Abstract
PC-cell derived growth factor (PCDGF) is an 88-kDa growth factor originally purified from the highly tumorigenic teratoma PC cell line and corresponds to the epithelin/granulin precursor. In teratoma cells, PCDGF expression was shown to be essential for tumorigenicity. We have reported that PCDGF was expressed in estrogen receptor-positive (ER+) human mammary epithelial cells in an estrogen-dependent fashion. In this study, we have investigated PCDGF expression in human mammary epithelial cell lines ranging from immortalized nontumorigenic cells to ER+ and ER− breast carcinoma cells. Northern and Western blot analyses indicated that PCDGF mRNA and protein expression was low in nontumorigenic cells and increased in human breast carcinomas cell lines in a positive correlation with their tumorigenicity. Treatment of the ER− MDA-MB-468 cells with anti-PCDGF neutralizing antibody resulted in a dose-dependent inhibition of their proliferation, suggesting that secreted PCDGF acted as an autocrine growth factor for breast carcinoma cells. We then examined the in vitro and in vivo growth properties of MDA-MB-468 cells, where PCDGF expression had been inhibited by antisense PCDGF cDNA transfection. Inhibition of PCDGF expression resulted in a reduced proliferation rate in vitro and a 60–80% reduction in colony formation. Tumor formation in vivo was dramatically inhibited in antisense cells with a 90% inhibition of tumor incidence and tumor weight. These results demonstrate the importance of PCDGF overexpression for the proliferation and tumorigenicity of ER− breast carcinomas and suggest that PCDGF overexpression may play an important role in human breast cancer.
Breast cancer is the most common malignancy among women worldwide, and, overall, 15% of all women will be diagnosed with breast cancer during their lifetime (1). The occurrence of human breast cancer is associated with the overexpression, and/or amplification of a number of genes including the ones encoding growth factors and growth factor receptors (2). Steroid hormones and peptide growth factors that play an important role in the development of the normal breast also are involved in carcinogenesis of its epithelium and progression of breast cancer (3). The autocrine growth factor hypothesis, where growth factors and growth factor receptors are overexpressed in tumor cells, was proposed to explain the decreased response to exogenous growth factors that is associated with the loss of growth regulation of transformed cells (4–7). In breast carcinoma cells, these include epidermal growth factor receptor (EGFR)/transforming growth factor α autocrine pathway involved in both normal gland growth and early stages of breast tumorigenesis (8–11). Several reports have shown that the type 1 family tyrosine kinase cell surface receptors, such as EGFR and c-erbB2, often are overexpressed in several breast tumors. Their overexpression has been correlated with treatment relapse and poor prognosis of the disease (12–14). Clinically, the anti-erbB2 antibody is presently used to treat patients with metastatic breast cancer overexpressing erbB2 receptor (15, 16). In addition, insulin-like growth factors I and II (IGF-I, IGF-II) and IGF-I receptor have also been implicated in the acquisition of growth advantage by breast cancer cells (17, 18). In addition, these various studies have pointed to the importance of identifying autocrine growth factor pathways being overexpressed in breast cancer cells as they progress toward a more malignant phenotype and determining their role in tumor growth.
PC cell-derived growth factor (PCDGF), also called epithelin/granulin precursor, is an 88-kDa secreted glycoprotein purified from the conditioned medium of the highly malignant mouse teratoma-derived cell line PC for its ability to stimulate its proliferation in an autocrine fashion (19). Amino acid and nucleotide sequencing indicated that PCDGF was identical to the precursor of epithelins and granulins, a group of double cysteine-rich 6-kDa polypeptides that either promote or inhibit cell growth, depending on the cell types (20–23). It originally was thought that the epithelin/granulin precursor has to be processed into the 6-kDa epithelins or granulins to be biologically active (24). However, several groups, including ours, have reported that the intact precursor was biologically active to stimulate the proliferation of fibroblast cells as well as epithelial cells (19, 25, 26). Cell surface binding sites for 125I-PCDGF with an apparent molecular mass of 120 kDa have been characterized by Scatchard analysis and by affinity labeling of iodinated PCDGF in several cell lines of mesenchymal and epithelial origins (27). Study of teratoma-derived cell lines with increasing tumorigenicity has shown that PCDGF expression increased with tumorigenicity of the cells. Moreover, it was demonstrated that inhibition of PCDGF expression by antisense PCDGF cDNA transfection in the highly tumorigenic PC cells led to a complete inhibition of tumor formation when the cells were injected in syngeneic mice C3H (28). These data indicated that overexpression of PCDGF was associated with the cell tumorigenicity and that PCDGF was a tumorigenic autocrine growth factor.
Recently, we have reported that PCDGF was expressed in estrogen receptor-positive (ER+) human breast cancer cells MCF-7 and T47D and that PCDGF expression was stimulated by 17-β estradiol in a time- and dose-dependent fashion in these ER+ cells (29). These studies led us to assume that PCDGF, in an autocrine fashion, mediated the growth of human breast cancer cells. Based on these data, experiments were carried out here to examine the expression and function of PCDGF in highly malignant, ER-negative (ER−) human breast cancer cells and to determine whether PCDGF contributes to the tumorigenicity of human breast cancer cells. Our studies demonstrate that, in ER− human breast cancer cells, PCDGF expression is elevated and that inhibition of PCDGF expression by antisense cDNA transfection inhibited human breast cancer cell growth both in vitro and in vivo.
Materials and Methods
Cell Culture.
