Abstract
Aluminum (Al) toxicity is a major constraint for crop production in acid soils, although crop cultivars vary in their tolerance to Al. We have investigated the potential role of citrate in mediating Al tolerance in Al-sensitive yeast (Saccharomyces cerevisiae; MMYO11) and canola (Brassica napus cv Westar). Yeast disruption mutants defective in genes encoding tricarboxylic acid cycle enzymes, both upstream (citrate synthase [CS]) and downstream (aconitase [ACO] and isocitrate dehydrogenase [IDH]) of citrate, showed altered levels of Al tolerance. A triple mutant of CS (Δcit123) showed lower levels of citrate accumulation and reduced Al tolerance, whereas Δaco1- and Δidh12-deficient mutants showed higher accumulation of citrate and increased levels of Al tolerance. Overexpression of a mitochondrial CS (CIT1) in MMYO11 resulted in a 2- to 3-fold increase in citrate levels, and the transformants showed enhanced Al tolerance. A gene for Arabidopsis mitochondrial CS was overexpressed in canola using an Agrobacterium tumefaciens-mediated system. Increased levels of CS gene expression and enhanced CS activity were observed in transgenic lines compared with the wild type. Root growth experiments revealed that transgenic lines have enhanced levels of Al tolerance. The transgenic lines showed enhanced levels of cellular shoot citrate and a 2-fold increase in citrate exudation when exposed to 150 μm Al. Our work with yeast and transgenic canola clearly suggest that modulation of different enzymes involved in citrate synthesis and turnover (malate dehydrogenase, CS, ACO, and IDH) could be considered as potential targets of gene manipulation to understand the role of citrate metabolism in mediating Al tolerance.
Aluminum toxicity is one of the major factors limiting crop productivity in acid soils. The root apex is considered the primary site of Al-induced injury, and inhibition of root elongation is one of the most visible symptoms of Al toxicity. Although most plants are sensitive to Al, several crop species exhibit genetic variation in their ability to tolerate Al. One possible mechanism of Al tolerance is the chelation of Al by organic anions within root cells or in the rhizosphere (Taylor, 1991). Exudation of a variety of low Mr organic anions such as citrate, malate, or oxalate has been reported in several crop species upon exposure to Al (Miyasaka et al., 1991; Basu et al., 1994; Pellet et al., 1995; Larsen et al., 1998). Although a rapid release of organic anions (malate2-) was observed in near isogenic, Al-tolerant wheat (Triticum aestivum) lines (Delhaize et al., 1993), several lines of evidence suggest that a lag phase may also exist between exposure of roots to Al and excretion of organic anions. Citrate exudation from roots of rye was observed only after 120 h of exposure (Li et al., 2000). Similarly, an Al-induced, de novo synthesis of malate leading to enhanced malate efflux 24 h after exposure was demonstrated in Al-tolerant wheat cv Katepwa (Basu et al., 1994). Delayed exudation of organic anions could possibly be due to alteration of cellular metabolism leading to biosynthesis and release of anions.
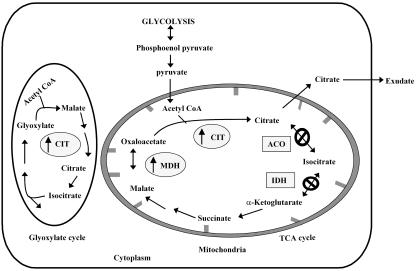
Among the various organic acids, citrate is most commonly cited to be involved in ameliorating the toxic effects of Al. Accumulation and/or efflux of citrate can be enhanced by increased citrate production or by reduced citrate catabolism (Neumann et al., 2000). Enhanced citrate synthesis could be achieved by increasing the activities of enzymes involved in citrate synthesis such as citrate synthase (CS), malate dehydrogenase (MDH), and phosphoenolpyruvate carboxylase. Citrate turnover could be reduced by decreasing the activities of enzymes involved in breakdown of citrate such as aconitase (ACO) and isocitrate dehydrogenase (IDH; Fig. 1).
Figure 1.
Metabolic map of TCA and glyoxylate cycles, indicating positions of genes that could be modulated for altering citrate metabolism. Up-regulation of genes involved in citrate synthesis (↑) and down-regulation of genes involved in citrate catabolism (Φ) would enhance citrate synthesis and/or its accumulation in cells.
CS is a key enzyme involved in condensation of oxaloacetate (OAA) and acetyl CoA to produce citrate. This biochemical reaction plays an important role in the Krebs cycle, in β-oxidation of fatty acids, and also in photo-respiratory glycolate pathways. As an important intermediate in various biochemical reactions, such as amino acid synthesis and fatty acid synthesis, citrate biosynthesis and catabolism are strictly regulated (Ryan and Delhaize, 2001). It is therefore important to undertake a holistic approach that considers enzymes and metabolites both upstream and downstream of citrate, while attempting to engineer citrate metabolism.
This study was conducted to investigate the role of citrate metabolism in mediating Al tolerance in both yeast and plant model systems. Yeast (Saccharomyces cerevisiae) is an excellent system for studies on metal toxicity and resistance (MacDiarmid and Gardner, 1998). Genes involved in Al tolerance (HSP150, SED1, and ALU1-P) have been identified in yeast (Jo et al., 1997; Ezaki et al., 1998), and the functions of several plant genes (BCB, NtGDI1, and ATPase) have been elucidated by complementation of yeast mutants (Ezaki et al., 1999; Hamilton et al., 2001). The availability of disruption mutants defective in various tricarboxylic acid (TCA) and glyoxylate cycle genes (McCammon, 1996; Przybyla-Zawislak et al., 1999) prompted us to use yeast to test the role of specific genes and gene products in citrate metabolism and Al tolerance (Table I; Fig. 1). The function of TCA cycle genes appears to be conserved between yeast and plants. For example, the genes for different subunits of tobacco (Nicotiana tabacum) NAD-dependent IDH were sufficiently conserved to complement yeast IDH mutant (Lancien et al., 1998). Thus, yeast TCA cycle mutants should be a useful model system for understanding citrate metabolism in Al tolerance.
Table I.
Yeast strains defective in various TCA and glyoxylate cycle genes used in this study are listed below
The genetic background of all mutants listed below is same as the WT, MMYO11 (Przybyla-Zawislak et al., 1999).
