited pale green leaves and a delay in growth and flowering (data not shown). In contrast, the next generation of DPa-overexpressing plants exhibited no phenotypic alteration, indicating that DPa alone had no effect on the growth and development.
Two lines of E2Fa-overexpressing plants with a high and low level of expression were crossed with a DPa-overexpressing plant. Seedlings of both crossed lines (designated H-F3D and L-F3D, respectively, for high and low levels of transgene expression) had small and downward curled cotyledons (data not shown). The H-F3D line exhibited severely delayed growth and almost all the plants died when 15 to 20 cm in height before or during flowering. All leaves of this line curled downward, and at later stages, developed chlorosis and spontaneous lesions reminiscent of the symptoms observed on a geminivirus-infected tobacco plant (Fig. 1, B and C). In contrast, the L-F3D line grew relatively normally in the early stages, but formed curled leaves similar to those of H-F3D plants at the adult stage (Fig. 1D). Other interesting phenotypes were observed in the flowers of both lines: petals of a rounder shape and pistils of a shorter length than wild-type flowers (Fig. 1, E and F).
Figure 1.
Phenotypes of 35S-E2Fa-DPa transgenic tobacco plants. A, Six-week-old 35S-β-glucuronidase (GUS) plant as a control. B, Nine-week-old 35S-E2Fa-DPa plant (H-F3D line) with a high level of expression of the transgenes. Leaves are partially curled downward and are rounder relative to the control plant. C, Sixteen-week-old H-F3D line. Leaves, especially upper leaves, develop chlorosis and spontaneous lesions. D, L-F3D line of 35S-E2Fa-DPa transgenics. Leaves are partially curled. The left shoot, which was formed at a later growth stage, has leaves with an extremely curled surface. E, Phenotype of flowers of a control 35S-GUS (left) and an L-F3D (right) plant. The round shape of the carpel of the L-F3D line contrasts with the sharp features of a control flower. F, Inner architecture of 35S-GUS (left) and L-F3D (right) flowers. Arrowheads indicate positions of stigma. The L-F3D line has a considerably shorter pistil than the control.:H-F3D and L-F3D, Lines of E2Fa-DPa double transformants with a high and low level of expression of the E2Fa transgene, respectively.
To confirm whether the phenotypical disorders in these crossed lines are linked to the expression levels of both transgenes, we performed reverse transcriptase (RT)-PCR analysis with primers specific for the E2Fa and DPa cDNAs, and a tobacco PCNA gene, an endogenous E2F target in tobacco. Whereas phenotypically normal leaves from L-F3D expressed low levels of the E2Fa and DPa genes, the deformed leaves from this line expressed high levels of both transgenes, comparable with the H-F3D line (Fig. 2A). The RT-PCR analysis further demonstrated that the endogenous PCNA gene was more active in the phenotypically altered leaves of both crossed lines. These results suggest that the transgene expression in the L-F3D line is developmentally regulated probably via an epigenetic effect by which the transgenes are reactivated at a later stage, and furthermore, that the phenotypical disorders observed are caused by the overexpression of E2Fa and DPa.
Figure 2.
Expression analyses of E2Fa and DPa transgenes. A, Semi-quantitative analysis by RT-PCR. First strand cDNAs were synthesized using 5 μg of total RNA isolated from mature leaves of the indicated plants. PCR was conducted with 12 cycles for 18S rRNA and 24 cycles for the other transcripts. Fractionated PCR products were visualized by staining with ethidium bromide. L-F3D and H-F3D indicate 35S-E2Fa-DPa transgenic lines with a low and high level of transgene expression. L-F3D-1 was derived from phenotypically normal leaves, whereas L-F3D-1′ and L-F3D-2 were from severely curled leaves. B, Electrophoretic mobility shift assay (EMSA) for E2F site-binding activity. Nuclear extracts were prepared from mature leaves of the indicated plants and were incubated with the indicated double-stranded oligonucleotide probes. te2f-1 and te2f-α contain different E2F-binding sequences and mte2f-1 contains a mutated sequence of te2f-1. The arrowhead indicates the position of a complex of E2Fa/DPa with the probe DNA.
The functional expression of the transgenes was further confirmed by EMSA with nuclear extracts prepared from mature leaves of the crossed and the original single transgenic lines. Nuclear extracts from the H-F3D line exhibited a high level of activity for binding to te2f-1 and te2f-α probes, each containing a different E2F site (Kosugi and Ohashi, 2002c), but not to the mutant probe, mte2f-1, despite an elevated activity of a nonspecific binding to all the probes, which was likely to be induced by the ectopic expression of E2Fa-DPa (Fig. 2B). This result indicates that the H-F3D line expresses a functional E2Fa/DPa complex, which causes the phenotypic difference from single E2Fa or DPa transgenic lines.
Ectopic Expression of S Phase-Specific Genes in E2Fa/DPa-Overexpressing Plants
To test whether in E2Fa-DPa transgenic plants, the expression of endogenous target genes other than the PCNA gene is affected, several tobacco genes, including cell cycle-regulated genes, were analyzed by northern-blot analysis. Fully expanded mature leaves of the control 35S-GUS and single transgenic plants showed no detectable or only a low level of expression of cell cycle-regulated genes; S phase-specific genes associated with DNA synthesis (PCNA and RNR) and chromosome assembly (histone H1 and histone H3), and M phase-specific genes (B-type cyclins; Fig. 3). Only the E2Fa-DPa double transgenic line (H-F3D) expressed ectopically high levels of DNA synthesis genes and moderate levels of histone genes, but not M phase and other cell cycle-unrelated genes involved in glycolysis (GAPDH) and photosynthesis (Cab). Also in immature leaves ranging from 10 to 15 mm, the plant expressed higher levels of these S phase genes than the control and other single E2Fa or DPa transgenic lines. In lines expressing low levels of E2Fa-DPa (L-F3D), moderate levels of PCNA transcripts were expressed in mature leaves, and the levels of B-type cyclin transcripts were similar to those of the control plants in mature and immature leaves (data not shown).
