Abstract
The aim of this study was to evaluate the putative role of the sucrosyl-galactosides, loliose [α-d-Gal (1,3) α-d-Glc (1,2) β-d-Fru] and raffinose [α-d-Gal (1,6) α-d-Glc (1,2) β-d-Fru], in drought tolerance of perennial ryegrass and to compare it with that of fructans. To that end, the loliose biosynthetic pathway was first established and shown to operate by a UDP-Gal: sucrose (Suc) 3-galactosyltransferase, tentatively termed loliose synthase. Drought stress increased neither the concentrations of loliose and raffinose nor the activities of loliose synthase and raffinose synthase (EC 2.4.1.82). Moreover, the concentrations of the raffinose precursors, myoinositol and galactinol, as well as the gene expressions of myoinositol 1-phosphate synthase (EC 5.5.1.4) and galactinol synthase (EC 2.4.1.123) were either decreased or unaffected by drought stress. Taken together, these data are not in favor of an obvious role of sucrosyl-galactosides in drought tolerance of perennial ryegrass at the vegetative stage. By contrast, drought stress caused fructans to accumulate in leaf tissues, mainly in leaf sheaths and elongating leaf bases. This increase was mainly due to the accumulation of long-chain fructans (degree of polymerization > 8) and was not accompanied by a Suc increase. Interestingly, Suc but not fructan concentrations greatly increased in drought-stressed roots. Putative roles of fructans and sucrosyl-galactosides are discussed in relation to the acquisition of stress tolerance.
One of the strategies employed by plants to survive drought stress includes the synthesis of protective compounds, which may act by stabilizing membranes and proteins or mediating osmotic adjustment (Bohnert et al., 1995; Hare et al., 1998; Hoekstra et al., 2001). Included among these protective compounds are the water-soluble carbohydrates (WSCs), Glc, Suc, raffinose, myoinositol, and fructans.
Raffinose family oligosaccharides (RFOs) such as raffinose and stachyose accumulate during seed development and are thought to play a role in the desiccation tolerance of seeds (Blackman et al., 1992; Brenac et al., 1997). Raffinose also accumulates in vegetative tissues under drought stress (Taji et al., 2002). RFO biosynthesis requires the presence of galactinol, which is formed by galactinol synthase (GolS; EC 2.4.1.123) from UDP-Gal and myoinositol. Galactinol is the galactosyl donor for the biosynthesis of raffinose from Suc by raffinose synthase (RafS; EC 2.4.1.82). Because galactinol has not been assigned any function in plants other than acting as galactosyl donor for RFOs synthesis, GolS potentially catalyzes a metabolic key step for RFO synthesis. In a recent study, two drought-responsive GolS genes were identified among seven in Arabidopsis (Taji et al., 2002). Overexpression of one of them caused an increase in endogenous galactinol and raffinose as well as an improvement in drought tolerance.
In addition to GolS, myoinositol 1-phosphate synthase (INPS; EC 5.5.1.4) is another enzyme that may control the levels of galactinol and raffinose. It represents the point of entry into the RFO biosynthetic pathway because it diverts carbon from Glc-6-phosphate to myoinositol-1-phosphate, which is then used by inositol monophosphatase to produce myoinositol, the galactinol precursor. When potato (Solanum tuberosum) antisense INPS transformants were analyzed, they showed strongly reduced levels of myoinositol, galactinol, and raffinose in their leaves (Keller et al., 1998). In ice plants (Mesembryanthemum crystallinum), which have remarkable tolerance against drought, high salinity, and low temperatures, salinity elevated INPS transcript levels (Ishitani et al., 1996; Nelson et al., 1998). In Vigna umbellata plants, myoinositol increased during drought stress, but this increase was not accompanied by an increase in INPS activity (Wanek and Richter, 1997). The only report on the effect of drought stress on INPS gene expression concerns potato plants; INPS transcript levels were unaffected by drought stress (Keller et al., 1998).
Fructans (polyfructosyl-Suc) are a further group of reported candidates for drought protectants. In several species, fructans are either accumulated (Volaire and Lelièvre, 1997; De Roover et al., 2000), modified in chain length (Thomas, 1991; Volaire et al., 1998; Thomas and James, 1999), or reduced (Spollen and Nelson, 1994) during desiccation. A role for fructan in drought resistance was also suggested by Hendry (1993), who stated that the appearance of fructan-producing taxa corresponded with a climatological shift toward seasonal drought and that the distribution of present-day fructan flora corresponds with regions of seasonal drought. Additional evidence for a role of fructans in drought tolerance was provided by the finding that transgenic tobacco (Nicotiana tabacum) and transgenic sugar beet (Beta vulgaris) plants that accumulated low fructan levels were slightly more drought tolerant than wild-type plants (Pilon-Smits et al., 1995, 1999). Interestingly, recent reports point to the possibility that fructans may directly stabilize membranes under stress conditions (Ozaki and Hayashi, 1996; Demel et al., 1998; Hincha et al., 2000; Vereyken et al., 2001; Hincha et al., 2002).
Suc:fructan 6-fructosyltransferase (6-SFT, EC 2.4.1.10) is one of the enzymes involved in grass fructan biosynthesis (Sprenger et al., 1995). In barley (Hordeum vulgare), both 6-SFT and INPS genes are up-regulated by (a) cold temperature, (b) Suc and Glc supplied to excised leaves in the dark, and (c) leaf excision followed by illumination. It is hypothesized that Glc released as a by-product of fructan synthesis may induce the INPS gene expression (Wei et al., 2001).
Temperate forage grasses such as Dactylis glomerata, Festuca arundinacca, or perennial ryegrass (Lolium perenne) cannot maintain growth and development under prolonged and intense drought, but must be able to remain alive during a limited water deficit period to recover actively after rehydration (Volaire and Lelièvre, 1997; Volaire et al., 1998). Stress responses of growing tissues differ from those of mature tissues, with younger tissues being more resistant to water deficit (Barlow et al., 1980; West et al., 1990). Enclosed leaf bases and apices, which represent the surviving organs, are protected from evaporative stress within the older sheaths. They cannot tolerate a complete dehydration. Consequently, resistant grass genotypes exhibit a combination of adaptative responses to delay dehydration in the surviving organs. Monosaccharides, Suc, and fructans have been shown to accumulate in elongating leaf bases (Thomas, 1991; Volaire et al., 1998; Thomas and James, 1999). However, accumulation of other recognized protective compounds such as myoinositol, galactinol, and raffinose has never been investigated in these grass species.