Human mammary epithelial cell lines MCF-10A, MCF-7, T47D, MDA-MB-468, and MDA-MB-453 were obtained from the American Type Culture Collection. Human breast epithelial cell line MCF-10A was maintained in a 1:1 mixture of DMEM and Ham's F-12 medium (DME/F12) supplemented with 20 ng/ml mouse epidermal growth factor (Upstate Biotechnology, Lake Placid, NY), 100 ng/ml cholera toxin, 10 μg/ml insulin, 500 ng/ml hydrocortisone, and 5% horse serum (30). Other cell lines were cultivated in DME/F12 medium supplemented with 5% FBS.
Northern Blot Analysis of PCDGF mRNA Expression.
MCF-10A, MCF-7, T47D, MDA-MB-468, and MDA-MB-453 cells were cultivated in media described above. After reaching 70% confluence, the media were changed to estrogen-depleted medium consisting of phenol red-free α-modified Eagle's medium (α-MEM) supplemented with 5% charcoal-stripped FBS (PFMEM) for 48 h as described (29). RNA isolation and Northern blot analysis were conducted as described (29).
Immunoprecipitation and Western Blot Analyses.
PCDGF protein expression secreted in the culture medium was determined by a combination of immunoprecipitation and Western blot analyses using anti-human PCDGF polyclonal antibody in the presence of protease inhibitors as described (28, 29). The conditions for immunoprecipitation and concentration of antibody to be used in the assay had been previously determined to ensure that immunoprecipitation of all of the PCDGF present in the conditioned medium be achieved (28). After collecting the conditioned medium, the cell number was determined with a hemocytometer after detaching the cells with a solution of 1 mg/ml trypsin and 1 mM EDTA. For comparative studies of PCDGF expression with each cell line, the amount of conditioned medium to analyze for measuring PCDGF expression was normalized to the same cell number (4 × 106 cells).
Quantitative analysis of the Northern and Western blotting was performed with an imaging densitometer.
Stable Transfection of PCDGF cDNA in the Antisense Orientation.
Antisense PCDGF cDNA expression vector was constructed by ligating in the antisense orientation a partial human PCDGF cDNA (−31 bp to 374 bp) into the EcoRI–BamHI restriction enzyme sites of pcDNA3 mammalian expression vector (Invitrogen). A total of 10 μg of antisense PCDGF cDNA plasmid constructs was transfected into MDA-MB-468 cells cultivated in 100-mm tissue culture dishes by means of Lipofectamine (GIBCO) in DME/F12 containing G418 (800 μg/ml). MDA-MB-468 cells transfected with pcDNA3 empty vector plasmid DNA were used as control. Stable clones were isolated after 3 wk and screened by Northern blot and later by Western blot analysis to measure the level of expression of PCDGF mRNA and protein.
[3H]Thymidine Incorporation Assay.
MDA-MB-468 empty vector control and antisense PCDGF cDNA transfected cells were plated in 24-well plates in DME/F12 medium supplemented with 5% FBS at a density of 105 cells per well. Twenty four hours later, the medium was changed to serum-free DME/F12 medium. After another 24 h, the medium was replaced with fresh serum-free DME/F12 medium either alone or in the presence of factors to be assayed, depending on the experiments and as indicated in Results. [3H]Thymidine (1 μCi/ml) was added 24 h later. After 5 h of incubation, cells were washed twice with cold PBS and then incubated with 10% ice-cold trichloroacetic acid containing 10 mM thymidine for 15 min at 4°C to precipitate DNA (19). The cells then were washed twice with cold PBS and lysed with 0.5 M NaOH. The radioactivity of the samples was counted by liquid scintillation counter.
For the experiments of neutralization of secreted PCDGF, highly purified anti-human PCDGF antibody preparation was used. This anti-PCDGF antibody was purified by using the following method. IgG fraction first was prepared from anti-human PCDGF antiserum by precipitation with 33% ammonium sulfate. The pellet was reconstituted in PBS, dialyzed against PBS. IgG fraction then was affinity-purified by chromatography on a PCDGF-Sepharose column. Purified antibodies were eluted at acidic pH. Protein concentration of the fraction was determined by the micro bicinchoninic acid method. SDS/PAGE analysis of the eluted antibody preparation followed by protein staining indicated the presence of a single band corresponding to IgG.
For measuring the effect of anti-PCDGF antibody on the growth of MDA-MB-468 cells, we used the thymidine incorporation assay described above. Anti-PCDGF IgG was added for 24 h before adding [3H]thymidine as described above. Detailed experiments demonstrating the specificity of the antibody for inhibiting PCDGF action and no other growth factors have been carried out with MCF-7 cells (data not shown).
Colony Formation in Soft Agar.
A total of 2,000 cells were suspended in 0.4% low melting agarose (Life Technologies, Rockville, MD) dissolved in 1 ml of serum-free DME/F12 medium and plated on top of 1 ml underlayer of 0.8% agarose in the same medium in 6-well culture plates. Cells received 1 ml serum-free DME/F12 medium alone or in the presence of 200 ng/ml human PCDGF. The media were changed every 3 days. After 3 wk of incubation at 37°C in a 5% CO2 incubator with humidified atmosphere, the colonies were visualized by staining with 1 ml of p-iodonitrotetrazolium violet (Sigma) for 24 h. Colonies with more than 15 cells were counted.
In Vivo Tumorigenicity Study.
A total of 2 × 105 antisense and empty vector transfected cells were inoculated s.c. in 100 μl of serum-free DME/F12 medium through 26-gauge needle behind the anterior forelimb of 4-wk-old athymic mice obtained from Harlan–Sprague–Dawley. Mice were maintained in a pathogen-free facility. The appearance and size of the tumors were examined daily. Mice were killed 4 wk after injection to measure tumor weight.
Statistical Analysis.
All experiments were repeated three times, and data were expressed as mean ± SD. Two-tailed Student's t test was used for statistical analysis of the data. P < 0.05 was taken as the level of significance.