Figure 3.
Activation of S phase-specific genes in E2Fa-DPa transgenic plants. Total RNA was isolated from mature and immature leaves of the indicated transgenic plants and 20 μg was subjected to northern-blot analysis. The E2Fa-DPa plant is from the H-F3D line. GAPDH, Glyceraldehyde 3-P dehydrogenase; cab, light-harvesting chlorophyll a/b-binding protein.
It is not clear whether the S phase genes such as histones H1 and H3 are direct targets for E2F in plants, although histone genes have been observed to be regulated directly by E2F in mammalian cells (Yagi et al., 1995; Ren et al., 2002; Weinmann et al., 2002). Because we failed to find consensus binding sites of E2F in plant histone promoters, these histone genes may be indirectly activated through S phase entry induced by E2Fa-DPa.
Altered Tissue and Cell Specificity of the S Phase- But Not the M Phase-Specific Genes in E2Fa-DPa Transgenic Plants
A number of cell cycle-regulated genes in plants are strictly expressed in the cell cycle-dependent manner in meristematic tissues, as evident from the patch-like pattern of expression in shoot apical meristematic regions (Fobert et al., 1994). We examined whether the tissue- and cell-specific expression of cell cycle-regulated genes is affected in apical meristematic regions of the E2Fa-DPa plants by in situ hybridization analysis. Apical shoot tips of the control 35S-GUS plants were observed to express the PCNA and B-type cyclin (cyc29) mRNAs in specific cells in a patch-like pattern (Fig. 4, A and C). In shoot tips from the E2Fa-DPa plants, the cell cycle-specific expression of the PCNA mRNA was changed to a constitutive pattern (Fig. 4B), which correlates with the expression pattern of the E2Fa and DPa transgenes driven by the CaMV 35S promoter. In contrast to the S phase-specific genes, the expression pattern of the cyc29 mRNA was similar between the control and the E2Fa-DPa plants (Fig. 4, C and D). These results indicate that constitutive overexpression of E2Fa and DPa abrogates the cell cycle-dependent expression of S phase genes but not M phase genes.
Figure 4.
In situ hybridization analysis for PCNA and cyclin B transcripts in the apical meristematic region of E2Fa-DPa plants. A and B, Detection with digoxygenin (DIG)-labeled antisense PCNA RNA. C and D, Detection with DIG-labeled antisense RNA for cyc29, a B-type cyclin gene from tobacco. A and C, Cross-section of the apical meristematic region of a 35S-GUS tobacco plant as a control. B and D, Cross-section of a 35S-E2Fa-DPa transgenic line, H-F3D.
Endoreplication and Cell Division Stimulated in E2Fa-DPa Transgenic Plants
To assess whether the ectopic S phase entry induced by E2Fa/DPa confers an ectopic cell division or endoreplication in the transgenic plants, we observed microscopically cell morphology in mature and immature leaves. Cells in immature leaves of the E2Fa-DPa (H-F3D and L-F3D) and the control 35S-GUS plants were similar in size and number (Fig. 5, G–I). In contrast, mature leaves of these two E2F transgenic lines contained smaller and increased numbers of cells than the control plants, despite that the leaf size was similar between them (Fig. 5, A–C). The smaller cell size of both transgenic lines was more striking in the palisade tissues of the outer region of the leaves, especially at the edge (data not shown). This observation suggests that the E2Fa-DPa expression stimulates cell division in a cell type-specific manner.
Figure 5.
Altered phenotypes of mesophyll cells of E2Fa-DPa plants. A through C, Slices of mature leaves observed under white light. Pieces cut from mature leaves (about 12 cm in length) were sliced into 60-μm-thick sections. The leaves were from corresponding positions of plants with 14 to 18 leaves. D through F, Nuclei of mature leaves. The same slices, stained with 4′,6-diamidino-2-phenylindole (DAPI), were observed with an epifluorescent microscope. G through I, Nuclei of immature leaf sections stained with DAPI. A, D, and G, 35S-GUS tobacco plant as a control. B, E, and H, 35S-E2Fa-DPa transgenic line, H-F3D. C, F, and I, 35S-E2Fa-DPa transgenic line, L-H3D. Note that in the E2Fa-DPa plants, there was a decrease in cell size and increases in cell number and nuclear size (in H-F3D), and increased numbers of idioblastic cells.
Interestingly, the spongy tissues of the transgenic lines had an increased number of idioblastic cells (Fig. 5, A–C). Crystal idioblasts deposited with calcium oxalate crystals, appearing dark under a light microscope, have been observed in various tissues and plant species (for review, see Franceschi, 2001). The idioblastic cells that were observed in these transgenic plants were over 10-fold more numerous and often larger in size like embryonic calli in the mature leaves than those in the 35S-GUS plants (data not shown), suggesting that idioblasts are associated with the cell cycle.
