Abstract
Plants possess two alternative biochemical pathways for sucrose (Suc) degradation. One involves hydrolysis by invertase followed by phosphorylation via hexokinase and fructokinase, and the other route—which is unique to plants—involves a UDP-dependent cleavage of Suc that is catalyzed by Suc synthase (SuSy). In the present work, we tested directly whether a bypass of the endogenous SuSy route by ectopic overexpression of invertase or Suc phosphorylase affects internal oxygen levels in growing tubers and whether this is responsible for their decreased starch content. (a) Oxygen tensions were lower within transgenic tubers than in wild-type tubers. Oxygen tensions decreased within the first 10 mm of tuber tissue, and this gradient was steeper in transgenic tubers. (b) Invertase-overexpressing tubers had higher activities of glyceraldehyde-3-phosphate dehydrogenase, lactate dehydrogenase, and alcohol dehydrogenase, and (c) higher levels of lactate. (d) Expression of a low-oxygen-sensitive Adh1-β-glucuronidase reporter gene construct was more strongly induced in the invertase-overexpressing background compared with wild-type background. (e) Intact transgenic tubers had lower ATP to ADP ratios than the wild type. ATP to ADP ratio was restored to wild type, when discs of transgenic tubers were incubated at 21% (v/v) oxygen. (f) Starch decreased from the periphery to the center of the tuber. This decrease was much steeper in the transgenic lines, leading to lower starch content especially near the center of the tuber. (g) Metabolic fluxes (based on redistribution of 14C-glucose) and ATP to ADP ratios were analyzed in more detail, comparing discs incubated at various external oxygen tensions (0%, 1%, 4%, 8%, 12%, and 21% [v/v]) with intact tubers. Discs of Suc phosphorylase-expressing lines had similar ATP to ADP ratios and made starch as fast as wild type in high oxygen but had lower ATP to ADP ratios and lower rates of starch synthesis than wild type at low-oxygen tensions typical to those found inside an intact tuber. (h) In discs of wild-type tubers, subambient oxygen concentrations led to a selective increase in the mRNA levels of specific SuSy genes, whereas the mRNA levels of genes encoding vacuolar and apoplastic invertases decreased. (i) These results imply that repression of invertase and mobilization of Suc via the energetically less costly route provided by SuSy is important in growing tubers because it conserves oxygen and allows higher internal oxygen tensions to be maintained than would otherwise be possible.
Oxygen access to internal tissues can be a problem in plants. Oxygen falls to low levels within metabolically active, dense, or bulky plant tissues, even when external oxygen concentrations are high. Low internal oxygen concentrations have been reported in growing tubers (Geigenberger et al., 2000), developing seeds (Quebedeaux and Hardy, 1976; Porterfield et al., 1999; Gibon et al., 2002; Rolletscheck et al., 2002), fruits (Magness, 1920; Banks, 1983; Ke et al., 1995), roots (Lushuk and Salveit, 1991; Thomson and Greenway, 1991), and in phloem tissue (van Dongen et al., 2003). Based on studies in growing wild-type potato (Solanum tuberosum) tubers, Geigenberger et al. (2000) concluded that falling internal oxygen leads to: (a) a restriction of glycolysis and respiration and a decrease in adenylate levels; (b) a widespread decrease in biosynthetic activity, which decreases ATP consumption; and (c) a switch to pathways that consume less ATP. They pointed out that saving ATP could be an important metabolic adaptation to decrease oxygen consumption and prevent the tissue from driving itself into anoxia.
There are two alternative routes of Suc degradation in plants. One involves irreversible hydrolysis (ΔG0′ = -29.3 kJ mol-1) into Glc and Fru via invertase, with a low Km for Suc (7–15 mm; Avigad, 1982). Glc and Fru are subsequently phosphorylated by various hexo- and fructokinases (Renz and Stitt, 1993), using ATP or UTP as energy donors. The other route is unique to plants and involves a UDP-dependent cleavage of Suc to UDP-Glc and Fru that is catalyzed by Suc synthase (SuSy; ap Rees, 1984; Huber and Akazawa, 1986; Kruger, 1997) in a readily reversible reaction in vivo (Geigenberger and Stitt ,1993). The Km (Suc) of SuSy is relatively high (40–200 mm; Avigad, 1982), and the activity of the enzyme is limited by the concentrations of Suc and UDP in the cytosol (Loef et al., 1999). The UDP-Glc that is formed by SuSy is converted to Glc1P and UTP in an inorganic pyrophosphate (PPi)-dependent reaction, catalyzed by UDP-Glc pyrophosphorylase (UGPase). The energy conserved as UTP can be recycled for use to drive the phosphorylation of Fru via fructokinase.
The two pathways of Suc degradation to hexose-phosphates differ in their energy costs. Although breakdown of a molecule of Suc via invertase requires two molecules of ATP, breakdown via SuSy and UGPase requires only one molecule of PPi (Huber and Akazawa, 1986; Stitt, 1998). The overall energy cost of the SuSy pathway is even lower if we assume that it recycles PPi, which is produced as a waste product in many biosynthetic reactions. Interestingly, SuSy genes in maize (Zea mays) typically show an up-regulation by low oxygen (Springer et al., 1986; Sachs et al., 1996; Zeng et al., 1998), whereas invertase genes are strongly repressed (Zeng et al., 1999). During periods of low oxygen, SuSy activity increases, whereas that of invertase declines (Guglielminetti et al., 1995; Zeng et al., 1999), with SuSy predominating as the main enzyme active in Suc breakdown in roots (Ricard et al., 1998). Intriguingly, invertase is expressed early and SuSy later in development of potato tubers (Appeldoorn et al., 1997) and seeds of maize (Tsai et al., 1970) and bean (Phaseolus vulgaris; Weber et al., 1997), when tissues become larger. This is discussed in the literature in respect to the role of Suc and Glc in signaling (Koch, 1996; Weber et al., 1997; Borisjuk et al., 1998, 2002). The possible consequences for the oxygen balance in the tissue have not been investigated.
In view of the possible implications for oxygen consumption, we decided to investigate if Suc degradation via SuSy allows maintenance of increased internal oxygen levels and improved storage metabolism in tubers. Ectopic overexpression of invertase (Sonnewald et al., 1997; Trethewey et al., 1998) or Suc phosphorylase (Trethewey et al., 2001) has been explored as a strategy to improve Suc breakdown in potato tubers. It was expected that substitution of these enzymes, which catalyze an irreversible breakdown of Suc and have a lower Km for Suc than SuSy (Avigad, 1982; Silverstein et al., 1967) would stimulate Suc breakdown. Their introduction led to increased rates of Suc degradation and increased levels of glycolytic intermediates but unexpectedly resulted in a stimulation of respiration and lower starch content. One possible explanation for these changes is that the altered levels of sugars, especially the decrease of Suc, disrupt sugar-signaling mechanisms that are required to allow efficient starch synthesis (Tiessen et al., 2002; Geigenberger, 2003a). However, the rates of starch synthesis found in labeling experiments with isolated tuber discs did not differ between wild type and transformants (Trethewey et al., 1999; Fernie et al., 2002), indicating that this is not the sole or major reason for the unexpectedly low starch content of the transformant tubers. In the following experiments, we show that overexpression of invertase or Suc phosphorylase leads to a strong decrease in the internal oxygen tension in growing tubers, which has marked consequences for metabolism that explain the decrease in their starch content.
RESULTS
Ectopic Expression of Invertase or Suc Phosphorylase Leads to Steeper Oxygen Gradients within Growing Tubers
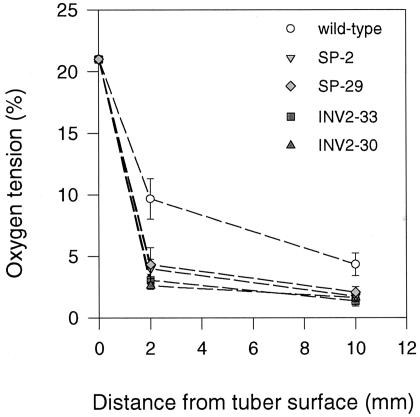
To investigate whether the bypass of the endogenous SuSy route affects internal oxygen tensions in growing tubers, oxygen concentrations were measured within tubers of the transgenic plants expressing invertase or Suc phosphorylase via the tuber-specific B33 patatin promoter (Fig. 1). At 2 and 10 mm below the periderm, oxygen concentrations were measured directly by inserting an oxygen micro-electrode (tip diameter < 1 mm) along a transverse axis into the tuber. In the wild type, oxygen levels showed large gradients from the surface toward the center of the tuber. Oxygen fell from 21% (v/v) at the tuber surface to 9.7% (v/v) at 2 mm and to 4.3% (v/v) at 10 mm below the periderm (Fig. 1). Similar gradients were seen in tubers of different sizes (approximately 10–30 g fresh weight), but absolute values for oxygen concentrations were generally lower when tuber size increased (data not shown). This is probably due to a decrease in the surface to volume ratio under these conditions. The results are similar to the oxygen gradients reported earlier in growing wild-type tubers (Geigenberger et al., 2000). Expression of invertase led to significantly steeper gradients, with oxygen falling from 21% (v/v) at the tuber surface to 2% to 3% (v/v) at 2 mm and to 1% to 2% (v/v) at 10 mm below the periderm (P < 0.05 using the Student's t test). Expression of Suc phosphorylase also led to significantly steeper oxygen gradients compared with the wild type, falling from 21% (v/v) at the surface to approximately 4% (v/v) at 2 mm and approximately 2% (v/v) at 10 mm below the tuber periderm (P < 0.05 using the Student's t test).
Figure 1.
Oxygen gradients within invertase or Suc phosphorylase-expressing potato tubers analyzed using a microelectrode. Oxygen tensions were measured at the tuber surface and at different depth below the tuber periderm (2 and 10 mm). Oxygen tensions are plotted in relation to the distance from the tuber surface. Data are means ± se of separate tubers from different plants (n = 6 for wild type, n = 6 for INV2-30, n = 4 for INV2-33, n = 3 for SP-2, and n = 3 for SP-29). Error bars are not shown when they are smaller than the symbol.