| Strain | Genotype | Properties |
|---|---|---|
| MMYO11 | Matα ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 | Wild-type parental strain |
| Δcit1 | cit1::LEU2 | Defective in mitochondrial citrate synthase CIT1 |
| Δcit2 | cit2::URA3 | Defective in glyoxysomal citrate synthase CIT2 |
| Δcit3 | cit3::URA3 | Defective in mitochondrial citrate synthase CIT3 |
| Δcit12 | cit1:: LEU2 cit2::URA3 | Defective in CIT1 and CIT2 |
| Δcit13 | cit1:: LEU2 cit1::URA3 | Defective in CIT1 and CIT3 |
| Δcit23 | cit2:: URA3 cit3::URA3 | Defective in CIT2 and CIT3 |
| Δcit123 | cit1:: LEU2 cit2::URA3 cit3::URA3 | Defective in CIT1, CIT2, and CIT3 |
| Δaco1 | aco1::URA3 | Defective in mitochondrial aconitase ACO1 |
| Δidh12 | idh1::URA3 idh2::HIS3 | Defective in subunits 1 and 2 of mitochondrial isocitrate dehydrogenase, IDH1 and IDH2 |
In addition, we also tested the hypothesis that increased synthesis and accumulation of citrate can mediate Al tolerance in plants using a transgenic approach. We used an important oilseed crop, canola (Brassica napus cv Westar), because the cultivars of this species are sensitive to acid soils and Al toxicity (Clune and Copeland, 1999). The potential use of an overexpression strategy to regulate citrate synthesis and mediate Al tolerance was first suggested by de la Fuente et al. (1997). They demonstrated that overexpression of CS from Pseudomonas aeroginosa in the cytoplasm of tobacco and Papaya sp. led to enhanced citrate efflux from roots and thereby enhanced Al tolerance in transgenic lines. In contrast, Delhaize et al. (2001) reported that overexpression of the same P. aeroginosa CS in the cytosol of tobacco neither resulted in enhanced exudation of citrate nor increased Al tolerance. Koyama et al. (1999, 2000) reported similar results to those of de la Fuente et al. (1997), where overexpression of an Arabidopsis mitochondrial CS (At-mtCS) in carrot (Daucus carota) cell lines and a carrot mitochondrial CS in Arabidopsis lead to increased Al tolerance. Despite a growing body of literature on this subject, a clear relationship between overexpression of CS and Al tolerance is not well established. In eukaryotes, two isoforms of CS (glyoxysomal and mitochondrial) are observed. Adopting an overexpression strategy targeting CS to the mitochondria would be an ideal approach to increase citrate biosynthesis in plants, because factors influencing citrate synthesis and accumulation would be potentially well regulated in mitochondria. Hence, we overexpressed an At-mtCS in canola and evaluated the transgenic lines for levels of CS activity, citrate exudation from roots, and their performance under Al-toxic conditions.
RESULTS
Effect of Al on Growth of Wild-Type (WT) Yeast and Induction of Genes Involved in Citrate Metabolism
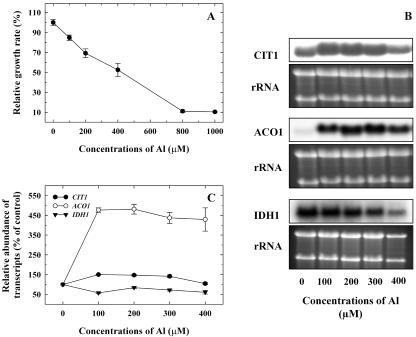
A progressive reduction in growth was observed in WT yeast when exposed to increasing concentrations of Al, with a 50% inhibition at 400 μm Al (Fig. 2A). Northern analysis of WT yeast exposed to Al (0–400 μm) showed that the transcript level of CIT1 increased by 40% to 50% from 100 to 300 μm Al, and no significant increase in transcript abundance was observed at 400 μm Al. Transcript abundance for ACO1 increased by 430% to 477% of control at 100 to 400 μm Al, whereas the IDH1 transcript level was down-regulated to 60% of control by increasing concentrations of Al (Fig. 2, B and C).
Figure 2.
The effect of Al (0–1,000 μm) on WT yeast (MMYO11). A, Reduction in growth of WT grown in LPP medium for 20 h, with different concentrations of Al. Vertical bars represent se (n = 3). B, Transcript levels of CIT1, ACO1, and IDH1 in WT yeast. Total RNA isolated from yeast cells exposed to different concentrations of Al for 20 h was probed with 32P-labeled fragments of CIT1, ACO1, and IDH1. C, Relative abundance of CIT1, ACO1, and IDH1 transcripts based on densitometry quantification of the RNA.
Cellular and Extracellular Citrate Content in WT and Disruption Mutants
Cellular (Fig. 3A) and extracellular (Fig. 3B) citrate contents were estimated in different yeast mutants with altered citrate metabolism. These include strains with all possible combinations of single, double, and triple mutations in three genes, CIT1, CIT2, and CIT3, encoding distinct CS isozymes. Two of the isozymes (CS1 and CS3) are mitochondrial, whereas the other isozyme (CS2) functions in the peroxisome. In addition, strains lacking the next two Krebs cycle isozymes were also tested. The Δaco1 strain lacked ACO1, whereas the Δidh12 strain lacked both subunits of the NAD+-dependent IDH. Single mutants of CS (Δcit1, Δcit2, and Δcit3) showed no significant reduction in cellular citrate content compared with the WT (8.50 ± 0.65 nmol 108 cells-1 mL-1), whereas the double and triple mutants (Δcit12, Δcit13, Δcit23, and Δcit123) showed a significant reduction (2-fold) compared with the WT yeast. Interestingly, the mutants disrupted for the genes downstream of citrate (Δaco1 and Δidh12) showed a significant increase (3- to 6-fold) in citrate levels compared with WT.
Figure 3.
Cellular (A) and extracellular (B) citrate content in WT yeast and disruption mutants with altered citrate metabolism. Cultures of different genotypes grown in LPP medium for 20 h were used for estimation of citrate. Vertical bars represent se (n = 3).
No significant reduction in citrate efflux from cells of single mutants of CS was observed, whereas citrate exuded from double and triple mutants of CS was reduced 2-fold compared with the WT yeast (7.7 ± 0.9 nmol 109 cells-1 mL-1). Citrate released from cells of Δaco1 and Δidh12 was significantly higher (7- to 10-fold) compared with WT.
Effect of Al on Disruption Mutants of Yeast
When yeast cells were challenged with Al, the single mutants (Δcit1, Δcit2, and Δcit3) did not show any significant difference in their growth compared with WT yeast (Fig. 4A). Likewise, the double mutants, Δcit12, Δcit13 did not show any difference in their growth response compared with WT (Fig. 4B), whereas Δcit23 and the triple mutant of CS, Δcit123, showed increased sensitivity at 200 and 400 μm Al. At 400 μm Al, growth of Δcit123 was reduced by 67%, whereas the WT showed a reduction by 43% (Fig. 4C). The Δaco1 and Δidh12 mutants, which had higher levels of cellular and extracellular citrate accumulation, showed reduced sensitivity to Al. The mutant disrupted for ACO1 showed a 30% better growth, whereas Δidh12 mutant showed a 19% better growth compared with WT yeast, at different concentrations of Al (Fig. 4C).
Figure 4.
Effect of Al on relative growth rate (percentage of control) of WT and disruption mutants with altered citrate metabolism. A, Single CS mutants (Δcit1, Δcit2, and Δcit3). B, Double CS mutants (Δcit12, Δcit13, and Δcit23). C, Triple mutant of CS, and ACO- and IDH-deficient mutants (Δcit123, Δaco1, and Δidh12). Cells of the specific genotypes were grown in LPP medium with different concentrations of Al for 18 to 20 h at 30°C, and A600 was measured. Vertical bars represent se (n = 3).