To observe the nuclear morphology, these leaf sections were stained with DAPI. In immature leaves of the H-F3D plants, the nuclei were indistinguishable in size from those of the control 35S-GUS plants (Fig. 5, G–I). Also, nuclei in meristematic regions of shoot and root apical tips were similar in size in these plants (data not shown). In contrast, mature leaves of the H-F3D plants contained considerably larger nuclei than the 35S-GUS plants (Fig. 5, D and E). The enlarged nuclei were more abundant in cells in the spongy tissue and epidermal cells, including trichomes, than in the palisade tissue, suggesting the presence of tissue specificity in the E2Fa/DPa-activated endoreplication. In the L-F3D plants, nuclear size, unlike cellular size, in the mature leaves was apparently similar to the control (Fig. 5F).
We then measured the nuclear DNA content in the mature leaves. The amount of genomic DNA isolated from the E2Fa-DPa H-F3D line was approximately 8-fold that of the 35S-GUS plants and the E2Fa or DPa single transgenic line (Fig. 6A). We also measured ploidy levels in the mesophyll cells by flow cytometric analysis. Almost all nuclei in mature leaves of the 35S-GUS plant had a ploidy level of 2C. In contrast, the L-F3D line exhibited an increased number of nuclei with a ploidy level of 4C, and the H-F3D line had even higher ploidy levels, varying from 2C to 32C, with the most abundant population having 4C (Fig. 6B). These results indicate that the ectopic E2Fa-DPa expression causes endoreduplication by stimulating DNA synthesis in terminally differentiated tissues.
Figure 6.
DNA content and ploidy level. A, DNA content of mature leaves. Total DNA was isolated from mature leaves (200 mg) of the indicated transgenic plants, 35S-GUS (control), and 35S-DPa, -E2Fa, and -E2Fa-DPa/H-F3D. DNA content was calculated as micrograms relative to milligrams of protein isolated from the same leaf. Bars represent mean values of triplicate data. B, Flow cytometric analysis of nuclear DNA content of mature leaves. Nuclei from the indicated transgenic plants were stained with DAPI and the degree of fluorescence was represented as DNA content relative to the main peak of 35S-GUS plants (control) given a ploidy level of 2C.
Induction of Callus Formation on Cultured Leaf Strips Independent of Hormones
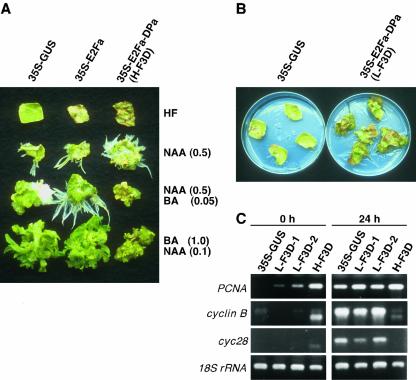
We examined the effect of plant hormones stimulating cell growth and division on in vitro cultures of leaf strips from E2Fa-DPa plants. Leaf strips from the E2Fa or DPa single transgenic line induced regeneration of roots, callus, or shoots on a medium containing α-naphthalene acetic acid (NAA), or combinations of NAA and 6-benzylaminopurine (BA), similar to those of the control 35S-GUS plant (Fig. 7A). Unlike these plants, leaf strips of the H-F3D line did not induce shoot regeneration on a medium containing 0.1 μg mL–1 NAA and 1.0 μg mL–1 BA, but continued to form embryonic calli (Fig. 7A), whereas those of the L-F3D line normally regenerated shoots (data not shown), indicating that a higher level of E2F activity inhibits the regeneration of shoots. A more prominent difference between the E2Fa-DPa lines and others was observed in the leaf strips cultured in the absence of the hormones. The single transgenic lines and the control plant formed no visible callus on the hormone-free medium, although incidentally formed small calli were observed on some leaf strips (data not shown). In contrast, leaf strips from the E2Fa-DPa plants formed greater numbers of green calli at the cut edges of the strips on the hormone-free medium (Fig. 7, A and B), although the growth of the callus was slow; the time required for the formation was three to four times longer than in leaf strips cultured with the hormones. This indicates that overexpression of E2Fa-DPa is sufficient to induce the formation of callus in culture. It was interesting that the H-F3D leaf tissues, except the formed calli, died at an early stage of the culture (Fig. 7A), suggesting that a high level of E2F activity induces a cell death of the leaves cultured in the absence of phytohormones.
Figure 7.
Hormone-independent callus growth of in vitro-cultured leaf sections of E2Fa-DPa plants. A, Effect of hormone combinations on regeneration of callus, roots, and shoots. Strips of mature leaves of 35S-GUS, -E2Fa, and -E2Fa-DPa/H-F3D transgenic tobacco plants were cultured with 0.5× Murashige and Skoog medium containing the indicated hormones at the given concentrations for 4 weeks. B, Callus growth in the L-F3D line on hormone-free medium. Leaf strips from 35S-GUS and -E2Fa-DPa/L-F3D plants were cultured with 0.5× Murashige and Skoog medium without hormones for 8 weeks. C, Expression of cell cycle genes in leaf strips cultured with hormone-free medium. Leaf strips from the indicated plants were cultured with 0.5× Murashige and Skoog medium without hormones for 0 or 24 h. Expression of the S phase (PCNA) and M phase (cyclin B and cyc29) genes was semiquantified by RT-PCR analysis.
The calli of the H-F3D line stopped showing cell proliferation at an early stage, while 1 to 3 mm in size, whereas calli of the L-F3D line continued to grow (Fig. 7B). To examine the difference in the growth competence of the calli of both lines of transgenic plants, RT-PCR was conducted to detect the expression of S and M phase-specific genes in the in vitro cultured leaf strips. The PCNA transcript was expressed not only in leaf strips of both lines of transgenic plants, but also in the control 35S-GUS plant when the strips were cultured with the hormone-free medium for 24 h (Fig. 7C). Although B-type cyclin transcripts were also produced on culturing the leaf strips of the 35S-GUS and L-F3D plants, leaf strips from the H-F3D line had fewer transcripts of the cyclin genes (Fig. 7C). These results suggest that the growth arrest of the H-F3D calli is due to impaired induction of the B-type cyclin genes in the in vitro culture system.