Under non-stressed conditions, perennial ryegrass plants accumulate small amounts of raffinose and loliose (Pavis et al., 2001; Amiard et al., 2003). Loliose [α-d-Gal (1,3) α-d-Glc (1,2) β-d-Fru] is a galactosyl trisaccharide, structurally similar to raffinose [α-d-Gal (1,6) α-d-Glc (1,2) β-d-Fru]. Loliose has been found in different species of Festuca and Lolium where its chemical structure was first elucidated (MacLeod and McCorquodale, 1958a, 1958b; Chatterton et al., 1993). Its role and its synthetic pathway are unknown. The main objective of this study was to investigate whether drought stress could induce loliose and/or raffinose synthesis in addition to fructan synthesis in perennial ryegrass. To this end, we first established the loliose synthetic pathway and cloned the GolS and INPS genes. Second, we studied (a) loliose accumulation by measuring loliose synthase activity and loliose concentrations; (b) raffinose accumulation by measuring RafS activity, GolS gene expression, and galactinol and raffinose concentrations; (c) INPS gene expression and myoinositol concentrations; and (d) fructan accumulation during drought stress and after rewatering. We here report that in perennial ryegrass, the synthesis of fructans but not that of raffinose and loliose is up-regulated by drought stress. We further report on a novel enzyme, tentatively termed loliose synthase, which is an α-galactosyl transferase producing loliose from UDP-Gal and Suc.
RESULTS
Loliose Tissue Localization and Loliose Synthase Characterization
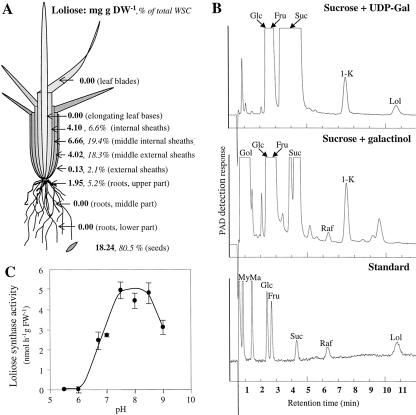
Loliose was not homogeneously distributed in perennial ryegrass plants (Fig. 1A). The highest concentration was found in seeds where it amounted to almost 2% of the dry weight, and 80.5% of the WSC, the other sugars being Suc, Glc, and Fru. In vegetative tissues, loliose was found exclusively in leaf sheaths and the upper part of the roots. In leaf sheaths, the highest concentration was found in the middle internal sheaths. Loliose was neither detected in leaf blades nor in immature growing leaves.
Figure 1.
Loliose localization and biosynthesis pathway in perennial ryegrass. A, Loliose repartition in the different tissues of perennial ryegrass. Values are mean of three replicates and indicate the loliose concentration (milligrams per gram dry weight). B, HPAEC-PAD profiles from incubations of desalted crude extract from leaf sheaths of perennial ryegrass with 50 mm Suc and either 5 mm UDP-Gal (top panel) or 5 mm galactinol (middle panel) at pH 8.5 for 24 h. The bottom panel show a mixed standard with myoinositol (My), mannitol (Ma), Glc, Fru, Suc, raffinose (Raf), and loliose (Lol). 1-K, 1-Kestotriose. C, pH-dependent activity profile of loliose synthase assayed with 50 mm Suc and 5 mm UDP-Gal. The enzyme activity was assayed at different pH from 5.5 to 9.0 using the following buffer solutions: pH 5.5 to 6.7, MES buffer; pH 6.7 to 8, HEPES buffer; and pH 8 to 9, HEPBS buffer. Vertical bars indicate ± se (n = 3).
The biosynthesis of galactosyl-Suc trisaccharides has been shown to proceed by pathways involving either UDP-Gal (for planteose and umbelliferose synthesis) or galactinol (for raffinose synthesis) as galactosyl donors, with Suc being always the galactosyl acceptor (for review, see Keller and Pharr, 1996). Thus, to determine the loliose biosynthetic pathway, Suc was incubated with UDP-Gal or galactinol, respectively. Incubation of desalted crude extract of leaf sheaths with Suc and UDP-Gal yielded loliose but no raffinose (Fig. 1B, top panel), whereas incubation with Suc and galactinol yielded raffinose but no loliose (Fig. 1B, middle panel). UDP-Gal is therefore the galactosyl donor for loliose synthesis, and galactinol is the galactosyl donor for raffinose synthesis in perennial ryegrass leaf sheaths. Additionally, the extract was also able to use Suc for 1-kestotriose synthesis (Suc:Suc 1-fructosyltransferase activity; Fig. 1B, top and middle panels) as well as to hydrolyze Suc to Glc and Fru (invertase activity). The pH dependency showed a broad optimum between 7.5 and 8.5 in HEPES and HEPBS buffers (Fig. 1C).
Effect of Drought and Rewatering on Water Relations and Growth
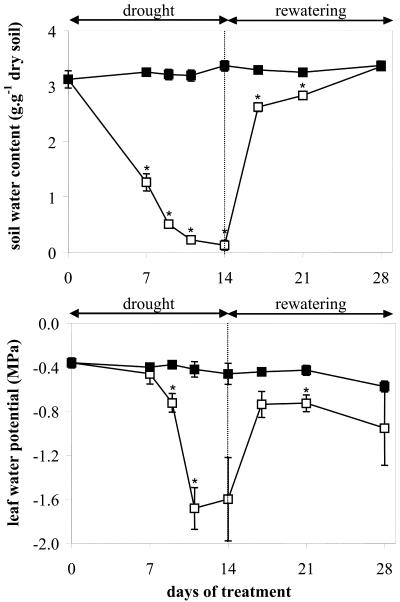
The water content of the soil was measured to determine the drought rate. Under control conditions, the water content of the soil remained fairly constant. Under stress conditions, the soil water content dropped from 3 to 1.2 g g-1 dry soil during the first 7 d of drought and still decreased during the 7 following d to reach almost zero (Fig. 2). By contrast, the leaf water potential did not change during the first week of drought but started to decrease sharply afterward from -0.4 to less than -1.6 MPa. During the rewatering period, the soil water content increased immediately from 0.1 to 2.6 g g-1 dry soil in 3 d and reached the same value as the control soil (3.3 g g-1 dry soil) 11 d later. The leaf water potential increased also sharply in parallel with the soil water content during the early period of rewatering from -1.6 to -0.7 MPa but remained stable afterward.
Figure 2.
Time courses of soil water content and leaf water potential in samples that were either well watered (control, black squares) or subjected to 14 d of drought followed by rewatering (drought-stressed, white squares). Vertical bars represent ± se (n = 3). The asterisk indicates significant differences between control and drought-stressed samples at P < 0.05.