Effect of Al on Growth of CIT1-Overexpressing Transformants and Their Citrate Levels
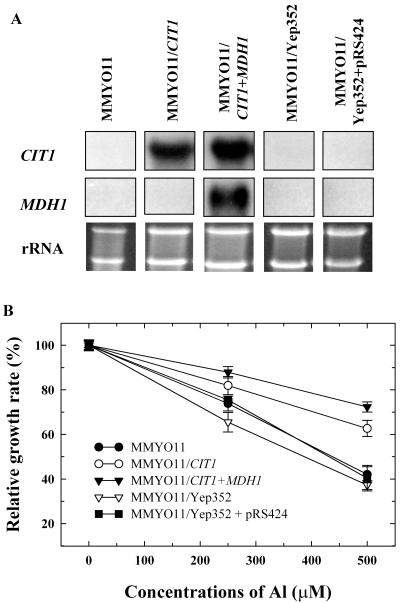
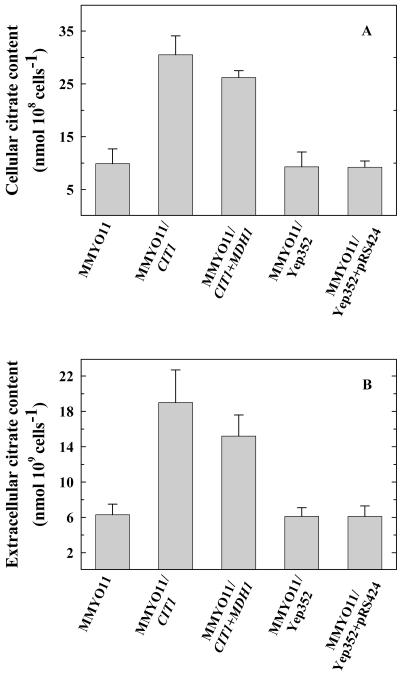
The gene encoding the major mitochondrial isoform of CS, CIT1, was overexpressed alone and with mitochondrial MDH gene, MDH1, in WT yeast. MDH is the enzyme involved in synthesizing OAA from malate. We therefore postulated that overexpression of MDH might supply additional OAA for citrate synthesis. Northern analysis of yeast transformants overexpressing CIT1 (MMYO11/CIT1) and CIT1 + MDH1 (MMYO11/CIT1 + MDH1) showed similar levels of increase in CIT1 transcript (Fig. 5A). When challenged with 500 μm Al, the transformants MMYO11/CIT1 and MMYO11/CIT1 + MDH1 showed significantly better growth (20% and 30%, respectively) compared with the WT and vector controls (Fig. 5B). Also, a significant increase (2.5- to 3-fold) in both cellular and extracellular citrate levels was observed in MMYO11/CIT1 and MMYO11/CIT1 + MDH1 transformants relative to the WT (Fig. 6, A and B). Interestingly, citrate levels were not higher with simultaneous overexpression of CIT1/MDH1 as in MMYO11/CIT1 + MDH1 compared with MMYO11/CIT1.
Figure 5.
Overexpresssion of mitochondrial CS, CIT1 (MMYO11/CIT1), and MDH, MDH1 (MMYO11/CIT1 + MDH1), in WT yeast. A, Northern-blot analysis of total RNA isolated from WT and yeast transformants overexpressing CIT1 and CIT1 + MDH1 and vector plasmids, Yep352 and Yep352 + pRS424 (probed with radiolabeled 32P-labeled CIT1 and MDH1 fragments). B, Effect of Al on growth of WT yeast and yeast transformants. Vertical bars represent se (n = 3).
Figure 6.
Cellular (A) and extracellular (B) citrate content in WT yeast and yeast transformants overexpressing CIT1, CIT1 + MDH1, and vector plasmids, Yep352 and Yep352 + pRS424. Cultures of different genotypes grown in LPP medium for 20 h at 30°C were used for estimation of citrate. Vertical bars represent se (n = 3).
Development of Transgenic Plants Overexpressing an At-mtCS
Transformation of canola cv Westar with pACS121-Hm (Fig. 7A) using an Agrobacterium tumefaciens-mediated system yielded 12 independent transgenic lines. The presence of the transgene, At-mtCS, was confirmed in four of these lines using genomic PCR analysis (primer positions used in genomic PCR are indicated in Fig. 7A). Amplification of an expected approximately 0.7-kb fragment was observed in transgenic lines and positive control when amplified with cauliflower mosaic virus (CaMV)-F and AtCS-R primers, but not in the WT and negative controls. When amplified with CaMV-F and HPTII-R primers, two bands of expected sizes (approximately 0.3 and 2.8 kb) were observed in transgenic lines and the positive control. Transgenic lines CS1 and CS12 were fertile and were raised to obtain homozygous seeds for further studies (Fig. 7B).
Figure 7.
Construction of transgenic plants overexpressing CS. Details of the construct (A), genomic DNA PCR analysis (B) used in transformation of canola with At-mtCS. A, Diagrammatic representation of pACS121-Hm. The gene, At-mtCS, is expressed under the control of a constitutive 35S promoter. The construct has selectable markers for NPT II and HPT II genes. The two primer pairs used in genomic DNA PCR amplification are indicated as arrows in the construct. B, Genomic DNA PCR analysis of transformants confirming the presence of At-mtCS transgene. Genomic DNA was isolated from WT and two independent transgenic lines (CS1 and CS12) and was used as template for PCR with two primer pairs indicated above. 35S-F/AtCS-R, Expected amplification size of 0.7 kb; 35S/HPT II-R, expected amplification sizes of 0.3 and 2.8 kb. Positive (+) control includes plasmid DNA (pACS121-Hm), and negative (-) control includes PCR reactions with no DNA.
Northern Analysis and CS Activity
Transgenic lines were analyzed for enhanced levels of the CS transcript (Fig. 8A) and presence of the HPT II transcript by northern analysis. The transgenic lines showed up to a 2-fold increase in accumulation of the mt-CS transcript (approximately 1.6 kb) compared with the WT. The At-mtCS probe hybridizes to the mt-CS of both Arabidopsis and canola. Due to the short exposure time used (2 h), the endogenous CS transcript in the WT was only visible as a faint band, whereas the band intensity was stronger in CS1 and CS1 for the same exposure time. When the same membrane was stripped and reprobed with HPT II, the presence of HPT II transcript (1.8 kb) was observed only in transgenic lines (data not shown). The enhanced level of CS expression was also confirmed in the roots and shoots of T2 plants and several of T2 progenies, with At-mtCS probe in T1 and homozygous T2 generation (data not shown).
Figure 8.
Analysis of transcript (A) and enzyme levels (B) in WT and transgenic lines. A, Northern analysis of total RNA isolated from young leaves of WT, CS1, and CS12. RNA blots were hybridized with [32P]dCTP labeled CS probe (1.6 kb). Ethidium bromide staining (EtBr) and rRNA probe are included as a loading control. B, CS enzyme activity was measured in young leaves of WT and transgenic lines. Vertical bars represent se (n = 3).
CS enzyme activity was measured in the WT and transgenic lines (Fig. 8B). Transgenic lines CS1 and CS12 showed a significant (2.5- to 4-fold) increase in CS enzyme activity (1.8 ± 0.005 and 3.2 ± 0.1 μmol CoA used min-1 mg-1 protein respectively) compared with the WT (0.8 ± 0.003 μmol CoA used min-1 mg-1 protein).
Enhanced Citrate Efflux and Increased Resistance of Transgenic Canola to Al
When exposed to toxic concentrations of Al, transgenic lines CS1 and CS12 showed better performance in the presence of Al (Fig. 9A). At 50 μm, a stimulatory effect of Al on root elongation was observed in CS1 and CS12 lines, whereas a growth reduction of 10% was observed in WT. At higher concentrations of Al, root elongation was greater in transgenic lines than in the WT. At 100 μm Al, no significant change in root elongation was observed in the transgenic lines compared with their controls (0 μm Al), whereas the WT showed a reduction of 9% in relative root growth. Larger differences in root elongation were observed at 150 and 200 μm Al. At 150 μm Al, root growth was reduced by 50% in WT, whereas CS1 and CS12 lines showed a reduction of 17% and 20%, respectively. At 200 μm Al, the transgenic lines showed a root growth inhibition of 65% to 70% their controls (0 μm Al), whereas the root growth of WT was reduced by 85% compared with its control.
Figure 9.