DISCUSSION
Morphological Change Caused by Ectopic E2F-DP Expression in Plants
Our previous analyses have demonstrated that Arabidopsis expresses two DP (DPa and DPb) and three E2F (E2Fa-c/AtE2F1-3) homologs with an overall similarity to animal E2Fs. E2Fa and E2Fb acquire the function as potent transcriptional activators by stimulating DNA binding and nuclear import upon interacting with DPa (Kosugi and Ohashi, 2002c). This synergistic effect of E2F and DP functions was also observed in this study using transgenic tobacco plants. Double transgenic plants but not single transformants for E2Fa or DPa exhibited altered phenotypes and the ectopic activation of target genes. Outstanding phenotypes observed in the E2Fa-DPa double transgenic plants included a delay in growth and a curling of the leaves. These phenotypes are more prominent in H-F3D, a line in which the E2Fa and DPa transgenes are highly expressed, than in a low-level expressor like L-F3D. Similar phenotypes have been observed in double transgenic Arabidopsis plants containing E2Fa and DPa; growth arrest early in development and leaves curled along their proximal-distal axis (De Veylder et al., 2002). We also tried to generate similar double transgenic lines of Arabidopsis, but failed due to embryonic lethality or growth arrest early in development (S. Kosugi and Y. Ohashi, unpublished data). The growth inhibitory effect of the overexpressed E2Fa-DPa seems to be exerted more strongly in Arabidopsis plants.
The phenotype accompanying leaf curving and chlorosis observed in the H-F3D line is similar to that observed in tobacco (Nicotiana benthamiana) plants infected with geminiviruses such as tomato golden mosaic virus (TGMV) and squash leaf curl virus (von Arnim and Stanley, 1992; Pascal et al., 1993). A replication protein AL1 encoded by TGMV, as well as TGMV infection itself, can induce ectopic expression of PCNA in the mature leaves, as the E2Fa/DPa complex does. It has been speculated that the replication protein sequesters an Rb-related protein (RBR) that forms a complex with E2F and relieves an RBR-mediated repression (Nagar et al., 1995; Kong et al., 2000). However, it is unknown whether the phenotypes and S phase gene activation observed in the E2Fa-DPa plants are due to the relief of RBR repression or the effect of direct activation by E2Fa-DPa.
Unlike the E2Fa-DPa transgenic Arabidopsis, all of the L-F3D and a few of the H-F3D tobacco plants continued to grow until the flowering stage. All flowers produced in the L-F3D plants displayed phenotypes of shorter pistils and rounder-shaped petals than wild-type flowers, despite that the CaMV 35S promoter directs a ubiquitous expression and phenotypes of other organs such as the size of petals and anthers were less affected. These observations suggest that the activity for driving S phase entry by E2Fa and DPa can modulate organ size or morphology by regulating growth of the organs. The organ and tissue specificity of the morphological change may be determined by other factors modulating the E2Fa/DPa activity or by mitotic factors, which are abundant in the organs showing altered phenotypes. If the E2F activity or modulating effects are distributed differently among tissues or species, or in different environments, plant architecture could be at least partly responsible for the control of cell proliferation mediated by E2F activity.
Enhanced Endoreduplication and Cell Division by Overexpression of E2F in Terminally Differentiated Tissues
In animals, overexpression of E2F induces S-phase entry followed by apoptosis (Johnson et al., 1993; Qin et al., 1994; Shan and Lee, 1994; Asano et al., 1996; Du et al., 1996). Ectopic E2F expression in transgenic plants also induces S phase entry accompanied by activation of S phase-specific genes, but not the subsequent typical apoptosis. Although the H-F3D line developed spontaneous lesions and chlorosis at the adult stage and died before or during flowering, apoptosis-like features in the nuclei and nuclear DNA were not observed (data not shown). Instead, overexpression of E2F-DP led to continuous DNA synthesis without cell division (endoreduplication) and ectopic cell division in planta rather than apoptosis and cell transformation. Endoreduplication is a frequently and naturally occurring phenomenon in plants and animals, depending on species, tissues, and environments (Joubes and Chevalier, 2000; Edgar and Orr-Weaver, 2001). The enhanced endoreduplication observed in mature leaves of the transgenic tobacco seems to be caused by a combination of the increased activity of E2Fa-DPa and a limited expression of mitotic genes. In the H-F3D line, the level of the B-type cyclin gene expression was similar to that in the control plant, in contrast to the activated expression of S phase genes. It is likely that the enhanced endoreduplication in mature leaves of the transgenic plants is attributable to the sustained S phase state followed by continuous S phase-gene activation, which is caused by an imbalance in the activity for S and M phase entry. A recent study showing that ectopic expression of a B-type cyclin in trichome of Arabidopsis increases cell division rather than enhances endoreduplication supports our notion (Schnittger et al., 2002b).
On the other hand, there was an increase in the number of small cells in the mesophyll tissue of the E2Fa-DPa plants, despite that the size and developmental stage of the leaves observed was similar between the control and transgenic plants. This indicates that the enhanced E2F activity induces ectopic cell division in tobacco. It has been shown that E2Fa-DPa transgenic Arabidopsis lines display ectopic cell division in the cotyledons and hypocotyls (De Veylder et al., 2002). Depending on the cell and tissue type, or environment, E2F activity may be sufficient to induce S and M phase entry. However, E2F/DP could inhibit cell expansion because even the L-F3D tobacco plant that exhibited pronounced ectopic cell division caused no increase in the leaf size rather a decrease in the cell size.