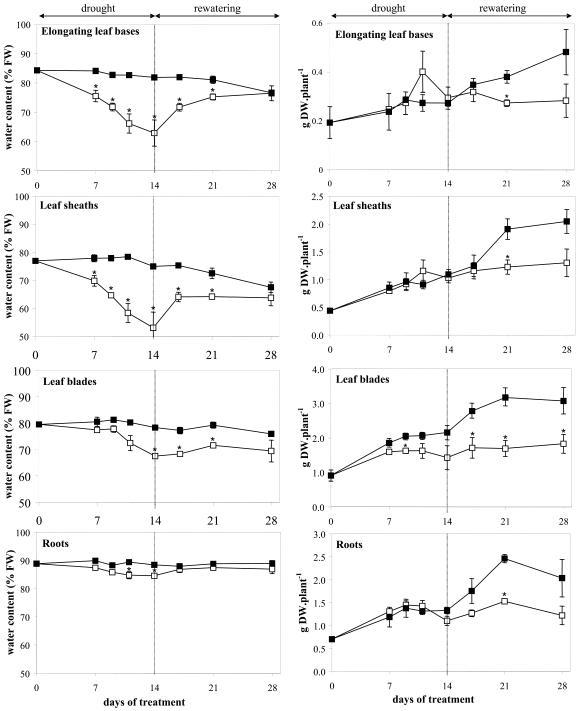
Under control conditions, the water content of the leaf tissues and the roots remained fairly constant (Fig. 3). Under stress conditions, the water content of elongating leaf bases and leaf sheaths showed a 25% to 30% decrease during the 14 d of drought, whereas that of leaf blade and roots declined only by 16% and 6%, respectively. However, no significant difference was found on a dry weight basis between control plants and drought-stressed plants. During the rewatering period, the water content in each tissue of drought-stressed plants increased again progressively to reach values that were not significantly different from those in control plants. The dry weight of drought-stressed plant tissues remained stable after rewatering, whereas it increased in control plants, leading to 30% to 45% differences in dry weight between drought-stressed plants and control plants at the end of the rewatering period. The number of tillers per plant increased progressively during the experiment but was always similar between droughtstressed and control plants (data not shown).
Figure 3.
Water content and biomass of the different plant parts of perennial ryegrass that were either well watered (control, black squares) or subjected to 14 d of drought followed by rewatering (drought-stressed, white squares). Vertical bars represent ± se (n = 3). The asterisk indicates significant differences between control and drought-stressed samples at P < 0.05.
Effect of Drought and Rewatering on Fructan, Suc, and Monosaccharide Concentrations
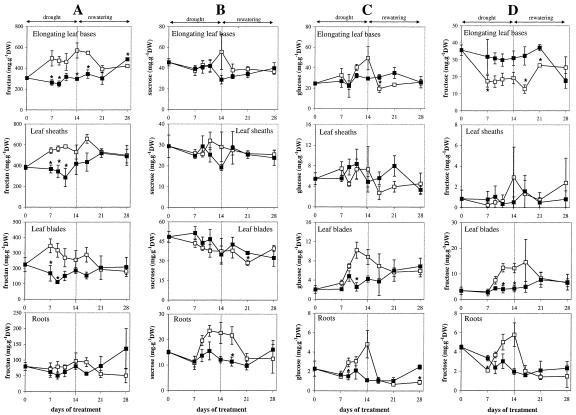
Under stress conditions, fructan concentrations increased significantly in all leaf tissues. The most pronounced increase was observed in elongating leaf bases where a 2-fold higher concentration was reached at the end of the drought period (amounting to 60% of the dry weight) as compared with control plants (Fig. 4A). During the rewatering period, fructan concentrations first increased (in mature leaves) or remained stable (in elongating leaf bases) before declining in all leaf tissues of drought-stressed plants to come back to values similar to those in control plants. In roots, fructan concentrations did not change significantly during the experiment, neither in control nor in drought-stressed plants.
Figure 4.
Concentrations of fructans (A), Suc (B), Glc (C), and Fru (D) in the plant parts of perennial ryegrass that were either well watered (control, black squares) or subjected to 14 d of drought followed by rewatering (drought-stressed, white squares). Vertical bars represent ± se (n = 3). The asterisk indicates significant differences between control and drought-stressed samples at P < 0.05.
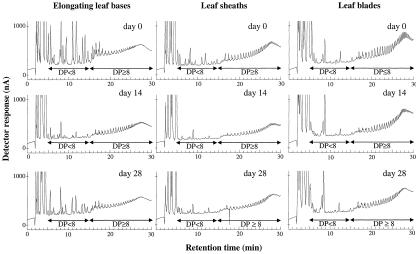
To determine whether the fructan accumulation in leaf tissues during drought was due to low or high degree of polymerization (DP) fructans, WSC samples were analyzed by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD; Fig. 5). To compare the relative proportions of low and high DP fructans, the same amounts of total fructan were injected for each type of tissue. At the beginning of the experiment (d 0), elongating leaf bases contained a higher proportion of low DP fructans, whereas in leaf sheaths and in leaf blades, high DP fructans were predominant. During drought, the proportion of low DP fructans decreased strongly in elongating leaf bases and only weakly in leaf sheaths, leading to a greater proportion of high DP fructans after drought stress (d 14) in those tissues. By contrast, the proportion of low DP fructans increased slightly in leaf blades. After rewatering (d 28), the proportion of low DP fructans increased again in elongating leaf bases, whereas it remained unchanged in leaf sheaths and leaf blades.
Figure 5.
Chromatograms of WSCs in the plant parts of perennial ryegrass that were subjected to 14 d of drought followed by 14 d of rewatering. Fructans from elongating leaf bases (0.15 mg), from leaf sheaths (0.20 mg), and from leaf blades (0.20 mg) were applied to the CarboPac PA100 column. Glc, Fru, and Suc were eluted between 2 and 5 min, fructans with low DP (<8) between 6 and 15 min and high DP fructans (DP < 8) between 16 and 30 min, according to the identifications obtained by Pavis et al. (2001).
Contrary to fructans, Suc levels were not significantly affected in leaf tissues, whereas they almost doubled in roots from d 7 to 14 of drought stress and declined again after rewatering (Fig. 4B).
Glc concentrations did not change significantly in elongating leaf bases of drought-stressed plants (Fig. 4C). Fru concentrations, however, declined by 50% during drought stress and increased after rewatering (Fig. 4D). In leaf sheaths, both Glc and Fru concentrations remained unchanged throughout the experiment. In leaf blades and in roots, hexose concentrations increased by at least 2-fold during drought and decreased after rewatering.
Effect of Drought Stress and Rewatering on Myoinositol, Galactinol, Raffinose, and Loliose Metabolism
Loliose and raffinose concentrations were determined at the beginning and the end of drought and rewatering, respectively. In roots, the concentrations were below the detection limit, so only data from leaf tissues are presented. The RafS activities were measured in elongating leaf bases and in leaf sheaths (Table I). Raffinose was barely detectable in elongating leaf bases, representing less than 0.1% of the dry weight. In leaf sheaths and leaf blades, it represented 0.2% and 0.4% of the dry weight, respectively, at the beginning of the experiment. At the end of the drought stress, raffinose concentrations declined more in mature leaf tissues of stressed plants than those of control plants. Concomitantly, RafS activity decreased in leaf sheaths under both conditions. Neither raffinose concentrations nor RafS activities were significantly affected by the rewatering treatment in mature leaf tissues. In elongating leaf bases, however, where raffinose concentrations and RafS activities were the lowest, raffinose concentrations were significantly affected by rewatering because it was higher in drought-stressed plants than in control plants. These higher raffinose concentrations in elongating leaf bases at the end of the rewatering period probably resulted from the higher RafS activities detected in this tissue at the end of the drought period compared with those in control plants. Among mature leaf tissues, loliose was specifically located in the sheaths. Loliose did not accumulate in this tissue during drought stress (Table I). Loliose synthase activity was detected in leaf sheaths but at an extremely low level (<0.1 nkat mg-1 protein) and did not show any significant change during drought stress (data not shown).
