Abstract
The Chlamydomonas reinhardtii cia3 mutant has a phenotype indicating that it requires high-CO2 levels for effective photosynthesis and growth. It was initially proposed that this mutant was defective in a carbonic anhydrase (CA) that was a key component of the photosynthetic CO2-concentrating mechanism (CCM). However, more recent identification of the genetic lesion as a defect in a lumenal CA associated with photosystem II (PSII) has raised questions about the role of this CA in either the CCM or PSII function. To resolve the role of this lumenal CA, we re-examined the physiology of the cia3 mutant. We confirmed and extended previous gas exchange analyses by using membrane-inlet mass spectrometry to monitor16O2,18O2, and CO2 fluxes in vivo. The results demonstrate that PSII electron transport is not limited in the cia3 mutant at low inorganic carbon (Ci). We also measured metabolite pools sizes and showed that the RuBP pool does not fall to abnormally low levels at low Ci as might be expected by a photosynthetic electron transport or ATP generation limitation. Overall, the results demonstrate that under low Ci conditions, the mutant lacks the ability to supply Rubisco with adequate CO2 for effective CO2 fixation and is not limited directly by any aspect of PSII function. We conclude that the thylakoid CA is primarily required for the proper functioning of the CCM at low Ci by providing an ample supply of CO2 for Rubisco.
Carbonic anhydrase (CA) catalyzes the reversible hydration of CO2 to bicarbonate (Eq. 1).
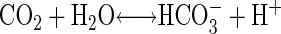 |
This enzyme is important for both photosynthesis and respiration, together with other reactions requiring carboxylation or decarboxylation. Multiple CAs are thought to be necessary for the proper functioning of some CO2-concentrating mechanisms (CCMs) by assisting in the accumulation of HCO3- within the cell and by localized dehydration of this pool to generate CO2 for use by the enzyme Rubisco in photosynthesis (Badger and Price, 1994; Raven, 1997; Kaplan and Reinhold, 1999; Moroney and Somanchi, 1999; Badger and Spalding, 2000; Moroney et al., 2001). In eukaryotic microalgae like Chlamydomonas reinhardtii, a periplasmic CA may assist in the diffusion of CO2 across the cellular membrane into the cytosol, although the results of Van and Spalding (1999) suggest that this may be of limited importance.
Within the cell, CO2 is converted to bicarbonate, possibly by another CA (Moroney et al., 1985; Sültemeyer et al., 1989, 1991; Palmqvist et al., 1994). Bicarbonate and/or CO2 in the cytosol are then transported or diffuse into the chloroplast stroma where the high pH favors formation of bicarbonate. Because Rubisco can only catalyze the fixation of CO2, any bicarbonate in the chloroplast must be dehydrated before it can be used for photosynthesis, and an additional CA is thought to be needed for this process to avoid CO2 limitation of Rubisco (Pronina and Semenenko, 1990; Pronina and Borodin, 1993; Raven, 1997). C. reinhardtii and many other microalgae have their Rubisco localized within the chloroplast in a structure called the pyrenoid. If a chloroplast-localized CA is randomly distributed throughout the stroma, then any CO2 generated away from Rubisco could easily diffuse back out of the chloroplast, reducing the efficiency of the CCM as was demonstrated in cyanobacteria (Price and Badger, 1989). Therefore it has been suggested that a CA may be specifically located within the pyrenoid (Badger et al., 1998).
Twenty years ago, the first high CO2-requiring mutant designated ca-1-12-1C (gene locus CA1) of C. reinhardtii was isolated from light-sensitive, acetate-requiring mutants (Spreitzer and Mets, 1981; Spalding et al., 1983). Physiological studies revealed that this mutant has reduced internal CA activity, a high photosynthetic CO2 compensation point, oxygen-sensitive photosynthesis, and high rates of glycolate production and that it accumulates internal inorganic carbon (Ci; Spalding et al., 1983). These data were interpreted as showing that the mutant is photosynthetically impaired due to a limitation in CO2 availability for the enzyme Rubisco. The impairment was attributed to a defective CCM caused by the loss of an internal CA, thereby identifying the first CCM-related protein in C. reinhardtii. The ca-1-12-1C mutant was later shown to be a knockout in the cah-3 gene (Funke et al., 1997), which is the same gene that is defective in the cia3 mutant in our study (Moroney et al., 1986; Karlsson et al., 1998). Additional physiological and immunological studies have corroborated the results of Spalding et al. (1983) and have demonstrated the presence of a chloroplast-localized CA (Husic et al., 1989; Sültemeyer et al., 1990; Husic and Marcus, 1994; Karlsson et al., 1995; Sültemeyer et al., 1995).
In the late 1990s, the Cah3 or ctCA1 cDNA was sequenced, and its polypeptide was identified as an α-type CA located within the lumen of thylakoid membrane in the chloroplast (Karlsson et al., 1998). Additional studies demonstrated that this CA copurifies with photosystem II (PSII; Park et al., 1999; Villarejo et al., 2002). Because the cia3 mutant has reduced PSII activity at low Ci levels, it has been hypothesized that the absence of a thylakoid CA may cause photosynthesis to be limited by PSII function rather than by CO2 utilization. Two papers have been published within the last year to support this hypothesis. Villarejo et al. (2002) clearly demonstrated that purified thylakoids from this mutant require bicarbonate for maximum function of the water-oxidizing complex of PSII. They also showed that cia3 cells have twice as many PSII complexes as the wild type, but only enough Mn2+ to supply one-half of the PSII cores with complete Mn clusters. Finally, their work on intact cells showed that the mutant is sensitive to high-light treatments in agreement with the original observations of Spalding et al. (1983). van Hunnik and Sültemeyer (2002) also studied purified thylakoid membranes and found that mutant preparations were impaired in ATP synthesis compared with the wild type. These new results have reinforced the idea that the thylakoid CA is not important for the CCM. Instead, it affects PSII activity or the proton gradient.
