Abstract
The Arabidopsis type II peroxiredoxin (PRXII) family is composed of six different genes, five of which are expressed. On the basis of the nucleotide and protein sequences, we were able to define three subgroups among the PRXII family. The first subgroup is composed of AtPRXII-B, -C, and -D, which are highly similar and localized in the cytosol. AtPRXII-B is ubiquitously expressed. More striking is the specific expression of AtPRXII-C and AtPRXII-D localized in pollen. The second subgroup comprises the mitochondrial AtPRXII-F, the corresponding gene of which is expressed constitutively. We show that AtPRXII-E, belonging to the last subgroup, is expressed mostly in reproductive tissues and that its product is addressed to the plastid. By in vitro enzymatic experiments, we demonstrate that glutaredoxin is the electron donor of recombinant AtPRXII-B for peroxidase reaction, but the donors of AtPRXII-E and AtPRXII-F have still to be identified.
Plants generate reactive oxygen species (ROS) such as the superoxide anion, hydrogen peroxide, or the hydroxyl radical as by-products of electron transport chains in chloroplast and mitochondria, photorespiration in the peroxisome, and cell wall oxidases and peroxidases (Dat et al., 2000). ROS are necessary for plants, because they participate in signal transduction (Karpinski et al., 1999; Orozco-Cardenas et al., 2001; Mullineaux and Karpinski, 2002) and play a role in response to pathogen attack (Wojtaszek, 1997; Bolwell, 1999; Dat et al., 2000). However, biotic or abiotic stress may promote ROS generation and break the redox balance of the cell. To protect macromolecules such as lipids, proteins, or nucleic acids from damage caused by ROS, cells contain a large variety of antioxidant enzymes that include catalase, superoxide dismutase, ascorbate- and glutathione-dependent peroxidases, and the more recently described peroxiredoxin (PRX) family. The PRX family was first described as alkyl hydroperoxide reductase C (Jacobson et al., 1989) and later as thiol-specific antioxidant in Brewer's yeast (Saccharomyces cerevisiae) and Escherichia coli (Chae et al., 1994). In contrast to other peroxidases, PRX enzymes do not have redox cofactors such as metal or prosthetic groups. They reduce hydrogen peroxides and alkyl peroxides to water and alcohols, respectively, by using reducing equivalents. These reducers are derived specifically from thiol-containing donor molecules such as thioredoxin (TRX; Chae et al., 1994; Kwon et al., 1994; Kang et al., 1998), glutaredoxin (GRX; Rouhier et al., 2001, 2002), and the flavin containing AhpF, a subunit of the Salmonella typhimurium alkyl hydroperoxide reductase highly similar to TRX reductase (Jacobson et al., 1989; Tartaglia et al., 1990). Members of the PRX family have now been identified in a wide variety of organisms ranging from archae and eubacteria to eukaryotes, including vertebrates and plants.
All PRX proteins contain a conserved Cys in their N-terminal part and some of them possess a second conserved Cys residue. The strictly conserved Cys residue is oxidized during the mechanism of ROS scavenging (Schroder and Ponting, 1998; Seo et al., 2000). To obtain regenerated PRX, the oxidized Cys residue forms a disulfide bridge, which is next reduced by disulfide oxidoreductases. On the basis of the number and position of conserved Cys residues, the PRX proteins are classified in several sub-families (Choi et al., 1999). Phylogenetic analysis using a wide range of PRX amino acid sequences and including related sequences of unidentified function (Verdoucq et al., 1999), allows the division of PRX into four main groups. The 2Cys-PRX group corresponds to PRX containing two conserved Cys residues, the 1Cys-PRX group includes PRX with only one conserved Cys residue, and the type II PRX family is composed of another type of PRX with two conserved Cys residues. In addition, a fourth group of PRX, called type Q PRX, has also been described and contains homologs of the E. coli bacterioferritin-associated protein (Jeong et al., 2000; Kong et al., 2000).
In plants, the four types of PRX have been described (Baier and Dietz, 1996; Horling et al., 2002; Konig et al., 2002). Two Arabidopsis genes encode chloroplastic 2Cys-PRX (Dietz et al., 2002). All plant homologs present a transit peptide suggesting a chloroplastic localization of the mature proteins, and several publications have reported the characterization and involvement of 2Cys-PRX from barley (Hordeum vulgare) and spinach (Spinacia oleracea; Baier and Dietz, 1996, 1997, 1999), Chinese cabbage (Brassica campestris L. subsp. pekinensis; Cheong et al., 1999), or Arabidopsis (Baier et al., 2000; Konig et al., 2002) in the protection of chloroplasts against ROS. 1Cys-PRX has also been studied in diverse plant species such as rice (Oryza sativa), Arabidopsis, buckwheat (Fagopyrum esculentum), or barley (Stacy et al., 1996, 1999; Haslekas et al., 1998; Lee et al., 2000; Lewis et al., 2000) and are described as being related to seed dormancy (Lewis et al., 2000) or at least to antioxidant activity in seeds (Lee et al., 2000). A type Q PRX described in Sedum lineare is also proposed to act in vivo as an antioxidant (Kong et al., 2000). One Arabidopsis gene encodes a PRX-Q protein homologous to the S. lineare PRX-Q (Horling et al., 2002).
In this paper, we describe the most recently discovered PRXII family. In Arabidopsis, it is composed of six members that define three different subgroups. Using RT-PCR and plant reporter gene studies, we present the differential expression patterns of AtPRXII. We also report the detection of AtPRXII proteins in different organs and in three distinct cellular compartments. We discuss the in vitro activity characteristics of AtPRXII and demonstrate that at least one of them is reduced by the GRX system.
RESULTS
Characterization of Arabidopsis Type II PRX Gene and Protein Sequences
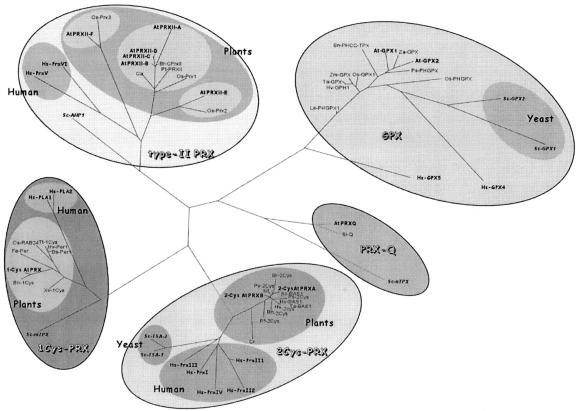
Searching for PRX homologs encoded by the nuclear genome of Arabidopsis, we found 10 potential open reading frames (ORFs). To build a phylogenetic tree, we used in addition all PRX sequences available for human, Brewer's yeast, and plants (Fig. 1). On this phylogenetic tree, built using the DARWIN program (Gonnet et al., 1992), the sequences are grouped in four different families, as previously shown (Verdoucq et al., 1999; Horling et al., 2002). We choose in this paper to use the names proposed by Horling et al. (2002) for the four PRX families. All of the human and yeast sequences are perfectly associated within the four groups defined by the Arabidopsis members (Fig. 1). A search for additional sequences in the National Center for Biotechnology Information non-redundant database and in The Arabidopsis Information Resource database using less stringent criteria showed that PRXs present low but significant similarity with glutathione peroxidases (GPX) in a limited region of the sequences. This exhaustive search allowed us to detect all potential members of the Arabidopsis PRX family and to define members of each group.
Figure 1.