Table I.
Concentrations of raffinose and loliose and activities of raffinose synthase in leaf tissues of perennial ryegrass subjected to 14 d of drought stress, followed by 14 d of rewatering
Values are means ± se (n = 3). Asterisk indicates significant differences between control and drought-stressed samples at P < 0.05. u.d.l., Under the detection limit; n.d., not determined.
| Leaf Tissue | d 0 | d 14 (End of Drought) | d 28 (End of Rewatering) | ||
|---|---|---|---|---|---|
| Control | Control | Drought-Stressed | Control | Drought-Stressed | |
| Raffinose (mg g-1 dry wt) | |||||
| Elongating leaf bases | u.d.l. | 0.33 ± 0.25 | 0.09 ± 0.21 | 0.01 ± 0.12 | 0.68 ± 0.14* |
| Leaf sheaths | 1.62 ± 0.35 | 0.34 ± 0.26 | 0.00 ± 0.14 | 0.88 ± 0.34 | 0.91 ± 0.23 |
| Leaf blades | 4.15 ± 0.23 | 1.56 ± 0.25 | 0.37 ± 0.56 | 1.46 ± 0.07 | 1.27 ± 0.77 |
| Raffinose synthase activity (nkat mg-1 proteins) | |||||
| Elongating leaf bases | 2.28 ± 0.89 | 2.93 ± 1.33 | 7.47 ± 2.37 | 2.80 ± 0.09 | 0.00 ± 0.87* |
| Leaf sheaths | 29.60 ± 7.27 | 20.46 ± 10.38 | 9.67 ± 4.43 | 20.74 ± 8.84 | 9.39 ± 6.58 |
| Leaf blades | n.d | n.d | n.d | n.d | n.d |
| Loliose (mg g-1 dry wt) | |||||
| Elongating leaf bases | u.d.l. | u.d.l. | u.d.l. | u.d.l. | u.d.l. |
| Leaf sheaths | 1.92 ± 0.71 | 0.60 ± 1.14 | 0.07 ± 0.35 | 1.49 ± 0.78 | u.d.l. |
| Leaf blades | u.d.l. | u.d.l. | u.d.l. | u.d.l. | u.d.l. |
On the basis of known INPS and GolS sequence data, specific DNA primers were designed and used for reverse transcription-PCR amplification of homologous sequences from perennial ryegrass leaf sheaths. The deduced amino acid sequence of INPS (EMBL accession no. AY154382) is highly homologous to known INPS sequences from monocotyledonous species (Yoshida et al., 1999). The deduced amino acid sequence of GolS (EMBL accession no. AY154380) is highly homologous to known GolS sequences from soybean (Glycine max), zucchini (Cucurbita pepo; Kerr et al., 1993), rice (Oryza sativa; Takahashi et al., 1994), common bugle (Ajuga reptans; Sprenger and Keller, 2000), and Arabidopsis (Taji et al., 2002).
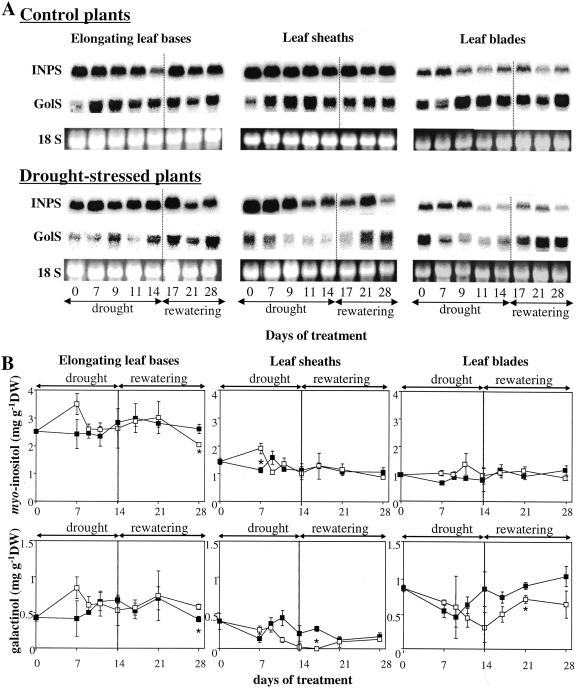
INPS and GolS expression as well as myoinositol and galactinol concentrations were determined in leaf tissues during drought stress and rewatering (Fig. 6). INPS expression was not affected by drought and rewatering in elongating leaf bases, whereas it decreased in leaf sheaths and in leaf blades, compared with that in control plants where it was rather stable. At the end of the rewatering period, INPS expression in leaf blades and leaf sheaths was lower than in plants grown under normal conditions. The concentration of myoinositol did not change significantly in these two leaf tissues throughout the experiment. It increased in elongating leaf bases during the first 7 d of drought stress and declined thereafter.
Figure 6.
Northern-blot analysis of INPS and GolS gene expression (A) and concentrations of myoinositol and galactinol (B) in the leaf tissues of perennial ryegrass plants that were either well watered (control, black squares) or subjected to 14 d of drought followed by rewatering (drought-stressed, white squares). A, Twenty micrograms of total RNA per lane was fractionated on a denaturing agarose gel, blotted to a nylon membrane, and hybridized with32P-labeled INPS and GolS cDNA probe, respectively. Loading of an equal amount of total RNA in each lane was verified by UV fluorescence of ethidium bromide-stained gel (the band corresponding to 18S rRNA was shown). B, Vertical bars represent ± se (n = 3). The asterisk indicates significant differences between control and drought-stressed samples at P < 0.05.
GolS gene expression was much less affected by drought stress in elongating leaf bases than in leaf sheaths and leaf blades where it was strongly down-regulated. In all tissues, rewatering caused an increase in GolS expression. The galactinol concentrations correlated positively with the changes in GolS expression; no significant change in elongating leaf bases and a decrease in leaf sheaths and leaf blades were observed.