Ectopic Expression of Invertase or Suc Phosphorylase Leads to Induction of Anaerobic Proteins like Alcohol Dehydrogenase (ADH) and Lactate Dehydrogenase (LDH) in Growing Tubers
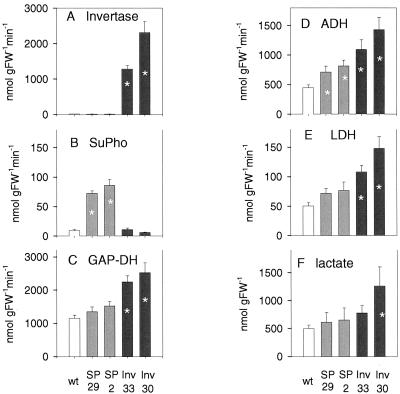
Because oxygen concentrations fell to very low levels within transgenic tubers, almost approaching zero, we investigated whether this was accompanied by increased expression of fermentative enzymes or accumulation of the respective fermentation products (Fig. 2). The activities of ADH (Fig. 2D) and LDH (Fig. 2E), representing enzymes involved in ethanolic and lactic fermentation, respectively, were present in wild-type tubers, which confirms previous findings (Geigenberger et al., 2000) and were further increased in invertase (up to 3-fold) and Suc phosphorylase-expressing tubers (up to 1.7-fold) of similar size (approximately 18–20 g fresh weight). The larger increase in invertase-expressing lines is consistent with the stronger decrease in oxygen in these tubers (compare with Fig. 1). Also GAP-DH, which is a wellknown anaerobic protein (Sachs et al., 1980; Dennis et al., 2000), showed increased activity in the transgenic tubers (Fig. 2C), following a similar pattern to ADH and LDH. Because it is well known that these enzymes are induced by low-oxygen concentrations (Dennis et al., 2000; Klok et al., 2002), these findings provide independent evidence for a decrease in internal oxygen levels in the transgenic tubers.
Figure 2.
Enzyme activities and lactate levels in invertase and Suc phosphorylase-expressing tubers. Activities of: A, invertase; B, Suc phosphorylase; C, GAP-DH; D, ADH; E, LDH; and levels of: F, lactate. Results are means ± se (n = 4 separate tubers from different plants). Error bars are not shown when they are smaller than the symbol. Significant changes from the wild type are marked with an asterisk (P < 0.05 using the Student's t test).
The induction of LDH was accompanied by an increase in lactate levels in the transgenic tubers, which was, however, only significant for line Inv2-30 (Fig. 2F). There were only slight changes in ethanol levels in the transformants; however, these were not consistent across lines—a fact that may be due to very large fluctuations in ethanol concentrations between individual tubers (data not shown).
Ectopic Expression of Invertase Leads to Enhanced Expression of a Low-Oxygen-Responsive ADH-β-Glucuronidase (GUS) Reporter Gene Construct in Growing Tubers
Detailed studies demonstrate that Adh1 is progressively induced when oxygen is decreased over a wide range of subambient concentrations in Arabidopsis (Dolferus et al., 1994), in maize root tips (Saglio et al., 1988; Johnston et al., 1989), seedlings (Andrews et al., 1993), and protoplasts (Howard et al., 1987; Walker et al., 1987). Taking advantage of this, a non-invasive approach was used to provide independent evidence that expression of invertase leads to decreased oxygen levels within potato tubers. In this approach, a construct containing the low-oxygen-sensitive promoter of the Arabidopsis Adh1 gene fused to a reporter gene encoding GUS (Dolferus et al., 1994) was introduced into wild-type and Inv2-30 potato plants. Line Inv2-30 was chosen for super-transformation because it showed the highest invertase expression (Fig. 2A) and a strong decrease in internal oxygen levels (see Fig. 1). From each genotype, 11 independent transgenic lines were selected showing expression of the reporter gene construct.
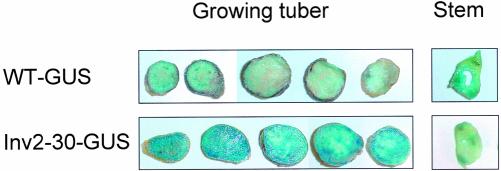
Figure 3 shows GUS staining in representative lines when smaller tubers (approximately 5–10 g fresh weight) were compared. In lines expressing GUS in the wild-type background, GUS activity was highest in the vascular bundles of stems and tubers, and much weaker staining was observed in tuber parenchyma tissue. This contrasts with lines expressing GUS in the Inv2-30 background, which showed strong GUS staining also in the tuber parenchyma tissue in addition to the vascular bundles. Similar results were obtained across all transformant lines. When tubers became larger (20–40 g fresh weight), GUS staining increased in the tuber parenchyma of wild-type GUS lines, whereas no further increase was observed in Inv-GUS transformants (data not shown). Independently of the genetic background, GUS staining was similar in the parenchyma and vascular tissues of the stems (Fig. 3). The specific induction of GUS expression in the parenchyma tissue of small tubers in lines with an Inv2-30 background is consistent with oxygen being decreased in these lines as a consequence of overexpression of invertase via the B33 promoter. Surprisingly, no clear gradient in GUS staining was observed within tuber transects; however, this could be due to bleeding of the product of the GUS reaction across cells.
Figure 3.
Expression of an ADH1-GUS reporter-gene construct introduced into INV2-30 and wild-type potato plants. Whole tubers (approximately 5–10 g fresh weight) were cut into 1-mm-thick slices from top to base, which were subsequently stained for GUS activity. A typical example is shown. Staining of the respective stem slices is shown in parallel.
At the moment, we cannot exclude that factors other than low oxygen led to increased GUS expression in the transgenic lines. The Arabidopsis Adh1-gene is also induced by low temperature, dehydration, and wounding (Dolferus et al., 1994). Despite these possibilities, the strong staining of vascular tissues in stems and tubers (Fig. 3) is in agreement with recent studies documenting low-oxygen concentrations in the vascular bundles of stems (van Dongen et al., 2003).
Ectopic Expression of Invertase or Suc Phosphorylase Leads to a Decrease in the ATP to ADP Ratio inside Growing Tubers, Which Can Be Reversed by Incubating Discs in 21% (v/v) Oxygen
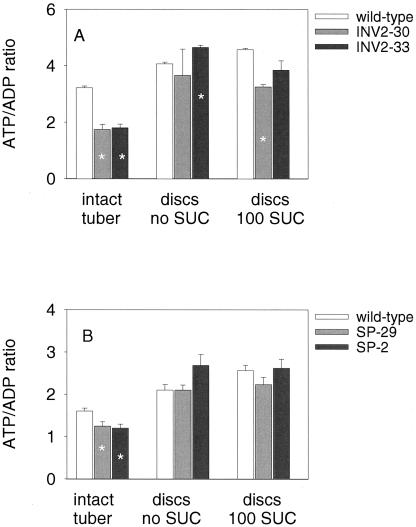
To investigate whether the decrease in internal oxygen levels affects energy metabolism in transgenic tubers, ATP to ADP ratios were analyzed in tissue sampled rapidly (within 2 s) from the center of an intact tuber. The ATP to ADP ratio was relatively low in growing wild-type tubers (see also Geigenberger et al., 2000) and decreased further after expression of invertase (Fig. 4A) or Suc phosphorylase (Fig. 4B). The UTP to UDP ratio decreased in parallel (data not shown). The wild-type values differ between Figure 4, A and B, because the data of these two figures derive from two different experiments with different batches of plants. A similar decrease in ATP to ADP ratio was observed in a second experiment with invertase-expressing tubers (data not shown) and Suc phosphorylase-expressing tubers (see below).
Figure 4.
Ectopic expression of invertase (A) or Suc phosphorylase (B) affects ATP to ADP ratios in growing tubers. Freshly cut slices of growing potato tubers were either immediately (within 2 s) quenched in liquid nitrogen (intact tuber) or incubated in aerated buffer with or without 100 mm Suc for 2 h before rapid quenching in liquid nitrogen, and then extracted to measure nucleotide levels. Data in A and B derive from two different experiments with different batches of plants. Data are means ± se (n = 3–4). Error bars are not shown when they are smaller than the symbol. Significant changes from the wild-type are marked with an asterisk (P < 0.05 using the Student's t test).
The decrease in adenylate energy state could be reversed by incubating freshly cut discs from invertase (Fig. 4A) and Suc phosphorylase-expressing (Fig. 4B) tubers (1 mm thick, 8-mm diameter) in aerated buffer solutions for 2 h. Addition of 100 mm Suc had no substantial effect on ATP to ADP ratios. This confirms that the low cellular energy state in intact tubers and the additional decrease of the energy state in the transformant tubers is due to low internal oxygen levels.
We used line SP-2 to investigate in more detail the short-term changes in ATP to ADP ratios after exposure of discs to air (results are means ± se, n = 3): In discs frozen in liquid nitrogen within 2 s of harvesting, the ATP to ADP ratio was lower in the transformant (1.4 ± 0.1) than wild-type (2.1 ± 0.2) material. With 2 min of exposure to air after cutting the discs, the ATP to ADP ratio recovered, reaching higher values in SP-2 (3.8 ± 0.5) than in wild type (3.0 ± 0.3). After 10 min, ATP to ADP ratios increased further to 4.5 ± 0.5 in SP-2 and 4.2 ± 0.5 in the wild type. These results demonstrate that tissue has to be quenched immediately after sampling from potato tubers to allow accurate measurements of adenine nucleotide levels.