Results
Comparison of PCDGF mRNA and Protein Expression in Human Breast Cancer Cell Lines.
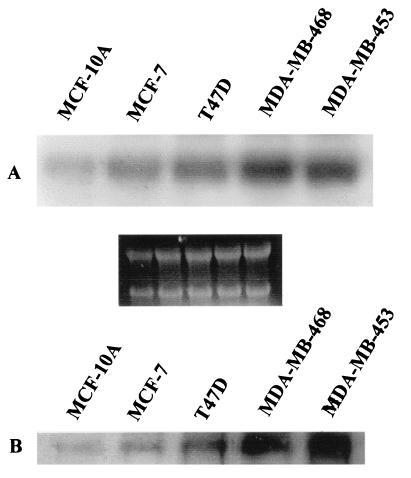
We previously have reported that PCDGF was expressed by ER+ human breast cancer cells and that its expression was stimulated by 17-β estradiol in a time-and dose-dependent fashion (29). In the present paper, we compared PCDGF expression in several human mammary epithelial cells. They included the nontumorigenic, immortalized human mammary epithelial cell line MCF-10A; the ER+ MCF-7 and T47D human breast carcinoma cell lines, and the ER− malignant breast carcinoma cell lines MDA-MB-468 and MDA-MB-453. As shown in Fig. 1A, PCDGF mRNA was expressed in all human carcinoma cell lines tested but with a different degree. PCDGF mRNA expression was the lowest in the nontumorigenic MCF-10A cells. It increased 3.5-fold in ER+ MCF-7 and T47D cells and 8.7- and 7-fold in ER− MDA-MB-468 and MDA-MB-453 cells, respectively.
Figure 1.
Comparison of PCDGF mRNA and protein expression in human mammary epithelial cells. (A) mRNA expression. Cells were cultivated in the culture media described in Materials and Methods until they reached 70% confluence. The culture medium was changed to estrogen-depleted PFMEM medium 48 h before RNA was extracted. Then, 20 μg of total RNA was analyzed by Northern blot to measure PCDGF expression (A). Ethidium bromide staining of RNA was used as internal loading standard. (B) PCDGF protein expression. When cells reached 70% confluence in the culture conditions described above, the medium was replaced by fresh medium for 24 h. The conditioned medium was then collected. The cell number was then determined in parallel for each cell line so that samples analyzed could be normalized to the same cell number (106 cells) from each cell line tested to determine PCDGF protein expression, as described in Materials and Methods.
The expression of PCDGF protein was examined in the conditioned medium of all cell lines tested because PCDGF is secreted immediately after being synthesized, as described in Materials and Methods. As shown in Fig. 1B, PCDGF protein expression followed the same pattern as PCDGF mRNA. PCDGF protein expression was very low in the immortalized, nontumorigenic MCF-10A and was significantly elevated in ER− MDA-MB-468 and -453 cells when compared with ER+ cells MCF-7 and T47D and to the immortalized MCF-10A cells. When compared with the MCF-10A cells, PCDGF expression increased by 3-fold in MCF-7 cells and by more than 10-fold for MDA-MB-468 and for MDA-MB-453 cells. The data would suggest that PCDGF mRNA and protein expression in these human breast cancer cells is positively correlated with the degree of tumorigenicity of the cells.
Effect of Anti-PCDGF Neutralizing Antibody on the Growth of MDA-MB-468 Human Breast Cancer Cells.
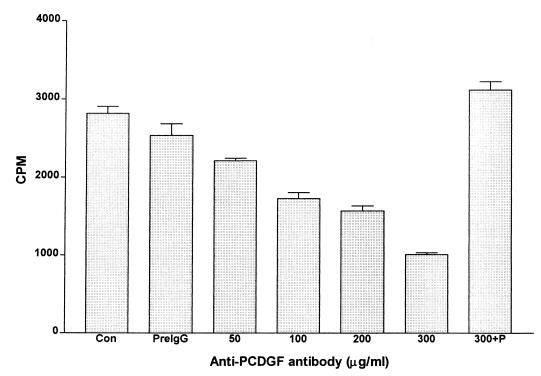
Because MDA-MB-468 cells synthesize and secrete PCDGF into their culture medium, experiments were carried out to examine whether PCDGF produced and secreted by the cells was required for their proliferation. For this purpose, we determined the effect of adding neutralizing anti-human PCDGF IgG into the culture medium on the cell proliferation. This anti-PCDGF antibody had been purified by affinity purification of anti-PCDGF IgG on a PCDGF-Sepharose column. As shown in Fig. 2, treatment of MDA-MB-468 cells with affinity-purified anti-PCDGF antibody resulted in a dose-dependent inhibition of cell proliferation. A maximal inhibition of 70% was observed at an antibody concentration of 300 μg/ml (P < 0.001), indicating the importance of this factor on the proliferation of MDA-MB-468 cells. In contrast, the same concentration of rabbit preimmune IgG had no inhibitory effect on cell proliferation. Exogenous addition of 100 ng/ml of human PCDGF to cells maintained in the presence of 300 μg/ml of anti-PCDGF IgG completely abolished the growth inhibitory effect of the antibody, indicating the specificity of the antibody toward PCDGF. In these conditions, the [3H]thymidine incorporation was similar to the one in control untreated cells (Fig. 2). Taken together, these results suggest that PCDGF secreted by the MDA-MB-468 cells stimulates their proliferation in an autocrine fashion.
Figure 2.