Our aim was to test the two competing hypotheses for the in vivo role of the thylakoid CA and to determine whether it is impaired PSII or thylakoid function rather than CO2 availability for Rubisco that limits photosynthesis in the cia3 mutant at low Ci levels. We were able to directly measure PSII function in vivo using gas-inlet mass spectrometry to monitor gross 16O2 evolution and to compare this with simultaneous measurements of net CO2 uptake and net O2 evolution and with quantum yields of PSI (ΦPSI) and PSII (ΦPSII). We also measured metabolite pool sizes to determine whether RuBP regeneration or use was limiting as Ci levels decline in the media. This is the first study, to our knowledge, to directly measure PSII function in intact cells of cia3 (instead of using fluorescence) and to compare this with other aspect of photosynthesis.
RESULTS
Effect of External Ci Concentration on Photosynthesis
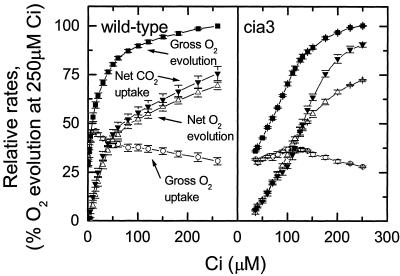
We measured the water splitting activity of PSII in vivo by monitoring gross16O2 evolution from wild-type and cia3 mutant C. reinhardtii cells, and we compared this with net CO2 uptake, gross O2 uptake, and net O2 evolution. In Ci draw-down experiments (Fig. 1), when Ci was near 200 μm, net O2 evolution and gross O2 uptake were a similar proportion of gross O2 evolution in both the mutant and wild type, although net CO2 uptake was proportionally greater in the mutant. As Ci decreased, gross O2 uptake initially increased and then decreased in both cell types, but the inflection occurred at higher Ci concentrations in the mutant. In addition, wild-type cells reached a minimum external Ci concentration near 1 μm compared with about 35 μm for the mutant. Maximum rates of net O2 evolution at saturating Ci and 300 μmol m-2 s-1 irradiance were similar in the mutant and wild type. The differential Ci responses between the wild type and cia3 mutant shown in Figure 1 are consistent with the previous phenotypes of both the cia3 and ca-12-1-C mutants (Spalding et al., 1983; Moroney et al., 1986). However, one notable difference between the experiments reported here and previous studies are that these physiological differences are apparent in cultures grown only at elevated CO2 (4%), rather than needing the imposition of a induction period of growth at low CO2 to discern clear differences.
Figure 1.
Rates of gross O2 evolution (▪), gross O2 uptake (○), net O2 evolution (▵), and net CO2 uptake (▾) in response to external Ci concentration. Data are expressed as a percentage of the gross O2 evolution rate at 250 μm Ci (wild type = 2.6 μmol mg-1 Chl min-1, cia3 = 1.6 μmol mg-1 Chl min-1). Cultures were concentrated by centrifugation and resuspended to a final concentration of 15 μg Chl mL-1 in 20 mm HEPES, pH 7.0, and 30 μg mL-1 CA. Starting conditions were 400 μm Ci, 300 μmol photons m-2 s-1, and 20°C. Measurements were initiated by switching on the light and allowing the cells to draw down the external Ci and were continued until the Ci compensation point was reached. The rates at various Ci concentrations were collected from the continuous draw-down experiments, n = 3.
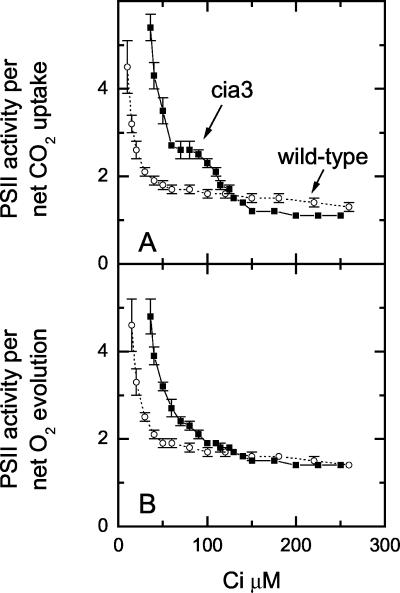
Using data from Figure 1, we calculated the ratio of PSII activity (measured as the rate of gross 16O2 evolution) to net CO2 uptake rate (Fig. 2A) and to net O2 evolution rate (Fig. 2B), and we plotted these ratios versus Ci concentration in the media. Both cia3 and wild-type cells have similar amounts of PSII activity relative to net CO2 uptake and net O2 evolution above 100 μm Ci. However, below 100 μm Ci, the cia3 mutant has more PSII activity per net CO2 uptake and per net O2 evolution than the wild type with a greater proportion of electrons being used for O2 uptake processes.
Figure 2.
PSII activity (measured as gross O2 evolution rate) divided by net CO2 uptake (A) or net O2 evolution (B) rate for wild-type (○) or mutant (▪) cells in response to external Ci concentration. Data are derived from Figure 1.