Ectopic Expression of Invertase or Suc Phosphorylase Leads to a Particularly Marked Inhibition of Starch Accumulation in the Center of Growing Tubers
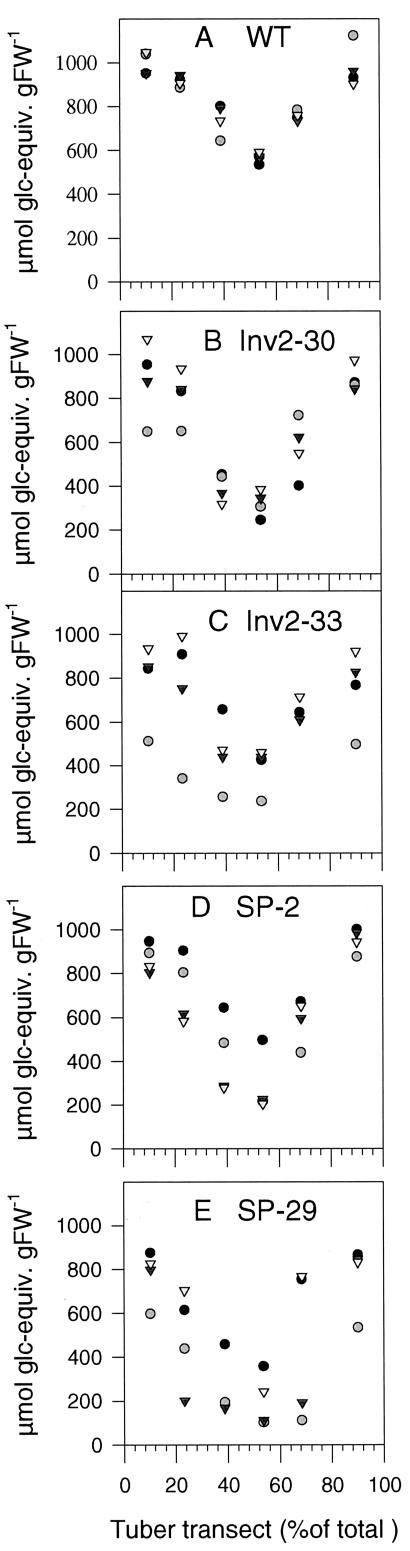
Previous studies showed that ectopic expression of invertase (Trethewey et al., 1998) or Suc phosphorylase (Trethewey et al., 2001) led to a decrease in starch content in tubers. In these earlier studies, overall starch levels were analyzed without taking into account possible gradients within tubers. Therefore, we analyzed starch profiles within tuber transects along a transverse axis (fresh weight of tubers of approximately 30 g). Even in wild-type tubers (Fig. 5A), starch levels decreased by 40% from the periphery (approximately 1,000 μmol g fresh weight-1) to the center of the tuber (approximately 600 μmol g fresh weight-1). Internal starch gradients were more marked in invertase (lines Inv2-30 and Inv2-33, corresponding to Fig. 5, B and C), or Suc phosphorylase-expressing tubers (lines SP-2 and SP-29, corresponding to Fig. 5, D and E), decreasing 3- to 4-fold from the periphery (500–1,000 μmol g fresh weight-1) to the center (100–300 μmol g fresh weight-1). There is some scatter between individual graphs (especially in Fig. 5C) because the graphs show individual tubers from different plants.
Figure 5.
Starch profiles across invertase or Suc phosphorylase-expressing tubers. To investigate starch levels in tuber transects, a cork borer was forced through the middle, removed, and the tissue plug rapidly forced out and simultaneously sliced into approximately 1-mm-thick discs, which fell directly into liquid nitrogen. A, Wild-type; B, Inv2-30; C, Inv2-33; D, SP-2; and E, SP-29. Data are from four individual tubers per line. Total transects lengths were 3.3, 3.2, 3.4, 3.5, and 3.2 cm for wild type, Inv2-30, Inv2.-33, SP-2, and SP-29, respectively.
Inhibition of Starch Synthesis in Response to Suc Phosphorylase Expression Is Dependent on the Oxygen Concentration in Tuber Discs
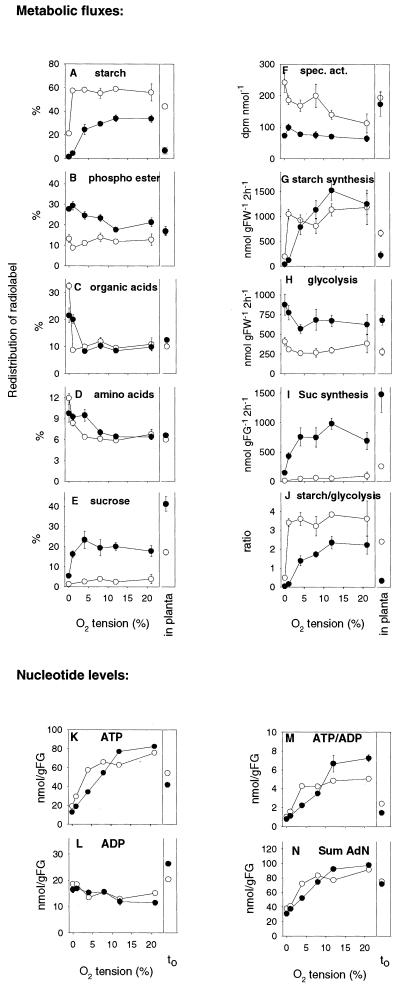
The above results show a striking similarity between internal oxygen and starch gradients, which suggest a link between starch synthesis and oxygen tension in tubers. We used line SP-2 to investigate in more detail whether the decrease in starch levels in the transgenic tubers is an indirect effect due to the lower oxygen concentrations. Thin tissue discs (1 mm thick, 8-mm diameter) were prepared from the center of wild-type and Suc phosphorylase-expressing tubers and incubated in 1 mm [U-14C]Glc in the presence of 0%, 1%, 4%, 8%, 12%, or 21% (v/v) oxygen by bubbling premixed gases through the medium. After 2 h, redistribution of radiolabel into starch, phosphoester, organic acids, amino acids, and Suc was analyzed, and starch synthetic, glycolytic, and Suc synthetic fluxes calculated (Fig. 6, A–J). For comparison, distribution of radiolabel and metabolic fluxes was also investigated after injection of [U-14C] Glc into intact tubers in planta. Previous studies have shown that this approach cannot be used for invertase-expressing tubers because in this case, incoming 14C-Glc is mixing with large endogenous pools, leading to complex and massive isotopic dilution effects (Trethewey et al., 1999). Therefore, we did not use invertase lines in this experiment.
Figure 6.
Metabolism of [U-14C]Glc and nucleotide levels in wild-type (white symbols) and Suc phosphorylase-expressing (black symbols) tubers and tuber discs. [U-14C]Glc was either injected directly into intact tubers (in planta) or was supplied to tuber slices incubated at different oxygen tensions. Freshly cut slices of growing potato tubers were incubated in a medium containing 1 mm Glc under continuous aeration using a stream of premixed gases containing 0%, 1%, 4%, 8%, 12%, or 21% (v/v) oxygen for 2 h as in Geigenberger et al. (2000) before slices were washed and extracted to determine label distribution into different fractions (A–E) or frozen rapidly to determine nucleotide levels (K–N). Percentages of label metabolized to starch (A), phosphorylated esters (B), organic acids (C), amino acids (D), and Suc (E) are shown. The specific activity of the hexose phosphate pool (F) was estimated by dividing the label retained in the phosphate ester pool by the summed carbon of the hexose phosphates (data not shown). The specific activity of the hexose phosphate pool and label incorporation into the relevant fractions were used to calculate the absolute rate of starch synthesis (G), glycolytic flux (the sum of the flux to the organic acids and amino acids; H), and the rate of Suc synthesis (I). J, Relative rates of starch synthesis and glycolysis. Nucleotide data are summarized in ATP (K), ADP (L), ATP to ADP ratio (M), and sum of ATP and ADP (N). Freshly cut slices of growing potato tubers immediately (within 2 s) quenched in liquid nitrogen (t0) are shown for comparison. The results are means ± se (n = 4). Error bars are not shown when they are smaller than the symbol.
There were no significant changes in the uptake of [U-14C]Glc between wild type and transformant or in response to changes in external oxygen tension (data not shown). Figure 6, A to E, show the percentage distribution of radiolabel that was metabolized to other compounds. In wild-type discs incubated at 21% (v/v) oxygen, the largest portion of label was converted to starch (approximately 60%; Fig. 6A), which is consistent with earlier studies (see Geigenberger et al., 1997). Label allocation to starch remained at a constant high level as the oxygen concentration was decreased from 21% to 1% (v/v) and decreased sharply as the oxygen concentration was decreased to zero, where only 20% (v/v) of the label was incorporated into starch (Fig. 6A). The proportion recovered in phosphoesters (mainly hexosephosphates; Fig. 6B), organic acids (Fig. 6C), and amino acids (Fig. 6D) decreased only marginally when oxygen was decreased from 21% to 1% (v/v) and increased in zero oxygen. The increase in zero oxygen was especially marked in the case of organic acids, which rose 4-fold from approximately 8% to 32% (v/v; Fig. 6C). Redistribution of label into Suc decreased slightly when oxygen was decreased to 4% (v/v) and more dramatically when oxygen was decreased to zero (Fig. 6E).
In discs of Suc phosphorylase-expressing tubers incubated at 21% (v/v) oxygen, less of the label was distributed to starch (Fig. 6A) and more of the label was distributed to phosphoesters (Fig. 6B) and Suc (Fig. 6E) than in wild-type discs (see also Fernie et al., 2002). When oxygen was decreased, there was a stronger decrease of starch labeling in the transformant than in the wild type. In the transgenic line, label incorporation into starch started to decrease at 8% (v/v) oxygen and decreased dramatically at 1% (v/v) and zero oxygen, where there was almost no labeling of starch. Low oxygen also led to differential effects in the labeling of phosphoesters between wild type and transformants, with label in phosphoesters decreasing in the wild type and increasing in line SP-2 (Fig. 2B). Although in the wild type oxygen had to be decreased to zero before label in organic acids and amino acids increased, this occurred at 1% (v/v) oxygen in the transformant.