The effect of Al on root growth (A), cellular citrate content in roots (B) and shoots (C), and citrate efflux (D) on WT and transgenic lines overexpressing At-mtCS. A, Relative root growth (percentage of 0 μm Al) of WT and transgenic lines exposed to Al for 48 h (n = 10). B, Citrate content in root tissues of WT and transgenic lines, CS1 and CS12. C, Citrate levels in shoots of WT and transgenic lines. Root and shoot tissues from WT and homozygous seedlings of transgenic lines were collected and used in citrate estimation (n = 3). D, Root exudates from seedlings of WT, CS1, and CS12 were analyzed for levels of citrate released in the presence (150 μm Al) or absence of Al. Vertical bars represent se. (n = 3).
Because the transgenic lines had the Atmt-CS expressed under control of the CaMV promoter, we expected higher levels of cellular citrate content and citrate exudation from roots of CS1 and CS12 under control conditions. But the amount of citrate (shoots and root exudates) in these lines was not significantly different from that of the WT at 0 μm Al (Fig. 9, B–D). The root citrate level was not different between WT and transgenic lines at 150 μm Al, although a significant difference was observed between WT and CS12 at 0 μm Al (Fig. 9B). However, when exposed to 150 μm Al, the citrate content of shoots was significantly increased (1.7- to 1.9-fold) in CS1 and CS12 compared with the WT (3.5 ± 0.7 μmol mg-1 protein; Fig. 9C). The amount of citrate exuded from the roots of CS1 (1.7 ± 0.2 μmol g -1 dry weight) and CS12 (1.2 ± 0.2 μmol g -1 dry weight) was 1.7- to 2.4-fold higher than WT (0.7 ± 0.2 μmol g -1 dry weight; Fig. 9D). These results indicate that overexpression of mitochondrial CS in canola results in an Al-induced increase in exudation of citrate levels and Al tolerance in the transgenic lines.
DISCUSSION
Several lines of evidence suggest that Al has a strong effect on the TCA cycle and glycolytic pathway. Modulation in activities of several enzymes involved in synthesis and catabolism of citrate has been reported in yeast and plants (Hoffland et al., 1992; Neumann et al., 2000; Zatta et al., 2000; Neumann and Martinoia, 2002). In our studies, exposure of yeast, MMYO11 to Al resulted in induction of CIT1 and ACO1 and down-regulation of IDH1 transcript levels (Fig. 2, B and C). Metabolic engineering of biochemical pathways involved in organic acid metabolism would provide us an opportunity to understand the role of these pathways in Al tolerance.
Genetic manipulation is relatively easy in yeast due to the availability of mutants, ease of handling, and short experimental periods. Our data from yeast mutants with altered citrate metabolism (defective in TCA and glyoxylate cycles genes) revealed that accumulation of citrate in cellular and extracellular pools can mediate Al tolerance. When measuring metabolite levels in yeast, it is important to measure both cellular and extracellular levels, because these metabolites are likely to be transported out of their site of synthesis and exuded out of cells. Citrate is efficiently transported out of mitochondria by tricarboxylate carrier proteins (Barbier-Brygoo et al., 2000) and excess citrate in the cytoplasm is generally released out of cells to prevent cytoplasmic acidiosis (Massonneau et al., 2001).
In the present study, cellular and extracellular citrate levels were not significantly different in single mutants (Δcit1, Δcit2, and Δcit3) compared with WT. These mutants were not hypersensitive to Al, based on their growth performance compared with the WT. Cellular and extracellular levels of citrate were also unaffected. This could reflect the compensatory effects from other isoforms of CS. For instance, CIT2 mRNA levels were increased by 6- to 10-fold in CIT1-deficient mutant (Liao et al., 1991). Moreover, there could be alterations in levels of other metabolites in these single mutants (such as malate or succinate) that could have contributed to alleviation of Al toxicity. For example, when Kispal et al. (1988) inactivated a mitochondrial isoform of CS, increased levels of both citrate and malate were observed. In our experiment, the double mutants of CS (Δcit12, Δcit13, and Δcit23) showed significant reductions in their citrate content (cellular and extracellular) compared with the WT. However, no differences were observed in the growth of Δcit12 and Δcit13, whereas Δcit23 showed increased sensitivity to Al. The reason for the discrepancy in the citrate content and Al sensitivity in the double mutants could not be explained from our data. Mutants defective in CS are affected in the transport of several organic anions (citrate, isocitrate, and malate) across mitochondrial membranes (Sandor et al., 1994). Hence, alterations in metabolism involving internal compartmentation and transport of these organic anions across membranes could potentially affect the sensitivity of these mutants to Al. The triple mutant, Δcit123, showed a significant reduction in both intracellular and extracellular citrate levels, and also showed reduced growth in the presence of Al compared with WT.
Increased accumulation of citrate can also be mediated by reduced activity of enzymes involved in citrate turnover (Massonneau et al., 2001). In our study, Δaco1 and Δidh12 mutants accumulated higher levels of citrate (cellular and extracellular) and showed improved Al resistance. These mutants showed only a 13% and 24% reduction in growth respectively, at 400 μm Al, whereas the WT showed a 43% reduction in growth at the same Al concentration (400 μm Al). Previous evidence suggested that an Al-induced reduction in levels of ACO leads to increased exudation of citrate in Al-tolerant maize (Zea mays cv Cateto-Columbia; Pineros et al., 2002) and from lupin roots under phosphate-deficient conditions (Neumann et al., 1999). Reduction of IDH activities in mutant carrot cell lines capable of growing under phosphate deficiency also showed enhanced citrate exudation (Takita et al., 1999; Kihara et al., 2003). It would be interesting to investigate what other organic anions accumulate in Δaco1 and Δidh12 mutants that might contribute to improved Al resistance observed in our studies.
Results from our yeast overexpression studies demonstrated that cellular and extracellular citrate levels can be enhanced in yeast by overexpressing a mitochondrial CIT1 either alone or with a mitochondrial MDH1. The transformants MMYO11/CIT1 and MMYO11/CIT1 + MDH1 showed a 2- to 3-fold increase in citrate content and reduced Al sensitivity compared with WT yeast. MMYO11/CIT1 + MDH1 was expected to synthesize and exude more citrate than MMYO11/CIT1 (we postulated reduced substrate limitations). However, citrate contents in both transformants were not significantly different. The difference in sensitivity of MMYO11/CIT1 + MDH1 compared with MMYO11/CIT1 again suggests that there could be other metabolites (perhaps malate) that attributed for the enhanced Al tolerance.
Enhanced levels of organic acid synthesis or efflux is often accompanied by reduced growth under control conditions or requires specific culture conditions for enhanced synthesis and exudation to occur (Ryan and Delhaize, 2001). This could possibly be due to energy constraints caused by loss of carbon from the cells that exude organic anions (Taylor, 1991). It is important to mention that neither the yeast disruption mutants (Δaco1 and Δidh12) nor the yeast-overexpressing transformants (MMYO11/CIT1 and MMYO11/CIT1 + MDH1) revealed any apparent growth defects when grown in low phosphate medium (LPP). Our investigation with yeast disruption mutants and yeast overexpressing a mitochondrial CS clearly illustrates the role of citrate in alleviating Al stress and indicates that altering metabolic pathways involved in organic acid synthesis could enhance citrate accumulation.
Changes in TCA cycle function have wide-ranging effects. Transcriptional profiling using microarray analysis of responses to TCA cycle dysfunction (McCammon et al., 2003) has revealed that the expression of more than 400 gene changes in response to defects in one of 15 yeast TCA cycle genes. Gene expression also changes in response to altered TCA cycle metabolites. Citrate and malate are two of the TCA cycle metabolites to which nuclear gene expression may be responding. Hence, these metabolites may be exerting physiological effects intracellularly as well as extracellularly. The yeast cultures in these experiments were grown under fermentative conditions (McCammon et al., 2003) in which the TCA cycle is not essential and the genes are repressed as much as 10-fold (DeRisi et al., 1997). It will be interesting to test how oxidative growth conditions, which induce expression of the TCA cycle enzymes, affect Al tolerance and cellular citrate levels.