In the leaves of E2Fa-DPa tobacco plants, there was a greater increase in the number of cells on the palisade side in the regions toward the edge (outer region), indicating that cell proliferation is stimulated more in the outer palisade tissue than in the inner region and spongy tissue. Taken together with the fact that the enlargement of nuclei is remarkable in spongy tissue of H-F3D plants, mitotic activity on the abaxial side is likely to be more limited than that on the adaxial side in the leaf. These observations further suggest that the phenotype of leaf curling is caused by an accelerated proliferation of adaxial mesophyll cells in the outer region and by repressed cell division on the abaxial side.
Hormone-Independent Callus Formation
The E2Fa-DPa double transgenic plants had the ability to form callus when their leaf sections were cultured with a hormone-free medium. Our study using RT-PCR analyses demonstrates that the culturing of leaf sections in the absence of hormones, probably in combination with the wounding effect, induces the re-entry of terminally differentiated cells into the cell cycle, as shown by the elevated expression of the PCNA and B-type cyclin transcripts in the cultured leaf strips. Leaf sections from wild-type tobacco incidentally formed small calli when cultured in the absence of hormones, but there was no further callus growth (data not shown). These observations suggest that the ectopic expression of E2Fa and DPa stimulates the growth of calli but not the initiation. We speculate that once a callus has formed on cultured leaf strips, mitotic activity is constantly provided, but activity for S phase entry is not sufficient to stimulate further growth of the callus. Calli produced in the H-F3D line stopped growing at an early stage, apparently in correlation with the impaired expression of B-type cyclin transcripts in the cultured H-F3D leaves. Considering that the H-H3D plants exhibited delayed growth and a lower degree of ectopic cell division than the L-F3D plants, a higher level of E2Fa-DPa expression may inhibit expression of mitotic genes.
Our observations suggest that a high level of E2F activity induces S phase entry and enhances endoreduplication in terminally differentiated tissues, and that additional mitotic activity to enter M phase could stimulate continuous cell cycle progression. It has been reported that the overexpression of Arabidopsis D-type cyclin species stimulates growth in tobacco plants (Cockcroft et al., 2000) and cytokinin-independent growth of calli (Riou-Khamlichi et al., 1999) or enhanced cell division of trichomes (Schnittger et al., 2002a) in Arabidopsis. Because some D-type cyclins have been implicated in regulating the G1-S and the G2-M phase transition, an increase in activities to enter the S and M phases would be necessary for enhanced cell proliferation in plants. In addition to the promotion of S phase entry using E2F-DP transgenes, further stimulation of M phase entry by manipulating mitotic regulators such as B-type cyclins and their transcriptional regulators may improve the growth rate of plants. Our observations further indicate that an enhanced E2F activity drives cell proliferation and endoreduplication in a cell type-specific manner. The morphological change observed on some specific tissues and organs of the transgenic plants could attribute to the cell type-specific effect of E2F. These suggest that the activity driving S phase entry is closely linked to plant growth and development.
MATERIALS AND METHODS
Plasmid Construction
For plant transformation constructs, XbaI-XhoI fragments from the E2Fa (AtE2F3) and DPa cDNA clones (Kosugi and Ohashi, 2002c) were inserted into the SpeI-XhoI sites of a binary vector pBI-CEPT5, which was generated by replacing a HindIII-SacI fragment of the pBI101 vector (Clontech, Palo Alto, CA) with the CaMV 35S promoter linked with the downstream multi-cloning sites from the pCEP5 vector (Kosugi and Ohashi, 2000).
Plant Transformation and in Vitro Culture of Leaf Strips
The binary vectors were introduced into Agrobacterium tumefaciens LBA4404 and were used for transformation of tobacco (Nicotiana tabacum) as described previously (Kosugi et al., 1991). Kanamycin-resistant transgenic tobacco plants were screened by northern analysis for expression of the transgenes. Mature leaves were excised from transgenic plants, cut into pieces 1.5 to 2.0 cm square after being sterilized with 3% (w/v) hypochlorite solution, and cultured on 0.5× Murashige-Skoog salt medium (Murashige and Skoog, 1962) containing 3% (w/v) Suc and/or a combination of NAA as an auxin and BA as a cytokinin. The plates were cultured for 4 to 8 weeks at 28°C in the dark.
RT-PCR Analysis
First strand cDNA was synthesized with 5 μg of total RNA, as described previously (Kosugi and Ohashi, 2002b). RT-PCR was performed using 1-μL aliquots of the 50 μL of the first strand cDNA products. PCR products were amplified for 24 cycles for the E2Fa, DPa, and PCNA mRNAs and for 12 cycles for 18S rRNA, separated by electrophoresis on 0.8% (w/v) agarose gel, and visualized by staining with ethidium bromide. PCR products were amplified in a liner manner at least within 24 cycles.
EMSAs
The preparation of nuclear extracts from tobacco leaves and EMSAs were performed as previously described (Kosugi and Ohashi, 1997). The double-stranded oligonucleotide probes used for EMSAs, te2f-1, mte2f-1, and te2f-α were reported elsewhere (Kosugi and Ohashi, 2002a). te2f-1 (5′-TCGAGTTTTCCCGCCTTTTTTCCCGCCTTGTCGA) and te2f-α (5′-TCGAGTTTTGGCGGCTTTTTTGGCGGCTTGTCGA) contained different E2F-binding sequences, TTTCCCGC and TTTGGCGG, respectively. mte2f-1 (5′-TCGAGTTTTCCAACCTTTTTTCCAACCTTGTCGA, in which mutated nucleotides are underlined), is a mutant of te2f-1.
