Abstract
We have studied the expression of 1l-myoinositol-1-phosphate synthase (MIPS; EC 5.5.1.4) in developing organs of Phaseolus vulgaris to define genetic controls that spatially regulate inositol phosphate biosynthesis. MIPS, the pivotal biosynthetic enzyme in inositol metabolism, is the only enzyme known to catalyze the conversion of glucose 6-phosphate to inositol phosphate. It is found in unicellular and multicellular eukaryotes and has been isolated as a soluble enzyme from both. Thus, it is widely accepted that inositol phosphate biosynthesis is largely restricted to the cytosol. Here, we report findings that suggest the enzyme is also expressed in membrane-bound organelles. Microscopic and biochemical analyses detected MIPS expression in plasma membranes, plastids, mitochondria, endoplasmic reticula, nuclei, and cell walls of bean. To address mechanisms by which the enzyme could be targeted to or through membranes, MIPS genes were analyzed for sorting signals within primary structures and upstream open reading frames that we discovered through our sequence analyses. Comprehensive computer analyses revealed putative transit peptides that are predicted to target the enzyme to different cellular compartments. Reverse transcriptase PCR experiments suggest that these putative targeting peptides are expressed in bean roots and leaves.
Inositol metabolism is essential for the development of plants, animals, and some microorganisms. Metabolites of inositol (a six carbon cyclitol) such as phosphoinositides and phytate function as potent regulators of signal transduction for a wide variety of hormones, growth factors, and neurotransmitters (York et al., 1999; Loewus and Murthy, 2000; Irvine and Schell, 2001). Inositol phosphate, the immediate precursor of free inositol, is synthesized via the internal cyclization of Glc 6-phosphate. The overall reaction mechanism consists of a tightly coupled oxidation and reduction (Sherman et al., 1969; Loewus and Loewus, 1983). 1l-Myoinositol-1-phosphate synthase (MIPS; EC 5.5.1.4) is the only enzyme known to catalyze this reaction. MIPS is found in diverse organisms, both eukaryotic and prokaryotic, suggesting that the pathway for inositol 1-phosphate biosynthesis from Glc 6-phosphate arose early in the evolution of life (Majumder et al., 1997; Bachhawat and Mande, 2000). The properties and generally accepted catalytic mechanisms of the enzyme are similar in all organisms where such assessment has been undertaken (Loewus and Murthy, 2000). Alignment of MIPS amino acid sequences from diverse organisms including Arabidopsis, bean (Phaseolus vulgaris), Brewer's yeast (Saccharomyces cerevisiae), and Entamoeba histolytica revealed remarkable evolutionary conservation of the primary structure (Majumder et al., 1997). Genome sequencing projects have provided additional evidence for this striking conservation with deduced primary structures from organisms such as Caenorhabditis elegans, fruitfly (Drosophila melanogaster), Leishmania major, and Chlamydomonas reinhardtii.
Although the essential roles of inositol in many cellular processes including membrane formation, cell wall biogenesis, stress response, and signal transduction have been well documented, less is known of the cellular mechanisms that regulate its complex metabolic flux. To identify spatial and temporal controls that regulate the biosynthesis of inositol phosphate, we have isolated and studied MIPS genes and gene products from Arabidopsis and bean using yeast inositol mutants and yeast MIPS polyclonal antibody (Johnson, 1994; Johnson and Burk, 1995; Johnson and Sussex, 1995; Wang and Johnson, 1995; Johnson and Wang, 1996). Here, we report microscopic and biochemical findings concerning the compartmentalization of MIPS (i.e. inositol phosphate biosynthesis) in organs of bean.
RESULTS
MIPS Expression during Root Development
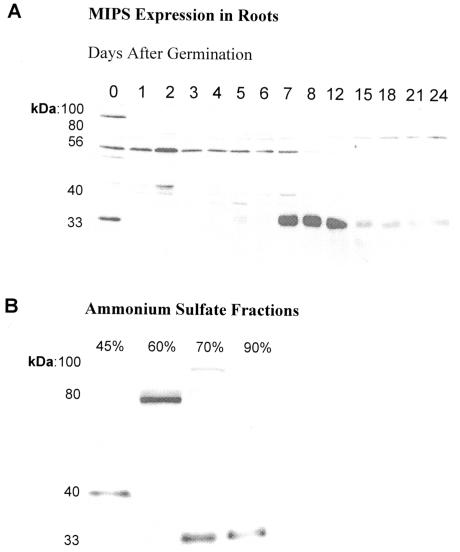
MIPS expression was monitored during root development using western-blot analyses. Isoforms of the enzyme were identified and partially purified from roots of 8-d-old plants (Fig. 1, A and B). Three of these forms (80, 56, and 33 kD) were previously shown to be temporally and spatially regulated during bean (Johnson and Wang, 1996) and Arabidopsis (Johnson, 1994; Johnson and Sussex, 1995) development. In plants and in animals, the existence of multiple isoforms of particular enzymes often reflects the number of subcellular compartments in which the same catalytic reaction is required (Gottlieb, 1982). Thus, we hypothesized that the number of MIPS isoforms detected might reflect the distribution of the enzyme to other cellular compartments in addition to the cytosol and chloroplast (Johnson and Wang, 1996). To address this hypothesis, we first conducted immunolocalization studies.