DISCUSSION
Loliose Biosynthetic Pathway and Its Role in Drought Tolerance of Perennial Ryegrass
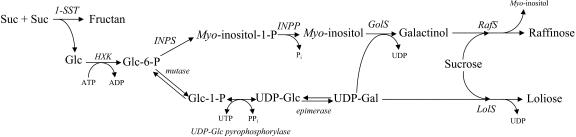
The in vitro synthesis of loliose was obtained by incubation of desalted enzyme extracts from perennial ryegrass leaf sheaths with UDP-Gal and Suc proceeding according to the reaction: UDP-Gal + Suc → loliose + UDP. The enzyme, tentatively termed loliose synthase, can therefore be classified as a UDP-Gal:Suc 3-galactosyltransferase (EC 2.4.1.?). It exhibits a broad pH optimum between 7.5 and 8.5, suggesting a cytosolic location. The biosynthesis of the two other galactosyl-Suc, umbelliferose (Hopf and Kandler, 1974) and planteose (Dey, 1980; Hopf et al., 1984), have also been shown to proceed with UDP-Gal as galactosyl donor. The optimal activities for umbelliferose- and planteose-synthesizing enzymes were obtained at pH 7.5 and 6.2, respectively. The same perennial ryegrass leaf sheath enzyme preparation was also able to produce raffinose when UDP-Gal was replaced by galactinol in the incubation medium. This reaction (galactinol + Suc → raffinose + myoinositol) is catalyzed by RafS (Lehle and Tanner, 1973; Bachmann et al., 1994; Peterbauer et al., 2002). On the basis of our current knowledge, the biosynthetic pathways for loliose and raffinose in perennial ryegrass are summarized in Figure 7.
Figure 7.
Biosynthetic pathways of loliose and raffinose in perennial ryegrass. HXK, Hexokinase; Glc-1-P, Glc 1-phosphate; Glc-6-P, Glc 6-phosphate; INPP, myoinositol 1-phosphate phosphatase; LolS, loliose synthase; Pi, inorganic phosphate, PPi, inorganic pyrophosphate; and 1-SST, Suc:Suc 1-fructosyltransferase.
In perennial ryegrass, loliose does not act as a transport carbohydrate because it was not detected in the phloem sap collected by the aphid stylectomy technique (unpublished data). It does not seem to represent a carbon storage compound either, which could be mobilized after defoliation to sustain regrowth (Pavis et al., 2001). Loliose was never detected in the leaf growth zone or in the root tips. Instead, it was found in leaf sheaths and in the oldest part of the roots, which contain less water than meristematic tissues (Amiard et al., 2003). Besides this specific location of loliose in plants at the vegetative stage, seeds store up to 80.5% of the WSC in the form of loliose, as shown in the present study, the other carbohydrates being Suc, Glc, and Fru. The aim of this study was to test the hypothesis that loliose acts as a desiccation protectant in leaves and roots of perennial ryegrass subjected to drought stress. Loliose does not seem to fulfill this role, however, because it did not accumulate in these tissues in response to water deficit, and the loliose synthase activity did not show any significant change during drought. The possibility that loliose may play important roles in seed desiccation or in other stress situations such as anoxia, low temperature, and high salinity cannot be ruled out.
Raffinose Biosynthetic Pathway and Its Role in Drought Tolerance of Perennial Ryegrass
The fact that transgenic plants, which overexpressed AtGolS cDNAs and accumulated galactinol and raffinose, showed improved drought tolerance provides direct evidence that the stress-inducible GolS gene controls the level of RFOs and that galactinol and raffinose play important roles in drought stress tolerance (Taji et al., 2002). Raffinose, which was also detected in small amounts in perennial ryegrass shoots and roots at the vegetative stage (Pavis et al., 2001), did not accumulate under drought stress. Raffinose metabolism was differentially affected by drought, depending on the leaf tissue type. In elongating leaf bases, raffinose metabolism was less affected than in mature leaves where it was reduced by a combination of decreased RafS activity and reduced levels of GolS and INPS transcripts. Raffinose does not seem, therefore, to play a role in desiccation tolerance of perennial ryegrass. Recently, seven GolS genes were isolated from Arabidopsis (Taji et al., 2002). They responded differently to drought stress.
Role of Fructan in Drought Tolerance of Perennial Ryegrass
The increased carbohydrate concentrations in shoots and roots of perennial ryegrass subjected to water deficit can easily be explained by the fact that growth is limited to a much higher extent than photosynthesis (Arcioni et al., 1985; Chaves, 1991). Exposure to a short-term drought (14 d) resulted in the accumulation of large amounts of fructans in elongating leaf bases and in leaf sheaths, as already reported for plants of the same species subjected to a long-term drought (57 d; Thomas and James, 1999). This increase was mainly due to the accumulation of high-DP fructans (DP>8) and is consistent with their proposed role as drought protectants maintaining membrane integrity. Recently, high-DP plant fructans were shown to prevent membrane damage by interacting with the head groups of phospholipids (Vereyken et al., 2001).
It is well known that drought stress induces a shift in the partitioning of photosynthetic products in favor of Suc (Hare et al., 1998), probably achieved by the activation of Suc phosphate synthase (Toroser and Huber, 1997). In fructan-accumulating plants, Suc may not only play a role as a substrate for fructan synthesis, but it may also act as an effector inducing fructan-synthesizing genes (Müller et al., 2000). In the present study, fructan accumulation in perennial ryegrass leaves was not accompanied by a Suc increase, contrary to previous results (Sprenger et al., 1995; Vijn et al., 1997; Thomas and James, 1999; De Roover et al., 2000). Moreover, and somewhat unexpectedly, Suc concentrations greatly increased in perennial ryegrass roots, but fructan accumulation was not enhanced. These contradictory results suggest that factors other than Suc are likely to affect the expression of fructan-synthesizing genes, an assumption that is also supported by a recent study where attached and detached barley leaves were exposed to continuous light and Suc concentrations increased similarly, but 6-SFT mRNA increased only in detached leaves. Additionally, Suc concentrations remained high in cold-treated plants, but 6-SFT gene expression was down-regulated (Wei et al., 2001). Further research is needed to identify the factors involved in fructan regulation and their signaling pathways.
Interplay between Fructan and Sucrosyl-Galactoside Pathways
A recent study showing homologous expression patterns for 6-SFT and INPS genes in barley leaves led to the proposition that the Glc produced from fructan synthesis may stimulate INPS gene activity (Wei et al., 2001). Under drought stress conditions, however, fructan levels increased with no concomitant rise in INPS transcripts. INPS gene expression was even down-regulated. INPS transcripts have already been reported to decline in response to water stress (Keller et al., 1998).
On the basis of current knowledge, RFO metabolism operates in families (Cucurbitaceae, Lamiaceae, and Scrophulariacea) where fructans do not accumulate (Keller and Pharr, 1996). Vice versa, RFO metabolism might not function in species that accumulate fructans. Interestingly, in perennial ryegrass, fructans, loliose, and raffinose were all detected in leaf sheaths, albeit at different levels, with fructans being much more concentrated than loliose and raffinose. Drought stress increased fructan levels but had no effect on loliose or raffinose levels. In the same species, loliose but not fructans and raffinose are stored in dry seeds. Hence, fructans may play a role in inducing drought tolerance in vegetative tissues of perennial ryegrass, whereas loliose may be important in inducing desiccation tolerance in dry seeds during development. Insights into the regulation of both pathways together with the drought sensitivity of the working enzymes should help to understand the basis of the apparent discrepancy between fructan and sucrosyl-galactoside pathways.