In Figure 6, A to E, label distribution in intact tubers also was analyzed. Expression of Suc phosphorylase led to similar changes in label distribution between starch, organic acids, and amino acids as in discs incubated at low (1%–4% [v/v]) external oxygen. Labeling of phosphoesters and Suc was different in discs compared with intact tubers. This could be due to changes in the internal Suc and hexose-phosphate pools during incubation of discs, which have been frequently observed in earlier experiments (Geigenberger et al., 1997).
The absorbed [U-14C] Glc will mix with internal unlabeled pools, so movement of label will not necessarily reflect fluxes into the various pools (Geigenberger et al., 1997). Label in the phosphoester fraction (see Fig. 6B) was divided by the total carbon found in hexose phosphates (data not shown) to calculate the specific activity of the hexose phosphate pool (Fig. 6F; for a discussion of the assumptions involved in these calculations, see Geigenberger et al., 1997). Expression of Suc phosphorylase led to a strong increase in the size of the internal hexose-phosphate pool (data not shown) and a lower specific activity of the hexose-phosphates (Fig. 6F). This reflects a higher rate of mobilization of internal unlabeled carbohydrates, presumably due to more effective breakdown of Suc via Suc phosphorylase. To estimate the absolute rate of starch synthesis (Fig. 6G), label in starch was divided by the specific activity of the hexose-phosphate pool. In discs incubated with oxygen concentrations in the range between 8% and 21% (v/v), the rate of starch synthesis was slightly faster in the transformant discs than in wild-type discs. When oxygen was decreased below 8% (v/v), starch synthesis was more severely inhibited in Suc phosphorylase-expressing tuber discs than in wild-type discs. The flux to starch was also estimated from the labeling experiment with intact tubers. In the case of intact tubers, the rates of starch synthesis resembled those in tuber discs incubated at low (approximately 1%) external oxygen, and expression of Suc phosphorylase led to a strong decrease of the rate of starch synthesis.
To estimate glycolytic flux (Fig. 6H), label in organic acids and amino acids was summed and divided by the specific activity of the hexose-phosphate pool. Compared with the wild type, Suc phosphorylase-expressing tuber discs had higher rates of glycolysis at 21% (v/v) external oxygen. Decreasing oxygen in the range between 20% and 1% (v/v) did not lead to a restriction of glycolysis in discs of the transformants. When oxygen was decreased to 1% (v/v) or below, there was a stronger increase of glycolytic flux in the transformant than in wild-type discs in absolute terms, probably reflecting increased fermentative activity. Again, the results obtained in intact tubers resembled those for discs incubated in low oxygen (Fig. 6H). Expression of Suc phosphorylase led to a dramatic increase in the rate of Suc synthesis in discs at high external oxygen levels, which was less marked at low oxygen (Fig. 6I).
The ratio between the rate of starch synthesis and the rate of glycolysis is shown in Figure 6J. Expression of Suc phosphorylase led to a general decrease in starch synthesis relative to glycolysis, but the decrease was much stronger in discs incubated at low oxygen or in intact tubers. At 0% to 1% (v/v) external oxygen, the decrease was dramatic, leading to starch to glycolysis ratios of almost zero in the transformant.
The Decrease in Adenylate Energy State in Response to Suc Phosphorylase Expression Is Dependent on the Oxygen Concentration in Tuber Discs
The labeling studies presented in Figure 6, A to J, show that Suc phosphorylase expression leads to a decreased rate of starch synthesis in the presence of low-oxygen tensions but not at high oxygen. To investigate whether this could be due to changes in the adenylate energy state under these conditions, we analyzed ATP and ADP levels in samples taken in parallel (Fig. 6, K–N).
Expression of Suc phosphorylase led to significantly higher ATP levels (Fig. 6K) and a higher ATP to ADP ratio in discs incubated at high external oxygen (12% and 21% [v/v]). However, when oxygen was decreased below 8% (v/v), ATP level and ATP to ADP ratio were more severely reduced in Suc phosphorylase-expressing tuber tissue than in the wild type, leading to a progressively lower adenylate energy state. Expression of Suc phosphorylase led to a similar decrease of the ATP to ADP ratio of intact tubers, which resembled discs incubated at 1% to 4% (v/v) external oxygen. These changes in adenylate energy state reflect the changes in the rate of starch synthesis (compare Fig. 6, G with M), indicating that the low-oxygen-induced inhibition of starch synthesis in the transformant is attributable to a decrease in the cellular energy state under these conditions.
Invertase Is Repressed and Specific SuSy Genes and Adh1 Are Induced When Discs from Wild-Type Tubers Are Incubated at Low External Oxygen Concentrations
The results presented so far suggest that a bypass of the endogenous SuSy route leads to impaired metabolic performance in hypoxic conditions. This occurs because: (a) Increased oxygen consumption leads to lower tissue oxygen levels, and (b) The transformants perform less effectively than wild-type tubers at low tissue oxygen tensions. The question is raised of whether expression of the endogenous genes encoding SuSy and invertase in tubers is regulated to allow SuSy to substitute for invertase when the oxygen concentration decreases.
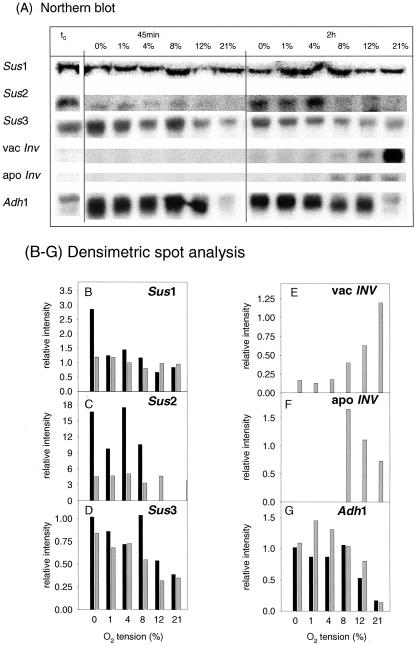
Recent studies with maize roots demonstrate that specific SuSy genes are induced and invertase gene repressed upon hypoxia (Zeng et al., 1999). To investigate whether there is a similar response in growing potato tubers, we incubated wild-type tuber discs in various oxygen concentrations (0%, 1%, 4%, 8%, 12%, and 21% [v/v]) for 45 min or 2 h and analyzed steady-state mRNA levels of the potato SuSy genes Sus1 (Salanoubat and Belliard, 1989), Sus2 (Ehlers Loureiro, 1999), and Sus3 (Fu and Park, 1995), and the genes encoding vacuolar (Zrenner et al., 1996) and apoplastic invertase in potato tubers (Hedley et al., 1993). For comparison, expression of the low-oxygen-sensitive potato Adh1 gene (Matton et al., 1990) was monitored. The changes in transcript levels are documented in Figure 7A (showing the northern blot) and are also expressed as relative intensities on an arbitrary scale after densitometric spot analysis (Fig. 7, B–G).
Figure 7.
Steady-state mRNA levels of Adh1 and specific SuSy and invertase genes in intact wild-type tubers (t0) and wild-type tuber slices incubated in buffer under continuous aeration using premixed gases as in Figure 6. Freshly cut slices of growing potato tubers were either immediately (within 2 s) quenched in liquid nitrogen (intact tuber) or incubated in aerated buffer for 45 min (black bars) or 2 h (gray bars) before rapidly quenching in liquid nitrogen, and RNA was extracted. A, Northern blot. B to G, Densitometric spot analysis of: B, Sus1; C, Sus2; D, Sus3; E, vacInv; F, apoInv; and G, Adh1. B to G, Relative intensities on an arbitrary scale which are corrected for the UV signal of the rRNA on the blotted membrane.
Low oxygen led to a selective increase in the mRNA levels of Sus2 and Sus3, which resembled the increase of Adh1 (Fig. 7, A, C, D, G). Sus1 mRNA levels were not substantially altered (Fig. 7, A, B), except an approximately 2-fold increase at 45 min in zero oxygen. Induction of Sus2 and Sus3 was already evident after 45 min of incubation and increased progressively with decreasing oxygen tensions. The mRNA levels of Sus2 and Sus3 were also high in intact tubers, resembling discs under low oxygen (Fig. 7A). Both Sus2 and Sus3 mRNA levels showed a further dramatic increase when intact tubers were submerged for 24 h (data not shown). We do not think that these changes in gene expression reflect wound responses because: (a) Expression of wound-inducible Sus1 was largely unchanged, and (b) Expression of Sus2 and Sus3 also increased in intact tubers under low oxygen.
Low oxygen led to a reciprocal decrease in mRNA levels, down to the limits of detection, of genes encoding vacuolar and apoplastic invertase (Fig. 7, A, E, and F). Expression of invertase was not detectable in intact tubers and in discs incubated at low oxygen but was clearly induced upon incubation of discs in 8% to 21% (v/v) external oxygen.