Figure 1.
Isoforms of MIPS are differentially expressed during root development. A, Western-blot analyses of bean root proteins during development detected five cross-reacting proteins including the 80-, 56-, and 33-kD proteins previously characterized (Johnson and Wang, 1996). Antigen capture immunoassays (Weiler et al., 1960), ammonium sulfate fractionations, and enzyme assays suggest these proteins are isoforms of MIPS. Each lane contains total proteins (50 μg). B, MIPS was partially purified from roots harvested 8 d after germination. Ammonium sulfate precipitates of 45%, 60%, 70%, and 90% produced 27, 64, 42, and 56 nmol inositol phosphate h-1 mg-1 protein, respectively.
Immunolocalization
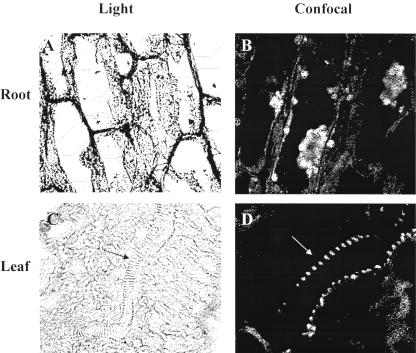
Immunohistochemical experiments provided valuable overviews of MIPS expression in cells of bean roots (Fig. 2, A and B) and leaves (Fig. 2, C and D). The enzyme is expressed in intracellular structures and in cell walls (Fig. 2B). In addition, it is highly expressed in the vascular system of leaves (Fig. 2D) where many inositol-containing compounds and other inositol metabolic enzymes have been identified (Gillaspy et al., 1995).
Figure 2.
Immunohistochemical studies profiled MIPS expression in organs of bean. Sections of 8-d-old bean roots (A and B) and leaves (C and D) were stained with toluidine blue and incubated with MIPS antibody and fluorescein isothiocyanate-conjugated secondary antibody. Shown are light (A) and confocal (B) micrographs of a longitudinal section of root and light (C) and confocal (D) micrographs of leaf section (arrows show vascular system). Root and leaf light micrographs were magnified 580× and 370×, respectively. Bar = 20 μm. Magnification of confocal micrographs could not be determined with the Nikon PCM 2000 microscope. Controls included unstained sections not treated with primary or secondary antibody to detect autofluorescence and stained sections incubated only with secondary antibody to detect nonspecific interactions.
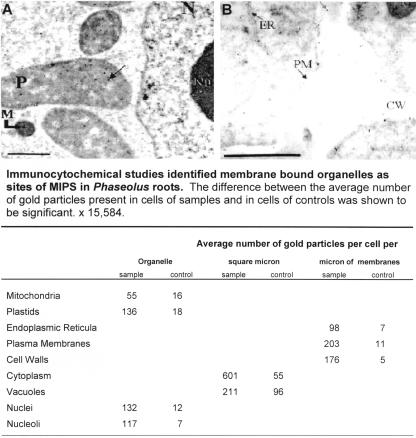
Immunocytochemistry was used to identity the subcellular structures. Micrographs of bean roots (Fig. 3, A and B) detail MIPS expression in plastids, mitochondria, endoplasmic reticula, plasma membranes, cell walls, nuclei, and nucleoli. The specificity of these results was verified by counting the number of gold particles present in each organelle in six samples (grids incubated with primary antibody and goat anti-rabbit 10-nm gold-conjugated secondary antibody, respectively) and in six controls (grids incubated with goat anti-rabbit 10-nm gold-conjugated secondary antibody only; Fig. 3). The difference, as determined by Tukey's honestly significant difference test (Daniel, 1995), was shown to be significant.
Figure 3.