Effect of External Ci Concentration on Metabolite Pools
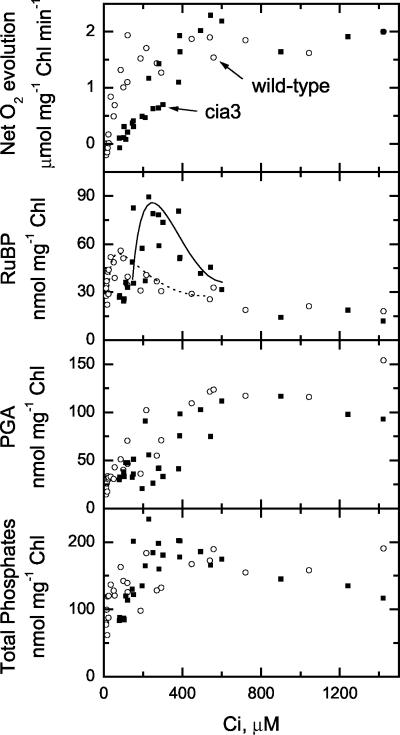
We rapidly killed cells during a Ci-draw-down curve such as that shown in Figure 1, and we assayed for ribulose-1,5-bisphosphate (RuBP), ribulose-5-phosphate (Ru-5-P), and 3-phosphoglyceric acid (PGA) content over a range of net O2 evolution and external Ci concentrations. The level of Ru-5-P was near the limit of detection, but no differences between mutant or wild-type cells were found, and we did not observe pool size changes for this metabolite among the samples we assayed (data not shown). However, RuBP pool sizes initially increased and then decreased as external Ci decreased (Fig. 3). The maximum pool sizes occurred near 250 μm Ci for cia3 cells and around 50 μm Ci for the wild type. Between 200 and 600 μm Ci, RuBP pool sizes were higher in the mutant than the wild type, although above 600 μm Ci, pool sizes were similar. PGA pool sizes showed roughly the inverse pattern seen for RuBP, thereby maintaining a relatively constant total phosphate pool size (determined as 2*RuBP+PGA) above 200 μm Ci (Fig. 3). Total phosphate pool sizes were similar between cia3 and wild-type cells, and pool sizes declined for both cell types below 200 μm Ci.
Figure 3.
Photosynthetic rates and metabolite pools in response to external Ci concentration for wild-type (○) or mutant (▪) cells. Cultures were concentrated by centrifugation and were resuspended to a final concentration of 15 μg Chl mL-1 in 20 mm HEPES pH 7.0 and 30 μg mL-1 CA. Ci draw-down experiments were conducted similar to those shown in Figure 1, and cell samples were killed by rapidly drawing them into a syringe containing trifluoroacetic acid (TFA; 10% final concentration) at a particular Ci concentration. A number of samples at different Ci levels could be collected from a single Ci drawn-down experiment. All measurements were made at 300 μmol m-2 s-1 light and 20°C.
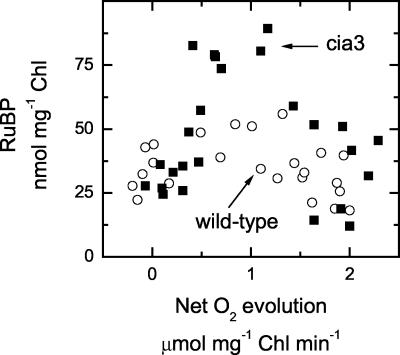
We also plotted data from Figure 3 to express RuBP pool size across a range of net O2 evolution rates. At low and high rates of net O2 evolution, RuBP pool sizes are similar between cia3 and wild-type cells (Fig. 4). However, at intermediate net O2 evolution rates, RuBP pool sizes were larger in the mutant than the wild type. This corresponds with the range of external Ci concentrations where net O2 evolution is lower and RuBP pool sizes are higher in the mutant than in the wild type.
Figure 4.
RuBP pool size versus photosynthetic rate (expressed as net O2 evolution) for wild-type (○) or mutant (▪) cells. Data are taken from the values presented in Figure 3.
Oxygen Sensitivity of Photosynthesis
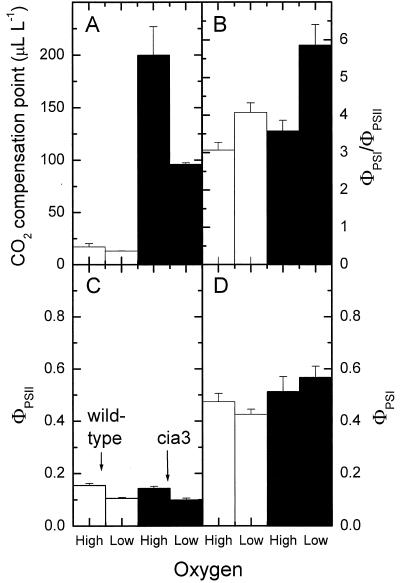
We measured the CO2 compensation point and the ΦPSI and ΦPSII at 2% and 21% O2. The CO2 compensation point for wild-type cells was very low (near 20 μL L-1) and similar at both oxygen levels (Fig. 5A). In contrast, the cia3 mutants had a compensation point around 200 μL L-1 at 21% O2 and around 95 μL L-1 at 2% O2. The ratio of ΦPSI to ΦPSII was higher at low O2 for both cell lines (Fig. 5B). Interestingly, the ΦPSI/ΦPSII ratios were 3 or above for both wild type and mutant, reaching up to near 6 for the mutant at low O2. The reasons for this are not clear, but these ratios at the CO2 compensation point are clearly higher than those obtained at saturating Ci (1–1.5; Fig. 6). This may indicate a greater engagement of cyclic electron flow under limiting carbon conditions. The high ΦPSI/ΦPSII at low O2 was primarily due to a low ΦPSII (Fig. 5C), although ΦPSII was similar for both wild-type and cia3 cells. ΦPSI was also similar between the wild type and mutant with both remaining essentially constant at both high and low O2 (Fig. 5D).