Northern-Blot Analysis
Total RNA was isolated from mature and immature leaves of tobacco with Trizol reagent, according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). RNA (20 μg) was fractionated in 1% (w/v) formaldehyde-agarose gels and was transferred to a nylon filter (Hybond N+; Amersham Biosciences, Piscataway, NJ) as described (Sambrook et al., 1989). Hybridization was performed at 68°C in a solution containing 0.2 m Na2HPO4, pH 7.2, 1 mm EDTA, 7% (w/v) SDS, and 1% (w/v) blocking reagent (Roche Diagnostics, Mannheim, Germany). Filters were washed twice in a buffer containing 20 mm Na2HPO4 (pH 7.2) and 0.5% (w/v) SDS at room temperature and then once more at 68°C. DNA probes were prepared from gel-purified fragments, which were amplified by RT-PCR with primers specific for tobacco cDNAs based on sequence information deposited in databases, and were labeled with α-32P-dCTP using a Rediprimed Labeling kit (Amersham Biosciences). The cDNA fragments corresponded to the entire coding sequences of the E2Fa, DPa, and tobacco histone H1 (H1c12, GenBank accession no. L29456) cDNAs and nucleotides 246 through 778 for tobacco PCNA (AJ012662), 166 through 977 for RNR2 (X92443), 22 through 630 for histone H3 (B015760), 280 through 854 for GAPDH/GapC (M14419), 43 through 756 for light-harvesting/cab (X64198), and 1,190 through 1,542 and 1,087 through 1,667 for B-type cyclins (Nt-CYM, D89635, and Ntcyc29; D50737). A cDNA fragment of an Arabidopsis 18S rRNA (X16077) consisting of nucleotides 659 through 1,108 was used for detection of the tobacco 18S rRNA to verify the equal loading of RNA samples.
In Situ Hybridization
Shoot tips from 35S-GUS and 35S-E2Fa-DPa plants were fixed with 4% (w/v) paraformaldehyde. The preparation of paraffin sections and in situ detection using DIG-labeled RNA probe were conducted as described (Kouchi et al., 1995). DIG-labeled sense and antisense RNAs were synthesized in vitro by transcription of linearized pGEM-3Zf(+) plasmids (Promega, Madison, WI) containing the cDNAs for PCNA, histone H4, and cyc29 from tobacco, all of which corresponded to those used for northern analysis, and were labeled using a DIG RNA labeling kit (SP6/T7; Roche Diagnostics). Nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Roche Diagnostics) were used as a substrate for alkaline phosphatase-conjugated anti-DIG antibody (Roche Diagnostics). The color reaction was conducted at 25°C for 6 h (for histone H4), 10 h (for PCNA), and 16 h (for cyc29).
Observation of Nuclei in Leaf Sections
Strips of mature and immature leaves from 35S-GUS and 35S-E2Fa-DPa transgenic plants were embedded in 5% (w/v) agar and sliced into 60-μm thick cross-sections using a microslicer (DTK-1000; Dohan-EM, Kyoto). The sections were incubated with 20% (w/v) ethanol containing 1 μg mL–1 DAPI for 5 min. Nuclei were observed using an epifluorescent microscope (model AX70; Olympus, Tokyo).
DNA Measurement
Nuclear DNA was isolated from 200 mg of mature leaves with a Nucleon PhytoPure kit according to the manufacturer's instructions (Amersham Biosciences). The isolated DNA was quantified using a spectrophotomer and simultaneously by a fluorometrical method with DAPI, and was normalized relative to the protein content of the leaves sampled.
Flow Cytometric Analysis
Leaf strips from 35S-GUS and 35S-E2Fa-DPa plants were chopped in Galbraith's buffer (Galbraith et al., 1983) containing 1 μg mL–1 DAPI with a razor blade to release nuclei. Filtrates obtained with a 100-μm nylon mesh were analyzed using a Ploidy Analyzer (Partec, Münster, Germany).
Acknowledgments
We thank Dr. Tetsuji Kakutani for advice on the culture, transformation, and crossing of Arabidopsis plants. We are grateful to Drs. Akemi Tagiri and Taka Murakami for tips on sectioning of tissues and in situ hybridization technique.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.025080.