Effect of anti-PCDGF neutralizing antibody on the growth of MDA-MB-468 human breast cancer cells. MDA-MB-468 cells were cultivated in a 24-well plate at 105 cells per well in DME/F12 medium supplemented 5% FBS. After 24 h, the medium was changed to serum-free DME/F12 medium in the presence or absence of anti-PCDGF or control IgG. Cells received nothing as control (con), 300 μg of rabbit preimmune IgG (preIgG), or increasing concentrations of anti-human PCDGF antibody, as indicated in the figure. A triplicate set of wells treated with 300 μg/ml of anti-PCDGF IgG was also treated with 100 ng/ml PCDGF (300 + P). After 24 h, [3H]thymidine at 1 μCi/ml was added for 5 h. Cells were washed twice with PBS, treated with 10% TCA, and lysed with 0.2 M NaOH, as described in Materials and Methods. Radioactivity in lysates was determined with a liquid scintillation counter.
Inhibition of PCDGF Protein Expression by Antisense PCDGF cDNA Stable Transfection.
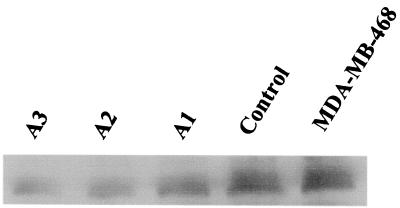
Based on the above results, experiments were carried out to examine the in vitro and in vivo growth properties of MDA-MB-468 cells, where PCDGF expression had been inhibited by transfection of antisense PCDGF cDNA. A 405-bp human PCDGF cDNA fragment (−31 bp to 374 bp) was ligated in the antisense orientation into the mammalian expression vector pcDNA3. The resulting construct was transfected into MDA-MB-468 cells, as described in Materials and Methods. Control MDA-MB-468 cells were transfected with empty pcDNA3 vector. In both cases, transfected cells were selected for growth in medium containing G418 (800 μg/ml). Colonies resistant to G418 were isolated 3 wk later from antisense-transfected cells and from empty vector-transfected control cells and were analyzed as described in Materials and Methods. Fifteen colonies from antisense PCDGF cDNA-transfected cells were isolated. Antisense clones generally grew slowly and formed fewer colonies than empty vector-transfected control cells (data not shown). Inhibition of PCDGF protein expression in antisense-transfected cells and in control cells was examined by Western blot analysis using anti-PCDGF antibody. The results of three representative clones are shown in Fig. 3. Western blot analysis using anti-PCDGF antibody showed that antisense PCDGF cDNA transfection dramatically inhibited PCDGF protein expression in MDA-MB-468 cells. PCDGF expression was 80% lower in the antisense clones no. 2 and 3 (A2, A3) than in the empty vector control cells, whereas in clone no. 1 (A1), PCDGF expression was reduced by 50%. The clones A1 and A3, representative of antisense clones with intermediate and strong inhibition of PCDGF expression, were used for further studies.
Figure 3.
Inhibition of PCDGF protein expression by antisense PCDGF cDNA stable transfection. A 405-bp human PCDGF cDNA fragment (−31 to +374 bp) was ligated into the mammalian expression vector pcDNA3. The resulting construct was stably transfected into MDA-MB-468 cells. Transfected cells were selected in the presence of G418 (800 μg/ml). Clones were picked up and cultivated in DME/F12 supplemented with 5% FBS. To measure PCDGF expression in antisense and control cells, cells were cultivated in DME/F12 medium supplemented with 5% FBS. When cells reached confluency, medium was replaced by fresh culture medium. After 24 h, the conditioned medium was collected and protease inhibitors were added. Cells were counted The conditioned medium was collected 24 h later and normalized by cell number. Immunoprecipitation and Western blot analysis were performed with an anti-human PCDGF antibody to measure PCDGF expression in the medium. A1, A2, and A3 are different clones of antisense PCDGF cDNA-transfected MDA-MB-468 cells.
Growth Properties of Antisense Cells in Vitro.
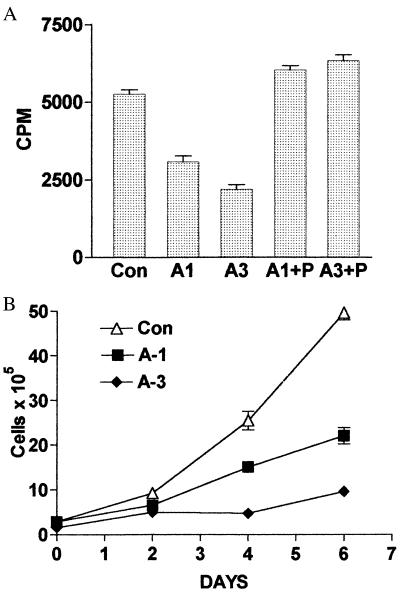
The growth properties of A1 and A3 clones and of empty vector control cells were first examined by measuring [3H]thymidine incorporation into DNA. As shown in Fig. 4A, both antisense PCDGF cDNA-transfected clones showed a reduced proliferation ability when compared with control cells. Furthermore, the effect on cell proliferation correlated well with the degree of inhibition of PCDGF expression in the antisense cells. The proliferation of A3 cells, which showed the strongest inhibition of PCDGF expression was reduced by 60% (P < 0.005), whereas proliferation of A1 cells was inhibited by 40% (P < 0.01). Interestingly, when adding PCDGF exogenously to the culture medium of antisense cells, DNA synthesis in A1 and A3 cells was fully restored to the level observed with empty vector control cells (Fig. 4A). These data indicated that the reduced growth ability of A1 and A3 cells was specifically because of the inhibition of PCDGF expression by the antisense cDNA transfection, further emphasizing the important role of PCDGF on the MDA-MB-468 cell growth.
Figure 4.