Figure 5.
Oxygen sensitivity of the CO2 compensation point (A) and the ΦPSII (C) and ΦPSI (D) for wild-type (white columns) or cai3 mutant (black columns) cells. The ΦPSI/ΦPSII is presented in B. Cultures were concentrated onto a glass fiber filter by gentle centrifugation and were measured in the gas phase at 2% (Low) and 21% (High) O2, 300 mol m-2 s-1 light, and 20°C in the presence of CA.
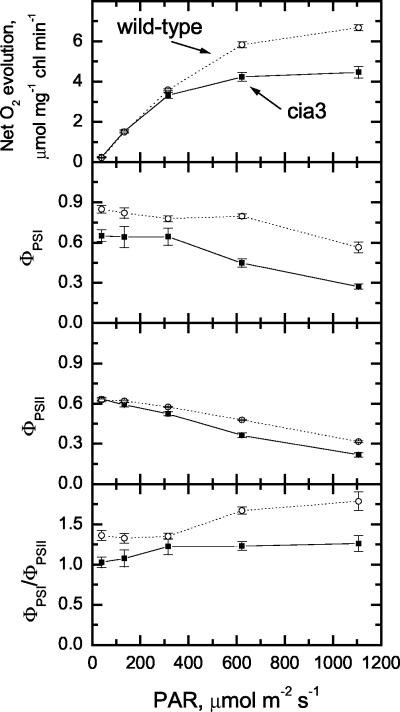
Figure 6.
Light response of photosynthesis and the ΦPSI and ΦPSII in wild-type (○) and mutant (▪) cells. Cultures were centrifuged and resuspended in 20 mm HEPES, pH 7.0, to a final concentration of 15 μg Chl mL-1. Ci levels were maintained around 2 mm Ci, no CA was added, and the measurement temperature was 20°C, n = 3.
Light Response of Photosynthesis
We measured the net O2 evolution rate, ΦPSI, and ΦPSII for wild-type and cia3 cultures across a range of light levels while maintaining about 2 mm Ci in the media. Net O2 evolution was similar for the wild type and cia3 mutant when light levels were equal to or lower than 300 μmol m-2 s-1 (Fig. 6). However, at 600 μmol m-2 s-1 and above, net O2 evolution was much lower than in the wild type. Because these measurements were made in a closed chamber, O2 levels in the solution ranged from about 5 μm at the start of the measurements (low light) to about 450 μm at the end (high light; data not shown), and this or the long assay period needed to complete each dataset, may have affected the mutant and wild-type cells differently. Villarejo et al. (2002) found no difference between wild-type and cia3 cells at high light (for short exposures), but Spalding et al. (1983) found a reduced maximum rate in ca-1-12-1C mutants grown at high light and low CO2. ΦPSI was lower in the mutant, especially at or above 600 μmol m-2 s-1, however, ΦPSII was similar between mutant and wild-type cells (Fig. 6). These low ΦPSI values resulted in a low ratio of ΦPSI to ΦPSII in the cia3 mutants at most light intensities measured.
DISCUSSION
We have confirmed and extended previous gas exchange analyses of the C. reinhardtii mutant cia3 (which lacks a CA in the thylakoid lumen) by using membrane-inlet mass spectrometry to simultaneously monitor16O2,18O2, and CO2 fluxes in vivo. Consistent with previous studies, our results show that at low Ci, PSII activity (gross O2 evolution), CO2 uptake, net O2 evolution, and gross O2 uptake rates in the mutant are reduced relative to the wild type. In addition, wild-type cells were able to consume almost all of the Ci in the surrounding media, whereas cia3 cells could only draw down Ci to about 35 μm. However, net CO2 uptake exceeded net O2 evolution quite significantly in the cia3 above 150 μm Ci (Fig. 1), and this may be due to the excessive Ci accumulation (5-fold higher than wild type) that is characteristic of the ca-1-12-1C mutant (Spalding et al., 1983). However, the different phenotypes observed here, although consistent with the originally reported phenotype of the mutant, were obtained with cells grown at 4% CO2 without the need for a low-CO2 induction period to enhance the differences between wild type and mutant. The fact that these differences are apparent with noninduced cells indicates that the thylakoid CA contributes to the Ci utilization efficiency of the high-CO2-grown wild type.
Interestingly, gross O2 uptake rates reached maximum levels around 10 μm Ci for wild-type cells and 100 μm for mutant cells (Fig. 1). Changes in gross O2 uptake in the light are heavily influenced by changes in Rubisco oxygenation, so it is likely that Rubisco in mutant cells experience a higher ratio of O2 to CO2 at a much higher external Ci level than the wild type. The extra O2 sensitivity of the mutant is also supported by a higher CO2 compensation point, a large effect of O2 concentration on the CO2 compensation point in the mutant (Fig. 5A), and by the larger amounts of glycolate produced by the mutant observed by others (Spalding et al., 1983). The subsequent decrease in gross O2 uptake seen at very low Ci is most likely due to deactivation of Rubisco through decarbamylation of the active site, although declining RuBP levels may play a part. All of these data suggest that the absence of a CA in the thylakoid lumen results primarily in an impaired CCM, which supports CO2 fixation by Rubisco. However they do not exclude the possibility of reduced PSII or a limited capability for ATP regeneration in addition to a less effective CCM as has been suggested by previous studies (van Hunnik and Sültemeyer, 2002; Villarejo et al., 2002).