Immunocytochemical analyses identified membrane-bound organelles as subcellular locations of MIPS. Bean root sections were incubated with MIPS primary antibody and goat anti-rabbit 10-nm gold-conjugated secondary antibody and photographed with a Zeiss 10A TEM microscope. Controls were incubated with goat anti-rabbit 10-nm gold-conjugated secondary antibody only. As shown in A and B, gold particles are present in plastids (P), mitochondria (M), the nucleus (N), nucleolus (Nu), endoplasmic reticula (ER), plasma membrane (PM), and the cell wall (CW). Bar = 1 μm.
Subcellular Fractionation
To biochemically corroborate the microscopic studies, organelles (mitochondria, plastids, endoplasmic reticula, plasma membranes, and chloroplasts) were isolated from roots and leaves and assayed for purity and enrichment using organelle-specific marker enzymes (Tables I and II). MIPS activity as determined by the end-product method (Chen and Charalampous, 1966) and the rapid colorimetric method (Barnett et al., 1970) was detected in all isolated organelles. Yeast soluble and insoluble (microsomal) fractions were used as positive and negative controls, respectively, because earlier studies indicated that MIPS was found in soluble cellular fractions (Donahue and Henry, 1981; Johnson and Henry, 1989). MIPS expression was detected in both controls.
Table 1.
Solubilized purified organelles exhibit MIPS activity
MIPS specific activity was determined in 0.003 mg of organelle protein using periodate oxidation to measure release of inorganic phosphate from inositol phosphate. One unit of MIPS is defined as 1 nmol of inositol phosphate produced per hour. Total activity, Nanomoles of inositol phosphate produced per hour (units × 10-3). Specific activity, Nanomoles of inositol phosphate per hour per 0.003 mg of protein (units/mg × 10-3).
| Fraction ([Protein] = 0.003 mg) | Total Activity | Specific Activity |
|---|---|---|
| units | units mg-1 | |
| Root | ||
| Plastids | 43 | 14.333 |
| Mitochondria | 38 | 12.666 |
| Microsomes | 27 | 9.000 |
| Plasma membranes | 64 | 21.333 |
| Leaf | ||
| Chloroplasts | 12 | 4.000 |
| Mitochondria | 40 | 13.333 |
| Plasma membranes | 67 | 22.333 |
Table II.
Solubilized purified organelles exhibit MIPS activity
To assess organelle purity and enrichment, marker enzymes for endoplasmic reticula (antimycin insensitive NADH cytochrome C reductase), mitochondria (cytochrome C oxidase), plastids (nitrite reductase), plasma membranes (vanadate-sensitive Ca2+ ATPase), and microbodies (catalase) were assayed using 0.010 mg of protein for each fraction. Contamination between organelles was estimated by comparing the specific activity (S.A.) of the marker enzymes in each fraction with the specific activity of the marker enzyme in its designate organelle.
| Fraction ([Protein] = 0.010 mg)
|
Specific Activity
|
||||
|---|---|---|---|---|---|
| Cytochrome C Reductase | Cytochrome C Oxidase | Nitrite Reductase | Ca 2+ ATPase | Catalase (Eref = 3.45 μmol) | |
| μmol Cyt C red min-1mg-1 | μmol Cyt C ox min-1mg-1 | nmol NO-2red min-1mg-1 | μmol Pi h-1mg-1 | μmol H2O2dec min-1 | |
| Root | |||||
| Plastids | 0.0296 | 0.0156 | 93.310 | 0.04875 | 0.0276 |
| Mitochondria | aua | 0.4116 | 30.890 | 0.01623 | 0.0690 |
| Microsomes | 0.3300 | au | au | 0.08125 | 0.0759 |
| Plasma membranes | au | au | au | 0.22100 | au |
| Leaf | |||||
| Chloroplasts | 0.0222 | 0.0254 | 98.590 | 0.05177 | 0.0414 |
| Mitochondria | 0.0222 | 0.2685 | 22.098 | 0.04719 | 0.0483 |
| Plasma membranes | au | 0.0137 | au | 0.25588 | 0.0449 |
Activity undetectable
Computer Analyses
MIPS has a very highly conserved primary structure and has been isolated from numerous organisms, yet such a broad distribution was unsuspected, and no mechanisms for localization to cellular compartments other than cytosol and chloroplasts have been reported (Majumder et al., 1997). We sought a causal explanation from comprehensive computer analyses of four genomic copies of MIPS, one from bean and three from Arabidopsis.