We also overexpressed a mitochondrial CS from Arabidopsis (At-mtCS) in canola cv Westar. Between the two isoforms of CS in eukaryotes (glyoxysomal and mitochondrial), modulating the mitochondrial isoform of CS might be a better strategy to test whether enhanced synthesis and exudation of citrate could be achieved by overexpressing CS. The mitochondria is the major site of function for CS in eukaryotes. Allosteric inhibition of CS by ATP would be low in mitochondria due to the low ATP to ADP ratio typically maintained in the organelle. Moreover, compartmentation of TCA cycle enzymes within the mitochondria not only ensures the availability of substrates but also physical proximity of substrates required for proper functioning of CS. A number of multi-enzyme complexes involving sequential enzymes termed “metabolons” exist in the TCA cycle, and their interactions are highly specific (Sumegi and Srere, 1984; Przybyla-Zawislak et al., 1999). Formation of such complexes affects the kinetics of enzymes involved in the complex (Datta et al., 1985). For example, in vitro studies on free enzymes of mitochondrial MDH or CS showed 2-fold reduced activity compared with the enzyme complex, mt-CS/mt-MDH. The interaction of mt-CS was specific only to mt-MDH and not to cytosolic MDH (Igor and Srere, 1998). Cellular compartmentalization is therefore a key phenomenon to carry out orderly and controlled reactions.
In this study, transgenic canola overexpressing At-mtCS showed increased CS expression at the transcript level and enhanced CS activity, leading to an increase in levels of cellular (shoots) citrate and citrate exudation from roots of transgenic lines relative to WT. Interestingly, the increase in shoot citrate content and exudation of citrate were observed only upon exposure to 150 μm Al, even though At-mtCS was expressed under the control of a constitutive promoter (CaMV). It is not clear why the root citrate level in transgenic lines, CS1 and CS12 was not increased in the presence of Al. In Al-tolerant cultivars of soybean (Glycine max), significant difference was observed in the citrate levels of root tips and not in roots, when exposed to Al (Silva et al., 2001). Although CS activity in CS1 and CS12 was not significantly different, citrate exudation was significantly higher in CS1 than in CS12. Our data suggest that citrate produced in the transgenic lines are exuded out of the roots upon exposure to Al but do not indicate a clear quantitative relationship between citrate synthesis and citrate export from transgenic lines.
It is important to note that our transgenic lines did not have any difference in growth and development compared with WT. A reduction in mitochondrial CS activity (up to 30% of the WT levels) in transgenic potato resulted in a normal phenotype, but this reduction in CS activity was a limiting factor during flowering because the transgenic potato plants had deformed ovules (Landschütze et al., 1995).
The transgenic canola lines overexpressing mtCS showed reduced sensitivity to Al. At 50 and 100 μm Al, root elongation rates were higher in the transgenic lines compared with the control (0 μm Al). This could be due to alleviation of H+ toxicity by Al as suggested by Clune and Copeland (1999). At higher concentrations of Al (150 and 200 μm), the transgenic lines showed a 20% to 25% better growth compared with the WT. Enhanced Al tolerance due to Al-induced increase in citrate exudation in transgenic canola clearly indicates that mitochondrial CS can play a major role in citrate metabolism and tolerance to Al.
Overexpression of a gene for CS from P. aeroginosa in cytoplasm of tobacco and Papaya sp. resulted in enhanced levels of cellular citrate and citrate exudation from roots of transgenic lines compared with the WT, with a concomitant increase in Al tolerance (de la Fuente et al., 1997) and phosphate acquisition (López-Bucio et al., 2000) by the transgenic lines. In contrast, Delhaize et al. (2001) generated transgenic lines by overexpressing the same P. aeroginosa CS gene in the cytosol of tobacco. Their transgenic lines showed up to 100-fold enhanced CS protein levels but had no increase in enzyme activity. The transgenic lines did not exhibit enhanced levels of citrate and were not Al tolerant. Delhaize et al. (2001) suggested that very high expression levels of P. aeroginosa CS in their transgenic lines could have inactivated the transgenic proteins through incorrect folding of the bacterial protein or formation of inactive protein aggregates in plants. Transgenic wheat lines with higher levels of luciferase (luc) expression in a T1 generation showed significant reduction in luc expression in their subsequent generations, compared with the T1 lines with moderate levels of luc expression (Bourdonm et al., 2002).
Several other studies have also suggested that an overexpression strategy can be used to enhance the synthesis and exudation of organic anions. For instance, transgenic alfalfa (Medicago sativa) overexpressing a nodule-specific MDH showed enhanced exudation of organic anions from their roots and enhanced Al tolerance (Tesfaye et al., 2001). Koyama et al. (1999) indicated the importance of CS by overexpressing an Arabidopsis mtCS in carrot cells. In addition, Koyama et al. (2000) showed that transgenic Arabidopsis lines overexpressing a carrot mtCS showed 60% increase in citrate efflux, performed better under toxic concentrations of Al, and also showed enhanced ability to grow in P-limited soils.
Despite a number of published studies on the topic, a holistic approach to understand citrate metabolism in relation to Al tolerance has not yet been undertaken. Our work with yeast and transgenic canola overexpressing At-mtCS has enabled us to explore the complexity of citrate metabolism. Our results clearly demonstrate that CS represents only a part of the complex system and that genetic manipulation of several enzymes involved in citrate metabolism (such as MDH, CS, ACO, and IDH) can be potentially used to increase citrate synthesis and its accumulation in cells (Fig. 1). Citrate levels could be modulated by up-regulation of MDH and CS by an overexpression strategy or by down-regulation of ACO and IDH using an antisense approach (perhaps, with an inducible promoter). These approaches might be adopted to generate a significant improvement in Al tolerance in plants. Because cellular metabolism involves an array of enzymes that are interrelated and function in coordination with each other, metabolic profiling of these transformants could help us to further understand the coordinated synthesis of other organic anions.
MATERIALS AND METHODS
Yeast Strains and Media
Strains of yeast (Saccharomyces cerevisiae) used in this study are listed in Table I. Most strains harboring disruptions in different TCA cycle and glyoxylate cycle genes were constructed previously (Przybyla-Zawislak et al., 1999). Double and triple mutant strains were constructed by crossing single mutant strains and screening random meiotic haploid products for the appropriate genotypes. Genotypes were confirmed by PCR using primers flanking the disruption sites, by phenotypic analysis, and by complementation analysis using marker strains (Przybyla-Zawislak et al., 1999). Yeast cultures were grown for 18 to 20 h at 30°C in yeast peptone dextrose medium (1% [w/v] yeast extract, 2% [w/v] peptone, and 2% [w/v] d-Glc). For experiments involving determination of Al response or estimation of citrate levels, cells were harvested from cultures by centrifugation at 3,000 rpm for 2 min, washed three times with sterile water, and suspended in 1 mL of sterile water. An initial OD600 of 0.05 was used in all experiments.
Overexpression of CIT1 and CIT1/MDH1 in MMYO11
To construct yeast overexpressing CIT1 and MDH1, strain MMYO11 was transformed with Yep352-CIT1 (Kispal et al., 1989) singly or in combination with pRS424-MDH1 (Small and McAlister-Henn, 1997) using the lithium acetate method (Chen et al., 1992). The CIT1-overexpressing transformants (MMYO11/CIT1) were selected on Uracil- plates. The CIT1 + MDH1overexpressing transformants (MMYO11/CIT1 + MDH1) were selected on Uracil-, Trp- plates. Increase in CIT1 and MDH1 transcripts were confirmed by northern analysis using yeast [32P]CTP-labeled CIT1 and MDH1 as probes.