Phylogenetic tree of proteins homologous to PRX. The DARWIN software (Gonnet et al., 1992) was used to generate this tree. The first letters correspond to the initials of the organism genus and species names. GenBank accession numbers are indicated: 1Cys-PRX: Brewer's yeast, Sc-mTPx (P34227); human (Homo sapiens), Hs-PLA1 (P30041) and Hs-PLA2 (XP_116186.1); Arabidopsis, 1-CysAtPRX (At1g48130); buckwheat, Fe-Per (AAF12782.1); barley, Hv-Per1 (P52572); Triticum turgidum, Tt-1Cys (AAG50024.1); Bromus secalinus, Bs-Per1 (P52571); rice, Os-RAB24 (P52573); oilseed rape (Brassica napus), Bn-1Cys (AAF61460.1); Xerophyta viscosa, Xv-1Cys (AAL88710.1). 2Cys-PRX: Brewer's yeast, Sc-TSA1 (P34760) and Sc-TSA2 (Q04120); human, Hs-PrxI (NP_002565.1), Hs-PrxII1 (P32119), Hs-PrxII2 (XP_068926.3), Hs-PrxIII (AAH08435.1), and Hs-PrxIV (NP_006397.1); Arabidopsis, 2-CysAtPRXA (At3g11630) and 2-CysAtPRXB (At5g06290); Chlamydomonas reinhardtii, Cr (AAG30934.1); Riccia fluitans, Rf-2Cys (CAB82860.1); barley, Hv-BAS1 (Q96468) and Hv-2Cys (S49173); wheat (Triticum aestivum), Ta-BAS1 (P80602); oilseed rape, Bn-2Cys (AAG30570.1); tobacco (Nicotiana tabacum), Nt (CAC84143.2); spinach, So-BAS1 (O24364); bean (Phaseolus vulgaris), Pv-2Cys (CAC17803.1); pea (Pisum sativum), Ps-2Cys (CAC48323.1); Brassica rapa, Br-2Cys (AAF00001.1). PRX-Q: Brewer's yeast, Sc-nTPX (P40553); Arabidopsis, AtPRXQ (At3g26060); S. lineare, Sl-Q (BAA90524.1). Type-II PRX: Brewer's yeast, Sc-AHP1 (P38013); human, Hs-PrxV (AAF04856.1) and Hs-PrxVI (XP_060391.1); Arabidopsis, AtPRXII-A (At1g65990), AtPRXII-B (At1g65980), AtPRXII-C (At1g65970), AtPRXII-D (At1g60740), AtPRXII-E (At3g52960), and AtPRXII-F (At3g06050); B. rapa, Br-CPrxII (AAD33602.1); peppers (Capsicum annuum), Ca (AAL35363.2); hybrid aspen (Populus tremula × Populus tremuloides), Pt-PRXII (AAL90751.1); rice, Os-Prx1 (AAG40130.1), Os-Prx2 (BAA82377.1), and Os-Prx3 (BAA88530.1). GPX: Brewer's yeast, Sc-GPX1 (NP_012899.1) and ScGPX2 (NP_009803.1); human, Hs-GPX4 (P36969) and Hs-GPX5 (NP_003987.2); Zantedeschia aethiopica, Za-GPX (AAC78466.1); oilseed rape, Bn-PHCC-TPX (AAM12502.1); Arabidopsis, AtGPX1 (At4g31870.1), AtGPX2 (At2g25080.1); maize (Zea mays) Zm-GPX (AAM88847.2); pea, Ps-PHGPX (O24296); rice, Os-GPX1 (AAM47493.1) and Os-PHGPX (expressed sequence tag [EST], CAC17628); tomato (Lycopersicon esculentum), Le-PHGPX1 (O24031); wheat, Ta-GPX (CAA09194.1); and barley, Hv-GPH1 (CAB59895.1).
Homologs of the Brewer's yeast AHP1 protein, the last discovered yeast PRX (Jeong et al., 1999; Lee et al., 1999; Verdoucq et al., 1999), form the type II PRX group, which is the focus of this study. It includes human and plant sequences. At least in plants, the type II PRX group is more complex than the three other groups. The Arabidopsis genome contains six different genes belonging to this group (Fig. 1). AtPRXII-F was detected during the analysis of the Arabidopsis mitochondrial proteome (Kruft et al., 2001). The ORF encodes 199 amino acids that includes an N-terminal extension of 28 residues (Kruft et al., 2001). AtPRXII-E has an ORF encoding 234 amino acids with an N-terminal extension of 70 amino acids that could code for a transit peptide predicted to address the protein to mitochondria or plastids depending on the prediction programs used. AtPRXII-B, -C, and -D are closely related and will be analyzed in detail in this study. Each gene has an ORF of 162 amino acids, which is exactly the same length as the PRXII described in poplar (Populus spp.; Rouhier et al., 2001) and B. rapa (Choi et al., 1999).
AtPRXII-B and AtPRXII-C are present as a tandem repeat. AtPRXII-A is on the same BAC F12P19 at a distance of approximately 10 kb, whereas AtPRXII-D is also on chromosome I, but further away. AtPRXII-E and AtPRXII-F are located on chromosome III. The coding sequences of AtPRXII-A, -B, -C, and -D are interrupted by two introns at the same positions, whereas AtPRXII-E has no intron. The AtPRXII-F coding sequence is interrupted by three introns at positions unrelated to those of AtPRXII-A, -B, -C, and -D. Thus, based on the exon/intron structure, the AtPRXII family can be divided into three subgroups.
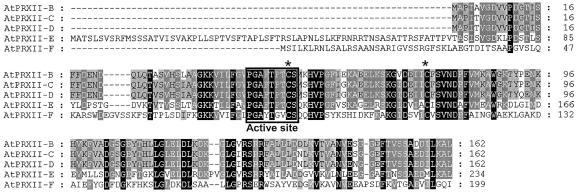
Due to an error in the Arabidopsis genome annotation, AtPRXII-D was previously described as a pseudogene (Horling et al., 2002), but the isolation of a partial cDNA in our laboratory allowed us to correct the annotation. AtPRXII-A shares homologies to these sequences, but the predicted protein is far larger (560 amino acids) due to a C-terminal domain homologous to numerous Arabidopsis sequences without known function. An alignment of the deduced amino acid sequences of these five Arabidopsis type II PRXs is shown in Figure 2. Each of these type II PRXs have the characteristic feature of all of the PRXII sequences analyzed so far. They contain one strictly conserved Cys residue (Cys-51 in AtPRXII-B, -C, and -D) with the surrounding consensus sequence: P[G/L][A/D]FT[P/F][T/V]C[S/P/T] (Rouhier et al., 2001). In addition, all of them possess a second Cys residue separated from the first one by the same number of residues.
Figure 2.
Multiple sequence alignment of Arabidopsis AtPRXII proteins. The alignment of the protein sequences of AtPRXII-B, AtPRXII-C, AtPRXII-D, AtPRXII-E, AtPRXII-F, and of the N-terminal part of AtPRXII-A was obtained using the ClustalW software. The active site is underlined, and the two conserved Cys residues are marked by an asterisk.
It is not known if the AtPRXII-A protein is correctly predicted because no EST or complete cDNA are available at the present time. Therefore, this protein is not further analyzed in this study. AtPRXII-B, -C, and -D are highly similar, showing between 93% to 99% identity. They share about 60% identity with the amino acid sequence of AtPRXII-E without the N-terminal extension, whereas they only share 50% similarity with the predicted mature form of AtPRXII-F. Thus, the amino acid sequence of AtPRXII allowed us to define three subgroups corresponding to those already defined by the exon/intron structures of the genes. We were able to find rice homologs of each sub-group, suggesting that this organization is common to all higher plants (Fig. 1).
AtPRXII-B and -E Present in Vitro Peroxidase Activities But Are Reduced in Different Ways
To test biochemical activities of AtPRXII, we produced His-tagged recombinant proteins using the bacterial-expressing vector pET16b. Because the amino acid sequences of AtPRXII-B, -C, and -D are so similar, we decided to restrict our biochemical study to AtPRXII-B. AtPRXII-E was cloned as a truncated form, Δ70AtPRXII-E, lacking the 70 N-terminal amino acids corresponding to the putative transit peptide.
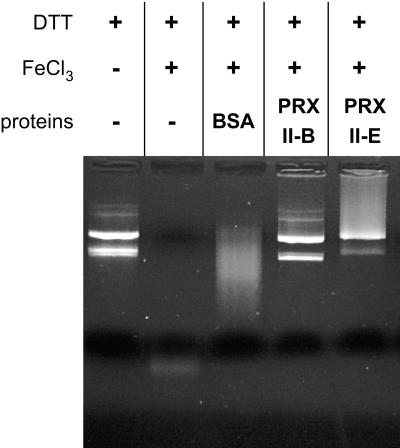
To test the in vitro peroxidase activity of these different AtPRXII, we used the classical assay of plasmid DNA protection against ROS generated by the Fenton reaction induced by this metal-catalyzed system. Dithiothreitol (DTT) and FeCl3 were incubated with 1 μg of plasmid DNA at room temperature in the presence or absence of 20 μm of recombinant proteins. After 5 h of incubation, the DNA mixed without PRX was completely degraded, as was DNA incubated with bovine serum albumin (BSA). In contrast, DNA incubated with AtPRXII-B or Δ70AtPRXII-E was efficiently protected (Fig. 3). Nevertheless, we observed a difference in the pattern of DNA protection between AtPRXII-B and Δ70AtPRXII-E, because the plasmid super-coiled form is much less abundant using Δ70AtPRXII-E than with AtPRXII-B. These results are in good agreement with the DTT-dependent H2O2-reducing activities obtained by Horling et al. (2003). They showed that AtPRXII-E has a DTT-dependent activity three times less efficient than that obtained for AtPRXII-B. Thus, a low protection of DNA by Δ70AtPRXII-E could be attributed to its lower peroxidase activity, as compared with that of AtPRXII-B.
Figure 3.
Type II AtPRX-dependent inactivation of DNA degradation by metal catalyzed oxidants. One microgram of pBluescript plasmid was incubated with a mixture of 10 μm FeCl3 and 1 mm DTT. The addition of AtPRXII-B or Δ70AtPRXII-E proteins led to the protected DNA.