MATERIALS AND METHODS
Plant Material and Drought Stress Conditions
Perennial ryegrass (Lolium perenne L. cv Bravo) plants were grown in a greenhouse with a photoperiod of 16 h of natural light supplemented by a photosynthetic photon flux density of 110 μmol m-2 s-1 (Phyto tubes, Claude, GTE, Puteaux, France). The thermoperiod was 24°C (day) and 18°C (night). Seeds were germinated on water and transferred to plastic pots filled with perlite (three plants per pots) after 2 weeks. Nutrient solution, previously described by Gonzalez et al. (1989), was applied every 2nd d. Six weeks later, the supply of nutrient solution was stopped for one-half of the pots (treated plants), whereas the other half was still watered with nutrient solution (control plants; d 0–14, drought period). Fourteen days later, the treated plants were rewatered with nutrient solution (d 14–28, rewatering period). Measurements were made on d 0, 7, 9, 11, and 14 (drought) and d 17, 21, and 28 (rewatering).
Leaf Water Potential Measurements
Measurements were made just before the light period on one excised laminae (the youngest mature leaf) per plant (three measurements for each triplicate), using a Scholander-type pressure chamber following the procedure described by Turner (1981).
Harvesting Procedure
For each time point, triplicate pots containing three plants each were harvested. Plants were divided into four parts: roots, sheaths of mature leaves, blades of mature leaves together with the emerged part of the elongating leaf, and elongating leaf bases. One part of the harvested tissues was used immediately for enzyme extraction, whereas the remainder was frozen, stored at -80°C for RNA extraction, or freeze-dried for soluble carbohydrate extraction.
Extraction and Analysis of WSC
Soluble carbohydrates were extracted from 100 mg of freeze-dried tissues as described previously by Morvan-Bertrand et al. (2001). Aliquots of carbohydrate extracts (100 μL) were passed through minicolumns (Mobicols from MoBITec, Göttingen, Germany) packed, from bottom to top, with 120 μL of Amberlite CG-400 II, formiate-form (Fluka, Buchs, Switzerland), 100 μL of polyvinylpolypyrrolidone (Sigma-Aldrich, St. Louis), and 120 μL of Dowex 50W X8–400 H+-form (Sigma-Aldrich) to remove charged compounds (Bachmann et al., 1994). Glc, Fru, Suc, and fructans were separated and quantified by HPLC on a cation exchange column (Sugar-PAK, 300 × 6.5 mm, Millipore Waters, Milford, MA) eluted at 0.5 mL min-1 and 85°C with 0.1 mm CaEDTA in water, using mannitol as internal standard and refractive index detection (Guerrand et al., 1996). Loliose, raffinose, myoinositol, and galactinol were analyzed by HPAEC-PAD (DX-300, Dionex, Sunnyvale, CA) on a Carbopac PA1 column (4 × 250 mm) eluted at room temperature with sodium acetate (25 mm) in 150 mm NaOH (1 mL min-1) using mannitol as internal standard. Fructan chromatograms were obtained by HPAEC-PAD on a Carbopac PA100 column (4 × 250 mm; Dionex) eluted with a sodium acetate gradient in 150 mm NaOH (1 mL min-1) as described by Pavis et al. (2001). Glc, Fru, and Suc eluted between 2 and 5 min, fructans with low DP (<8) between 6 and 15 min, and high DP fructans (DP < 8) between 16 and 30 min, according to the identifications obtained by Pavis et al. (2001).
Enzyme Extraction
Freshly harvested tissues (300 mg) were ground in 840 μL of extraction buffer (50 mm MES, pH 6.3, 1 mm EDTA, 0.01% [v/v] Triton X-100, and 1 mm phenylmethylsulfonyl fluoride) at 4°C. After centrifugation (12,000g, 5 min), samples were desalted by gel filtration on Sephadex G-50 equilibrated with incubation buffer (75 mm HEPES, pH 8.5, and 5 mm dithiothreitol).
Loliose Synthase Characterization
The loliose synthetic pathway was determined by testing different galactosyl donors, galactinol (purified from cucumber [Cucumis sativus] according to Pharr et al. [1987]), UDP-Gal, and raffinose (Sigma-Aldrich). The pH optimum of the enzyme reaction was determined using MES buffer (pH 5.5–6.7 adjusted with NaOH), HEPES buffer (pH 6.7–8 adjusted with NaOH), and HEPBS buffer (pH 8–9 adjusted with NaOH) with 50 mm Suc and 5 mm UDP-Gal as substrates.
Enzyme Assays
Enzyme activities were determined at 30°C and pH 8.5 with 50 mm Suc and 5 mm UDP-Gal or 5 mm galactinol for loliose synthase and RafS, respectively. Assays were stopped after 24 h by boiling. For control reactions, UDP-Gal or galactinol was omitted. Products of the reactions were quantified by HPAEC-PAD as described above.
RNA Isolation, Reverse Transcription-PCR Amplification, Cloning, DNA Sequencing, and Probe Labeling
Plant tissues were ground in liquid nitrogen and suspended in warm (80°C) solution consisting of 750 μL of phenol and 750 μL of extraction buffer (0.1 m LiCl, 100 mm Tris-HCl, 10 mm EDTA, and 1% [w/v] SDS, pH 8.0). After shaking, 750 μL of chloroform:isoamylalcohol (24:1, v/v) was added, and the solution was centrifuged for 5 min (4°C) at 20,000g. Total RNA was precipitated with LiCl (final concentration 2 m) overnight at 4°C. After centrifugation for 30 min (4°C) at 20,000g, the pellet was suspended in 250 μL of DEPC-water and 250 μL of phenol:chloroform:isoamylalcohol (25:24:1, v/v), mixed, and centrifuged for 5 min. RNA in the supernatant was precipitated again with 1 mL of absolute ethanol and 50 μL of sodiumacetate buffer (3 m; pH 5.6) overnight at -20°C. After centrifugation for 20 min (4°C) at 20,000g, the pellet was washed with 75% (v/v) ethanol, and total RNA was suspended in 50 μL final buffer (25 mm EDTA and 0.1% [w/v] SDS).