References
- Albani D, Mariconti L, Ricagno S, Pitto L, Moroni C, Helin K, Cella R (2000) DcE2F, a functional plant E2F-like transcriptional activator from Daucus carota. J Biol Chem 275: 19258–19267 [DOI] [PubMed] [Google Scholar]
- Asano M, Nevins JR, Wharton RP (1996) Ectopic E2F expression induces S phase and apoptosis in Drosophila imaginal discs. Genes Dev 10: 1422–1432 [DOI] [PubMed] [Google Scholar]
- Chaboute ME, Clement B, Philipps G (2002) S phase and meristem-specific expression of the tobacco RNR1b gene is mediated by an E2F element located in the 5′ leader sequence. J Biol Chem 277: 17845–17851 [DOI] [PubMed] [Google Scholar]
- Chaboute ME, Clement B, Sekine M, Philipps G, Chaubet-Gigot N (2000) Cell cycle regulation of the tobacco ribonucleotide reductase small subunit gene is mediated by E2F-like elements. Plant Cell 12: 1987–2000 [PMC free article] [PubMed] [Google Scholar]
- Cockcroft CE, den Boer BG, Healy JM, Murray JA (2000) Cyclin D control of growth rate in plants. Nature 405: 575–579 [DOI] [PubMed] [Google Scholar]
- DeGregori J (2002) The genetics of the E2F family of transcription factors: shared functions and unique roles. Biochim Biophys Acta 1602: 131–150 [DOI] [PubMed] [Google Scholar]
- de Jager SM, Menges M, Bauer UM, Murra JA (2001) Arabidopsis E2F1 binds a sequence present in the promoter of S-phase-regulated gene AtCDC6 and is a member of a multigene family with differential activities. Plant Mol Biol 47: 555–568 [DOI] [PubMed] [Google Scholar]
- Del Pozo JC, Boniotti MB, Gutierrez C (2002) Arabidopsis E2Fc functions in cell division and is degraded by the ubiquitin-SCF(AtSKP2) pathway in response to light. Plant Cell 14: 3057–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GT, de Almeida Engler J, Ormenese S, Maes S, Naudts M, Van Der Schueren E, Jacqmard A et al. (2002) Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa-DPa transcription factor. EMBO J 21: 1360–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Xie JE, Dyson N (1996) Ectopic expression of dE2F and dDP induces cell proliferation and death in the Drosophila eye. EMBO J 15: 3684–3692 [PMC free article] [PubMed] [Google Scholar]
- Dyson N (1998) The regulation of E2F by pRB-family proteins. Genes Dev 12: 2245–2262 [DOI] [PubMed] [Google Scholar]
- Edgar BA, Orr-Weaver TL (2001) Endoreplication cell cycles: more for less. Cell 105: 297–306 [DOI] [PubMed] [Google Scholar]
- Egelkrout EM, Mariconti L, Settlage SB, Cella R, Robertson D, Hanley-Bowdoin L (2002) Two E2F elements regulate the proliferating cell nuclear antigen promoter differently during leaf development. Plant Cell 14: 3225–3236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelkrout EM, Robertson D, Hanley-Bowdoin L (2001) Proliferating cell nuclear antigen transcription is repressed through an E2F consensus element and activated by geminivirus infection in mature leaves. Plant Cell 13: 1437–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fobert PR, Coen ES, Murphy GJ, Doonan JH (1994) Patterns of cell division revealed by transcriptional regulation of genes during the cell cycle in plants. EMBO J 13: 616–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V (2001) Calcium oxalate in plants. Trends Plant Sci 6: 331. [DOI] [PubMed] [Google Scholar]
- Galbraith DWHK, Maddox JM, Ayres NM, Sharma DP, Firoozabadi E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220: 1049–1051 [DOI] [PubMed] [Google Scholar]
- Gaubatz S, Lindeman GJ, Ishida S, Jakoi L, Nevins JR, Livingston DM, Rempel RE (2000) E2F4 and E2F5 play an essential role in pocket protein-mediated G1 control. Mol Cell 6: 729–735 [DOI] [PubMed] [Google Scholar]
- Ishida S, Huang E, Zuzan H, Spang R, Leone G, West M, Nevins JR (2001) Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol Cell Biol 21: 4684–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DG, Schwarz JK, Cress WD, Nevins JR (1993) Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature 365: 349–352 [DOI] [PubMed] [Google Scholar]
- Joubes J, Chevalier C (2000) Endoreduplication in higher plants. Plant Mol Biol 43: 735–745 [DOI] [PubMed] [Google Scholar]
- Kong LJ, Orozco BM, Roe JL, Nagar S, Ou S, Feiler HS, Durfee T, Miller AB, Gruissem W, Robertson D et al. (2000) A geminivirus replication protein interacts with the retinoblastoma protein through a novel domain to determine symptoms and tissue specificity of infection in plants. EMBO J 19: 3485–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y (1997) PCF1 and PCF2 specifically bind to cis elements in the rice proliferating cell nuclear antigen gene. Plant Cell 9: 1607–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y (2000) Cloning and DNA-binding properties of a tobacco ethylene-insensitive3 (EIN3) homolog. Nucleic Acids Res 28: 960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y (2002a) E2F sites that can interact with E2F proteins cloned from rice are required for meristematic tissue-specific expression of rice and tobacco proliferating cell nuclear antigen promoters. Plant J 29: 45–59 [DOI] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y (2002b) E2Ls, E2F-like repressors of Arabidopsis that bind to E2F sites in a monomeric form. J Biol Chem 277: 16553–16558 [DOI] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y (2002c) Interaction of the Arabidopsis E2F and DP proteins confers their concomitant nuclear translocation and transactivation. Plant Physiol 128: 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Suzuka I, Ohashi Y, Murakami T, Arai Y (1991) Upstream sequences of rice proliferating cell nuclear antigen (PCNA) gene mediate expression of PCNA-GUS chimeric gene in meristems of transgenic tobacco plants. Nucleic Acids Res 19: 1571–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi H, Sekine M, Hata S (1995) Distinct classes of mitotic cyclins are differentially expressed in the soybean shoot apex during the cell cycle. Plant Cell 7: 1143–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavia P, Jansen-Dürr P (1999) E2F target genes and cell-cycle checkpoint control. Bioessays 21: 221–230 [DOI] [PubMed] [Google Scholar]