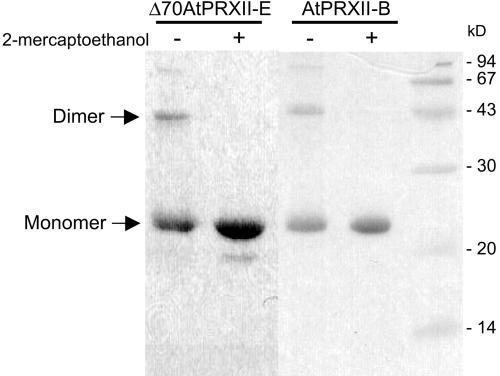
The reaction mechanism of 2Cys-PRX includes homodimer formation. In contrast, the activity of type II PRXs analyzed so far is associated with monomers. We tested the ability of the recombinant AtPRXII to form dimers. Recombinant proteins were analyzed directly after purification under nonreducing conditions or were incubated under reducing conditions with 5% (v/v) β-mercaptoethanol and then submitted to SDS-PAGE (Fig. 4). Under nonreducing conditions, a dimer of about 44 kD and a monomeric form of about 22 kD were observed with His-tagged AtPRXII-B. The dimeric form completely disappeared in the presence of β-mercaptoethanol. ΔAtPRXII-E protein also formed a dimer in nonreducing conditions. Thus the recombinant Arabidopsis type II PRXs are able to form disulfide bonds between two molecules. Nevertheless, the amount of dimers is not increased when PRXIIs are incubated with H2O2 at 0.5 to 50 mm (data not shown). In addition, no dimers were detected in crude plant extracts under nonreducing conditions by western blot using sera raised against AtPRXII-B or AtPRXII-E (data not shown). Although we were not able to demonstrate the existence of these dimers in planta, we cannot exclude that this property of the AtPRXII proteins to form dimers may not play a regulating role under certain conditions.
Figure 4.
Effect of 2-mercaptoethanol on type II AtPRX dimerization. Recombinant AtPRXII-B and truncated Δ70AtPRXII-E proteins were boiled for 5 min in a loading buffer in the presence (+) or absence (-) of the reducing agent 2-mercaptoethanol. About 5 μg of recombinant proteins was loaded in each lane on a 12.5% (w/v) SDS PAGE and stained by Coomassie Blue. Positions of molecular size standards are indicated on the left in kilodaltons.
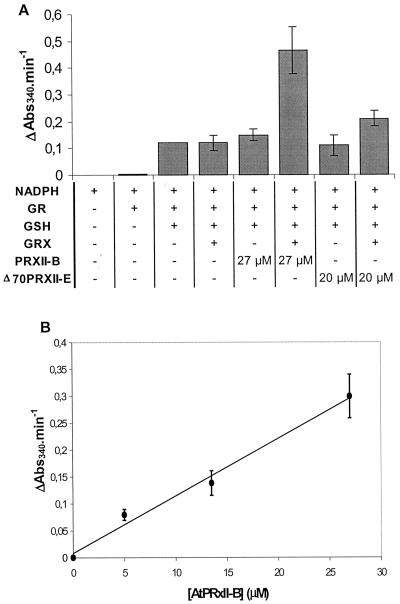
To further investigate the thiol dependence of the type II AtPRX peroxidase activity, we followed the oxidation of NADPH by the TRX or GRX system. The TRX system consists of Arabidopsis TRX reductase (Jacquot et al., 1994) and Arabidopsis TRX h2 (GenBank accession no. S58123). With NADPH as the primary electron donor, it reduces insulin efficiently (data not shown) and acts as an efficient reducer of AHP1, the PRXII of Brewer's yeast (Verdoucq et al., 1999). We were not able to detect any peroxidase activity in the presence of this TRX system for either AtPRXII-B or Δ70AtPRXII-E. However, in the presence of a GRX system, composed of a commercial glutathione reductase associated with reduced glutathione (GSH) and a recombinant Arabidopsis GRX, the initial rate of NADPH oxidation increased as a function of the AtPRXII-B concentration (Fig. 5B). The turnover number of the peroxidase activity of AtPRXII-B was estimated to be 2.86 10-2 s-1 under the conditions of the experiment. The reduction system without GSH did not show any activity. The absence of either GRX or PRX led to a residual NADPH consumption (Fig. 5A), which is most probably due to GSH oxidation by hydrogen peroxide. No significant activity was observed with the recombinant truncated Δ70AtPRXII-E. These results suggest that GRX is the main electron donor to AtPRXII-B and that the AtPRXII-E enzyme has a thiol-dependent activity but its electron donor has still to be identified.
Figure 5.
Thiol peroxidase activity of AtPRXII-B followed by oxidation rate of NADPH. NADPH oxidation was monitored at 340 nm in a 500-μL reaction mixture containing 0.1 m potassium-phosphate buffer, pH 7, 2 mm EDTA, 1 mm H2O2, 0.5 mm NADPH, 0.5 unit of glutathione reductase, 0.5 mm GSH, and 10 μm GRX AtGRX1 and the indicated concentrations of AtPRXII-B. Rate of NADPH oxidation is shown by ΔAbs340 min-1. A, Initial rates of NADPH oxidation coupled to H2O2 reduction by various combinations of glutathione reductase (GR), GSH, GRX, and AtPRXII. NADPH oxidation measurements started 30 s after H2O2 addition. Rates of NADPH oxidation were constant for at least 10 min. B, Concentration-dependent peroxidase activity of AtPRXII-B. The background NADPH oxidation due to direct reduction of H2O2 by GSH was subtracted.
AtPRXII-E Is Chloroplastic, Whereas AtPRXII-B, -C, and -D Are Cytosolic
Rabbit antibodies raised against AtPRXII-B and Δ70AtPRXII-E recombinant proteins were produced, and their specificity was tested by immunodetection against different quantities of recombinant AtPRXII-B and Δ70AtPRXII-E. The serum raised against AtPRXII-B can recognize at least 5 ng of recombinant AtPRXII-B but does not show any signal with up to 80 ng of Δ70AtPRXII-E (data not shown). Thus the AtPRXII-B serum does not recognize AtPRXII-E, but we assume it can detect AtPRXII-C and -D as well as AtPRXII-B. This assumption is sustained by the high similarity of these three PRX and by the fact that the serum produced by Horling et al. (2002), raised against the recombinant AtPRXII-C, recognized recombinant AtPRXII-C and AtPRXII-B (Horling et al., 2002). The serum raised against Δ70AtPRXII-E shows a lower specificity: It recognized at least 5 ng of the recombinant Δ70AtPRXII-E but cross-reacts with 20 ng of AtPRXII-B.
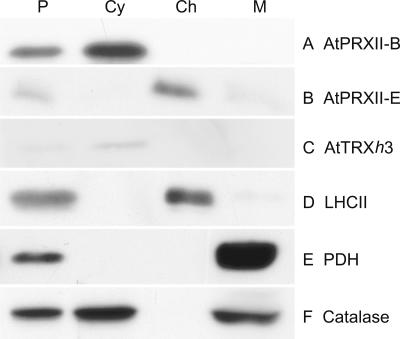
To get insights into the subcellular localizations of the AtPRXII, we performed immunoblots against subcellular fractions of Arabidopsis protoplasts. The purity of each subcellular fraction was tested using antibodies raised against proteins specifically located in the different organelles: the large subunit of the light-harvesting complex II from C. reinhardtii for the chloroplast, TRX h (AtTRXh3) for the cytosol, pyruvate dehydrogenase for the mitochondrion, and catalase for the peroxisome. No contamination was detected in chloroplast and cytosol fractions (Fig. 6, lines C and E). However, a very weak contamination by chloroplasts in the mitochondrial fraction can be observed (Fig. 6, line D). Anti-AtPRXII-E serum reveals a 19-kD band in total protoplast and chloroplast fractions (Fig. 6, line B). A very faint signal is also observed in the mitochondrial fraction, which is most probably due to the weak contamination of this fraction by chloroplasts. The chloroplast localization of AtPRXII-E was confirmed by import experiments of the radiolabeled protein into purified organelles. No import could be detected into potato (Solanum tuberosum) mitochondria, whereas processing of the major 29-kD protein, obtained by in vitro coupled transcription/translation, was observed after its incubation under light with pea chloroplasts (data not shown). A signal of 19 kD resistant to added proteinase K appeared at the same position as the Arabidopsis protein immunodetected in chloroplast extracts. Thus we concluded that AtPRXII-E is only located in the chloroplasts.
Figure 6.