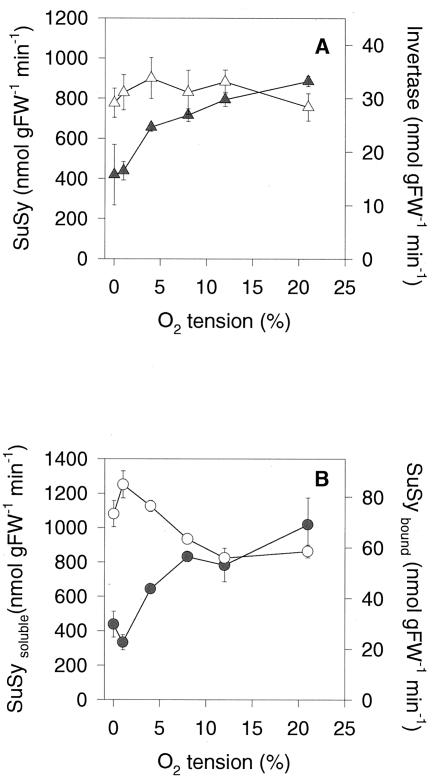
Analysis of enzyme activities revealed a 2-fold decrease of invertase activity in response to low oxygen after 8 h, whereas total SuSy activity was unaltered (Fig. 8A). However, there were changes in the subcellular distribution of SuSy activity. When oxygen was decreased, less SuSy activity was found in the microsomal fraction, whereas more SuSy activity was found in the soluble fraction (Fig. 8B). Binding of SuSy to the microsomal fraction has been found previously to be involved in the channeling of carbon toward cell wall synthesis (Winter et al., 1997), probably by interacting with membrane-bound cellulose synthase (Amor et al., 1995). Decreased SuSy membrane association at low-oxygen concentrations is in confirmation with an inhibition of cell wall biosynthesis under these conditions, which could be part of an adaptive response leading to an inhibition of biosynthetic processes to save ATP when oxygen is low (see Geigenberger et al., 2000).
Figure 8.
Changes in invertase and SuSy activities in tuber discs in response to low oxygen. A, Overall activities of acid invertase (black symbols) and SuSy (white symbols) after 8 h of incubation of discs at the different oxygen concentrations indicated in Figure 7. B, SuSy activity in the microsomal (black symbols) and soluble fraction (white symbols) of tuber discs incubated for 2 h at the different oxygen concentrations. The results are means ± se (n = 4). Error bars are not shown when they are smaller than the symbol.
DISCUSSION
Our results show that overexpression of invertase or Suc phosphorylase to bypass the energetically less expensive SuSy route leads to a strong decrease in internal oxygen tensions in growing tubers, a marked decrease in their energy state, and an inhibition of starch synthesis. These marked changes in metabolism are due to: (a) increased oxygen consumption, leading to lower tissue oxygen levels; and (b) a less effective metabolic performance at low tissue oxygen tension. It implies an important role for the plant-specific SuSy pathway in reducing the degree to which internal oxygen falls and in allowing better maintenance of metabolism under the low-oxygen tensions present within plant tissues.
Ectopic Overexpression of Invertase or Suc Phosphorylase to Bypass the Endogenous SuSy Route Leads to Steeper Oxygen Gradients within Tubers
We provide several independent lines of evidence that overexpression of Suc phosphorylase or invertase leads to lower oxygen tensions within growing tubers. First, direct measurements of oxygen concentrations within growing tubers reveal decreased oxygen levels compared with the wild type (Fig. 1). Second, analyses of enzyme activities reveal that enzymes that are known to be induced by low-oxygen conditions (anaerobic proteins; see Dennis et al., 2000) are increased relative to the wild type. The increase is stronger in invertase than in Suc phosphorylase-expressing lines and includes GAP-DH, ADH, and LDH (Fig. 2, C–E). Expression of SuSy, which is another anaerobic protein, is also increased in these lines (Trethewey et al., 1999; Fernie et al., 2002). Third, products of various fermentative pathways accumulate in the transgenic tubers. There is an increase in lactate levels in the line with the strongest invertase expression (Fig. 2D). The levels of Ala, which is known to accumulate as an early response to anoxia (Davies, 1980), increased 5-fold in invertase (Trethewey et al., 1998) and 3- to 4-fold in Suc phosphorylase-expressing tubers (Fernie et al., 2002). Also, succinate, a major end product of fermentative metabolism in plants (Davies, 1980), is strongly increased in invertase-expressing tubers (Roessner et al., 2001). Fourth, when the low-oxygen-responsive promoter of the Arabidopsis Adh1 gene was fused to a reporter gene encoding GUS (Dolferus et al., 1994) and transformed into wild-type and invertase-expressing potato plants, expression was highest in the tubers of invertase-expressing plants (Fig. 3). Although each approach clearly has its limitations, taken together, these different approaches provide cumulative evidence that oxygen is decreased in the transgenic tubers.
We propose that the decrease in internal oxygen levels is a direct consequence of increased oxygen consumption within the transgenic tubers. Expression of Suc phosphorylase or invertase leads to a 2- or 3-fold increase in respiration rates, respectively (Trethewey et al., 1998, 2001). Three factors could be responsible for this: (a) There is an increased energy cost when Suc is degraded via invertase (2 mol ATP mol Suc-1) or Suc phosphorylase (1 mol ATP mol Suc-1), compared with the endogenous SuSy route (1 mol PPi mol Suc-1). The energy cost of the SuSy pathway is actually zero when PPi is delivered as waste product from biosynthetic reactions, which does not need extra ATP (Stitt, 1998). (b) Expression of Suc phosphorylase (Fernie et al., 2002) or invertase (Trethewey et al., 1999) leads to increased rates of Suc degradation, activation of SPS, and an increase in Suc cycling, leading to an additional energy demand in tubers. In the case of Suc phosphorylase-expressing tubers, this energy demand has been calculated to be approximately 30% of the total ATP produced during respiration, compared with only 3% to 4% in the wild type (Fernie et al., 2002). (c) Suc phosphorylase and invertase-expressing tubers contain higher levels of glycolytic metabolites and increased activities of glycolytic enzymes (Trethewey et al., 1998, 2001; see also Fig. 3), which will support increased respiration rates.
A further interesting possibility is that the heterologous invertase and Suc phosphorylase proteins are not susceptible to feedback mechanisms that regulate the rate of Suc degradation in wild-type tubers, in particular, mechanisms that decrease Suc degradation when oxygen levels fall. In wild-type tubers, falling ATP and rising ADP may restrict fructokinase activity, leading to an increase of Fru and feedback inhibition of SuSy when oxygen is low (Geigenberger et al., 2000). The continued high rates of Suc degradation leading to high levels of glycolytic intermediates may interfere with the regulation network that allows the rate of respiration and metabolism to be reduced in low oxygen.
The Low-Oxygen Tensions within Transgenic Tubers Lead to a Decrease in Their Energy State and an Inhibition of Starch Synthesis
There was a marked decrease in the adenylate energy state in transgenic tubers compared with wild type (Figs. 4 and 6; Fernie et al., 2002), which was stronger in invertase than in Suc phosphorylase-expressing tubers. The low adenylate energy state in intact tubers was rapidly reversed when tuber slices were exposed to air or aerated solutions, providing evidence that it was due to the lower internal oxygen levels present within transgenic tubers. A strong correlation between oxygen levels and adenylate energy state has been demonstrated in earlier experiments with wild-type tubers (Geigenberger et al., 2000) and in the phloem (van Dongen et al., 2003).
There were steep starch gradients from the periphery toward the center of transgenic tubers (Fig. 5), similar to the gradients in oxygen concentration (Fig. 1). The spatial decrease in starch content was much steeper in the transformants than in wild-type tubers. The decrease in starch content in the transformants relative to the wild type was highest near the tuber center, where tissue oxygen levels fall below 2% (v/v). This is broadly in agreement with the measurements of the effect of low oxygen on the rate of starch synthesis in tuber discs, in which a marked inhibition was not found until oxygen dropped to about 4% and 1% (v/v) in discs from Suc phosphorylase and wild-type tubers, respectively (Fig. 6). Further, these labeling experiments showed that expression of Suc phosphorylase leads to a decreased rate of starch synthesis in intact tubers, which is reversed if the tuber discs are incubated at 21% (v/v) oxygen. This provides evidence that the inhibition of starch synthesis in intact transgenic tubers is attributable to decreased tissue oxygen tensions.
The immediate cause of the inhibition of starch synthesis in low oxygen is probably the low adenylate energy state. It has been demonstrated recently that antisense inhibition of the plastidic ATP to ADP translocator leads to an inhibition of starch accumulation in potato tubers (Tjaden et al., 1998), despite a large accumulation of Suc, Glc, and hexose-phosphates (Geigenberger et al., 2001). Conversely, incubation of discs with adenine to increase ATP levels leads to a stimulation of starch synthesis (Loef et al., 2001). These results imply that starch synthesis is restricted by the supply of ATP.
Previous studies show that low oxygen also leads to an increased sensitivity of potato tubers toward pathogens (Butler et al., 1990), with rotting starting in the center of the tuber where oxygen concentrations are low. This is probably due to repression of phenylalanine ammonium lyase and subsequent inhibition of phenylpropanoid synthesis in response to low oxygen (Geigenberger, 2003b; Geigenberger et al., 2000). Invertase overexpression in the cytosol also led to consistently lower oxygen levels within stored tubers (data not shown). When batches of harvested tubers were stored for 8 weeks at 20°C, all of the invertase-expressing tubers were irreversibly damaged due to pathogen attack and rotting, whereas all of the wild-type tubers survived without visible impairment (data not shown). Recent studies demonstrate that invertase-expressing tubers (Inv2-30 and Inv2-33) exhibit drastic susceptibility to Erwinia carotovora attack (Conrath et al., 2003).
Falling Internal Oxygen Leads to a Switch to the Plant-Specific SuSy Pathway of Suc Degradation, Which Consumes Less ATP and Utilizes Oxygen More Efficiently
In contrast to animals, plants lack specialized circulation systems, and oxygen falls to low levels within many plant tissues (see above). Based on studies in potato tubers, Geigenberger et al. (2000) concluded that saving ATP and oxygen by restricting metabolic activity provides an important metabolic strategy to defend a fall in internal oxygen levels and to avoid internal anoxia. A complementary strategy to a decrease in the rate of metabolism would be to prioritize metabolic pathways that conserve energy and, hence, reduce oxygen consumption. Our results imply that the use of the plant-specific SuSy pathway for Suc degradation is an important part of this defense strategy because it leads to improved energy efficiency, saving of ATP, and reduction of respiration, allowing higher internal oxygen tensions to be maintained than would otherwise be possible. This is consistent with invertase being repressed and SuSy being induced in response to low oxygen in maize roots (Zeng et al., 1999) and potato tubers (see Fig. 7).