Poly(A+) RNA was purified from total RNA isolated from leaf sheaths and reverse transcripted with oligo(dT) using reverse transcriptase (Invitrogen, Carlsbad, CA). cDNA was then amplified by PCR. Specific primers for GolS sequence amplification were designed according to conserved amino acid regions of known GolS sequences (Sprenger and Keller, 2000): 5′-TTC GCC TG GCC TAC TAC G-3′ and 5′-GGC GGC GGC GCA GTA GTG-3′. Specific primers for INPS sequence amplification were designed according to conserved amino acid regions of known INPS sequences from Poaceae species (Clustal multiple sequence alignment of oat [Avena sativa], rice [Oryza sativa], and maize [Zea mays]): 5′-ATG TTC ATC GAG AGC TTC-3′ and 5′-TGGATC CTG GTG CTG CTG AGC TC-3′. Amplification was achieved under the following conditions: 10 min at 95°C; 35 cycles of denaturation at 95°C for 30 s, annealing at 60°C (INPS) or 55°C (GolS) for 1 min, and elongation at 68°C for 2 min. PCR products were analyzed by agarose gel electrophoresis, cloned into pGEM-T Easy vector (Promega, Madison, WI) and were used for sequencing or32P-labeled probe synthesis using the Random Priming Labeling Kit (Roche Diagnostics, Indianapolis).
Northern Blotting
Total RNA, isolated as described above, was quantified according to A260 and loaded (20 μg lane-1) onto a 1% (w/v) agarose gel containing 2.2 m formaldehyde subjected to electrophoresis (80 V for 3 h). RNA was then transferred to a nylon transfer membrane (gene screen, PerkinElmer Life Sciences, Boston) by capillary blotting with 10× SSC buffer. Membranes were prehybridized for 3 h at 60°C in a Church buffer (0.25 m Na2HPO4, 7% [w/v] SDS, 2 mm EDTA, 20 mg mL-1 heparin, and 10 μg mL-1 salmon sperm denatured DNA). Membranes were hybridized with the INPS probe overnight at 60°C, and the INPS probe was removed from the blot. Finally, the blot was hybridized with the GolS probe.
Statistical Evaluation
The significance of the results was evaluated by Student's t test, where P < 0.05 was considered significant.
Acknowledgments
We thank the harvesting team for help and Dr. Jean-Louis Durand for the loan of the pressure chamber.
References
- Amiard V, Morvan-Bertrand A, Billard JP, Huault C, Prud'homme MP (2003) Fate of fructose supplied to leaf sheaths after defoliation of Lolium perenne L.: assessment by13C-fructose labelling. J Exp Bot 54: 1-13 [DOI] [PubMed] [Google Scholar]
- Arcioni S, Falcinelli M, Mariotti D (1985) Ecological adaptation in Lolium perenne L.: physiological relationships among persistence, carbohydrate reserves and water availability. Can J Plant Sci 65: 615-624 [Google Scholar]
- Bachmann M, Matile P, Keller F (1994) Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L. Plant Physiol 105: 1335-1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow EWR, Munns RE, Brady CJ (1980) Drought responses of apical meristems. In NC Turner, PJ Kramer, eds, Adaptation of Plants to Water and High Temperature Stress. John Wiley & Sons, New York, pp 191-205
- Blackman SA, Obendorf RL, Leopold AC (1992) Maturation proteins and sugars in desiccation tolerance of developing soybean seeds. Plant Physiol 100: 225-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7: 1099-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenac P, Horbowicz M, Downer SM, Dickermn AM, Smith ME, Obendorf RL (1997) Raffinose accumulation related to desiccation tolerance during maize (Zea mays L.) seed development and maturation. J Plant Physiol 150: 481-488 [Google Scholar]
- Chatterton NJ, Harrison PA, Thornley WR (1993) Loliose: a novel trisaccharide in leaves of Lolium and Festuca species. Plant Physiol 12: 113-116 [Google Scholar]
- Chaves MM (1991) Effects of water deficits on carbon assimilation. J Exp Bot 42: 1-16 [Google Scholar]
- Demel RA, Dorrepaal E, Ebskamp MJM, Smeekens JCM, de Kruijff B (1998) Fructans interact strongly with model membranes. Biochim Biophys Acta 1375: 36-42 [DOI] [PubMed] [Google Scholar]
- De Roover J, Vandenbranden K, Van Laere A, Van den Ende W (2000) Drought induce fructan synthesis and 1-SST (sucrose:sucrose fructosyltransferase) in roots and leaves of chicory seedlings (Cichorium intybus L.). Planta 210: 808-814 [DOI] [PubMed] [Google Scholar]
- Dey PM (1980) Biosynthesis of planteose in Sesamum indicum. FEBS Lett 114: 153-156 [Google Scholar]
- Gonzalez B, Boucaud J, Salette J, Langlois J, Duyme M (1989) Changes in stubble carbohydrate content during regrowth of defoliated perennial ryegrass (Lolium perenne L.) on two nitrogen levels. Grass Forage Sci 44: 411-415 [Google Scholar]
- Guerrand D, Prud'homme M-P, Boucaud J (1996) Fructan metabolism in expanding leaves, mature leaf sheaths and mature leaf blades of Lolium perenne: fructan synthesis, fructosyltransferase and invertase activities. New Phytol 134: 205-214 [Google Scholar]
- Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolytes accumulation during stress. Plant Cell Environ 21: 535-553 [Google Scholar]
- Hendry GAF (1993) Evolutionary origins and natural functions of fructans: a climatological, biogeographic and mecanistic appraisal. New Phytol 123: 3-14 [Google Scholar]
- Hincha DK, Hellwege EM, Heyer AG, Crowe JH (2000) Plant fructans stabilize phosphatidylcholine liposomes during freeze-drying. Eur J Biochem 267: 535-540 [DOI] [PubMed] [Google Scholar]
- Hincha DK, Zuther E, Hellwege EM, Heyer AG (2002) Specific effects of fructo- and gluco-oligosaccharides in the preservation of liposomes during drying. Glycobiology 12: 103-110 [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J (2001) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6: 431-438 [DOI] [PubMed] [Google Scholar]
- Hopf H, Spanfelner M, Kandler O (1984) Planteose synthesis in seeds of Sesamum indicum L. Z Pflanzenphysiol 114: 485-492 [Google Scholar]
- Hopf H, Kandler O (1974) Biosynthesis of umbelliferose in Aegopodium podagraria. Plant Physiol 54: 13-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani M, Majumder AL, Bornhouser A, Michalowski CB, Jensen RG, Bohnert HJ (1996) Coordinate transcriptional induction of myo-inositol metabolism during environmental stress. Plant J 9: 537-548 [DOI] [PubMed] [Google Scholar]
- Keller F, Pharr DM (1996) Metabolism of carbohydrates in sinks and sources: galactosyl-sucrose oligosaccharides. In E Zamski, AA Schaffer, eds, Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships. Marcel Dekker, New York, pp 157-183