AtPRXII sub-cellular localization. Arabidopsis total protoplast (P), cytosol (Cy), chloroplast (Ch), and mitochondrial (M) fractions were probed with antibodies directed against AtTRXh3, pyruvate dehydrogenase (PDH), catalase, and the light-harvesting complex II (LHCII) as controls. The same fractions were also probed with serum raised against AtPRXII-B or AtPRXII-E.
Using the serum raised against AtPRXII-B, signals were obtained in protoplast and cytosol fractions but not in those of mitochondria or chloroplasts (Fig. 6, line A). As shown using the serum raised against catalase (Fig. 6, line F), peroxisomes copurify with the mitochondrial fraction, whereas soluble peroxisomal proteins contaminate the cytosolic fraction. Because no signal is detected by the AtPRXII-B serum in the mitochondrial fraction, we conclude that AtPRXII-B and probably AtPRXII-C and -D are cytosolic proteins and are not located in the peroxisomes.
AtPRXII Have Different mRNA Accumulation Patterns
Expression of AtPRXII-B,-C,-D, -E, and -F in plant tissues was analyzed by RT-PCR. For each gene, two primers were designed for transcript amplification. Amplified products were cloned and sequenced to check for the specificity of amplification, except for AtPRXII-F. This demonstrated that each couple of primers is highly specific.
The expression of each gene was analyzed by reverse transcription followed by radioactive PCR (Fig. 7). This allowed us to amplify the sequence of interest and the control gene, actin 2 (GenBank accession no. U41998), which is supposed to be uniformly expressed in all tissues, in the same tube. Because the Act2 gene is not expressed in dry seeds, we used primers designed to amplify EM1 mRNA (Carles et al., 2002) as the internal control for seeds. Signals were then quantified using a phosphorimager, and the intensities were related to the intensity of the actin signal, enabling us to compare levels of expression between the different tissues (Fig. 7B). Because of the different efficiency of each couple of primers, it is not possible to compare the level of expression of the different genes. Nevertheless, the number of ESTs in the database gives some estimation of the relative expression, at least in the tissues used for the construction of the cDNA libraries: AtPRXII-A, no EST; AtPRXII-B, 30 ESTs; AtPRXII-C, 10 ESTs; AtPRXII-D, no EST; AtPRXII-E, 3 ESTs; and AtPRXII-F, 14 ESTs.
Figure 7.
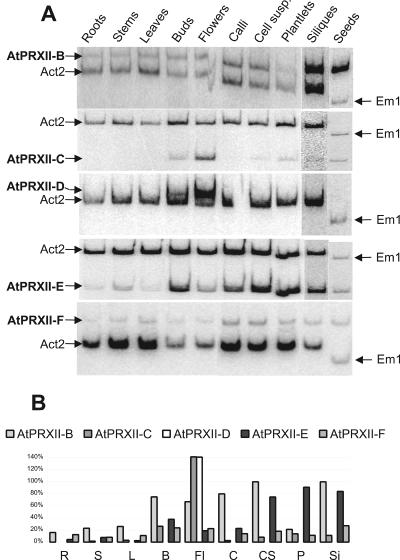
Expression of AtPRXII-B to -F in different plant organs. A, Semiquantitative RT-PCR was performed using gene-specific (AtPRXII) and reference gene (Act2 or EM1) primers. B, Signal intensities were measured using a phosphorimager and relative abundance of each AtPRXII transcript was calculated with Act2 signal intensity as reference. R, Roots; S, stems; L, leaves; B, buds; Fl, flowers; Si, siliques; and S, seeds.
AtPRXII-B mRNA is detected in all tissues but mostly in reproductive tissues such as buds, flowers, siliques, and seeds. AtPRXII-B is also strongly expressed in callus and cell suspensions. Concerning the AtPRXII-C transcripts, the strongest signals were obtained with buds and flowers, whereas a very faint signal is detected in all other tissues, except in roots. AtPRXII-D mRNA is present exclusively in buds and flowers. A signal corresponding to the AtPRXII-E transcripts was obtained with all tissues, but with a high predominance in buds, siliques, seeds, cell suspensions, and plantlets. AtPRXII-F transcripts are detected in all tissues without any significant difference of intensity (Fig. 7). Thus, the genes encoding PRXs have distinct tissue expression patterns.
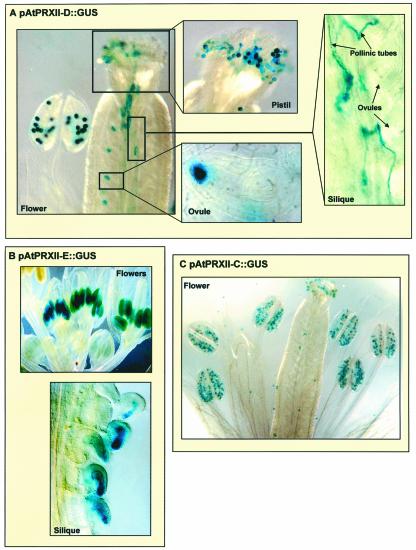
To get more insight into the expression patterns of AtPRXII genes, we analyzed their promoter activities using transgenic Arabidopsis plants carrying AtPRXII-B to -E promoter-β-glucuronidase (GUS) reporter gene fusion. 5′-Upstream regions of 1.465, 1.139, 1.141, and 1.831 kb from the gene initiation codon of AtPRXII-B, -C, -D, and -E, respectively, were used to generate transcriptional fusions with the GUS reporter gene. The constructs were introduced into wild-type Arabidopsis plants via Agrobacterium tumefaciens-mediated transformation. Independent primary transformants were obtained, and the T2 or T3 generations were analyzed for GUS expression. Five lines of T2 generation for AtPRXII-B, -C, and -E, respectively, and three homozygous lines of T3 generation for AtPRXII-D were analyzed. Concerning the expression of AtPRXII-C and -D, similar GUS staining for the different reporter lines, consistent with the RT-PCR data, was observed: AtPRXII-C is almost exclusively expressed in flower buds, and the GUS staining was restricted to mature pollen (Fig. 8C). In pAtPRXII-D::GUS plants, GUS activity is detected in mature pollen but also in germinating pollen, pollinic tubes, and fertilized ovules (Fig. 8A). To verify whether the staining observed in fertilized ovules in siliques was from pollinic tubes or from maternal material, we did reciprocal fertilizations using pollen of wild-type plants to fecundate transgenic pAtPRXII-D::GUS pistils, or pollen from transgenic pAtPRXII-D::GUS flowers to fertilize wild-type pistils. GUS staining in ovules was observed exclusively when fertilizing wild-type pistils with pAtPRXII-D::GUS pollen, leading to the conclusion that only the male material expressed the GUS gene.
Figure 8.
Histochemical localization of GUS activity in Arabidopsis plants. GUS activity was revealed by blue staining after incubation of 6- to 8-week-old plants in 5-bromo-4-chloro-3-indolyl-β-glucuronic acid.
Concerning pAtPRXII-B::GUS plants, we did not obtain consistent results with the different lines. One line exhibited blue staining in vascular tissues of stem and roots, whereas another line was stained in blue exclusively in young anthers. Some lines were not stained at all. No line gave a staining pattern coherent with the RT-PCR data. Most probably the 1,465 bp of the promoter did not contain all of the information responsible for in vivo expression of AtPRXII-B gene.
Looking at pAtPRXII-E::GUS plants, we observed blue staining in stamen of young flowers, the embryo sac of young seeds, and the albumen of older seeds from green or yellow siliques (Fig. 8B). These results are consistent with the expression pattern we obtained by RT-PCR. However, we did not observe blue staining in 10-d-germinating plantlets, whereas transcripts were detected by RT-PCR (Fig. 7). This may be due to a difference of stability of the AtPRXII-E transcripts in plantlets. The AtPRXII-E is weakly expressed, but the corresponding stable transcripts accumulate to a high level and thus are detectable by RT-PCR.
Protein Accumulation in Different Organs
Because AtPRXII-B, -C, and -D differ only by 0.06 kD from one another (Table I), we used two-dimensional electrophoresis to distinguish AtPRXII-B from the AtPRXII-C and -D proteins, using their predicted pI difference of 0.15. The mature form of AtPRXII-E predicted by PSORT (http://psort.nib-b.ac.jp; Nakai, 2000) is about 0.15 kD smaller than the three first AtPRXII and could then be distinguished by its size as well as by its pI which is 0.15 point more acidic than AtPRXII-B and 0.30 point from AtPRXII-C and -D (Table I). Thus in addition to one-dimensional western blots, we have used two-dimensional immunoblotting to detect the presence of the different PRXII in Arabidopsis plant organs.