These results further imply a specific role of PPi in conserving oxygen. The cytosol of the plant cell contains significant levels of PPi (Weiner et al., 1987; Geigenberger et al., 1993; Tiessen et al., 2002), which are maintained at high levels in hypoxic tissues (Dancer and ap Rees, 1989; Geigenberger et al., 2000; Gibon et al., 2002). PPi is utilized as an energy donor for Suc mobilization via SuSy and UGPase, for glycolysis via PFP, and for tonoplast energization via a PPi-dependent proton pump, each of these enzymes being induced by low oxygen (see Dennis et al., 2000, and refs. therein). Each PPi-dependent reaction actually duplicates an ATP-consuming reaction (Stitt, 1998), which in the case of invertase (see Fig. 7; Zeng et al., 1998) and the ATP-dependent proton pump (Gout et al., 2001) are repressed under low oxygen. When SuSy is bypassed by a route that utilizes ATP instead of PPi, tissue oxygen tension (Fig. 1) and cellular energy state decreased (Fig. 4). This provides evidence for an important role of PPi in recycling waste energy to fuel important central metabolic and cellular functions, thereby allowing both ATP and oxygen consumption to be decreased.
Suc Degradation via the SuSy Pathway Improves Metabolic Performance at Low Tissue Oxygen Levels
Experiments with tuber slices show that the reductions in cellular energy state and starch synthetic rates in response to Suc phosphorylase expression are also present in isolated tuber discs incubated in low external oxygen (0%, 1%, or 4% [v/v]). In these instances, changes in energy state and starch flux in transgenic versus wild-type tuber discs are unlikely to be due to differences in tissue oxygen concentrations. This indicates that a bypass of the SuSy pathway also leads to less effective metabolic performance at a given low tissue oxygen level, probably due to a decrease in the energy efficiency of Suc degradation (Fig. 6M). Interestingly, this is accompanied by an increased glycolytic flux and fermentative activity in zero oxygen (Fig. 6H) and is consistent with earlier studies on an Sus1:Sh1 double mutant in maize and an Sus1 antisense line in potato, indicating that decreased SuSy leads to impaired anoxic and postanoxic resistance in roots (Ricard et al., 1998; Biemelt et al., 1999).
Our results imply that a shift in the pathway of Suc degradation from invertase to SuSy allows a higher cellular energy state to be established in the presence of a lower respiratory or fermentative activity at a given low-oxygen tension. A reduction of cellular energy requirements and a concomitant suppression of ATP-generating pathways have been identified previously as important adaptive responses to low oxygen, both in animals (Hochachka et al., 1997) and plants (Geigenberger et al., 2000; Colmer et al., 2001). Crucially, recent reports in rice (Oryza sativa) document that prolonged anoxia leads to a decrease in the energy requirements for maintenance, including diminished ion transport presumably by closed K+ channels and a down-regulation of fermentation (Colmer et al., 2001). Furthermore, in potato tubers, falling internal oxygen led to a widespread decrease in biosynthetic activities and a restriction of respiration (Geigenberger et al., 2000). This general decrease in cellular energy requirements will be beneficial at low tissue oxygen levels because it primarily allows oxygen consumption to be decreased to avoid or delay internal anoxia and, second, because it depresses fermentation and accumulation of toxic intermediates when anoxic conditions develop.
MATERIALS AND METHODS
Plant Material
Potato plants (Solanum tuberosum L. cv Desirée, Saatzucht Fritz Lange, Bad Schwartau, Germany) were grown in well-aerated soil (3-L pots) supplemented with Hakaphos grün slow-release fertilizer (100 g per 230 L of soil; BASF, Ludwigshafen, Germany) in a growth chamber (350 μmol photons m-2 s-1 irradiance, 14-h day/10-h night regime, 20°C, 50% relative humidity), or in a greenhouse during the summer (16 h of light/8 h of dark, 20°C/18°C day/night, 60% relative humidity) with supplementing light as in Tiessen et al. (2002). Growing tubers from 10-week-old daily watered plants with high activities of SuSy, which is taken as an indicator for rapidly growing tubers (Merlo et al., 1993), were used for the experiments.
Generation of GUS Reporter Gene-Expressing Potato Plants and Analysis of Expression Patterns
Transformation of wild-type potato cv Desirée and the yeast (Saccharomyces cerevisiae) invertase-expressing line U-INV2-30 (Sonnewald et al., 1997) with the constructs ADH1-GUS (Dolferus et al., 1994) was performed as described by Rocha-Sosa et al. (1989). Transformants were selected on kanamycin-containing media (Dietze et al., 1995) before transfer to 2-L pots in the greenhouse. Initial screening of approximately 80 plants per parental line was carried out by slicing whole tubers into 1-mm-thick slices from top to base; these were subsequently stained, overnight at 37°C, for GUS activity in six-well macrotiter plates containing GUS staining buffer (Jefferson et al., 1987). From this initial screen, 11 lines were selected that showed reproducible GUS expression across similar-sized tubers. Potato stems were stained following the same protocol.
Analysis of Starch Transects
A cork borer (8-mm diameter) was forced through the middle, removed, and the tissue plug rapidly forced out and simultaneously sliced into approximately 1-mm-thick discs, which fell directly into liquid nitrogen (Geigenberger et al., 2000). For each sample, a single disc was taken every 5 mm down the transect (i.e. every fifth disc was taken), extracted, and starch levels analyzed as in Geigenberger et al. (1999).
Analysis of Oxygen Tensions in Potato Tubers
Intact tubers growing near the surface of the pot (where the oxygen concentration of the soil was above 18% [v/v]; data not shown) were excavated. The internal oxygen tension was measured 1 to 2 min later by introducing an O2 microelectrode (diameter of the tip < 1 mm; Toepffer Lab Systems, Goeppingen, Germany) into the tuber tissue.
Labeling Experiments with Tuber Slices
Tuber discs (8-mm diameter, 1-mm thickness) were cut directly from the center of growing tubers attached to the fully photosynthesizing mother plant, washed quickly with 10 mm MES (pH 6.5; KOH), pre-incubated for 45 min in buffer containing 2 mm Glc and 20 mm mannitol using 50-mL Falcon tubes in a water bath at 20°C (approximately eight discs in 20 mL), and [U-14C]Glc (final specific activity 18.5 KBq μmol-1; Amersham-Buchler, Freiburg, Germany) was added, and incubation was continued for another 2 h (Geigenberger et al., 2000). During the whole incubation and preincubation time, discs were aerated by a continuous stream of premixed gases containing 0%, 1%, 4%, 8%, 12%, and 21% (v/v) oxygen. The oxygen concentration in the solution was routinely checked using an oxygen electrode. After 2 h, discs were rapidly washed three times with buffer to remove external radioactivity and frozen in liquid nitrogen to analyze label distribution.
In Planta Labeling Experiments with Intact Tubers
Labeling experiments with intact tubers were performed as in Geigenberger and Stitt (2000). Tubers were excavated, taking care not to bend the stolons, a fine canal (1–2-mm diameter) was bored through the middle of each tuber using a metal hypodermic needle, and filled with 2 μm [U-14C]Glc (specific activity 7 MBq μmol-1), equivalent to approximately 40 to 50 kBq per tuber. After 2 h of incubation, a concentric ring of tissue (0.8-cm diameter) was removed for radiolabel analysis. During the whole experiment, tubers remained attached to their mother plants via their stolons.
Fractionation of 14C-Labeled Tissue Extracts
Discs were extracted with 80% (v/v) ethanol at 80°C (1 mL per two discs), re-extracted in two subsequent steps with 50% (v/v) ethanol (1 mL per two discs for each step), the combined supernatants dried under an air stream at 35°C, taken up in 1 mL of water (“soluble fraction”), and separated into neutral, anionic, and basic fractions by ion-exchange chromatography; the neutral fraction (2.5 mL) was freeze dried, taken up in 100 μL of water, and further analyzed by thin-layer chromatography (Geigenberger et al., 1997). To measure phosphate esters, samples (250 μL) of the soluble fraction were incubated in 50 μL of buffer (10 mm MES-KOH [pH 6.0]) with or without 1 unit of potato acid phosphatase (Grade II, Boehringer, Mannheim) for 3 h at 37°C, boiled for 2 min, and analyzed by ion-exchange chromatography (Geigenberger et al.,. 1997). The insoluble material left after ethanol extraction was homogenized, taken up in 1 mL of water, and counted for starch. In discs from growing tubers, starch accounts for over 90% of the label in the insoluble fraction (Geigenberger et al., 1994).
Metabolite and Nucleotide Analysis
Tissue slices (30 discs in approximately 80 mL of medium) were incubated using glass vessels allowing continuous aeration with premixed gases (see above). Slices were harvested as in Geigenberger et al. (2000) by pouring the medium immediately through a strainer and throwing the slices into liquid nitrogen within 1 s. Tissue slices from intact tubers were harvested using a cork borer and by quenching the slices immediately in liquid nitrogen. The frozen material was extracted with trichloroacetic acid, and metabolites and nucleotides were measured as in Geigenberger et al. (1998). The recovery of small, representative amounts of each metabolite through the extraction, storage, and assay procedures has been documented previously (see Hajirezaei and Stitt, 1991; Jelitto et al., 1992; Merlo et al., 1993; Geigenberger et al., 1994; Farré et al., 2001). Lactate and ethanol were measured according to Bergmeyer (1987).