The programs, PSORT (Prediction of Protein Sorting Signals and Localization Sites in Amino Acid Sequences; Nakai and Horton, 1999) and ExPASy (Expert Protein Analysis System), a proteomics server of the Swiss Institute of Bioinformatics, provided valuable information pertaining to sorting signals and common motifs within MIPS primary structures and upstream open reading frames (ORFs). PSORT analyses predict that all four MIPS primary structures are type II membrane proteins with most of the primary structure being associated with cytoplasm (Table III). A predicted conserved transmembrane motif (CEDSLLAAPIILDLVLLAELSTR), located approximately 68 amino acids from the carboxy terminus, is also predicted. Database entries for MIPS, in fact, reveal that this transmembrane motif is not only present, but is extraordinarily conserved in representatives from both the plant and animal kingdoms and in organisms as different as Brewer's yeast and human (Homo sapiens) despite the former presumption that MIPS is a cytosolic non-membrane-associated enzyme. The genomic sequences immediately 5′ of the translation start codons are not conserved in the one bean and three Arabidopsis MIPS genes examined (Fig. 4, A–D). A striking commonality in these upstream regions is, however, the presence of short ORFs that could potentially encode transit peptides capable of targeting MIPS isoforms to a variety of subcellular locations (Table III). The Phaseolus gene contains upstream ORFs interspersed with consensus RNA splice sites that predict five such peptides, each with a high probability of directing the enzyme to a different cellular compartment, including the nucleus, thylakoid membranes of chloroplast, and microbodies (Fig. 4A; Table III). Each Arabidopsis gene has a distinct set of predicted transit peptides. Chromosome 2 gene has three such sequences, the first targeting with highest probability to the microbody, the second to chloroplast (thylakoid membrane, stroma, and thylakoid space), and the third to the endoplasmic reticulum (Fig. 4B; Table III). Chromosome 4 MIPS gene has one targeting sequence for the nucleus and the microbody (Fig. 4C; Table III). Finally, the chromosome 5 MIPS gene has one such sequence with the most likely target being the mitochondrial intermembrane space (Fig. 4D; Table III). The ProfileScan computer program identified N glycosylation, protein kinase C phosphorylation, casein kinase II phosphorylation, and N myristoylation as possible posttranslational modifications for MIPS and some of its putative targeting peptides. Intriguingly, ProfileScan also ascribed a Wnt motif to bean putative transit peptide number 5 (Table III). Members of the Wnt family of secreted glycoproteins participate in many signaling events during development and play permissive roles during cell-fate assignment by interacting with a number of extracellular and cell-surface proteins (Arias et al., 1999).
Table III.
PSORT predicted locations for MIPS and some of its putative targeting peptides
| Amino Acid Sequence | Predicted Location (Percent Probability) |
|---|---|
| Primary Structures | |
| Phaseolus, Arabidopsis (chromosome 2), Arabidopsis (chromosome 4), and Arabidopsis (chromosome 5) | ER membrane (85), plasma membrane (44), chloroplast thylakoid membrane (21), and mitochondrial inner membrane (10) |
| CEDSLLAAPIILDLVLLAELSTR | Transmembrane region |
| Putative Targeting Peptides | |
| Phaseolus | |
| MCISRIIIKETKRKREVCHTCRPPLCFILLHFSFISEQTK | Mitochondrial intermembrane space (82), matrix (55), inner membrane (28), and outer membrane (28) |
| MCISRIIIKETKRKDCKREVCHTCREKI | Mitochondrial intermembrane space (81), and nucleus (72) |
| MRPPLCFILLHFSFLISEQTK | Chloroplast thylakoid (80), mitochondrial matrix (43), mitochondrial intermembrane (78), and microbody (64) |
| MNPAKNYNKRNQKEVKGKMRLRPPLCFILLHFSFLISEQTK | Nucleus (58), mitochondrial matrix (10), and chloroplast thylakoid membrane (10) |
| MNPAKDCKSREVCHTCRPPLCFILLHFSFLISEQTK | Extracellular (37), ER membrane (10), ER lumen (10), and Golgi body (10) |
| Arabidopsis (Chromosome 2) | |
| MHLDFIQTKVQKWRKIFYHLLW | Microbody (64), cytosol (45), and mitochondrial matrix (10) |
| MSCPTTTTASSSSSLGCLRVCSQIYKETCLHVNHATCLNPTSNYSLTFSNSTLLVVLVLSGLLDWARTISIPPITSKPATCPPSSVSRLYIHALVRRFEITHTTTKHKAFQNPEK | Chloroplast thylakoid membrane (82), stroma (75), thykaloid space (75), and mitochondri inner membrane (61) |
| MHLDFIQTKVQKWRKIFYHLLWCPTTTTASSSSSLGCLRVCSQIYKETCLHVNHATCLNPTSNYSLTFSNSTLLVVLVLSGLDWARTISIPPITSKPATCPPSSVSRLYIHALVRRFEITHTTTKHKAFQNPEK | ER (85), microbody (49), plasma membrane (44), and chloroplast thylakoid membrane (11) |
| Arabidopsis (Chromosome 4) | |
| MHWTNRDHQPVKKHKTHTPKPIQIPRKTTKFSFSSAK | Nucleus (81), microbody (64), and mitochondrial matrix (10) |
| Arabidopsis (Chromosome 5) | |
| MIDTNQSRSLFDVTLTLTYCHMYEVAK | Mitochondrial intermembrane space (81), mitochondrial matrix (50), inner membrane (22), and cytoplasm (45) |
Figure 4.