Effect of Al on Growth of Mutants and Transformants
While testing the growth response of different yeast strains to Al, a low phosphate medium (LPP) was used (Schott and Gardner, 1997). This is a synthetic complete minimal medium made with 0.67% (w/v) yeast nitrogen base (without amino acids; without phosphate). In addition, K2HPO4 is reduced to 100 μm, KCl added to 4.5 mm, and the pH of the medium is reduced to 3.5 to 3.6 to ensure solubility of Al. Treatment cultures (2 mL of LPP medium with 0–400 μm Al) with an initial OD600 of 0.05 were grown for 18 to 20 h at 30°C at 200 rpm in triplicates. The final OD600 was measured using a plate reader (μQuant, Biotek Instruments, Winooski, VT). The relative growth rate of each strain was expressed as a percentage of control (0 μm Al).
Al-Induced Changes in Transcript Levels in Yeast
Total RNA was isolated from 5-mL yeast cultures grown with varying concentrations of Al (0–300 μm) for 20 h by an enzymatic method using the RNAeasy mini kit (Qiagen, Ontario, Canada). Northern-blot analysis was done using [32P]CTP-labeled CIT1, ACO1, and IDH1 probes.
Estimation of Citrate Content
Yeast cells grown in 3 mL of LPP medium for 20 h were pelleted and washed three times with 3 mL of sterile water. The medium and the supernatant from each wash were pooled as the extracellular fraction. The cells were resuspended in 1 mL of sterile water, and cell density was determined by estimating OD600. Cells were again pelleted, and the supernatant was pooled into the extracellular fraction. Cells were finally suspended in 1 mL of 80% (v/v) ethanol in 15-mL screw-capped tubes and vortexed vigorously to lyse the cells. The cell suspension was boiled at 80°C for 20 min with frequent vortexing to ensure complete lysis of cells and denaturation of proteins. The cell debris with denatured proteins was pelleted, the supernatant was passed through a 0.45-μm filter, and the filtrate was used in the estimation of citrate content in cells. To estimate citrate content in the extracellular medium, the pooled supernatant was concentrated (from 13 to 1 mL) using an Evap-o-Vac (Cole-Parmer, Ontario, Canada) placed inside an incubator at 50°C. Enzymatic analysis of citrate was conducted with 100 μL of either samples or standards. The reaction mix consisted of 100 mm Tris-Cl (pH 8.2), 0.2 mm ZnCl2, 3 units mL-1 MDH, and 10 mm NADH. A stable initial A340 was noted, the reaction was started by the addition of citrate lyase (Sigma-Aldrich, St. Louis), and stable final A340 was recorded (Tompkins and Toffaletti, 1982; Delhaize et al., 1993).
Plant Transformation, Growth Conditions, and Selection of Transformants
Agrobacterium tumefaciens strain EHA101 carrying a binary vector pACS121-Hm was kindly provided by Dr. Hiroyuki Koyama (Gifu University, Japan) and was used in the transformation of canola (Brassica napus cv Westar). This binary vector had a full-length cDNA for At-mtCS (1.6 kb) under the control of the CaMV promoter (Fig. 6A) and two selectable marker genes encoding kanamycin resistance (NPT II) and hygromycin resistance (HPT II; Koyama et al., 1999). Canola cv Westar seeds were surface-sterilized and germinated on seed germination medium (one-half strength Murashige and Skoog + 1% [w/v] Suc, pH 5.8) as described by Basu et al. (2001). Cotyledonary petioles from 4- to 5-d-old seedlings were used as explants, and A. tumefaciens-mediated transformation was carried out (Moloney et al., 1989). Overnight-grown cultures (in yeast extract peptone with 50 μg mL-1 kanamycin + 25 μg mL-1 hygromycin) of A. tumefaciens strain EHA 101 carrying pACS121-Hm were used to infect the explants. Explants were cocultivated with A. tumefaciens in M1 medium (Murashige and Skoog medium with 3% [w/v] Suc and 0.005% [w/v] benzyladenine) for 2 d in the dark. Explants were transferred to M2 medium (M1 + 300 μg mL-1 timentin) for 1 week to recover and regenerate and then were transferred to M3 selection medium (M2 + 300 μg mL-1 timentin and 30 μg mL-1 kanamycin) for regeneration of calli to shoots. The putative transformants with green shoots were transferred to M4 medium (Murashige and Skoog medium with 0.005% [w/v] indole butyric acid, 300 μg mL-1 timentin, and 30 μg mL-1 kanamycin) for rooting. All tissue culture materials were maintained in a controlled chamber (16 h of light, 80 μmol m-2 s-1, and 20°C/8 h of dark).
Presumptive positive plants (T1) were transferred to soil (Metromix, Apache Seeds, Edmonton, Canada) and raised under controlled environment conditions (16 h of light and 300 μmol m-2 s-1 at 20°C/8 h of dark at 18°C). The presence of the transgene was tested using PCR on genomic DNA isolated from 12 independent lines. Among the four positive lines, two of these T1 plants were fertile. Thirty seeds from each of the T1 selfed-lines were surface-sterilized and germinated on seed germination medium with 150 μg mL-1 kanamycin. Seedlings (T2 plants), which remained green on antibiotic selection, were again tested by genomic PCR. Segregation ratio was determined based on number of green to bleached yellow seedlings. From each of the primary transformants (T1), 15 green T2 seedlings were transferred to soil and screened to identify homozygous lines. To select for homozygous lines in the T2 generation, about 20 to 30 seedlings from T2 progenies were screened by genomic PCR for the presence of transgene.
Genomic DNA PCR and Northern Analysis
For genomic PCR and Southern analyses, genomic DNA was isolated from shoots or roots of canola using Qiagen DNeasy mini kit. For genomic PCR analysis, genomic DNA was used as template DNA, and the sequences of primers used for amplification were as follows: 35S-F, 5′-CCACTGACGTAAGGGATGACG-3′; AtCS-R, 5′-AAGCCTCCAG ACTGGGCAGTA-3′; and HPT II-R 5′-GCCATCGGTCCAGACGGCC-3′.
RNA from shoots and roots were isolated using the Qiagen RNAeasy mini kit, separated by electrophoresis on agarose formaldehyde denaturing gels, and transferred to nitrocellulose membranes (Genescreen, NEN Research Products, Perkin Elmer Life Sciences Inc., Boston). Membranes were hybridized at 42°C overnight and washed under standard stringent conditions recommended by Genescreen. Hybridization probes were radioactively labeled with [32P]CTP using Oligolabelling kit (Amersham Biosciences, Uppsala).
CS Enzyme Assay
Roots and shoots were collected, frozen in liquid nitrogen, and stored at -70°C until used for enzyme assays. Approximately 1 g of tissue was ground in liquid nitrogen and homogenized with 2 mL of ice-cold extraction buffer (50 mm HEPES, 0.5% [w/v] Triton-X, 1 mm EDTA, 1 mm iodoacetamine, and 10% [w/v] glycerol). The extract was centrifuged for 10 min at 4°C, and the supernatant was collected and desalted by passing through PD-10 columns (Bio-Rad Laboratories, Hercules, CA) equilibrated with 10 mm HEPES and eluted with 2 mL of 10 mm HEPES. The reaction mix for CS enzyme assay consisted of 1 mm 5,5′-dithio-bis(2-nitrobenzoic acid) in Tris-Cl (pH 8.1), 10 mm acetyl CoA, and the enzyme. The reaction was started by the addition of 10 mm OAA, and increase in absorbance due to deacetylation of acetyl CoA was measured at 412 nm (Srere et al., 1963).