Analysis of RNA
Total RNA was extracted from potato tubers according to Logemann et al. (1987) and blotted to a nylon membrane following standard procedures (Sambrook et al., 1989). Radioactive hybridization probes were prepared by the random priming labeling system of Amersham-Buchler using P32-dCTP according to the manufacturer's instructions. The following hybridization probes were used: (a) a 430-bp EcoRI-StyI fragment of StSus1 (Fu and Park, 1995), (b) a 2.1-kb Asp718-Asp718 fragment of StSus2 (Ehlers Loureiro 1999), (c) a 185-bp EcoRI-EcoRI fragment of StSus3 (Fu and Park 1995), (d) a 2,063-bp EcoRI-NotI fragment of the potato gene encoding vacuolar invertase (Zrenner et al., 1996), (e) a 630-bp BamHI-BamHI fragment of the apoplastic potato invertase gene (Hedley et al., 1993), and (f) a 250-bp EcoRI-EcoRI fragment from the potato gene encoding the hypoxia-inducible ADH (Matton et al., 1990). Quantification was performed with an imaging system (Herolab, Wiesloch, Germany), and the signal of the specific transcript was normalized to the UV signal of the rRNA on the blotted membrane.
Analysis of Enzyme Activities
Enzymes were extracted according to Geigenberger and Stitt (1993), ADH and LDH were measured as in Geigenberger et al. (2000), and GAP-DH, soluble acid invertase, and SuSy were measured as in Geigenberger et al. (2001). Binding of SuSy activity to microsomal membranes was analyzed as in Winter et al. (1997).
Acknowledgments
We wish to thank Mark Stitt (MPI Molecular Plant Physiology, Golm, Germany) for his support and interest in this work, stimulating discussions, and helpful comments on the manuscript. We are grateful to Lothar Willmitzer (MPI Molecular Plant Physiology, Golm, Germany) for support and providing the Suc phosphorylase-expressing plants, Uwe Sonnewald (IPK, Gatensleben, Germany) for providing the invertase-expressing lines, Liz Dennis (Commonwealth Scientific and Industrial Research Organization, Plant Industry, Canberra, Australia) for the gift of the Arabidopsis ADH1-GUS construct, Norman Brisson (Département de Biochemie, Université de Montreal) for providing the potato Adh1 cDNA, and Rita Zrenner (MPI Molecular Plant Physiology, Golm, Germany) for providing the potato invertase cDNAs. We are grateful to Ute Roessner (MPI Molecular Plant Physiology, Golm, Germany) for technical help during harvest of material, to Björn Junker (MPI Molecular Plant Physiology, Golm, Germany) for photographic work, and to John Lunn (MPI Molecular Plant Physiology, Golm, Germany) for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (grant nos. Ge 878/1–1 and Ge 878/1–3 to K.L.B. and P.G.).
References
- Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP (1995) A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA 92: 9353-9357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DL, Cobb BG, Johnson JR, Drew MC (1993) Hypoxic and anoxic induction of alcohol dehydrogenase in roots and shoots of seedlings of Zea mays. Plant Physiol 101: 407-414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appeldoorn NJG, de Bruijn SM, Koot-Gronsveld EAM, Visser RGF, Vreugdenhil D, van der Plas LHW (1997) Developmental changes of enzymes involved in conversion of sucrose to hexose phosphate during early tuberisation of potato. Planta 202: 220-226 [Google Scholar]
- ap Rees T (1984) Sucrose metabolism. In DH Lewis, ed, Storage carbohydrates in Vascular Plants. Cambridge University Press, Cambridge, UK pp 53-73
- Avigad G (1982) Sucrose and other disaccharides. In TA Loewus, W Tanner, eds, Encyclopedia of Plant Physiology. Springer-Verlag, Heidelberg, pp 217-347
- Banks NH (1983) Evaluation of methods for determining internal gases in banana fruit. J Exp Bot 34: 871-879 [Google Scholar]
- Bergmeyer HU (1987) Methods of Enzymatic Analysis. VCH, Weinheim, Germany
- Biemelt S, Hajirezaei MR, Melzer M, Albrecht G, Sonnewald U (1999) Sucrose synthase activity does not restrict glycolysis in roots of transgenic potato plants under hypoxic conditions. Planta 210: 41-49 [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Walenta S, Rolletschek H, Mueller-Klieser W, Wobus U, Weber H (2002) Spatial analysis of plant metabolism: sucrose imaging within Vicia faba cotyledons reveals specific developmental patterns. Plant J 29: 521-530 [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Walenta S, Weber H, Mueller-Kliesig W, Wobus U (1998) High resolution mapping of glucose concentrations in developing cotyledons of Vicia faba in relation to mitotic activity and storage processes: glucose as a possible developmental trigger. Plant J 15: 583-591 [Google Scholar]
- Butler W, Cook L, Vayda ME (1990) Hypoxic stress inhibits multiple aspects of the potato tuber wound response. Plant Physiol 93: 264-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD, Huang S, Greenway H (2001) Evidence for down-regulation of ethanolic fermentation and K+ effluxes in the coleoptile of rice seedlings during prolonged anoxia. J Exp Bot 52: 1507-1517 [DOI] [PubMed] [Google Scholar]
- Conrath U, Linke C, Jeblick W, Geigenberger P, Quick WP, Neuhaus HE (2003) Enhanced resistance to Phytophthora infestance and Alternaria solani in leaves and tubers, respectively, of potato plants with decreased activity of the plastidic ATP/ADP transporter. Planta 217: 75-83 [DOI] [PubMed] [Google Scholar]
- Dancer JE, ap Rees T (1989) Effects of 2, 4-dinitrophenol and anoxia on the inorganic pyrophosphate content of the spadix of Arum maculatum and the root apices of Pisum sativum. Planta 178: 421-424 [DOI] [PubMed] [Google Scholar]
- Davies DD (1980) Anaerobic metabolism and the production of organic acids. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants, Vol. 2. Academic Press, pp 581-609 [Google Scholar]
- Dennis ES, Dolferus R, Ellis M, Rahman M, Wu Y, Hoeren FU, Grover A, Ismond KP, Good AG, Peacock WJ (2000) Molecular strategies for improved waterlogging tolerance in plants. J Ex Bot 51: 89-97 [PubMed] [Google Scholar]
- Dietze J, Blau A, Willmitzer L (1995) Agrobacterium-mediated transformation of potato (Solanum tuberosum). In I Potrikus, G Spangenberg, eds, Gene Transfer to Plants. Springer-Verlag, Berlin, pp 24-29
- Dolferus R, Jacobs M, Peacock WJ, Dennis L (1994) Differential interactions of promoter elements in stress responses of the Arabidopsis Adh gene. Plant Physiol 105: 1075-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers Loureiro M (1999) Role of SNF1 kinase and sucrose synthase in the metabolism of potato. PhD thesis. Freie Universität, Berlin
- Farré EM, Tiessen A, Roessner U, Geigenberger P, Trethewey RN, Willmitzer L (2001) Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, amino acids and sugar alcohols in potato tubers using a non-aqueous fractionation method. Plant Physiol 127: 685-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Tiessen A, Stitt M, Willmitzer L, Geigenberger P (2002) Altered metabolic fluxes result from shifts in metabolite levels in sucrose phosphorylase expressing potato tubers. Plant Cell Environ 25: 1219-1232 [Google Scholar]
- Fu H, Park W (1995) Sink- and vascular-associated sucrose synthase functions are encoded by different gene classes in potato. Plant Cell 7: 1369-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P (2003a) Regulation of sucrose to starch conversion in growing potato tubers. J Exp Bot 54: 457-465 [DOI] [PubMed] [Google Scholar]
- Geigenberger P (2003b) Response of plant metabolism to too little oxygen. Curr Opin Plant Biol 6: 247-256 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Fernie AR, Gibon Y, Christ M, Stitt M (2000) Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biol Chem 381: 723-740 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Hajirezaei M, Geiger M, Deiting U, Sonnewald U, Stitt M (1998) Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes for sucrose-starch interconversions, and increased levels of nucleotides in growing potato tubers. Planta 205: 428-437 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Langenberger S, Wilke I, Heineke D, Heldt HW, Stitt M (1993) Sucrose is metabolised by sucrose synthase and glycolysis within the phloem complex of Ricinus communis L. seedlings. Planta 190: 446-453 [Google Scholar]
- Geigenberger P, Merlo L, Reimholz R, Stitt M (1994) When growing potato tubers are detached from their mother plant there is a rapid inhibition of starch synthesis, involving inhibition of ADP-glucose pyrophosphorylase. Planta 193: 486-493 [Google Scholar]
- Geigenberger P, Reimholz R, Deiting U, Sonnewald U, Stitt M (1999) Decreased expression of sucrose phosphate synthase strongly inhibits the water stress-induced synthesis of sucrose in growing potato tubers. Plant J 19: 119-129 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Reimholz R, Geiger M, Merlo L, Canale V, Stitt M (1997) Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta 201: 502-518 [Google Scholar]
- Geigenberger P, Stamme C, Tjaden J, Schulz A, Quick PW, Betsche T, Kersting HJ, Neuhaus HE (2001) Tuber physiology and properties of starch from tubers of transgenic potato plants with altered plastidic adenylate transporter activity. Plant Physiol 125: 1667-1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M (1993) Sucrose synthase catalyses a readily reversible reaction in developing potato tubers and other plant tissues. Planta 189: 329-339 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M (2000) Diurnal changes in sucrose, nucleotides, starch synthesis and AGPS transcript in growing potato tubers that are suppressed by decreased expression of sucrose phosphate synthase. Plant J 23: 795-806 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Vigeolas H, Tiessen A, Geigenberger P, Stitt M (2002) Sensitive and high throughput metabolite assays for inorganic pyrophosphate, ADPGlc, nucleotide phosphates, and glycolytic intermediates based on a novel enzymic cycling system. Plant J 30: 221-235 [DOI] [PubMed] [Google Scholar]