Effect of inhibition of PCDGF expression on cell proliferation. (A) DNA synthesis: Antisense PCDGF cDNA-transfected cells A1, A3, and empty vector control MDA-MB-468 cells were plated into 24-well plates in triplicate in DME/F12 medium supplemented with 5% FBS at a density of 105 cells per well. The medium was changed to serum-free DME/F12 medium after 24 h in the absence or presence of 200 ng/ml PCDGF. [3H]Thymidine was added (1 μCi/ml) 24 h later. After 5 h of labeling, the cells were lysed with 0.5 M NaOH. The radioactivity of the samples was counted by liquid scintillation counter. The results are expressed as mean ± SD. (B) Long-term cell growth: Empty vector control and antisense PCDGF cDNA-transfected MDA-MB-468 cells were plated in triplicate at 105 cells per well into 6-well plates in DME/F12 medium supplemented with 5% FBS. After 24 h, the medium was changed to serum-free DME/F12 medium. Cell numbers were counted with a hemocytometer every 2 days until day 8, as described in Materials and Methods. The results are expressed as mean ± SD. Con, empty vector control cells; A1, antisense A1 clone; A3, antisense A3 clone; A1 + P, A1 treated for 24 h with 200 ng/ml PCDGF; A3 + P, A3 cells treated with PCDGF.
Experiments then were performed to compare the long-term growth of A1, A3 cells to the ones of control cells. As shown in Fig. 4B, A1 and A3 cells both grew slowly when compared with control cells in correlation with the degree of inhibition of PCDGF expression. The number of control cells after 6 days in culture was 5-fold higher than A3 cells and 2.5-fold higher than A1 cells, respectively. Moreover, the doubling time for antisense cells was significantly increased (4 days for A3 cells) when compared with the control cells (1 day), indicating a reduced proliferation rate when PCDGF expression was inhibited. As was observed in the DNA synthesis experiment, cultivation of A1 and A3 cells in the presence of PCDGF (200 ng/ml) fully restored growth of antisense cells to the level found in empty vector control cells. After 6 days, the cell number was 58 ± 6 × 105 cells and 55 ± 5 × 105 cells for A1 cells and A3 cells cultivated in the presence of PCDGF and 49 ± 2 × 105 cells for empty vector control cells.
Growth on Soft Agar of Antisense PCDGF cDNA and Empty Vector-Transfected Cells.
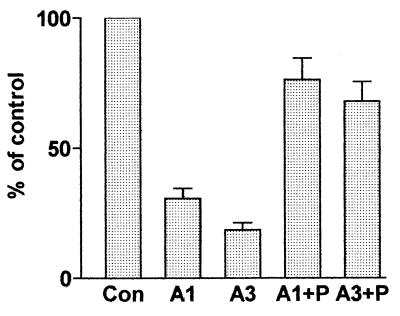
Because antisense cells showed reduced proliferative ability, we next examined whether inhibition of PCDGF would affect their ability to form colonies in soft agar, a property believed to be an in vitro parameter reflective of malignancy (31). As shown in Fig. 5, antisense cells formed fewer colonies than the control cells. The number of colonies formed by A3, the clone showing the highest inhibition of PCDGF expression, was inhibited by about 80% (P < 0.001). Addition of exogenous PCDGF (200 ng/ml) also stimulated soft agar growth of both A1 and A3 antisense cells to a level close to the one observed for empty vector control cells.
Figure 5.
Anchorage-independent growth of antisense and empty vector control MDA-MB-468 cells. Antisense cells A1, A3, and control cells were plated in triplicate at a density of 2,000 cells per well in 6-well plates in agarose containing serum-free medium with or without 200 ng/ml human PCDGF. The medium was changed every 3 days. Colonies were allowed to grow for 3 wk and then stained with p-iodonitrotetrazolium violet. Colonies with at least 15 cells were counted, and the results were expressed as percentage of control cells. The number of colonies for control cells was 206 ± 42. Con, empty vector control cells; A1, antisense A1 clone; A3, antisense A3 clone; A1 + P, A1 treated with 200 ng/ml PCDGF; A3 + P, A3 cells treated with 200 ng/ml PCDGF.
In Vivo Tumorigenicity Studies.
We next examined the tumorigenic properties of antisense and control cells in vivo. Antisense and control cells (2 × 105) were s.c. injected into female athymic mice to determine whether inhibition of PCDGF expression in breast carcinoma cells would affect tumor formation in nude mice. Tumor formation was monitored daily, and the mice were killed after 4 wk to examine the weight of tumor. These in vivo studies were repeated three times. The results of one representative experiment are provided in Table 1. All mice injected with control cells developed tumors (100% incidence). Tumors were visible as early as 7 days after injection for empty vector-transfected control MDA-MB-468 cells. In contrast, antisense cells showed both a reduced tumor incidence and tumor weight. Four mice developed tumor from A1 group (57% incidence) with an average weight about 80% lower than the control cells (P < 0.0001). Interestingly, only one of seven mice developed tumors in the group of mice injected with A3 cells (14% incidence). Taking all experiments into account, the average tumor weight with A3 cells was 0.05 ± 0.01 g. Even 8 wk after injection, A3 cells did not form other palpable tumors.
Table 1.
In vivo tumorigenicity of MDA-MB-468 cells transfected with antisense PCDGF cDNA and with empty vector
| Cells injected | Day of appearance | Mice with tumors | Weight (g ± SD) |
|---|---|---|---|
| Empty vector control | 7 | 7 /7 | 0.44 ± 0.18 |
| Antisense A1 | 15 | 3 /7 | 0.08 ± 0.03* |
| Antisense A3 | 23 | 1 /7 | 0.05 ± 0.01** |
Exponentially growing A1, A3, and empty vector-transfected control cells were inoculated s.c. at a density of 2 × 105 cells per mouse behind the anterior forelimb of athymic nude mice. The mice were sacrificed after 4 wk, and the tumors were collected and weighed. *, P < 0.0001; **, P < 0.00001.