Table I.
pl and molecular masses (kD) of AtPRXII predicted mature forms
| AtPRXII-B | AtPRXII-C | AtPRXII-D | AtPRXII-E | AtPRXII-F | |
|---|---|---|---|---|---|
| pl | 5.17 | 5.33 | 5.33 | 5.02 | 6.29 |
| kD | 17.43 | 17.41 | 17.47 | 17.28 | 18.3 |
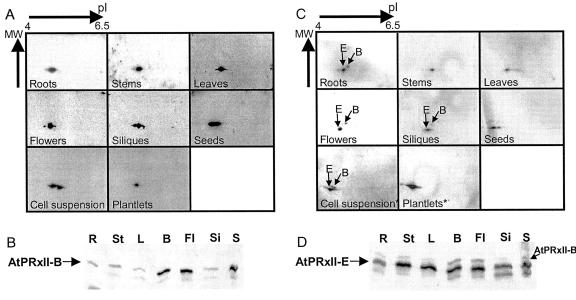
We first performed a one-dimensional western blot from an SDS-PAGE loaded with exactly 10 μg of proteins from the different tissues. Using the serum raised against AtPRXII-B, a stronger signal was observed with extracts from buds, flowers, and seeds than with proteins from other tissues (Fig. 9B). Probing the same membrane with the serum raised against AtPRXII-E (Fig. 9D), we observed the presence of AtPRXII-E in an approximately equal quantity in each tissue that was tested, except for roots where a weaker signal was seen. The serum also reacts with proteins of higher Mr that fit exactly the signals showed by anti-AtPRXII-B serum with the same membrane (Fig. 9D).
Figure 9.
Type II AtPRX protein accumulation patterns. The serum raised against AtPRXII-B (A and B) and AtPRXII-E (C and D) detected the type II PRX proteins in planta. A and C, Total protein extracts from different plant tissues were separated by two-dimensional electrophoresis with pI ranging from 4 to 6.5 (except for * annotated gels that were done with pI ranging from 3.5–10). B and D, Ten micrograms of proteins from each tissue was loaded on a 12.5% (w/v) PAGE. R, Roots; St, stems; L, leaves; B, buds; Fl, flowers; Si, siliques; and S, seeds.
With all tissue extracts, one or several signals were obtained on two-dimensional gels with the serum raised against AtPRXII-B (Fig. 9A). These different signals may correspond to the AtPRXII-B, -C, and -D proteins or to modified isoforms of AtPRXII-B, -C and/or -D.
Using serum raised against AtPRXII-E, two types of signal were obtained with two-dimensional blots (Fig. 9C): strong signals most probably corresponding to the mature AtPRXII-E, and weaker signals (indicated in Fig. 9C by a B) present at exactly the same position as those obtained with serum raised against AtPRXII-B and then corresponding to AtPRXII-B to -D. AtPRXII-E signals were observed with all tissues, leading to the conclusion that AtPRXII-E as well as AtPRXII-B and/or AtPRXII-C and/or AtPRXII-D are present in all plant tissues. The position of the AtPRXII-E spots, compared with the ones of AtPRXII-B to -D, coincides with the molecular mass and pI of the predicted mature AtPRXII-E (Table I; Fig. 9).
DISCUSSION
Higher Plants Present Three Subgroups of Type II PRX Genes
All eukaryotic PRXs can be classified in four groups, on the basis of their sequences, as shown in the tree of Figure 1. This classification fits well with our knowledge on the mechanisms of peroxide reduction which are different in each group. Among the PRXs, the type II PRXs are less studied, probably because the peroxidase activity of this family of proteins was only recently identified in yeast (Jeong et al., 1999; Lee et al., 1999; Verdoucq et al., 1999), Arabidopsis (Verdoucq et al., 1999; Horling et al., 2002, 2003), poplar (Rouhier et al., 2001), and Chinese cabbage (Choi et al., 1999). At least in plants, this type is also more complex, composed of several different members. The six Arabidopsis PRXII proteins are not grouped but are distributed into three subclasses corresponding to the AtPRXII-B, -C, and -D group and AtPRXII-F and AtPRXII-E forms. The genes encoding these subclasses show different exon/intron structures. At least one rice sequence is clearly associated with each Arabidopsis PRXII subtype, suggesting that the three subtypes are present in all higher plants.
AtPRXII Accumulate in Different Plant Organs and in Different Subcellular Compartments
We have analyzed in detail the expression pattern and protein accumulation of three cytosolic PRXIIs (AtPRXII-B, -C, and -D). AtPRXII-B is expressed in all tissues, and the protein also accumulates in all tissues. This pattern may well correspond to the defense function that is frequently associated with PRXs. Recently, Horling et al. (2003) showed an absence of induction of AtPRXII-B expression by high or low light but an induction of this gene in the presence of butyl hydroperoxide. They suggest that the protein may function in protecting the plasma membrane.
More striking is the almost exclusive expression in pollen of the two AtPRXII-C and -D genes. To our knowledge, it is the first report of PRX expression localized in flowers. Until now plant PRXs were mainly characterized in leaves and seeds (Baier and Dietz, 1996; Haslekas et al., 1998; Lewis et al., 2000; Rouhier et al., 2001; Horling et al., 2002, 2003). This particular expression pattern is reminiscent of the TRX regulation of the S-locus receptor kinase, implicated in cauliflower (Brassica oleracea) pollen auto-incompatibility (Cabrillac et al., 2001), and suggests a developmental function for these two proteins. In addition, this expression pattern may mean that the PRXII-C and/or -D proteins are implicated in the protection of pollen components. Pollen grains are exposed to an increase of free radical production during desiccation (Van Bilsen and Hoekstra, 1993) and PRXs constitute one of the molecular antioxidants able to alleviate oxidative stress in dried tissues (Hoekstra et al., 2001).
The gene encoding plastidial PRX AtPRXII-E is expressed constitutively but is overexpressed in anther tapetum and during seed formation, suggesting that the function of this protein is not limited to chloroplasts, but is also very active in other types of plastids. In particular, elaioplats are plastids present in anther tapetum and contain mainly globuli of neutral esters (Wu et al., 1997; Ting et al., 1998; Hernandez-Pinzon et al., 1999). AtPRXII-E could thus be involved in a protective mechanism preventing a high level of lipid peroxidation in elaioplasts. The gene coding for the mitochondrial PRX AtPRXII-F (Kruft et al., 2001) is ubiquitously expressed. The protein is probably implicated in the protection of mitochondria against ROS damage and may be reduced by the mitochondrial TRX system described by Laloi et al. (2001), as suggested by Horling et al. (2003).
AtPRXIIs Are Reduced by Different Systems
The cytosolic AtPRXII-B and -C, the plastidial AtPRXII-E, and mitochondrial AtPRXII-F are able to scavenge ROS in the presence of DTT (Horling et al., 2002, 2003; this paper). Looking for a more physiological reducing system, we tested TRX and GRX systems, both from Arabidopsis. AtPRXII-B is not reduced by the Arabidopsis TRX h2 system. In a previous study (Verdoucq et al., 1999), AtPRXII-B presented a weak TRX-dependent activity, using a TRX from C. reinhardtii. This system was also proved to be able to reduce a cytosolic poplar PRXII, but less efficiently than a GRX system (Rouhier et al., 2001). We cannot rule out that one of the eight cytosolic TRXs (Meyer et al., 2002) could be a physiological electron donor of AtPRXII-B. However, we showed that AtPRXII-B is efficiently reduced in vitro by the Arabidopsis GRX 1 system, suggesting that one of the numerous Arabidopsis GRXs is the natural electron donor of cytosolic PRXII. AtPRXII-C and AtPRXII-D accumulate in pollen and pollinic tubes, tissues where Arabidopsis TRX h4 is expressed (Reichheld et al., 2002). Therefore, AtTRXh4 is also a good candidate for the reduction of AtPRXII-C and/or -D.
The plastidial AtPRXII-E cannot be efficiently reduced by any of these systems. This is not surprising because, on the basis of the Arabidopsis genome, no gene was found to encode a GRX with a transit peptide. The lack of activity in the presence of the cytosolic TRX system is more surprising, because the substrate specificity of TRXs is rather low in vitro. For example, a C. reinhardtii cytosolic TRX was reported to be an excellent electron donor for a chloroplastic 2-Cys PRX from Arabidopsis (Goyer et al., 2002). Nevertheless, a systematic test with chloroplastic TRXs should be performed in the future.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis ecotype Columbia seeds were sterilized by incubating them in 70% (v/v) ethanol for 10 min and then sown on one-half-diluted Murashige and Skoog medium supplemented with 1% (w/v) Glc, 0.5 g L-1 MES, and 0.8% (w/v) agar or directly in soil. Seeds were cold treated for 3 to 4 d at 4°C, then germinated and grown under continuous light at 22°C. Arabidopsis cell suspensions were maintained in Murashige and Skoog medium supplemented with 3% (w/v) Suc, 50 μg L-1 kinetin, and 500 μg L-1 naphthaleneacetic acid and subcultured every 14 d. Calli were grown for 1 month on the same medium solidified with 0.8% (w/v) agar.