Root Elongation Experiments
WT and transgenic lines (T2 homozygous) were tested for their sensitivity to Al by the root growth elongation assay described by Basu et al. (2001). The seeds were germinated on a bed of wet sand. Seedlings (2 d old) were plated onto 1-mL graduated syringe tubes (with plungers removed and tops filled with cotton plug to support the seedlings). Syringes were mounted onto a plastic tray and floated in a 15-L aquarium filled with 10 L of full nutrient solution (FNS) at pH 4.2. Roots of seedlings grown over the syringes elongated in a straight line, enabling easy handling of the seedlings and accurate measurements of root lengths. After a day of recovery in FNS, syringes carrying the seedlings were transferred to FNS (pH 4.2) with or without Al. Seedlings were selected for uniform root length; initial root lengths were recorded and placed in 50 mL of treatment solution (FNS with or without Al) in a 50-mL disposable centrifuge tube. Seedlings were exposed to treatment solution for 48 h with constant shaking before the final root lengths were recorded.
Cellular Citrate Content in Shoot and Root Tissues
Shoot and root tissues (approximately 0.2 g) of WT and transgenic lines were ground with liquid nitrogen, homogenized with 1 mL of 80% (v/v) ethanol, and vortexed thoroughly. The samples were centrifuged for 2 min at 13,000 rpm, and 100 μL of the supernatant was taken for protein estimation. The remaining portion of the samples was then vortexed and boiled at 80°C for 15 min. Samples were centrifuged at 13,000 rpm for 5 min, the supernatant was collected and passed through 0.45-μm filters, and 100 μL was used in citrate assay as described above (Delhaize et al., 1993).
Estimation of Extracellular Citrate from Root Exudates
Seeds of WT and transgenic lines were surface-sterilized and plated on seed germination medium. Two-day-old seedlings (13–15 seedlings per vessel, plated on a 14′ mesh) were transferred to 75 mL of sterile FNS in magenta vessels (Basu et al., 1994). After a day in FNS, the mesh supporting the seedlings was then transferred to treatment solution (0 or 150 μm Al) under aseptic conditions and maintained for 2 d with constant shaking. The pH was checked every 8 h and was adjusted to 4.2 with 0.5 n HCl. Root exudates were collected after 2 d, and roots were harvested to obtain dry weight. The pH values of root exudates were adjusted to 2.0 with 0.5 n HCl to dissociate Al-citrate complexes and were passed through cation exchange columns with 1.5 mL of resin (Bio-Rad Laboratories). Samples were eluted with 20 mL of deionized water and concentrated to 1 mL. An aliquot of 100 μL of this sample was used for citrate assay as described earlier.
Experimental Design
All experiments included three to 10 independent replicates, and statistical analyses were performed using Sigmastat statistical analysis package (v1.0, Jandel Scientific, Chicago). Single- or two-factor ANOVA was performed, and a P value below 0.05 was considered statistically significant based on Student Newman-Keul's test or Dunnett's test. Experiments were repeated three to six times to ensure reproducibility of results.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Acknowledgments
We thank Dr. Hiroyuki Koyama (Gifu University, Japan) for providing with the construct (At-mtCS) for plant transformation.
This work was supported by the Natural Sciences and Engineering Research Council of Canada, by the Department of Biological Sciences, University of Alberta (Edmonton, Alberta, Canada), and by the National Institutes of Health (grant no. AG17477 to L.M.-H.).
References
- Barbier-Brygoo H, Vinauger M, Colcombet J, Ephritikhine G, Frachisse JM, Maurel C (2000) Anion channels in higher plants: functional characterization, molecular structure and physiological role. Biochim Biophys Acta 1565: 199-218 [DOI] [PubMed] [Google Scholar]
- Basu U, Godbold D, Taylor GJ (1994) Aluminum resistance in Triticum aestivum associated with enhanced exudation of malate. J Plant Physiol 144: 747-753 [Google Scholar]
- Basu U, Good A, Taylor GJ (2001) Transgenic Brassica napus plants overexpressing aluminum-induced mitochondrial manganese superoxide dismutase cDNA are resistant to aluminum. Plant Cell Environ 24: 1269-1278 [Google Scholar]
- Bourdonm V, Ladbrooke Z, Wickham A, Lansdale D, Harwood W (2002) Homozygous transgenic wheat plants with increased luciferase activity do not maintain their high level of expression in the next generation. Plant Sci 163: 297-305 [Google Scholar]
- Chen DC, Yang BC, Kuo TT (1992) One-step transformation of yeast in stationary phase. Curr Genet 21: 83-84 [DOI] [PubMed] [Google Scholar]
- Clune TS, Copeland L (1999) Effects of Al on canola roots. Plant Soil 216: 27-33 [Google Scholar]
- Datta A, Merz JM, Spivey HO (1985) Substrate channeling of oxalacetate in solid-state complexes of malate dehydrogenase and citrate synthase. J Biol Chem 260: 15008-15012 [PubMed] [Google Scholar]
- de la Fuente JM, Ramírez-Rodríguez V, Cabrera-Ponce JL, Herrera-Estrella L (1997) Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science 276: 1566-1568 [DOI] [PubMed] [Google Scholar]
- Delhaize E, Hebb DM, Ryan PR (2001) Expression of a Pseudomonas aeruginosa citrate synthase gene in tobacco is not associated with either enhanced citrate accumulation or efflux. Plant Physiol 125: 2059-2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.): II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol 103: 695-702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRisi J, Vishwanath RI, Brown PO (1997) Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278: 680-686 [DOI] [PubMed] [Google Scholar]
- Ezaki B, Gardner RC, Ezaki Y, Kondo H, Matsumoto H (1998) Protective roles of two Al-induced genes, HSP150 and SED1 of Saccharomyces cerevisiae in Al and oxidative stresses FEMS Microbiol Lett 159: 99-105 [DOI] [PubMed] [Google Scholar]
- Ezaki B, Sivaguru M, Ezaki Y, Matsumoto H, Gardner R (1999) Acquisition of aluminum tolerance in Saccharomyces cerevisiae by expression of the BCG or NtGDI1 gene derived from plants. FEMS Microbiol Lett 171: 81-87 [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Good AG, Taylor GJ (2001) Vacuolar H+-ATPase, but not mitochondrial F1F0-ATPase, is required for aluminum resistance in Saccharomyces cerevisiae. FEMS Microbiol Lett 205: 99-105 [DOI] [PubMed] [Google Scholar]
- Hoffland E, Van Den Boogaard R, Nelemans J, Findenegg G (1992) Biosynthesis and root exudation of citric and malic acids in phosphate-starved rape plants. New Phytol 122: 675-680 [Google Scholar]
- Igor M, Srere PA (1998) Interaction between citrate synthase and malate dehydrogenase-substrate channeling of oxaloacetate. J Biol Chem 273: 29540-29544 [DOI] [PubMed] [Google Scholar]
- Jo J, Jang Y, Kim K, Kim M, Kim I, Chung W (1997) Isolation of Alu1-P gene encoding a protein with aluminum tolerance activity from Arthrobacter viscosus. Biochem Biophys Res Commun 239: 835-839 [DOI] [PubMed] [Google Scholar]
- Kihara T, Ohno T, Koyama H, Sawafuji T, Hara T (2003) Characterization of NADP-isocitrate dehydrogenase expression in a carrot mutant cell line with enhanced citrate excretion. Plant Soil 248: 145-153 [DOI] [PubMed] [Google Scholar]