Discussion
Early stages of mammary neoplasia are sensitive to growth factor stimulation, whereas the most malignant metastatic stages overproduce significant levels of several growth factors and are refractory to their exogenous supplementation (32). Moreover, overexpression of several growth factors is considered a strong risk factor for mammary cancer (33). The results presented here have identified a growth factor PCDGF, also called epithelin/granulin precursor, as being overexpressed in ER− breast carcinoma cell lines and acting as an autocrine growth factor for these cells. PCDGF originally was identified through studies of the role of autocrine growth factors on the acquisition of tumorigenic properties in teratoma tumors (34). PCDGF was purified as a secreted growth factor from the highly tumorigenic teratoma PC cells (19). Biochemical characterization showed that it was an 88-kDa glycoprotein with a 20-kDa carbohydrate moiety, whereas amino acid sequencing showed that PCDGF corresponded to the precursor for epithelin/granulin, a family of 6-kDa double cysteine-rich polypeptides (24, 20). The 6-kDa epithelins, originally purified from rat kidney extracts, were shown to be dual growth modulators for a variety of epithelial cell lines (24, 35, 36). Cloning of the cDNA encoding the 6-kDa polypeptides indicated that they were encoded by a 63-kDa protein with several putative glycosylation sites containing 7½ 6-kDa repeats (24). Failure to show functionality of the expressed precursor in growth assay by these authors led to the conclusion that the precursor was inactive and had to be processed into the 6-kDa polypeptides to have biological activity (24). Our work with the highly tumorigenic teratoma cells provided biochemical identity of the precursor as an 88-kDa glycoprotein, demonstrated that the precursor could be secreted without processing and was biologically active as stimulating the proliferation of mesenchymal cells (19, 28). Biological function of the precursor as a growth factor was later confirmed for other fibroblastic cells and for epithelial cells (25, 26).
Interestingly, whether or not the processing is physiologically regulated and whether the processed forms occur naturally was never directly demonstrated. Apart from their extraction by chemical procedure from kidney or granulocytes, the only report of the existence of the processed forms in biological samples was in the patients urine (37), which would suggest that epithelins may result from a degradative process rather than from the physiologically regulated processing of the precursor. However, these questions remain to be explored. Among the activities reported for the epithelins, one of which is of interest to the studies presented here is the inhibition of DNA synthesis of MDA-MD-468 cells shown both for epithelins 1 and 2 (22). However, the doses shown to be effective to inhibit proliferation were very high (150 ng/ml corresponding to 20 nM), whereas epithelin had no effect at lower doses in the range corresponding to the affinity constant for binding to the epithelin receptors (22). This report together with the present data of the growth promoting activity of the precursor on the same cells raise an interesting point in that the 6-kDa product and the autocrine-produced precursor have opposite biological activities on the breast carcinoma cell line.
The signal transduction pathway of epithelin/granulin precursor has been investigated in embryo fibroblasts from mice null for the IGF-I receptor (26). It was shown that the growth factor stimulated DNA synthesis in these cells by means of activating mitogen-activated protein (MAP) kinase pathway. Our studies with mammary epithelial cells have shown similar activation of MAP kinase pathway in response to PCDGF (data not shown).
Concerning PCDGF expression, we show here that PCDGF (epithelin/granulin precursor) is widely expressed in human breast cancer cell lines and that the degree of its expression appears correlated with the tumorigenicity of the cells. MCF-10A, and ER+ MCF-7 and T47D cells have a low level of PCDGF, whereas ER− MDA-MB-468 and -453 cells that can proliferate in the absence of estrogen have a high PCDGF expression. At the present time, the mechanism of the overexpression of PCDGF in the ER− cells is not known. Preliminary promoter studies using a PCDGF proximal promoter ligated to a luciferase reporter gene indicate that luciferase activity was the lowest in MCF-10 A cells and increased in ER+ cells and in ER− cells (unpublished results), suggesting a loss of transcriptional control in the more malignant cells.
The data presented here indicate that PCDGF, by means of an autocrine pathway, stimulates the growth of the ER− MDA-MB-468 cells. This was first demonstrated by the fact that treatment of MDA-MB-468 cells with highly purified neutralizing anti-PCDGF antibody inhibited their growth in a dose-dependent fashion. The purity and specificity of the antibody used in the studies for PCDGF and no other growth factor had been demonstrated elsewhere by showing that only PCDGF and no other growth factors could prevent the growth inhibitory effect of the purified antibody (data not shown). The data of Fig. 2 also showed that exogenously added PCDGF completely prevented the inhibition of DNA synthesis in MDA-MB-468 cells caused by the addition of the anti-PCDGF antibody.
In addition, PCDGF added exogenously also stimulated DNA synthesis of MDA-MB-468 cells plated at low cell density to minimize the contribution of endogenous PCDGF. A 2-fold increase in the thymidine incorporation was observed at a PCDGF concentration of 200 ng/ml (data not shown).