Plant Transformation and GUS Staining
The AtPRXII-B promoter region was isolated from genomic DNA (ecotype Columbia) by PCR walking (Devic et al., 1997) using a reverse primer to introduce a unique SpeI site just upstream from the ATG codon. A fragment of 1.46 kb was isolated. Fragments of 1.14, 1.14, and 1.83 kb of the regions upstream from the ATG codon of AtPRXII-C, -D, and -E, respectively, were isolated by direct PCR on genomic DNA using primers to introduce unique HindIII and SalI sites for AtPRXII-C, XbaI and SalI sites for AtPRXII-D, and SpeI and SalI sites for AtPRXII-E. The DNA fragments were then digested by the corresponding enzymes and cloned into pGPTV-HYG binary vector. The resulting plasmids were introduced into Agrobacterium tumefaciens C18CIRifR. Arabidopsis were transformed with agrobacteria by the floral dip method (Clough and Bent, 1998). T1, T2, and T3 seedlings were selected in vitro on one-half-diluted Murashige and Skoog medium supplemented with 1% (w/v) Glc, 0.5 g L-1 MES, 0.8% (w/v) agar, and 25 μg mL-1 hygromycine under continuous light at 22°C. For the GUS assay, plants were cultivated either in vitro under the same conditions or grown in soil mixed with vermiculite in a greenhouse under continuous light at 22°C. GUS histochemical staining was performed according to Lagarde et al. (1996).
RNA Extraction and Radioactive Reverse Transcriptase PCR
Total RNA was extracted from frozen cell suspensions, calli, and each plant organ—siliques and seeds excepted—as described (Kay et al., 1987). Total RNA from siliques and seeds were extracted using the Invisorb Spin Plant-RNA Mini Kit (Invitek, Berlin, Germany) and following the procedure provided by the manufacturer. Five micrograms of total RNA were used for reverse transcription using the Moloney murine leukemia virus reverse transcriptase as described by the manufacturer's protocol (First Strand RT-PCR kit, ProSTAR, Stratagene, La Jolla, CA). One-fiftieth of the reverse transcription product was used for further PCR using in each reaction primers specific to distinct AtPRXII cDNA and primers specific to the control gene EM1 for dry seed cDNA and Act2 for other tissue cDNA. PCR was performed in the presence of 6.6 nm [α-32P]dCTP and 20 μm for the three other dNTPs. Fifteen cycles were performed to amplify AtPRXII transcripts. One-third of the reaction was run on a 5% (w/v) acrylamide/bis-acrylamide gel. Radioactive amplifications were visualized and quantified through PhosphorImager (Storm 640, Molecular Dynamics, Sunnyvale, CA). The sequences of the primers used for RT-PCR experiments are indicated in Table II.
Table II.
Sequences of primers used for RT-PCR experiments
| Primer Name | Primer Sequence (5′ to 3′) |
|---|---|
| RT-AtPRXIIB35 | CAAACAAGTCACAATGAAACCGC |
| RT-AtPRXIIB53 | TTTCATTTGTCTCCTTTCGTCAAC |
| RT-AtTPXIIC35 | CAATACAACAAGCGAAACGATAAA |
| RT-AtTPXIIC53 | GAAGGCATGGGGAAAAACATATCC |
| RT-AtPRXIID35 | AGATTCACAATACAACAAGCGAAT |
| RT-AtPRXIID53 | CTTCTATTTTCCCCTGTCTTCATAT |
| RT-AtTPXIIE35 | CCTCCACGCTTCCATCACGAACGC |
| RT-AtTPXIIE53 | CAGACTCCACTCTCTCCTACCTCG |
| RT-AtTPXIIF35 | AAGGATCCACTTTTTAGATCTGTCCTAAGATGA |
| RT-AtTPXIIF53 | AAGAATTCGGCGATGTCAATTCTAAAGCTAA |
| EM1ATG | AACCATGGCGTCAAAGCAACTG |
| EM1Stop | GGGGATCCGTAACTACTATCAC |
| Act2(5′) | GTTAGCAACTGGGATGATATGG |
| Act2(3′) | CAGCACCAATCGTGATGACTTGCCC |
As a control for genomic DNA contamination, PCR was carried out on RNA not treated by reverse transcriptase, with each couple of primers. No amplification was observed. Furthermore, different couples of primers we used are able to amplify genomic DNA leading to the production of DNA fragments of higher Mr (612, 1,390, 770, 1,010, and 1,503 bp for Act2, AtPRXII-B, AtPRXII-D, AtPRXII-E, and AtPRXII-F, respectively). But such an amplification was never observed.
Recombinant Protein and Antibody Productions
The AtPRXII-B ORF from the ATG codon to the stop codon, and the AtPRXII-E ORF from the codon coding for the amino acid A71 to the stop codon were amplified and cloned downstream from, and in phase with, a sequence coding for His tag in the pET16b plasmid. The construction with the incomplete reading frame of AtPRXII-E is called Δ70AtPRXII-E.
To overproduce AtPRXII in Escherichia coli, BL21(DE3) strains were cotransformed with pSBET (Schenk et al., 1995) and pET16b containing the AtPRXII constructs, and grown in 500 mL of liquid B medium up to A600 nm = 0.5, when production was induced by addition of 0.4 mm isopropyl-1-thio-β-d-galactopyranoside for 3 h at 37°C. Cells were then pelleted and stored at -80°C. Total proteins from bacteria were extracted using a hydraulic press (model 3968, Carver, Wabash, IN) and purified on a nickel affinity column as described by Verdoucq et al. (1999). The productions of antibodies from rabbit were done by Eurogentec using 400 μg of each purified AtPRXII-B and Δ70AtPRXII-E.
Plant Protein Extraction, Electrophoresis, and Immunodetection
For one-dimensional electrophoresis, proteins were extracted from different frozen plant organs according to the protocol described by Carles et al. (2002). Ten micrograms of plant proteins was separated on a SDS-12.5% (w/v) polyacrylamide gel. For two-dimensional electrophoresis, protein extractions were performed according to the protocol previously described (Grosset et al., 1990), with the following modifications: After the homogenization of frozen tissues in 10 mL of 50% (w/v) phenol in Tris-HCl (0.1 m, pH 8) containing 5% (v/v) 2-mercaptoethanol, the mixture was centrifuged for 20 min at 5,000 rpm. The phenol phase was then extracted again with 1 volume of Tris-HCl (0.1 m pH 8) containing 5% (v/v) 2-mercaptoethanol and was separated by centrifugation for 20 min at 5,000 rpm. Proteins from the phenolic phase were then precipitated by five volumes of cold acetone during 2 h at -20°C. The precipitate was collected by centrifugation, washed four times with acetone (-20°C), and dried under reduced pressure. Analytical two-dimensional electrophoresis were performed as described by Grosset et al. (1990).
Immunodetections were done as described by Mouaheb et al. (1998) with the following modifications: Wet membranes were incubated at room temperature for 1 h in Tris-buffered saline plus Tween 20 (TBS-T) buffer (20 mm Tris-HCl, 500 mm NaCl, and 0.05% [v/v] Tween 20, pH 7.6) containing 5% (w/v) lyophilized skimmed milk, washed once in 50 mL of TBS-T buffer one time for 15 min and twice for 5 min. The reaction with the first antibody was carried out for 2.5 h at room temperature in 20 mL of TBS-T buffer containing 1% (w/v) lyophilized skimmed milk and 20,000-fold diluted rabbit immunoserum directed against the different PRXs. The membranes were washed and incubated for 1 h at room temperature in 20 mL of TBS-T buffer containing 20,000-fold diluted goat anti-rabbit horseradish peroxidase conjugate (Bio-Rad Laboratories, Hercules, CA). Finally, antibodies were visualized with ECL+ Western Blotting detection reagent (Amersham Biosciences, Uppsala).
Subcellular Fractionation and in Vitro Import Experiments
Arabidopsis protoplasts were prepared from 3- to 4-d-old suspension cell cultures as previously described (Sakamoto et al., 2000). Typically, protoplasts obtained from 500 mL of culture were resuspended in 50 mL of extraction buffer (400 mm Suc, 50 mm Tris-HCl pH 7.5, 3 mm EDTA, 0.1% [w/v] BSA, and 2 mm DTT) and disrupted by filtrations through nylon meshes. Cell debris were eliminated by centrifugation for 5 min at 100g, and the supernatant was recentrifuged for 10 min at 2,000g. The chloroplast-enriched pellet was collected and purified as described (Sakamoto et al., 2000), and the supernatant was centrifuged at 16,000g for 15 min to pellet mitochondria. The supernatant (cytosol) was stored for further analysis. The mitochondrial pellets were purified on 18%–23%-40% (v/v) Percoll step gradients. The mitochondria were collected at the 23% to 40% interface and washed in the extraction buffer without BSA and DTT. All fractions were frozen at -80°C before SDS-PAGE.