- Kispal G, Rosenkrantz M, Guarente L, Srere PA (1988) Metabolic changes in Saccharomyces cerevisiae strains lacking citrate synthases. J Biol Chem 263: 11145-11149 [PubMed] [Google Scholar]
- Kispal G, Rosenkrantz M, Guarente L, Srere PA (1989) Metabolic changes in Saccharomyces cerevisiae strains lacking citrate synthase. J Biol Chem 264: 11204-11210 [PubMed] [Google Scholar]
- Koyama H, Kawamura A, Kihara T, Hara T, Takita E, Shibata D (2000) Overexpression of mitochondrial citrate synthase in Arabidopsis thaliana improved growth on a phosphorus limited soil. Plant Cell Physiol 41: 1030-1037 [DOI] [PubMed] [Google Scholar]
- Koyama H, Takita E, Kawamura A, Hara T, Shibata D (1999) Over expression of mitochondrial citrate synthase gene improves the growth of carrot cells in aluminum-phosphate medium. Plant Cell Physiol 40: 482-488 [DOI] [PubMed] [Google Scholar]
- Lancien M, Gadal P, Hodges M (1998) Molecular characterization of higher plant NAD-dependent isocitrate dehydrogenase: evidence for a heterometric structure by the complementation of yeast mutants. Plant J 16: 325-333 [DOI] [PubMed] [Google Scholar]
- Landschütze V, Willmitzer L, Müller-Röber B (1995) Inhibition of flower formation by antisense repression of mitochondrial citrate synthase in transgenic potato plants leads to a specific disintegration of the ovary tissues of flowers. EMBO J 14: 660-666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Degenhardt J, Tai CY, Stenzler LM, Howell SH, Kochian LV (1998) Aluminum-resistant Arabidopsis mutants that exhibited altered patterns of aluminum accumulaiton and organic acid release from roots. Plant Physiol 117: 9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Ma JF, Matsumoto H (2000) Pattern of aluminum-induced secretion of organic acids differs between rye and wheat. Plant Physiol 123: 1537-1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Small WC, Srere PA, Butow RA (1991) Intramitochondrial functions regulate nonmitochondrial citrate synthase (CIT2) expression in S. cerevisiae. Mol Cell Biol 11: 38-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Martínez de la Vega O, Guevara-García A, Herrera-Estrella L (2000) Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nat Biotechnol 18: 450-453 [DOI] [PubMed] [Google Scholar]
- MacDiarmid CW, Gardner RC (1998) Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J Biol Chem 273: 1727-1732 [DOI] [PubMed] [Google Scholar]
- Massonneau A, Langlade N, Leon S, Smutny J, Vogt E, Neumann G, Martinoia E (2001) Metabolic changes associated with cluster root development in white lupin (Lupinus albus L.): relationship between organic acid excretion, sucrose metabolism and energy status. Planta 213: 534-542 [DOI] [PubMed] [Google Scholar]
- McCammon MT (1996) Mutants of Saccharomyces cerevisiae with defects in acetate metabolism: isolation and characterization of Acn- mutants. Genetics 144: 57-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCammon MT, Epstein CB, Przybyla-Zawislak B, McAlister-Henn L, Butow RA (2003) Global transcription analysis of Krebs tricarboxylic acid cycle mutants reveals an alternating pattern of gene expression and effects on hypoxic and oxidative genes. Mol Biol Cell 14: 958-972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka SC, Buta JG, Howell RK, Foy CD (1991) Mechanism of aluminum tolerance in snapbeans: root exudation of citric acid. Plant Physiol 96: 737-743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney MM, Walker JM, Sharma KK (1989) High efficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Rep 8: 238-242 [DOI] [PubMed] [Google Scholar]
- Neumann G, Martinoia E (2002) Cluster roots: an underground adaptation for survival in extreme environment. Trends Plant Sci 7: 162-167 [DOI] [PubMed] [Google Scholar]
- Neumann G, Massonneau A, Langlade N, Dinkelaker B, Hengeler C, Romheld V, Martinoia E (2000) Physiological aspects of cluster root function and development in phosphorous-deficient white lupin. Ann Bot 85: 909-919 [Google Scholar]
- Neumann G, Massonneau A, Martinoia E, Romheld V (1999) Physiological adaptations to phosphorous deficiency during proteoid root development in white lupins. Planta 208: 373-382 [Google Scholar]
- Pellet DM, Grunes DL, Kochian LV (1995) Organic acid exudation as an aluminum tolerance mechanism in maize (Zea mays L.). Planta 196: 788-795 [Google Scholar]
- Pineros M, Magalhaes JV, Carvalho Alves VM, Kochian LV (2002) The physiology and biophysics of an aluminum tolerance mechanism based on root citrate exudation in maize. Plant Physiol 129: 1194-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla-Zawislak B, Gadde DM, Ducharme K, McCammon MT (1999) Genetic and biochemical interactions involving tricarboxylic acid cycle (TCA) function using a collection of mutants defective in all TCA cycle genes. Genetics 152: 153-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52: 527-560 [DOI] [PubMed] [Google Scholar]
- Sandor A, Johnson JH, Srere PA (1994) Cooperation between enzyme and transporter in the inner mitochondrial membrane of yeast: requirement for mitochondrial citrate synthase for citrate and malate transport in Saccharomyces cerevisiae. J Biol Chem 269: 29609-29612 [PubMed] [Google Scholar]
- Schott EJ, Gardner RC (1997) Aluminum-sensitive mutants of Saccharomyces cerevisiae. Mol Gen Genet 254: 63-72 [DOI] [PubMed] [Google Scholar]
- Silva IR, Smyth TJ, Raper CD, Carter TE, Rufty TW (2001) Differential aluminum tolerance in soybean: an evaluation of the role of organic acids. Physiol Plant 112: 200-210 [DOI] [PubMed] [Google Scholar]
- Small WC, McAlister-Henn L (1997) Metabolic effects of altering redundant targeting signals for yeast mitochondrial malate dehydrogenase. Arch Biochem Biophys 344: 53-60 [DOI] [PubMed] [Google Scholar]
- Srere PA, Brazil H, Gonen L (1963) The citrate condensing enzyme of pigeon breast muscle and moth flight muscle. Acta Chem Scand 17: 129-134 [Google Scholar]
- Sumegi B, Srere PA (1984) Complex I binds several mitochondrial NAD-coupled dehydrogenases. J Biol Chem 259: 15040-15045 [PubMed] [Google Scholar]
- Takita E, Koyama H, Hara T (1999) Organic acid metabolism in aluminum-phosphate utilizing cells of carrot (Daucus carota L.). Plant Cell Physiol 40: 489-495 [Google Scholar]
- Taylor JG (1991) Current views of the stress response: physiological basis of tolerance. Curr Topics Plant Biochem Physiol 10: 57-93 [Google Scholar]
- Tesfaye M, Temple SJ, Allan DL, Vance CP, Samac DA (2001) Overexpression of malate dehydrogenase in transgenic alfalfa enhances organic acid synthesis and confers tolerance to aluminum. Plant Physiol 127: 1836-1844 [PMC free article] [PubMed] [Google Scholar]
- Tompkins D, Toffaletti J (1982) Enzymic determination of citrate in serum and urine, with use of the Worthington “ultrafree” device. Clin Chem 28: 192-195 [PubMed] [Google Scholar]
- Zatta P, Lain E, Cagnolini C (2000) Effects of Al on activity of Krebs cycle enzymes and glutamate dehydrogenase in rate brain homogenate. Eur J Biochem 267: 3049-3055 [DOI] [PubMed] [Google Scholar]