To examine the role of PCDGF in the tumorigenic properties of the ER− breast cancer cells, we have used the antisense approach to determine whether inhibition of PCDGF expression in the ER− MDA-MB-468 breast carcinoma cells would result in reduced proliferation in vitro and malignancy in vivo. This approach had been successfully used to demonstrate the role of PCDGF overexpression in the tumorigenic properties of the mesenchymal mouse teratoma-derived PC cell line from which PCDGF was originally purified (28). This approach has been widely applied to study the role of autocrine growth factors on in vitro and in vivo cell growth properties (38–41), such as the effect of IGF-1 on the tumorigenicity of glioblastoma (41). The results presented here demonstrate that antisense inhibition of PCDGF expression in MDA-MB-468 cells resulted in an inhibition of cell proliferation. A 60% inhibition of proliferation was observed in A3 cells, the antisense clone showing the maximum inhibition of PCDGF expression when compared with empty vector-transfected control cells. When plated in soft agar, the antisense cells also formed 80% fewer colonies than empty vector-transfected MDA-MB-468 control cells. Exogenous addition of PCDGF stimulated the proliferation of the antisense cells (both stimulation of DNA synthesis and long-term increase in cell number) and fully restored it to the level found in empty vector control cells. Concerning the soft agar assay, where a 60% reduction of colony formation for the antisense cells had been measured, exogenous addition of PCDGF appeared only partially efficient in restoring the levels found in control cells. This is because of the inherent biochemical properties of the glycoprotein and the nature of the assay itself. PCDGF added to the medium, probably because of the presence of the large carbohydrate moiety and hydrophobic regions in its protein core bound to the agar, thereby reducing the effective concentration of PCDGF readily available to the cells for colony formation. This problem was not encountered with the empty vector control cells because they are able to continuously produce and secrete the factor. Moreover, analysis of the data by two tailed Student's t test indicated that the difference between control and A1 cells supplemented with PCDGF (A1 + P) had a P > 0.1 considered not significant, whereas the difference between A3 + P and control had a P value > 0.06, considered marginally significant.
The ability of PCDGF in these various assays to restore the growth of the antisense cells to levels found in control cells indicates that the reduced growth properties of the antisense cells specifically resulted from the inhibition of PCDGF expression in the transfected cells and not to changes independent of PCDGF expression.
The results of the in vivo tumorigenicity studies have indicated that the antisense cells displayed a dramatic inhibition in their ability to form tumors in nude mice. Antisense PCDGF cDNA-transfected cells displayed a dramatic decrease of both tumor incidence and tumor size when compared with the control cells when injected in nude mice. An 86% and 43% decrease in tumor incidence was observed for A3 and A1 cells, respectively. Moreover, even after 8 wk, the nude mice injected with the antisense cells that did not have tumors after 4 wk remained tumor-free (data not shown).
Although the data presented here mainly point out a role of PCDGF in breast carcinoma cell proliferation, it is also possible to assume that it may also play a role in other processes important for tumor formation, such as cell survival and angiogenic process (42). Moreover, antisense cells did not spread well on the culture dishes and maintained a rounded morphology, suggesting a possible involvement of PCDGF in the attachment of cells to the substratum or in cell matrix formation. This was also observed with the highly tumorigenic teratoma cells, where PCDGF expression had been inhibited (28). Further experiments are required to examine all of these possibilities.
We have shown here that the constitutive expression of PCDGF in estrogen-negative breast carcinoma cells is essential for their growth and tumorigenicity. It would be tempting to hypothesize that PCDGF overexpression may be involved in the transition from estrogen responsiveness to estrogen-independence observed in more malignant breast cancer.
Acknowledgments
We thank Drs. Dan Lindsay, Peter Gutteriez, and Angela Brodie for providing us with the cell lines, Dr. Jun You for his help in preparing PCDGF, Dr. Koichiro Tanaka for his advice in the antibody purification, and Dr. Jun Hayashi for his assistance with the in vivo studies. This work was supported by DAMD 17-96-1-6072 from U.S. Army Medical Research and Material Command and Grant RO1 CA58449 from the National Institutes of Health. R.L. is a recipient of a Dr. Frank J. Slama Endowed Predoctoral Fellowship.
Abbreviations
- ER
estrogen receptor
- IGF
insulin-like growth factor
- PCDGF
PC cell-derived growth factor
- PFMEM
phenol-red free α-MEM plus 5% charcoal-stripped FBS
References
- 1.Lopez-Otin C, Diamandis E P. Endocr Rev. 1998;19:365–396. doi: 10.1210/edrv.19.4.0337. [DOI] [PubMed] [Google Scholar]
- 2.Chrysogelos S A, Dickson R B. Breast Cancer Res Treat. 1994;29:29–40. doi: 10.1007/BF00666179. [DOI] [PubMed] [Google Scholar]
- 3.Dickson R B, Lippman M E. Endocr Rev. 1995;16:559–589. doi: 10.1210/edrv-16-5-559. [DOI] [PubMed] [Google Scholar]