Concerning in vitro import into isolated chloroplast and mitochondria, the precursor proteins were synthesized from the corresponding cDNA clones by coupled in vitro transcription/translation (TNT kit, Promega, Madison, WI) in the presence of [35S]Met. Chloroplasts were isolated from leaves of 10-d-old pea seedlings and import assays were carried out as described previously (Bruce et al., 1994). Mitochondria were isolated from cauliflower with a juice extractor as described (Fey et al., 1999). Import assays into mitochondria were carried out as described (Wischmann and Schuster, 1995).
Enzymatic Activities
Metal-catalyzed oxidation DNA cleavage protection assays were performed as previously described (Klimowski et al., 1997) with the following modifications. ROS were generated for 30 min at room temperature by incubation of 10 μm FeCl3 with 1 mm DTT. Reaction mixtures were incubated for 4.5 h at room temperature with 20 μm proteins and 1 μg of pBluescript plasmid in a final volume of 50 μL. BSA (Fraction V, Roche Diagnostics, Mannheim, Germany) was used as a negative control. DNA degradation was checked by electrophoresis on 1% (w/v) agarose gels.
AtPRXII GRX-dependent or TRX-dependent peroxidase activity was assayed as follows: After 5 min of preincubation, the reaction was initiated by the addition of 1 mm H2O2 to 0.5 mL of 0.1 m potassium phosphate buffer at pH 7 containing 2 mm EDTA, 0.5 mm NADPH (Sigma-Aldrich), different concentrations of AtPRXII, and the GRX system composed of 0.5 unit of glutathione reductase (Roche Diagnostics), 0.5 mm GSH (Sigma-Aldrich), and 10 μm Arabidopsis GRX AtGRX1, or the TRX system composed of 10 μm Arabidopsis TRX AtTRXh2 and 0.2 μm Arabidopsis TRX reductase AtNTRB. The consumption of NADPH was followed spectrophotometrically at 340 nm at 22°C. The TRX insulin disulfide reduction assays were performed as described (Laloi et al., 2001). These experiments were done two to five times, and comparable results were obtained.
Acknowledgments
We thank Dr. C. Laloi for the gift of recombinant NTRB, Dr. L. Verdoucq for pET-AtPRXII-B construct, Y. Chartier for technical help, Dr C. de Vitry for the gift of anti-light-harvesting complex II serum, and Dr. J.-P. Reichheld and Dr. R. Cooke for critical reading of the manuscript.
C.B. was the recipient of a fellowship from the Centre National de la Recherche Scientifique and from the Région Languedoc Roussillon.; E.H.M. was the recipient of a fellowship from the French Ministère de la Recherche et de la Technologie.
References
- Baier M, Dietz KJ (1996) Primary structure and expression of plant homologues of animal and fungal thioredoxin-dependent peroxide reductases and bacterial alkyl hydroperoxide reductases. Plant Mol Biol 31: 553-564 [DOI] [PubMed] [Google Scholar]
- Baier M, Dietz KJ (1997) The plant 2-Cys peroxiredoxin BAS1 is a nuclear-encoded chloroplast protein: its expressional regulation, phylogenetic origin, and implications for its specific physiological function in plants. Plant J 12: 179-190 [DOI] [PubMed] [Google Scholar]
- Baier M, Dietz KJ (1999) Protective function of chloroplast 2-cysteine peroxiredoxin in photosynthesis: evidence from transgenic Arabidopsis. Plant Physiol 119: 1407-1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier M, Noctor G, Foyer CH, Dietz KJ (2000) Antisense suppression of 2-cysteine peroxiredoxin in Arabidopsis specifically enhances the activities and expression of enzymes associated with ascorbate metabolism but not glutathione metabolism. Plant Physiol 124: 823-832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP (1999) Role of active oxygen species and NO in plant defence responses. Curr Opin Plant Biol 2: 287-294 [DOI] [PubMed] [Google Scholar]
- Bruce BD, Perry S, Froehlich J, Keegstra K (1994) In vitro import of proteins into chloroplasts. In Plant Molecular Biology Manual, Vol J1. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 1-15 [Google Scholar]
- Cabrillac D, Cock JM, Dumas C, Gaude T (2001) The S-locus receptor kinase is inhibited by thioredoxins and activated by pollen coat proteins. Nature 410: 220-223 [DOI] [PubMed] [Google Scholar]
- Carles C, Bies-Etheve N, Aspart L, Leon-Kloosterziel KM, Koornneef M, Echeverria M, Delseny M (2002) Regulation of Arabidopsis thaliana Em genes: role of ABI5. Plant J 30: 373-383 [DOI] [PubMed] [Google Scholar]
- Chae HZ, Chung SJ, Rhee SG (1994) Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem 269: 27670-27678 [PubMed] [Google Scholar]
- Cheong NE, Choi YO, Lee KO, Kim WY, Jung BG, Chi YH, Jeong JS, Kim K, Cho MJ, Lee SY (1999) Molecular cloning, expression, and functional characterization of a 2Cys-peroxiredoxin in Chinese cabbage. Plant Mol Biol 40: 825-834 [DOI] [PubMed] [Google Scholar]
- Choi YO, Cheong NE, Lee KO, Jung BG, Hong CH, Jeong JH, Chi YH, Kim K, Cho MJ, Lee SY (1999) Cloning and expression of a new isotype of the peroxiredoxin gene of Chinese cabbage and its comparison to 2Cys-peroxiredoxin isolated from the same plant. Biochem Biophys Res Commun 258: 768-771 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735-743 [DOI] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranova E, Van Montagu M, Inze D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57: 779-795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devic M, Albert S, Delseny M, Roscoe TJ (1997) Efficient PCR walking on plant genomic DNA. Plant Physiol Biochem 35: 331-339 [Google Scholar]
- Dietz KJ, Horling F, Konig J, Baier M (2002) The function of the chloroplast 2-cysteine peroxiredoxin in peroxide detoxification and its regulation. J Exp Bot 53: 1321-1329 [PubMed] [Google Scholar]
- Fey J, Vermel M, Grienenberger J, Marechal-Drouard L, Gualberto JM (1999) Characterization of a plant mitochondrial active chromosome. FEBS Lett 458: 124-128 [DOI] [PubMed] [Google Scholar]
- Gonnet GH, Cohen MA, Benner SA (1992) Exhaustive matching of the entire protein sequence database. Science 256: 1443-1445 [DOI] [PubMed] [Google Scholar]
- Goyer A, Haslekas C, Miginiac-Maslow M, Klein U, Le Marechal P, Jacquot JP, Decottignies P (2002) Isolation and characterization of a thioredoxin-dependent peroxidase from Chlamydomonas reinhardtii. Eur J Biochem 269: 272-282 [DOI] [PubMed] [Google Scholar]
- Grosset J, Meyer Y, Chartier Y, Kauffmann S, Legrand M, Fritig B (1990) Tobacco mesophyll protoplasts synthesize 1,3-β-glucanase, chitinases and “osmotins” during in vitro culture. Plant Physiol 92: 520-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslekas C, Stacy RA, Nygaard V, Culianez-Macia FA, Aalen RB (1998) The expression of a peroxiredoxin antioxidant gene, AtPer1, in Arabidopsis thaliana is seed-specific and related to dormancy. Plant Mol Biol 36: 833-845 [DOI] [PubMed] [Google Scholar]
- Hernandez-Pinzon I, Ross JH, Barnes KA, Damant AP, Murphy DJ (1999) Composition and role of tapetal lipid bodies in the biogenesis of the pollen coat of Brassica napus. Planta 208: 588-598 [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J (2001) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6: 431-438 [DOI] [PubMed] [Google Scholar]
- Horling F, König J, Dietz K-J (2002) Type II peroxiredoxin C, a member of the peroxiredoxin family of Arabidopsis thaliana: its expression and activity in comparison with other peroxiredoxins. Plant Physiol Biochem 40: 491-499 [Google Scholar]
- Horling F, Lamkemeyer P, Konig J, Finkemeier I, Kandlbinder A, Baier M, Dietz KJ (2003) Divergent light-, ascorbate-, and oxidative stress-dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis. Plant Physiol 131: 317-325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson FS, Morgan RW, Christman MF, Ames BN (1989) An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage: purification and properties. J Biol Chem 264: 1488-1496 [PubMed] [Google Scholar]
- Jacquot JP, Rivera-Madrid R, Marinho P, Kollarova M, Le Marechal P, Miginiac-Maslow M, Meyer Y (1994) Arabidopsis thaliana NADPH thioredoxin reductase: cDNA characterization and expression of the recombinant protein in Escherichia coli. J Mol Biol 235: 1357-1363 [DOI] [PubMed] [Google Scholar]