To use our gas exchange data to differentiate between PSII activity and CO2 utilization, we also determined the ratio of PSII activity to net CO2 uptake and net O2 evolution. If PSII activity is limiting photosynthesis in the mutant when external Ci is low, then PSII activity per net CO2 uptake or per net O2 evolution should be lower in the mutant than the wild type. However, the opposite was true. At low Ci, the PSII activity per net CO2 uptake and O2 uptake is equal to or higher than in the wild type (Fig. 2). Therefore, there is excess PSII activity for photosynthetic CO2 uptake in the mutant at low Ci.
Although gas exchange data from the mass spectrometer demonstrated that PSII activity is not limiting photosynthesis at low Ci, it was insufficient to assess the possibility of ATP limitation as suggested by van Hunnik and Sültemeyer (2002). To address this question, we measured metabolite pools sizes across a range of external Ci concentrations. ATP is consumed at two steps during the regeneration of RuBP, for the conversion of PGA to triose-phosphate and for the conversion of Ru-5-P to RuBP. ATP is also consumed during the partial recovery of phosphoglycolate produced during photorespiration. Therefore any limitation in ATP production should reduce the pool size of RuBP and increase the PGA pool. RuBP levels in the mutant were generally higher than in the wild type (Fig. 3) except at very low Ci levels (below 100 μm). The highest levels of RuBP only occur transiently at low Ci and/or low photosynthetic rates and do not represent a consistently larger metabolite pool size in the mutant (Figs. 3 and 4). This is consistent with the interpretation that as Ci declines, the ability to use RuBP is more limiting for photosynthesis than the ability to regenerate it. Therefore, it is likely that ATP synthesis is not the primary limitation for photosynthesis at low Ci as suggested by van Hunnik and Sültemeyer (2002). If chloroplastic ATP synthesis is impaired in the mutant, then the mitochondria must be able to compensate. Interestingly, RuBP levels decline rapidly as Ci levels and net O2 evolution near zero in both mutant and wild type. Although this could be interpreted as a due to a decline in PSII function and reducing power availability, we interpret this to be loss to glycolate excretion and a general down-regulation of photosynthesis that is occurring at very low Ci. These data are supported by the studies of Spalding et al. (1983), who showed that at low Ci, the mutant had elevated RuBP levels and that in air, glycolate is the major product of photosynthesis (90% of which is excreted) and by Kaplan and Berry (1981), who demonstrated that glycolate excretion increases dramatically as Ci becomes limiting for photosynthesis in wild-type cells.
In considering the results obtained here, it is worthwhile to consider the potential similarities and differences between the cia3 mutant examined here and the ca-1-12-1C mutant originally described by Spalding et al. (1983) and pleiotropic effects that may contribute to different phenotypes. When the ca-1-12-1C was originally analyzed and concluded to be a potential CO2 fixation mutant (Spreitzer and Mets, 1981), their preliminary studies revealed no alterations in PSI, PSII, or chlorophyll (Chl) and no other pleiotropic biochemical alterations. Moroney et al. (1986) also made it clear that the cia3 mutant was not apparently light sensitive in its growth at elevated CO2. Since these initial studies, it is probably wise to be aware that subsequent mutant strains representing these mutants could accumulate various pleiotropic alterations from additional mutations that could contribute to variable phenotypes. In this respect, it is important to note that the cia3 mutant described here does have a phenotype consistent with an impaired ability to use CO2 efficiently for photosynthesis.
The molecular basis of the cia3 mutant is biochemically different from the ca-1-12-1C mutant. Whereas the ca-1-12-1C mutant results from a nonsense mutation that eliminates the CA protein (Funke et al., 1997), the equivalent cia3 gene was found to contain two mutations in the region containing the chloroplast transit peptide (Karlsson et al., 1998). The presence of two point mutations in this same region would be a very rare event, indicating that one of the mutations may have been selected to due to some other deleterious effect of the first substitution. More importantly, the cia3 mutant synthesizes normal levels of the mature CA protein (Karlsson et al., 1998). However, despite any mutation in the mature coding region, the enzyme appeared to lack activity and labeling by an active site photoaffinity label, and the authors conclude that this must be due to mistargeting, misfolding, or incorrect cleavage of the mature protein. Considering this information, it is impossible to be absolutely certain that the ca-1-12-1C and the cia3 mutants should have exactly the same phenotypes.
Our in vivo results clearly demonstrate that in the cia3 mutant, the apparent loss of a CA activity in the thylakoid lumen impairs the ability of the CCM to supply CO2 to Rubisco at low Ci and not by limiting PSII function. However, there is strong evidence from thylakoid and PSII preparations that PSII function is somehow impaired. Villarejo et al. (2002) clearly demonstrated that there are nearly twice as many PSII reaction centers in the mutant cells and that addition of bicarbonate greatly increased the activity of cia3 PSII particle preparations and water oxidation complex (WOC) activity. Despite these gross differences in PSII content and activity, we were unable to detect meaningful differences from wild type in ΦPSII and only a small reduction in ΦPSI/ΦPSII in the mutant (Figs. 5 and 6). We also found similar rates of PSII activity (gross O2 evolution) at 1 mm Ci. Therefore, we believe that much of the excess PSII in the mutant is actually inactive. This is supported by the finding of Villarejo et al. (2002) that there is not enough manganese in the mutant to generate complete water-oxidizing complexes for each PSII center.