- 4.Aaronson S A. Science. 1991;254:1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- 5.Sporn M B, Roberts A B. Nature (London) 1985;313:745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- 6.Roberts A B, Sporn M B. Cancer Surv. 1985;4:683–705. [PubMed] [Google Scholar]
- 7.Sporn M B, Todaro G J. N Engl J Med. 1980;303:878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- 8.Shankar V, Ciardiello F, Kim N, Derynck R, Liscia D S, Merlo G, Langton B C, Sheer D, Callahan R, Bassin R H, et al. Mol Carcinog. 1989;2:1–11. doi: 10.1002/mc.2940020102. [DOI] [PubMed] [Google Scholar]
- 9.Ciardiello F, McGeady M L, Kim N, Basolo F, Hynes N, Langton B C, Yokozaki H, Saeki T, Elliott J W, Masui H, et al. Cell Growth Differ. 1990;1:407–420. [PubMed] [Google Scholar]
- 10.Sandgren E P, Luetteke N C, Palmiter R D, Brinster R L, Lee D C. Cell. 1990;61:121–135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick S L, Brightwell J, Wittliff J L, Barrows G H, Schultz G S. Cancer Res. 1984;44:3448–3453. [PubMed] [Google Scholar]
- 12.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz C C, Dantis L, Sklarin N T, Seidman A D, Hudis C A, Moore J, et al. J Clin Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 13.Weiner L M, Clark J I, Davey M, Li W S, Garcia de Palazzo I, Ring D B, Alpaugh R K. Cancer Res. 1995;55:4586–4593. [PubMed] [Google Scholar]
- 14.Arteaga C L, Osborne C K. Cancer Res. 1989;49:6237–6241. [PubMed] [Google Scholar]
- 15.Quinn K A, Treston A M, Unsworth E J, Miller M J, Vos M, Grimley C, Battey J, Mulshine J L, Cuttitta F. J Biol Chem. 1996;271:11477–11483. doi: 10.1074/jbc.271.19.11477. [DOI] [PubMed] [Google Scholar]
- 16.Matsui Y, Halter S A, Holt J T, Hogan B L, Coffey R J. Cell. 1990;61:1147–1155. doi: 10.1016/0092-8674(90)90077-r. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton M, Wolfman A. J Biol Chem. 1998;273:28155–28162. doi: 10.1074/jbc.273.43.28155. [DOI] [PubMed] [Google Scholar]
- 18.Treinies I, Paterson H F, Hooper S, Wilson R, Marshall C J. Mol Cell Biol. 1999;19:321–329. doi: 10.1128/mcb.19.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Gao G, Crabb J W, Serrero G. J Biol Chem. 1993;268:10863–10869. [PubMed] [Google Scholar]
- 20.Bhandari V, Palfree R G E, Bateman A. Proc Natl Acad Sci USA. 1992;89:1715–1719. doi: 10.1073/pnas.89.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bateman A, Bennett H P. J Endocrinol. 1998;158:145–151. doi: 10.1677/joe.0.1580145. [DOI] [PubMed] [Google Scholar]
- 22.Culouscou J, Carlton G W, Shoyab M. J Biol Chem. 1993;268:10458–10462. [PubMed] [Google Scholar]
- 23.Belcourt D R, Lazure C, Bennett H P. J Biol Chem. 1993;268:9230–9237. [PubMed] [Google Scholar]
- 24.Plowman G D, Green J M, Neubauer M G, Buckley S D, McDonald V L, Todaro G J, Shoyab M. J Biol Chem. 1992;267:13073–13078. [PubMed] [Google Scholar]
- 25.He Z, Bateman A. Cancer Res. 1999;59:3222–3229. [PubMed] [Google Scholar]
- 26.Xu S Q, Tang D, Chamberlain S, Pronk G, Masiarz F, Kaur S, Prisco M, Zanocco-Marani T, Baserga R. J Biol Chem. 1998;273:20078–20083. doi: 10.1074/jbc.273.32.20078. [DOI] [PubMed] [Google Scholar]
- 27.Xia X, Serrero G. Biochem Biophys Res Commun. 1998;245:539–543. doi: 10.1006/bbrc.1998.8498. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Serrero G. Proc Natl Acad Sci USA. 1998;95:14202–14207. doi: 10.1073/pnas.95.24.14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu R, Serrero G. Biochem Biophys Res Commun. 1999;256:204–207. doi: 10.1006/bbrc.1999.0253. [DOI] [PubMed] [Google Scholar]
- 30.Soule H D, Maloney T M, Wolman S R, Peterson W D, Jr, Brenz R, McGrath C M, Russo J, Pauley R J, Jones R F, Brooks S C. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 31.Boyd D D, Levine A E, Brattain D E, McKnight M K, Brattain M G. Cancer Res. 1988;48:2469–2474. [PubMed] [Google Scholar]
- 32.Dickson R B, Gottardis M M, Merlino G T. BioEssays. 1991;13:591–596. doi: 10.1002/bies.950131109. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen B, Keane M M, Johnston P G. Crit Rev Oncol Hematol. 1995;20:223–236. doi: 10.1016/1040-8428(94)00161-L. [DOI] [PubMed] [Google Scholar]
- 34.Serrero G, Zhou J, Mills D, LePak N. J Cell Physiol. 1991;149:503–511. doi: 10.1002/jcp.1041490321. [DOI] [PubMed] [Google Scholar]
- 35.Shoyab M, McDonald V L, Byles C, Todaro G J, Plowman G D. Proc Natl Acad Sci USA. 1990;87:7912–7916. doi: 10.1073/pnas.87.20.7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bateman A, Belcourt D, Bennett H, Lazure C, Solomon S. Biochem Biophys Res Commun. 1990;173:1161–1168. doi: 10.1016/s0006-291x(05)80908-8. [DOI] [PubMed] [Google Scholar]
- 37.Sparro G, Galdenzi G, Eleuteri A M, Angeletti M, Schroeder W, Fioretti E. Protein Expression Purif. 1997;10:169–174. doi: 10.1006/prep.1997.0726. [DOI] [PubMed] [Google Scholar]
- 38.Kenney N J, Saeki T, Gottardis M, Kim N, Garcia-Morales P, Martin M B, Normanno N, Ciardiello F, Day A, Cutler M L, et al. J Cell Physiol. 1993;156:497–514. doi: 10.1002/jcp.1041560309. [DOI] [PubMed] [Google Scholar]
- 39.Reddy K B, Yee D, Hilsenbeck S G, Coffey R J, Osborne C K. Cell Growth Differ. 1994;5:1275–1282. [PubMed] [Google Scholar]
- 40.Trojan J, Johnson T R, Rudin S D, Ilan J, Tykocinski M L, Ilan J. Science. 1993;259:94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- 41.Trojan J, Blossey B K, Johnson T R, Rudin S D, Tykocinski M, Ilan J, Ilan J. Proc Natl Acad Sci USA. 1992;89:4874–4878. doi: 10.1073/pnas.89.11.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J. Anticancer Res. 1996;16:2233–2239. [PubMed] [Google Scholar]