Computer analyses predict that MIPS and putative targeting peptides might localize to organelles identified as sites of inositol phosphate biosynthesis. Upstream regions of four MIPS genes (A–D) were analyzed for splice sites (nucleotides in bold type), Met residues (amino acids in bold type), stop codons, and organelle targeting ORFs. Negative numbers represent bases 5′ of published start sites (underlined amino acids). This information was used to generate putative targeting peptides for each gene (Table III). Bean MIPS is 90% identical to the Arabidopsis MIPS on chromosome 2, 96% identical to the one on chromosome 4, and 86% identical to MIPS on chromosome 5. PSORT (Table III) predicts the primary structures for all four MIPS can reside in the endoplasmic reticulum, plasma membrane, chloroplast thylakoid membrane, and mitochondria inner membrane. In addition, MIPS is predicted to be a type ll membrane protein with most of its structure associated with the cytosol.
Reverse Transcriptase (RT)-PCR
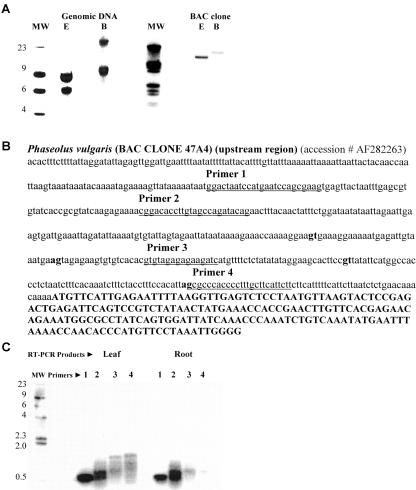
To systematically assess expression of the ORFs, the upstream region of the bean MIPS gene (Fig. 5B) was used to generate primers for RT-PCR experiments. Four forward primers (underlined sequence) and a reverse primer from within the MIPS coding sequence (third exon) were designed to detect appropriately spliced mRNAs that contain the upstream ORFs (Fig. 5, B–C). Sequencing of a leaf cDNA produced from primer 1 confirmed the existence of an appropriately spliced mRNA capable of producing peptide number 3 (Table III). Analysis of other products is currently in progress.
Figure 5.
Phaseolus has two MIPS genes. One of two bean MIPS genes was isolated from a bacterial artificial chromosome (BAC) library, characterized, sequenced, and used to design primers for RT-PCR experiments. A, Southern-blot analysis of genomic DNA and BAC DNA digested with restriction enzymes not present in the gene sequence, EcoRI (E) and Bam Hl (B), detected two hybridizing fragments (9 and 6 kb) in genomic DNA and one hybridizing fragment (9 kb) in the BAC DNA. The deduced amino acid sequence of the clone is 89% identical to the bean root cDNA used as probe for library screening (Wang and Johnson, 1995). These results suggest that there are two MIPS genes in the bean genome. B, Bean upstream region contains ORFs interspersed with consensus RNA splice sites (lowercase bold type) and the first exon (uppercase bold type). C, RT-PCR reactions were performed using a reverse primer (5′-CCTTGGCCC TACCCATGGC-3′) made from a sequence in the third exon and four different forward primers (underlined sequence). All lanes were loaded with RT-PCR reaction products (5 μL) generated from leaf or root mRNA.
DISCUSSION
We have used a variety of experimental approaches including microscopic analyses, organelle isolations, western-blot analyses, and enzyme assays to question MIPS presence (i.e. inositol phosphate biosynthesis) in membrane-bound cellular compartments. Although MIPS has only been isolated in its soluble form, it is clear that other forms exist and that they are associated with membrane-bound organelles. Given the numerous cellular compartments and genetic loci for MIPS, it is reasonable to speculate that a probable function for the enzyme is to help regulate the complex metabolic flux of inositol. The identification of several new inositol-utilizing pathways within organelles (York et al., 1999; Irvine and Schell, 2001) suggests these pathways may draw from the same inositol pool, necessitating large quantities of readily available inositol phosphate that can easily be supplied and regulated at the level of inositol phosphate biosynthesis, i.e. MIPS. Likewise, a transmembrane orientation for MIPS places the enzyme in a pivotal position from which to regulate both membrane and non-membrane-bound aspects of the complex metabolic flux of inositol. It has been known for some time that MIPS is coordinately regulated with membrane-bound phospholipid biosynthetic enzymes in Brewer's yeast (Carmen and Henry, 1999) even though there is no available evidence that suggests that this regulation is mediated through the physical interaction of MIPS with these enzymes. Others have also found the expression of MIPS in membrane-bound cellular compartments. Wong et al. (1987) detected MIPS expression and activity in the walls of all the vascular elements including cerebral capillaries of bovine brain, whereas Yoshida et al. (1999) discovered MIPS transcripts in the scutellum and aleurone layers of rice embryos.