- Gout E, Boisson A-M, Aubert S, Douce R, Bligny R (2001) Origin of the cytoplasmatic pH changes during anaerobic stress in higher plant cells: carbon-13 and phosphorous-31 nuclear magnetic resonance studies. Plant Physiol 125: 912-925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielminetti L, Perata P, Alpi A (1995) Effect of anoxia on carbohydrate metabolism in rice seedlings. Plant Physiol 108: 735-741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajirezaei MR, Stitt M (1991) Contrasting roles for pyrophosphate: fructose-6-phosphate phosphotransferase during aging of tissues from potato tubers and carrot storage tissues. Plant Sci 77: 177-183 [Google Scholar]
- Hedley PE, Machray GC, Davies HV, Burch L, Waugh R (1993) cDNA cloning and expression of a potato (Solanum tuberosum) invertase. Plant Mol Biol 22: 917-922 [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Land SC, Buck LT (1997) Oxygen sensing and signal transduction in metabolic defence against hypoxia: lessons from vertebrate facultative anaerobes. Comp Biochem Physiol 118A: 23-29 [DOI] [PubMed] [Google Scholar]
- Howard EA, Walker JC, Dennis ES, Peacock WJ (1987) Regulated expression of an alcohol dehydrogenase chimeric gene introduced into maize protoplasts. Planta 170: 535-540 [DOI] [PubMed] [Google Scholar]
- Huber SC, Akazawa T (1986) A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol 81: 1008-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901-3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelitto T, Sonnewald U, Willmitzer L, Hajirezaei MR, Stitt M (1992) Inorganic pyrophosphate content and metabolites in leaves and tubers of potato and tobacco plants expressing E. coli pyrophosphatase in their cytosol. Planta 188: 238-244 [DOI] [PubMed] [Google Scholar]
- Johnston J, Cobb BG, Drew MC (1989) Hypoxic induction of anoxia tolerance in roots of Zea mays. Plant Physiol 91: 837-841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke D, Yahia E, Hess B, Zhou L, Kader AA (1995) Regulation of fermentative metabolism in avocado fruit under oxygen and carbon dioxide stresses. J Am Soc Hortic Sci 120: 481-490 [Google Scholar]
- Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES (2002) Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell 14: 2481-2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE (1996) Carbohydrate modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 509-540 [DOI] [PubMed] [Google Scholar]
- Kruger NJ (1997) Carbohydrate synthesis and degradation. In DT Dennis, DH Turpin, DD Lefebvre, DB Layzell, eds, Plant Metabolism. Longman, Harlow, UK, pp 83-104
- Loef I, Stitt M, Geigenberger P (1999) Feeding orotate leads to a specific increase in uridine nucleotide levels, resulting in a stimulation of sucrose degradation and starch synthesis in discs of growing potato tubers. Planta 209: 314-323 [DOI] [PubMed] [Google Scholar]
- Loef I, Stitt M, Geigenberger P (2001) Increased adenine nucleotide levels modify the interaction between respiration and starch synthesis when adenine is fed to discs of growing potato tubers. Planta 212: 782-791 [DOI] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Ann Biochem 163: 16-20 [DOI] [PubMed] [Google Scholar]
- Lushuk JA, Salveit ME (1991) Effects of rapid changes in oxygen concentration on respiration in carrot roots. Physiol Plant 82: 559-568 [Google Scholar]
- Magness JR (1920) Composition of gases in intercellular spaces of apples and potatoes. Bot Gaz 70: 308-316 [Google Scholar]
- Matton DP, Constabel P, Brisson N (1990) Alcohol dehydrogenase gene expression in potato following elicitor and stress treatment. Plant Mol Biol 14: 775-783 [DOI] [PubMed] [Google Scholar]
- Merlo L, Geigenberger P, Hajirezaei M, Stitt M (1993) Changes of carbohydrates, metabolites and enzyme activities in potato tubers during development, and within a single tuber along a stolon-apex gradient. J Plant Physiol 142: 392-402 [Google Scholar]
- Porterfield DM, Kuang A, Smith PJS, Crispi ML, Musgrave ME (1999) Oxygen-depleted zones inside reproductive structures of Brassicaceae: implications for oxygen control of seed development. Can J Bot 77: 1439-1446 [PubMed] [Google Scholar]
- Quebedeaux B, Hardy RWF (1976) Oxygen concentration: regulation of crop growth and productivity. In RH Burris, CC Black, eds, CO2 Metabolism and Plant Productivity. University Park Press, Baltimore, pp 185-204
- Renz A, Stitt M (1993) Substrate specificity and product inhibition of different forms of fructokinase and hexokinases in developing potato tubers. Planta 190: 166-175 [Google Scholar]
- Ricard B, VanToai T, Chourey P, Saglio P (1998) Evidence for the critical role of sucrose synthase for anoxic tolerance of maize roots using a double mutant. Plant Physiol 116: 1323-1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Sosa M, Sonnewald U, Frommer W, Stratmann M, Schell J, Willmitzer L (1989) Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J 8: 23-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie AR (2001) Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 13: 11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolletscheck H, Borisjuk L, Koschorreck M, Wobus U, Weber H (2002) Legume embryos develop in a hypoxic environment. J Exp Bot 53: 1-9 [DOI] [PubMed] [Google Scholar]
- Sachs MM, Freeling M, Okimoto R (1980) The anaerobic proteins of maize. Cell 20: 761-767 [DOI] [PubMed] [Google Scholar]
- Sachs MM, Subbaiah CC, Saab IN (1996) Anaerobic gene expression and flooding tolerance in maize: glycolytic and XET genes and signal transduction. J Exp Bot 47: 1-15 [Google Scholar]
- Saglio PH, Drew MC, Pradet A (1988) Metabolic acclimation to anoxia induced by low (2–4 kPa partial pressure) oxygen pretreatment (hypoxia) in root tips of Zea mays. Plant Physiol 86: 61-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanoubat M, Belliard G (1989) The steady-state level of potato sucrose synthase mRNA is dependent on wounding, anaerobiosis and sucrose. Gene 84: 181-185 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Silverstein R, Voet J, Reed D, Abeles RH (1967) Purification and mechanism of action of sucrose phosphorylase. J Biol Chem 242: 1338-1345 [PubMed] [Google Scholar]
- Sonnewald U, Hajirezaei MR, Kossmann J, Heyer A, Trethewey RN, Willmitzer L (1997) Increased potato tuber size resulting from apoplastic expression of a yeast invertase. Nat Biotechnol 15: 794-797 [DOI] [PubMed] [Google Scholar]
- Springer B, Werr W, Starlinger P, Bennet DC, Zokolica M, Freeling M (1986) The shrunken gene on chromosome 9 of Zea mays L. is expressed in various plant tissues and encodes an anaerobic protein. Mol Gen Genet 205: 461-468 [DOI] [PubMed] [Google Scholar]
- Stitt M (1998) Pyrophosphate as an energy donor in the cytosol of plant cells: an enigmatic alternative to ATP. Bot Acta 111: 167-175 [Google Scholar]
- Thomson CJ, Greenway H (1991) Metabolic evidence for stelar anoxia in maize roots exposed to low O2 concentrations. Plant Physiol 96: 1294-1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiessen A, Hendriks JHM, Stitt M, Branscheid A, Gibon Y, Farré EM, Geigenberger P (2002) Starch synthesis in potato tubers is regulated by post-translational redox-modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell 14: 2191-2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden J, Möhlmann T, Kampfenkel K, Henrichs G, Neuhaus HE (1998) Altered plastidic ATP/ADP-transporter activity influences potato (Solanum tuberosum L.) tuber morphology, yield and composition of starch. Plant J 16: 531-540 [Google Scholar]
- Trethewey R, Riesmeier J, Willmitzer L, Stitt M, Geigenberger P (1999) Tuber-specific expression of a yeast invertase and a bacterial glucokinase in potato leads to an activation of sucrose phosphate synthase and the creation of a sucrose futile cycle. Planta 208: 227-238 [DOI] [PubMed] [Google Scholar]
- Trethewey RN, Fernie AR, Bachmann A, Fleischer-Notter H, Geigenberger P, Willmitzer L (2001) Expression of a bacterial sucrose phosphorylase in potato tubers results in a glucose-independent induction of glycolysis. Plant Cell Environ 24: 357-365 [Google Scholar]
- Trethewey RN, Geigenberger P, Hajirezaei M, Sonnewald U, Stitt M, Riesmeier J, Willmitzer L (1998) Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J 15: 109-118 [DOI] [PubMed] [Google Scholar]
- Tsai CY, Salamini F, Nelson OE (1970) Enzymes of carbohydrate metabolism in the developing endosperm of maize. Plant Physiol 46: 299-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JT, Schurr U, Pfister M, Geigenberger P (2003) Phloem metabolism and function have to cope with low internal oxygen. Plant Physiol 131: 1529-1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JC, Howard EA, Dennis ES, Peacock WJ (1987) DNA sequences required for anaerobic expression of the maize alcohol dehydrogenase gene. Proc Natl Acad Sci USA 84: 6624-6628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U (1997) Sugar import and metabolism during seed development. Trends Plant Sci 2: 169-174 [Google Scholar]
- Weiner H, Heldt HW, Stitt M (1987) Subcellular compartmentation of pyrophosphate and pyrophosphatase in leaves. Biochim Biophys Acta 893: 13-21 [Google Scholar]
- Winter H, Huber JL, Huber SC (1997) Membrane association of sucrose synthase: changes during graviresponse and possible control by phosphorylation. FEBS Lett 420: 151-155 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Wu Y, Avigne WT, Koch KE (1998) Differential regulation of sugar-sensitive sucrose synthases by hypoxia and anoxia indicate complementary transcriptional and posttranscriptional regulation. Plant Physiol 116: 1573-1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Wu Y, Avigne WT, Koch KE (1999) Rapid repression of maize invertases by low oxygen: invertase/sucrose synthase balance, sugar signaling potential and seedling survival. Plant Physiol 121: 599-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrenner R, Schüler K, Sonnewald U (1996) Soluble acid invertase determines the hexose-to-sucrose ratio in cold-stored potato tubers. Planta 198: 246-252 [DOI] [PubMed] [Google Scholar]