- Jeong JS, Kwon SJ, Kang SW, Rhee SG, Kim K (1999) Purification and characterization of a second type thioredoxin peroxidase (type II TPx) from Saccharomyces cerevisiae. Biochemistry 38: 776-783 [DOI] [PubMed] [Google Scholar]
- Jeong W, Cha MK, Kim IH (2000) Thioredoxin-dependent hydroperoxide peroxidase activity of bacterioferritin comigratory protein (BCP) as a new member of the thiol-specific antioxidant protein (TSA)/Alkyl hydroperoxide peroxidase C (AhpC) family. J Biol Chem 275: 2924-2930 [DOI] [PubMed] [Google Scholar]
- Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG (1998) Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J Biol Chem 273: 6297-6302 [DOI] [PubMed] [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P (1999) Systemic signalling and acclimation in response to excess excitation energy in Arabidopsis. Science 284: 654-657 [DOI] [PubMed] [Google Scholar]
- Kay R, Chan A, Daly M, Mc Pherson J (1987) Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 236: 1299-1302 [DOI] [PubMed] [Google Scholar]
- Klimowski L, Chandrashekar R, Tripp CA (1997) Molecular cloning, expression and enzymatic activity of a thioredoxin peroxidase from Dirofilaria immitis. Mol Biochem Parasitol 90: 297-306 [DOI] [PubMed] [Google Scholar]
- Kong W, Shiota S, Shi Y, Nakayama H, Nakayama K (2000) A novel peroxiredoxin of the plant Sedum lineare is a homologue of Escherichia coli bacterioferritin co-migratory protein (Bcp). Biochem J 351: 107-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig J, Baier M, Horling F, Kahmann U, Harris G, Schurmann P, Dietz KJ (2002) The plant-specific function of 2-Cys peroxiredoxin-mediated detoxification of peroxides in the redox-hierarchy of photosynthetic electron flux. Proc Natl Acad Sci USA 99: 5738-5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruft V, Eubel H, Jansch L, Werhahn W, Braun HP (2001) Proteomic approach to identify novel mitochondrial proteins in Arabidopsis. Plant Physiol 127: 1694-1710 [PMC free article] [PubMed] [Google Scholar]
- Kwon SJ, Park JW, Choi WK, Kim IH, Kim K (1994) Inhibition of metal-catalyzed oxidation systems by a yeast protector protein in the presence of thioredoxin. Biochem Biophys Res Commun 201: 8-15 [DOI] [PubMed] [Google Scholar]
- Lagarde D, Basset M, Lepetit M, Conejero G, Gaymard F, Astruc S, Grignon C (1996) Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J 9: 195-203 [DOI] [PubMed] [Google Scholar]
- Laloi C, Rayapuram N, Chartier Y, Grienenberger JM, Bonnard G, Meyer Y (2001) Identification and characterization of a mitochondrial thioredoxin system in plants. Proc Natl Acad Sci USA 98: 14144-14149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Spector D, Godon C, Labarre J, Toledano MB (1999) A new antioxidant with alkyl hydroperoxide defense properties in yeast. J Biol Chem 274: 4537-4544 [DOI] [PubMed] [Google Scholar]
- Lee KO, Jang HH, Jung BG, Chi YH, Lee JY, Choi YO, Lee JR, Lim CO, Cho MJ, Lee SY (2000) Rice 1Cys-peroxiredoxin over-expressed in transgenic tobacco does not maintain dormancy but enhances antioxidant activity. FEBS Lett 486: 103-106 [DOI] [PubMed] [Google Scholar]
- Lewis ML, Miki K, Ueda T (2000) FePer 1, a gene encoding an evolutionarily conserved 1-Cys peroxiredoxin in buckwheat (Fagopyrum esculentum Moench), is expressed in a seed-specific manner and induced during seed germination. Gene 246: 81-91 [DOI] [PubMed] [Google Scholar]
- Meyer Y, Vignols F, Reichheld JP (2002) Classification of plant thioredoxins by sequence similarity and intron position. Methods Enzymol 347: 394-402 [DOI] [PubMed] [Google Scholar]
- Mouaheb N, Thomas D, Verdoucq L, Monfort P, Meyer Y (1998) In vivo functional discrimination between plant thioredoxins by heterologous expression in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA 95: 3312-3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux P, Karpinski S (2002) Signal transduction in response to excess light: getting out of the chloroplast. Curr Opin Plant Biol 5: 43-48 [DOI] [PubMed] [Google Scholar]
- Nakai K (2000) Protein sorting signals and prediction of subcellular localization. Adv Protein Chem 54: 277-344 [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas M, Narvaez-Vasquez J, Ryan C (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13: 179-191 [PMC free article] [PubMed] [Google Scholar]
- Reichheld J-P, Mestres-Ortega D, Laloi C, Meyer Y (2002) The multigenic family of thioredoxin h in Arabidopsis thaliana: specific expression and stress response. Plant Physiol Biochem 40: 685-690 [Google Scholar]
- Rouhier N, Gelhaye E, Jacquot JP (2002) Glutaredoxin dependent peroxiredoxin from poplar: protein-protein interaction and catalytic mechanism. J Biol Chem 277: 13609-13614 [DOI] [PubMed] [Google Scholar]
- Rouhier N, Gelhaye E, Sautiere PE, Brun A, Laurent P, Tagu D, Gerard J, de Fay E, Meyer Y, Jacquot JP (2001) Isolation and characterization of a new peroxiredoxin from poplar sieve tubes that uses either glutaredoxin or thioredoxin as a proton donor. Plant Physiol 127: 1299-1309 [PMC free article] [PubMed] [Google Scholar]
- Sakamoto W, Spielewoy N, Bonnard G, Murata M, Wintz H (2000) Mitochondrial localization of AtOXA1, an Arabidopsis homologue of yeast Oxa1p involved in the insertion and assembly of protein complexes in mitochondrial inner membrane. Plant Cell Physiol 41: 1157-1163 [DOI] [PubMed] [Google Scholar]
- Schenk PM, Baumann S, Mattes R, Steinbiss HH (1995) Improved high-level expression system for eukaryotic genes in Escherichia coli using T7 RNA polymerase and rare ArgtRNAs. Biotechniques 19: 196-200 [PubMed] [Google Scholar]
- Schroder E, Ponting CP (1998) Evidence that peroxiredoxins are novel members of the thioredoxin fold superfamily. Protein Sci 7: 2465-2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo MS, Kang SW, Kim K, Baines IC, Lee TH, Rhee SG (2000) Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J Biol Chem 275: 20346-20354 [DOI] [PubMed] [Google Scholar]
- Stacy RA, Munthe E, Steinum T, Sharma B, Aalen RB (1996) A peroxiredoxin antioxidant is encoded by a dormancy-related gene, Per1, expressed during late development in the aleurone and embryo of barley grains. Plant Mol Biol 31: 1205-1216 [DOI] [PubMed] [Google Scholar]
- Stacy RA, Nordeng TW, Culianez-Macia FA, Aalen RB (1999) The dormancy-related peroxiredoxin anti-oxidant, PER1, is localized to the nucleus of barley embryo and aleurone cells. Plant J 19: 1-8 [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Storz G, Brodsky MH, Lai A, Ames BN (1990) Alkyl hydroperoxide reductase from Salmonella typhimurium: sequence and homology to thioredoxin reductase and other flavoprotein disulfide oxidoreductases. J Biol Chem 265: 10535-10540 [PubMed] [Google Scholar]
- Ting JT, Wu SS, Ratnayake C, Huang AH (1998) Constituents of the tapetosomes and elaioplasts in Brassica campestris tapetum and their degradation and retention during microsporogenesis. Plant J 16: 541-551 [DOI] [PubMed] [Google Scholar]
- Van Bilsen D, Hoekstra FA (1993) Decreased membrane integrity in aging Typha latifolia L. pollen (accumulation of lysolipids and free fatty acids). Plant Physiol 101: 675-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoucq L, Vignols F, Jacquot JP, Chartier Y, Meyer Y (1999) In vivo characterization of a thioredoxin h target protein defines a new peroxiredoxin family. J Biol Chem 274: 19714-19722 [DOI] [PubMed] [Google Scholar]
- Wischmann C, Schuster W (1995) Transfer of rps10 from the mitochondrion to the nucleus in Arabidopsis thaliana: evidence for RNA-mediated transfer and exon shuffling at the integration site. FEBS Lett 374: 152-156 [DOI] [PubMed] [Google Scholar]
- Wojtaszek P (1997) Oxidative burst: an early plant response to pathogen infection. Biochem J 322: 681-692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SS, Platt KA, Ratnayake C, Wang TW, Ting JT, Huang AH (1997) Isolation and characterization of neutral-lipid-containing organelles and globuli-filled plastids from Brassica napus tapetum. Proc Natl Acad Sci USA 94: 12711-12716 [DOI] [PMC free article] [PubMed] [Google Scholar]