CONCLUSIONS
On the basis of these and other data (Lackey et al., 2002), we propose that a complex repertoire of cellular mechanisms functions to spatially and temporally control inositol phosphate biosynthesis during plant growth and development. Tagged Arabidopsis MIPS genes will be used to test this hypothesis and to question the role(s) of MIPS in inositol-signaling events.
MATERIALS AND METHODS
Plant and Yeast Growth Conditions
Bean (Phaseolus vulgaris) seeds (Taylor's horticultural variety) were purchased from Asgrow Seed Co. (Kalamazoo, MI) and were grown aseptically in agar medium containing a Murashige and Skoog salt base without inositol (Sigma-Aldrich, St. Louis) in an environmental chamber maintained at 24°C with 16-h photoperiods. Wild-type Brewer's yeast (Saccharomyces cerevisiae) strain SH 477 (Mat a, ura3), was grown at 30°C.
Protein Isolations and Western-Blot Analyses
Soluble plant and yeast proteins were isolated, and protein concentrations were determined as previously described, respectively (Johnson and Henry, 1989; Johnson and Wang, 1996). MIPS partial purification included the preparation of crude extracts, streptomycin sulfate precipitations, and ammonium sulfate fractionations (Johnson and Wang, 1996). Brewer's yeast polyclonal antibody to MIPS was used for western blotting as detailed previously (Johnson and Henry, 1989; Johnson and Wang, 1996).
Microscopic Analyses of Tissues Harvested Eight Days after Germination
For immunohistochemical studies, bean roots and leaves were fixed, embedded in paraffin blocks, sectioned on a New World 820 microtome, and stained with 1% (w/v) toluidine blue O (Sigma-Aldrich) to block autofluorescence. After overnight incubation with MIPS antibody (1:500 dilution), sections were incubated with goat anti-rabbit IgG (whole-molecule) fluorescein isothiocyanate-conjugated secondary antibody (1:100 dilution; Sigma-Aldrich), mounted in Mowiol (Calbiochem, San Diego) and photographed with a confocal microscope (PCM 2000, Nikon, Tokyo). Controls included unstained sections not treated with primary or secondary antibody to detect autofluorescence and stained sections incubated only with secondary antibody to detect nonspecific interactions.
Bean roots subjected to immunocytochemistry were fixed, embedded in Lowcryl K4M (Polysciences, Warrington, PA) as described (Altman et al., 1984), and transferred to capsules for polymerization over a 15-W black-light UV lamp at a distance of 10 cm for 45 min at 4°C. After sectioning, samples were placed on 300-mesh formvar coated nickel grids, blocked, incubated (first, with MIPS primary antibody [1:500 dilution] and then goat anti-rabbit 10-nm gold-conjugated secondary antibody [1:100 dilution] for 16 h at room temperature), viewed, and photographed with a microscope (10A TEM, Zeiss, Jena, Germany). Primary antibody was omitted from control grids to determine the amount of nonspecific binding by the gold-conjugated secondary antibody.
Isolation of Organelles from Eight-Day-Old Plants and Enzyme Assays
Plastids were isolated from roots using two successive Percoll gradients, rinsed, solubilized, and assayed for purity and enrichment (Robinson and Barnett, 1988). Chloroplasts were purified from leaves and cotyledons as detailed previously (Johnson and Wang, 1996). Mitochondria were harvested from green and non-green tissues using the procedures of Dounce et al. (1987) and Moore and Proudlove (1987), respectively. Mitochondrial fractions from roots were isolated from two sequential 28% (v/v) Percoll gradients, whereas fractions from green tissues were subjected to two successive discontinuous Percoll gradients. Purified mitochondria were washed, solublized, and assayed for purity and intactness.
Microsomes, endoplasmic reticula, and plasma membranes were collected as described (Surowy and Sussman, 1986). Purified organelles were dialyzed against gradient buffer and concentrated (concentrator solution, Pierce, Rockford, IL). Yeast microsomes, a negative control, were extracted (Carman and Fischl, 1992), washed, and assayed for MIPS expression and activity.