- Lindeman GJ, Dagnino L, Gaubatz S, Xu Y, Bronson RT, Warren HB, Livingston DM (1998) A specific, nonproliferative role for E2F-5 in choroid plexus function revealed by gene targeting. Genes Dev 12: 1092–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar Z, Atanassova A, De Veylder L, Rombauts S, Inzé D (2000) Characterization of two distinct DP-related genes from Arabidopsis thaliana. FEBS Lett 486: 79–87 [DOI] [PubMed] [Google Scholar]
- Mariconti L, Pellegrini B, Cantoni R, Stevens R, Bergounioux C, Cella R, Albani D (2002) The E2F family of transcription factors from Arabidopsis thaliana: novel and conserved components of the retinoblastoma/E2F pathway in plants. J Biol Chem 277: 9911–9919 [DOI] [PubMed] [Google Scholar]
- Müller H, Bracken AP, Vernell R, Moroni MC, Christians F, Grassilli E, Prosperini E, Vigo E, Oliner JD, Helin K (2001) E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev 15: 267–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H, Helin K (2000) The E2F transcription factors: key regulators of cell proliferation. Biochim Biophys Acta 1470: M1–12 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Nagar S, Pedersen TJ, Carrick KM, Hanley-Bowdoin L, Robertson D (1995) A geminivirus induces expression of a host DNA synthesis protein in terminally differentiated plant cells. Plant Cell 7: 705–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Ishiguro K, Gaubatz S, Livingston DM, Nakatani Y (2002) A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 296: 1132–1136 [DOI] [PubMed] [Google Scholar]
- Paramio JM, Segrelles C, Casanova ML, Jorcano JL (2000) Opposite functions for E2F1 and E2F4 in human epidermal keratinocyte differentiation. J Biol Chem 275: 41219–41226 [DOI] [PubMed] [Google Scholar]
- Pascal E, Goodlove PE, Wu LC, Lazarowitz SG (1993) Transgenic tobacco plants expressing the geminivirus BL1 protein exhibit symptoms of viral disease. Plant Cell 5: 795–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XQ, Livingston DM, Kaelin WG Jr, Adams PD (1994) Deregulated transcription factor E2F-1 expression leads to S-phase entry and p53-mediated apoptosis. Proc Natl Acad Sci USA 91: 10918–10922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Parra E, Gutierrez C (2000) Characterization of wheat DP, a heterodimerization partner of the plant E2F transcription factor which stimulates E2F-DNA binding. FEBS Lett 486: 73–78 [DOI] [PubMed] [Google Scholar]
- Ramírez-Parra E, Xie Q, Boniotti MB, Gutierrez C (1999) The cloning of plant E2F, a retinoblastoma-binding protein, reveals unique and conserved features with animal G(1)/S regulators. Nucleic Acids Res 27: 3527–3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA, Dynlacht BD (2002) E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev 16: 245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JA (1999) Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283: 1541–1544 [DOI] [PubMed] [Google Scholar]
- Rossignol P, Stevens R, Perennes C, Jasinki S, Cella R, Tremousaygue D, Bergounioux C (2002) AtE2F-a and AtDP-a, members of the E2F family of transcription factors, induce Arabidopsis leaf cells to re-enter S phase. Mol Genet Genomics 266: 995–1003 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, and Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schnittger A, Schobinger U, Bouyer D, Weinl C, Stierhof YD, Hulskamp M (2002a) Ectopic D-type cyclin expression induces not only DNA replication but also cell division in Arabidopsis trichomes. Proc Natl Acad Sci USA 99: 6410–6415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittger A, Schobinger U, Stierhof YD, Hulskamp M (2002b) Ectopic B-type cyclin expression induces mitotic cycles in endoreduplicating Arabidopsis trichomes. Curr Biol 12: 415–420 [DOI] [PubMed] [Google Scholar]
- Sekine M, Ito M, Uemukai K, Maeda Y, Nakagami H, Shinmyo A (1999) Isolation and characterization of the E2F-like gene in plants. FEBS Lett 460: 117–122 [DOI] [PubMed] [Google Scholar]
- Shan B, Lee WH (1994) Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol Cell Biol 14: 8166–8173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R, Mariconti L, Rossignol P, Perennes C, Cella R, Bergounioux C (2002) Two E2F sites in the Arabidopsis MCM3 promoter have different roles in cell cycle activation and meristematic expression. J Biol Chem 277: 32978–32984 [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Fairchild B, Wen J, Lees JA (2001) The E2F6 transcription factor is a component of the mammalian Bmi1-containing polycomb complex. Proc Natl Acad Sci USA 98: 1519–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepoele K, Raes J, De Veylder L, Rouzé P, Rombauts S, Inzé D (2002) Genome-wide analysis of core cell cycle genes in Arabidopsis. Plant Cell 14: 903–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arnim A, Stanley J (1992) Determinants of tomato golden mosaic virus symptom development located on DNA B. Virology 186: 286–293 [DOI] [PubMed] [Google Scholar]
- Wang D, Russell JL, Johnson DG (2000) E2F4 and E2F1 have similar proliferative properties but different apoptotic and oncogenic properties in vivo. Mol Cell Biol 20: 3417–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann AS, Yan PS, Oberley MJ, Huang TH, Farnham PJ (2002) Isolating human transcription factor targets by coupling chromatin immunoprecipitation and CpG island microarray analysis. Genes Dev 16: 235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT, Nuckolls F, Giangrande P, Wright FA, Field SJ et al. (2001) The E2F1–3 transcription factors are essential for cellular proliferation. Nature 414: 457–462 [DOI] [PubMed] [Google Scholar]
- Yagi H, Kato T, Nagata T, Habu T, Nozaki M, Matsushiro A, Nishimune Y, Morita T (1995) Regulation of the mouse histone H2A: X gene promoter by the transcription factor E2F and CCAAT binding protein. J Biol Chem 270: 18759–18765 [DOI] [PubMed] [Google Scholar]