It is particularly intriguing that the WOC activity increases when PSII preparations of the cia3 mutant are treated with 1 mm bicarbonate (Villarejo et al., 2002) but that the 12 mm bicarbonate that can accumulate in the mutant in vivo (Spalding et al., 1983) does not increase PSII activity. It is possible that instead of activating PSII, bicarbonate may be protecting the WOC from excess damage. Under conditions of high light, protons generated by the WOC can accumulate locally and lower the pH, which can disrupt the Mn cluster (Virgin et al., 1988; Kramer et al., 1999). If a CA were in the same vicinity, it would catalyze the dehydration of bicarbonate, thereby consuming protons locally and simultaneously generating CO2 for Rubisco. This mechanism would also help to drive bicarbonate transport across the thylakoid membrane by creating a lumenal sink for bicarbonate. This is likely to be necessary because charged species like bicarbonate do not easily diffuse across membranes. However, even if bicarbonate can diffuse across the thylakoid membrane, it is unlikely that the uncatalyzed dehydration of bicarbonate in the lumen would be sufficient to protect the WOC because it is theoretically incapable of providing enough CO2 for Rubisco (Raven, 1997).
Villarejo et al. (2002) also found that the cia3 mutant was severely impaired by a 60-min high-light treatment but that the short-term light response was not different from wild-type cells. However, our results show reduced activity in the mutant above 600 μmol m-2 s-1 (Fig. 6). The propensity for cia3 mutant cells toward lower photosynthesis at high light even in the presence of 1 to 2 mm Ci is consistent with the need for a CA to catalyze the removal of local protons away from the WOC by dehydrating bicarbonate. In this context, it makes sense that the differences in PSII function seen in thylakoid and PSII preparations are only apparent in vivo under high light and not low Ci conditions. Under high light, generation of protons by the WOC activity of PSII would be maximized, and the need for a rapid supply of CO2 to Rubisco would be high.
The connection of the PSII-associated lumenal CA (Cah3 or ctCA1) and the functioning of the CCM remains to be established. The simplest possible explanation is that bicarbonate transported into the lumen is used to generate CO2 and that this can elevate CO2 around Rubisco. Several scenarios for where CO2 may be elevated have been previously explored and included either the pyrenoid or the whole chloroplast (Raven, 1997; Badger et al., 1998). Why this CA needs to be associated with PSII is unclear. Perhaps it originated as a mechanism of photoprotection for PSII and was subsequently capitalized on by the chloroplast to evolve a CCM through the development of effective diffusion barriers that would restrict CO2 efflux. However, it may also be possible to envisage dual targeting of the protein, with specific association with both the PSII complex and a region such as the pyrenoid, and it is the loss of pyrenoid CA activity that is responsible for the phenotype observed here.
MATERIALS AND METHODS
Algal Strains and Culture Conditions
The Chlamydomonas reinhardtii cell wall-less mutant 15 (cw15-CC-400) was obtained from the Chlamydomonas culture collection at Duke University. The cell wall-less mutant is a standard strain used in photosynthetic studies and is referred to as the wild type in this paper. The high-CO2 requiring, cell wall-less double mutant (cia3) was obtained from J. V. Moroney (Louisiana State University, Baton Rouge; Moroney et al., 1986). Cells were cultured at 18°C and 130 μmol m-2 s-1 light on a 16-h/8-h day/night cycle in minimal media (Sueoka, 1960) bubbled with 4% CO2. It should be noted that cells were not pre-adapted to low CO2 in any of the experiments and that the photosynthetic responses of the wild type (see Fig. 1) probably reflect the presence of a basal level of CCM activity.
Gas Exchange
CO2 and O2 concentrations were monitored in stirred aqueous suspensions of C. reinhardtii using an isotope ratio mass spectrometer (IsoPrime-EA, Micromass, Manchester, UK) attached to custom built thermostatted cuvettes with semipermeable plastic membrane inlets (Hoch and Kok, 1963; Radmer and Ollinger, 1980; Badger and Andrews, 1982). Cultures were concentrated immediately before measurement using low-speed centrifugation at 2,000g for 60 s and resuspended to a final concentration of 15 μg Chl mL-1 in 20 mm HEPES, pH 7.0, and 30 μg mL-1 CA. Ci levels in the liquid phase were measured by monitoring the CO2 level with the mass spectrometer and assuming that CO2 was in rapid equilibrium with HCO3- due to CA being present. Light was provided via a 1.5-cm diameter fiber optic cable attached to a KL1500 Schott lamp (Walz, Effeltrich, Germany) for 2- and 4-mL chambers and by a projector lamp for a 20-mL glass chamber. Light intensity was controlled with neutral density filters. When measuring 16O2 evolution and18O2 uptake, the assay buffer was sparged with N2 and degassed before the addition of18O2 in the cuvette. This method was used to directly measure16O2 evolution from the oxygen-evolving complex of PSII (Mehler and Brown, 1952; Badger, 1985). The CO2 compensation point was also determined via mass spectrometry in the gas phase by concentrating cells directly onto a 1.23 cm2 glass fiber filter disc to a final density of 15 μg Chl cm-2. The cell-covered disc was placed in a gas-tight cuvette after adding 20 μL of a 1 μg μL-1 solution of CA. The chamber was quickly flushed with N2, and the desired levels of O2 and CO2 were immediately added. The CO2 concentration in the cuvette was monitored in the light until there was no net CO2 uptake, and this value was taken as the compensation point.