- Keller R, Brearley CA, Trethewey RN, Müller-Röber B (1998) Reduced inositol content and altered morphology in transgenic potato plants inhibited for 1d-myo-inositol 3-phosphate synthase. Plant J 16: 403-410 [Google Scholar]
- Kerr PS, Pearlstein RW, Schweiger BJ, Becker-Manley MF (1993) Nucleotide sequences of galactinol synthase from zucchini and soybean. International Application Number PCT/US92/06057
- Lehle L, Tanner W (1973) The function of myo-inositol in the biosynthesis of raffinose: purification and characterisation of galactinol:sucrose-6-galactosyltransferase from Vicia faba seeds. Eur J Biochem 38: 103-110 [DOI] [PubMed] [Google Scholar]
- MacLeod A, McCorquodale H (1958a) Trisaccharides of Lolium and Festuca. Nature 20: 815-816 [DOI] [PubMed] [Google Scholar]
- MacLeod A, McCorquodale H (1958b) Water-soluble carbohydrates of seeds of the Gramineae. New Phytol 57: 168-182 [Google Scholar]
- Morvan-Bertrand A, Boucaud J, Le Saos J, Prud'homme MP (2001) Roles of the fructans from leaf sheaths and from the elongating leaf bases in the regrowth following defoliation of Lolium perenne L. Planta 213: 109-120 [DOI] [PubMed] [Google Scholar]
- Müller J, Aeschbacher RA, Sprenger N, Boller T, Wiemken A (2000) Disaccharide-mediated regulation of sucrose:fructan-6-fructosyltransferase, a key enzyme of fructan synthesis in barley leaves. Plant Physiol 123: 265-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Rammesmayer G, Bohnert HJ (1998) Regulation of cell-specific inositol metabolism and transport in plant salinity tolerance. Plant Cell 10: 753-764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K, Hayashi M (1996) Cryoprotective effects of cycloinulohexaose on freezing and freeze-drying of liposomes. Chem Pharm Bull 44: 2116-2120 [DOI] [PubMed] [Google Scholar]
- Pavis N, Chatterton NJ, Harrison PA, Baumgartner S, Praznik W, Boucaud J, Prud'homme MP (2001) Structure of fructans in roots and leaf tissues of Lolium perenne. New Phytol 150: 83-95 [Google Scholar]
- Peterbauer T, Mach L, Mucha J, Richter A (2002) Functional expression of a cDNA encoding pea (Pisum sativum L.) raffinose synthase, partial purification of the enzyme from maturing seeds, and steady-state kinetic analysis of raffinose synthesis. Planta 215: 839-846 [DOI] [PubMed] [Google Scholar]
- Pharr DM, Hendrix DL, Robbins NS, Gross KC, Sox HN (1987) Isolation of galactinol from leaves of Cucumis sativus. Plant Sci 50: 21-26 [Google Scholar]
- Pilon-Smits EAH, Ebskamp MJM, Paul MJ, Jeuken MJW, Weisbeek PJ, Smeekens SCM (1995) Improved performance of transgenic fructan-accumulating tobacco under drought stress. Plant Physiol 107: 125-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Smits EAH, Terry N, Sears T, van Dun K (1999) Enhanced drought resistance in fructan-producing sugar beet. Plant Physiol 37: 313-317 [Google Scholar]
- Spollen WG, Nelson CJ (1994) Response of fructan to water deficit in growing leaves of tall fescue. Plant Physiol 106: 329-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger N, Bortlik K, Brandt A, Boller T, Wiemken A (1995) Purification, cloning, and functional expression of sucrose:fructan 6-fructosyltransferase, a key enzyme of fructan synthesis in barley. Proc Natl Acad Sci USA 92: 11652-11656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger N, Keller F (2000) Allocation of raffinose family oligosaccharides to transport and storage pools in Ajuga reptans: the roles of two distinct galactinol synthases. Plant J 21: 249-258 [DOI] [PubMed] [Google Scholar]
- Taji T, Ohsumi C, Luchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi- Shinozaki K, Shinozaki K (2002) Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J 29: 417-426 [DOI] [PubMed] [Google Scholar]
- Takahashi R, Joshee N, Kitagawa Y (1994) Induction of chilling resistance by water stress, and cDNA sequence analysis and expression of water stress-regulated genes in rice. Plant Mol Biol 26: 339-352 [DOI] [PubMed] [Google Scholar]
- Thomas H (1991) Accumulation and consumption of solutes in swards of Lolium perenne during drought and after rewatering. New Phytol 118: 35-48 [Google Scholar]
- Thomas H, James AR (1999) Partitioning of sugars in Lolium perenne (perennial ryegrass) during drought and on rewatering. New Phytol 142: 292-305 [Google Scholar]
- Toroser D, Huber SC (1997) Protein phosphorylation as a mechanism for osmotic-stress activation of sucrose-phosphate synthase in spinach leaves. Plant Physiol 114: 947-955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner NC (1981) Technics and experimental approaches for the measurement of plant water status. Plant Soil 58: 339-366 [Google Scholar]
- Vereyken IJ, Chupin V, Demel RA, Smeekens SCM, De Kruijff B (2001) Fructans insert between the headgroups of phospholipids. Biochim Biophys Acta 1510: 307-320 [DOI] [PubMed] [Google Scholar]
- Vijn I, van Dijken A, Sprenger N, van Dun K, Weisbeek P, Wiemken A, Smeekens S (1997) Fructan of the inulin neoseries is synthesized in transgenic chicory plants (Cichorium intybus L.) harbouring onion (Allium cepa L.) fructan:fructan 6G-fructosyltransferase. Plant J 11: 387-398 [DOI] [PubMed] [Google Scholar]
- Volaire F, Lelièvre F (1997) Production, persistence, and water-soluble carbohydrate accumulation in 21 contrasting populations of Dactylis glomerata L. subjected to severe drought in the south of France. Aust J Agric Res 48: 933-944 [Google Scholar]
- Volaire F, Thomas H, Lelievre F (1998) Survival and recovery of perennial forage grasses under prolonged Mediterranean drought: I. Growth, death, water relations and solute content in herbage and stubble. New Phytol 140: 439-449 [DOI] [PubMed] [Google Scholar]
- Wanek W, Richter A (1997) Biosynthesis and accumulation of d-ononitol in Vigna umbellata in response to drought stress. Physiol Plant 101: 416-424 [Google Scholar]
- Wei J-Z, Chatterton NJ, Larson SR (2001) Expression of sucrose:fructan 6-fructosyltransferase (6-SFT) and myo-inositol 1-phosphate synthase (MIPS) genes in barley (Hordeum vulgare) leaves. J Plant Physiol 158: 635-643 [Google Scholar]
- West CP, Oosterhuis DM, Wullschleger SD (1990) Osmotic adjustment in tissues of tall fescue in response to water deficit. Environ Exp Bot 30: 149-156 [Google Scholar]
- Yoshida KT, Wada T, Koyama H, Mizobuchi-Fukuoka R, Naito S (1999) Temporal and spatial pattern of accumulation of the transcript of myo-inositol-1-phosphate synthase and phytin-containing particles during seed development in rice. Plant Physiol 119: 65-72 [DOI] [PMC free article] [PubMed] [Google Scholar]