Catalase (EC 1.11.1.6) and cytochrome C oxidase (EC 1.9.3.1) activities were monitored as described (Johnson and Wang, 1996). Antimycin-insensitive NADH cytochrome C reductase (EC 1.6.99.3) activity (Briskin et al., 1987) and nitrite reductase (EC 1.7.7.1) activity (Wray and Fido, 1989) were assayed spectrophotometrically. Vanadate-sensitive Ca2+ ATPase (EC 3.6.1.38) activity was identified using the procedure of Surowy and Sussman (1986). MIPS (EC 5.5.1.4) activity was assayed by the end-product method (Chen and Charalampous, 1966) and the rapid colorimetric method (Barnett et al., 1970). d-[1-14C]Glc 6-phosphate (specific activity 60.3 mCi mmol-1) and [1,2-3H]myoinositol (specific activity 370–740 GBq mmol-1) were obtained from PerkinElmer Life Sciences (Boston). Glc 6-phosphate, bacterial alkaline phosphatase, and phosphate standard were purchased from Sigma-Aldrich.
Isolation of Phaseolus MIPS Genomic Clone and Expression Studies
A bean MIPS cDNA (Wang and Johnson, 1995) was used to isolate two genomic clones from an indexed BAC library. DNA sequencing of one clone, both strands, was performed at the Iowa State University DNA Sequencing Facility (Ames, IA). Southern-blot analyses (Sambrook et al., 1989) and RT-PCR (Promega, Madison, WI) were performed according to instructions.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We are indebted to Dr. Susan Henry, Dr. Patricia McGraw, Dr. Sally Mackenzie, and Dr. Janis O'Donnell for yeast strains, antibody, bean BAC clones, and valuable discussion, respectively.
This work was supported by the National Science Foundation (grant no. MCB–9724117 to M.D.J.).
References
- Altman LG, Schneider BG, Papermaster DS (1984) Rapid embedding of tissue in lowicryl K4M for immunoelectron microscopy. J Histochem Cytochem 32: 1217-1223 [DOI] [PubMed] [Google Scholar]
- Arias AM, Brown AMC, Brennan K (1999) Wnt signalling: pathway or network? Curr Gen Dev 9: 447-454 [DOI] [PubMed] [Google Scholar]
- Bachhawat N, Mande SC (2000) Complex evolution of the inositol-1-phosphate synthase gene among archaea and eubacteria. Trends Genet 16: 111-113 [DOI] [PubMed] [Google Scholar]
- Barnett JEG, Brice RE, Corina DL (1970) A colorimetric determination of inositol monophosphates as an assay for d-glucose-6-phosphate-1l-myo-inositol-1-phosphate cyclase. Biochem J 119: 183-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin P, Leonard RT, Hodge TK (1987) Isolation of plasma membrane: membrane markers and general principles. Methods Enzymol 148: 542-558 [Google Scholar]
- Carman GM, Fischl AS (1992) Phosphatidylinositol synthase from yeast. Methods Enzymol 209: 305-312 [DOI] [PubMed] [Google Scholar]
- Carmen GM, Henry SA (1999) Phospholipid biosynthesis in the yeast Saccharomyces cerevisiae and interrelationship with other metabolic processes. Prog Lipid Res 38: 361-399 [DOI] [PubMed] [Google Scholar]
- Chen IW, Charalampous FC (1966) Biochemical studies on inositol: IX. d-inositol 1-phosphate as intermediate in the biosynthesis of inositol from glucose 6-phosphate, and characteristics of two reactions in this biosynthesis J Biol Chem 241: 2194-2199 [PubMed] [Google Scholar]
- Daniel W (1995) Tukey's honestly significant difference (HSD) test. In J Wiley, ed, Biostatistics: A Foundation for Analysis in the Health Science. John Wiley & Sons, New York, pp 295-591
- Donahue TF, Henry SA (1981) myo-Inositol-1 phosphate synthase. J Biol Chem 256: 7077-7085 [PubMed] [Google Scholar]
- Dounce R, Bourguignon J, Brouquisse R, Neuburger M (1987) Isolation of plant mitochondria: general principles and criteria of integrity. Methods Enzymol 148: 403-415 [Google Scholar]
- Gillaspy GE, Keddie JS, Oda K, Gruissem W (1995) Plant inositol monophosphatase is a lithium-sensitive enzyme encoded by a multigene family. Plant Cell 7: 2175-2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb LD (1982) Conservation and duplication of isozymes in plants. Science 216: 373-380 [DOI] [PubMed] [Google Scholar]