Chl a Fluorescence and P700 Absorbance Measurements
The ΦPSII and ΦPSI were determined simultaneously using two pulse-modulated fluorometers (PAM 101, Walz) attached to a multibranched fiber optic cable (as described by Siebke et al., 1997; Hanson et al., 2002). ΦPSII was calculated as the change in fluorescence (F) in the light divided by the maximum fluorescence in the light (F′m - Fs)/F′m; Genty et al., 1989) and ΦPSI was calculated from the observed change in absorbance at 830 nm (A830) in the light divided by the maximum absorbance change in the dark (Asat - A)/(Amax - A0; Klughammer and Schrieber, 1994; Siebke et al., 1997). A high-intensity far-red light flash (2-s pulse of 200 μmol m-2 s-1 generated with a KL1500 Schott lamp and 3-mm-thick RG9 filter) provided before the saturating white light flash in the dark was optimal (data not shown) for maximum oxidation of PSI in C. reinhardtii (see Siebke et al., 1997). Quantum yields were measured simultaneously with gas exchange measurements in the mass spectrometer cuvette, although liquid stirring was stopped for 2 s before each saturating flash to reduce noise.
Metabolite Assays
RuBP, PGA, and Ru-5-P pool sizes were determined from actively photosynthesizing C. reinhardtii cells rapidly killed by addition of TFA (von Caemmerer et al., 1983). Photosynthetic rate and Ci concentration were measured in a 20-mL glass cuvette as described above for aqueous cell suspensions at 20°C and a light intensity of 300 μmol photons m-2 s-1. At each Ci concentration, 4 mL of cells was rapidly drawn into a syringe containing 300 μL of TFA (7% final concentration) and immediately placed on ice. Samples were then centrifuged for 5 min at 4,000g, and the supernatant was dried using a SpeedVac concentrator (ThermoSavant, Holbrook, NY). The dried pellet was resuspended in 200 μL of milli-Q water, and the pH was neutralized to between 5 and 6 using 2 n KHCO3. Two 100- to 150-μL aliquots from each sample were centrifuged for 5 min (14,000g) and assayed for RuBP, PGA, and Ru-5-P in a 1-mL spectrophotometric assay (Badger et al., 1984), and the results of each pair were averaged. The assay buffer consisted of 100 mm Epps, pH 8.2 (pH 8.0 after sample addition), 20 mm MgCl2, 10 mm NaHCO3, 5 mm dithiothreitol, 150 μm NADH, 5 mm phosphocreatine, 6 IU of creatine phosphokinase, 6 IU of 3-phosphoglycerate kinase, 5 IU of glyceraldehyde-3-phosphate dehydrogenase, 5 IU of triose-phosphate isomerase, 5 IU of glycerol-3-phosphate dehydrogenase, and 25 μg of CA. Metabolites were assayed by the sequential addition of 2 mm ATP for PGA, 50 μg of pre-activated tobacco (Nicotiana tabacum) Rubisco for RuBP, and 2 IU of spinach (Spinacia oleracea) phosphoribulokinase for Ru-5-P.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Susanne von Caemmerer and John Andrews (Australian National University) for their help with the metabolite assays. We are also grateful for the fruitful discussions with Arsenio Villarejo (University of Umeå, Sweden), Eddy van Hunnik (Universidad National Autonoma de Mexico), and Dieter Sültemeyer (Universität Kaiserslautern, Germany). We acknowledge the contributions of two anonymous reviewers and Dr. Robert Spreitzer for suggested alterations to the final manuscript.
References
- Badger MR (1985) Photosynthetic oxygen-exchange. Annu Rev Plant Physiol 36: 27-53 [Google Scholar]
- Badger MR, Andrews TJ (1982) Photosynthesis and inorganic carbon usage by the marine cyanobacterium, Synechococcus sp. Plant Physiol 70: 517-523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD (1998) The diversity and co-evolution of Rubisco, plastids, pyrenoids and chloroplast-based CCMs in the algae. Can J Bot 76: 1052-1071 [Google Scholar]
- Badger MR, Price GD (1994) The role of carbonic anhydrase in photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 45: 369-392 [Google Scholar]
- Badger MR, Sharkey TD, von Caemmerer S (1984) The relationship between steady-state gas exchange of bean leaves and the levels of carbon-reduction-cycle intermediates. Planta 160: 305-313 [DOI] [PubMed] [Google Scholar]
- Badger MR, Spalding MH (2000) CO2 acquisition, concentration and fixation in cyanobacteria and algae. In S von Caemmerer, ed, Photosynthesis: Physiology and Metabolism. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 369-397
- Funke RP, Kovar JL, Weeks DP (1997) Intracellular carbonic anhydrase is essential to photosynthesis in Chlamydomonas reinhardtii at atmospheric levels of CO2: demonstration via genomic complementation of the high-CO2-requiring mutant ca-1. Plant Physiol 114: 237-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker N (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87-92 [Google Scholar]
- Hanson D, Andrews TJ, Badger MR (2002) Variability of the pyrenoid-based CO2 concentrating mechanism in hornworts (Anthocerotophyta). Funct Plant Biol 29: 407-416 [DOI] [PubMed] [Google Scholar]
- Hoch G, Kok B (1963) A mass spectrometer inlet system for sampling gases dissolved in liquid phases. Arch Biochem Biophys 101: 160-170 [DOI] [PubMed] [Google Scholar]