- Irvine RF, Schell MJ (2001) Back in the water: the return of the inositol phosphates. Nat Rev Mol Cell Biol 5: 327-338 [DOI] [PubMed] [Google Scholar]
- Johnson MD, Burk DH (1995) Isozyme of 1l-myo-inositol 1-phosphate synthase from Arabidopsis. Plant Physiol 109: 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Sussex LM (1995) 1l-myo-inositol 1-phosphate synthase from Arabidopsis thaliana. Plant Physiol 107: 613-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD (1994) The Arabidopsis thaliana myo-inositol phosphate synthase (EC 5.5.1.4). Plant Physiol 105: 1023-1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Henry SA (1989) Biosynthesis of inositol in yeast. J Biol Chem 264: 1274-1283 [PubMed] [Google Scholar]
- Johnson MD, Wang X (1996) Differentially expressed forms of 1-l-myo-inositol-1-phosphate synthase (EC 5.5.1.4) in Phaseolus vulgaris. J Biol Chem 271: 17215-17218 [DOI] [PubMed] [Google Scholar]
- Lackey KH, Pope PM, Johnson MD (2002) Biosynthesis of inositol phosphate in organelles of Arabidopsis thaliana. Southern Association of Agricultural Scientists Bull Biochem Biotechnol 15: 8-15 [Google Scholar]
- Loewus FA, Loewus MW (1983) myo-Inositol: its biosynthesis and metabolism. Annu Rev Plant Physiol 34: 132-161 [Google Scholar]
- Loewus FA, Murthy PPN (2000) myo-Inositol metabolism in plants. Plant Sci 150: 1-19 [Google Scholar]
- Majumder AL, Johnson MD, Henry SA (1997) 1l-myo-inositol-1-phosphate synthase. Biochim Biophys Acta 1348: 245-256 [DOI] [PubMed] [Google Scholar]
- Moore AL, Proudlove MO (1987) Isolation of plant mitochondria: general principles and criteria of integrity. Methods Enzymol 148: 403-415 [Google Scholar]
- Nakai K, Horton P (1999) PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci 24: 34-35 [DOI] [PubMed] [Google Scholar]
- Robinson C, Barnett LK (1988) Isolation and analysis of chloroplasts. In CH Show, ed, Plant Molecular Biology: A Practical Approach. IRL Press, Washington, DC, pp 67-78
- Sambrook JE, Fritsch F, Maniatis T (1989) Plasmid Vectors. In N Ford, C Nolan, M Ferguson, eds, Molecular Cloning. A Laboratory Manual, Ed 2, Vol 1. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, p 1.46 [Google Scholar]
- Sherman WR, Stewart M, Zinbo M (1969) Mass spectrometric study on the mechanism of d-glucose 6 phosphate-l-myo-inositol 1-phosphate cyclase. J Biol Chem 244: 5703-5708 [PubMed] [Google Scholar]
- Surowy TS, Sussman MS (1986) Immunological cross-reactivity and inhibitor sensitivities of the plasma membrane H+-ATPase from plants and fungi. Biochim Biophys Acta 848: 24-34 [Google Scholar]
- Wang X, Johnson MD (1995) An isoform of 1l-myo-inositol 1-phosphate synthase (EC 5.5.1.4) from Phaseolus vulgaris. Plant Physiol 110: 336 [Google Scholar]
- Weiler RJ, Hofstra D, Szentivanyi A, Blaisdell R, Talmage DW (1960) The inhibition of labeled antigen precipitation as a measure of γ-globulin. J Immunol 85: 130-137 [PubMed] [Google Scholar]
- Wong YHH, Kalmbach SJ, Hartman BK, Sherman WR (1987) Immunohistochemical staining and enzyme activity measurements show myo-inositol-1-phosphate synthase to be localized in the vasculature of brain. J Neurochem 48: 1434-1444 [DOI] [PubMed] [Google Scholar]
- Wray JL, Fido RJ (1989) Nitrate reductase and nitrite reductase. In PJ Lea, ed, Methods in Plant Biochemistry. Academic Press, London, pp 241-253
- York JD, Odom AR, Murphy R, Ives EB, Wente SR (1999) A phospholipase C dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science 285: 96-100 [DOI] [PubMed] [Google Scholar]
- Yoshida KT, Wada T, Koyama H, Mizobuchi-Fukuoka R, Naito S (1999) Temporal and spatial patterns of accumulation of the transcript of myo-inositol 1-phosphate synthase and phytin-containing particles during seed development in rice. Plant Physiol 119: 65-72 [DOI] [PMC free article] [PubMed] [Google Scholar]