- Husic HD, Kitayama M, Togasaki RK, Moroney JV, Morris KL, Tolbert NE (1989) Identification of intracellular carbonic-anhydrase in Chlamydomonas reinhardtii which is distinct from the periplasmic form of the enzyme. Plant Physiol 89: 904-909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husic HD, Marcus CA (1994) Identification of intracellular carbonic-anhydrase in Chlamydomonas reinhardtii with a carbonic anhydrase-directed photoaffinity label. Plant Physiol 105: 133-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A, Berry JA (1981) Glycolate excretion and the oxygen to carbon dioxide net exchange ratio during photosynthesis in Chlamydomonas reinhardtii. Plant Physiol 67: 229-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A, Reinhold L (1999) CO2 concentrating mechanisms in photosynthetic microorganisms. Annu Rev Plant Physiol Plant Mol Biol 50: 539-570 [DOI] [PubMed] [Google Scholar]
- Karlsson J, Clarke AK, Chen ZY, Hugghins SY, Park YI, Husic HD, Moroney JV, Samuelsson G (1998) A novel alpha-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J 17: 1208-1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J, Hiltonen T, Husic HD, Ramazanov Z, Samuelsson G (1995) Intracellular carbonic-anhydrase of Chlamydomonas reinhardtii. Plant Physiol 109: 533-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klughammer C, Schrieber U (1994) An improved method, using saturated light pulses, for determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 192: 261-268 [Google Scholar]
- Kramer DM, Sacksteder CA, Cruz JA (1999) How acidic is the lumen? Photosynth Res 60: 151-163 [Google Scholar]
- Mehler AH, Brown AH (1952) Studies on reactions of illuminated chloroplasts. III. Simultaneous photoproduction and consumption of oxygen studied with oxygen isotopes. Arch Biochem Biophys 38: 365-370 [DOI] [PubMed] [Google Scholar]
- Moroney JV, Bartlett SG, Samuelsson G (2001) Carbonic anhydrases in plants and algae. Plant Cell Environ 24: 141-153 [Google Scholar]
- Moroney JV, Husic HD, Tolbert NE (1985) Effect of carbonic anhydrase inhibitors on inorganic carbon accumulation by Chlamydomonas reinhardtii. Plant Physiol 79: 117-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney JV, Somanchi A (1999) How do algae concentrate CO2 to increase the efficiency of photosynthetic carbon fixation? Plant Physiol 119: 9-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney JV, Tolbert NE, Sears BB (1986) Complementation analysis of the inorganic carbon concentrating mechanism of Chlamydomonas reinhardtii. Mol Gen Genet 204: 199-203 [Google Scholar]
- Palmqvist K, Yu J-W, Badger MR (1994) Carbonic anhydrase activity and inorganic carbon fluxes in low- and high-Ci cells of Chlamydomonas reinhardtii and Scenedesmus obliquus. Physiol Plant 90: 537-547 [Google Scholar]
- Park YI, Karlsson J, Rojdestvenski I, Pronina N, Klimov V, Oquist G, Samuelsson G (1999) Role of a novel photosystem II-associated carbonic anhydrase in photosynthetic carbon assimilation in Chlamydomonas reinhardtii. FEBS Lett 444: 102-105 [DOI] [PubMed] [Google Scholar]
- Price GD, Badger MR (1989) Expression of human carbonic anhydrase in the cyanobacterium Synechococcus PCC7942 creates a high CO2-requiring phenotype. Plant Physiol 91: 505-513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronina N, Borodin VV (1993) CO2 stress and CO2 concentration mechanism: investigation by means of photosystem-deficient and carbonic anhydrase-deficient mutants of Chlamydomonas reinhardtii. Photosynthetica 28: 515-522 [Google Scholar]
- Pronina NA, Semenenko VE (1990) Membrane-bound carbonic anhydrase takes part in CO2 concentration in algal cells. In M Baltscheffsky, ed, Current Research in Photosynthesis, Vol 4. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 489-492 [Google Scholar]
- Radmer RJ, Ollinger O (1980) Light-driven uptake of oxygen, carbondioxide, and bicarbonate by the green-alga Scenedesmus. Plant Physiol 65: 723-729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA (1997) CO2-concentrating mechanisms: a direct role for thylakoid lumen acidification? Plant Cell Environ 20: 147-154 [Google Scholar]
- Siebke K, von Caemmerer S, Badger MR, Furbank RT (1997) Expressing an RbcS antisense gene in transgenic Flaveria bidentis leads to an increased quantum requirement for CO2 fixed in photosystems I and II. Plant Physiol 115: 1163-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding MH, Spreitzer RJ, Ogren WL (1983) Carbonic anhydrase-deficient mutant of Chlamydomonas reinhardtii requires elevated carbon-dioxide concentration for photoautotrophic growth. Plant Physiol 73: 268-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreitzer RJ, Mets L (1981) Photosynthesis-deficient mutants of Chlamydomonas reinhardtii with associated light-sensitive phenotypes. Plant Physiol 67: 565-569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N (1960) Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardtii. Proc Nat Acad Sci USA 46: 83-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sültemeyer D, Amoroso G, Fock H (1995) Induction of intracellular carbonic-anhydrases during the adaptation to low inorganic carbon concentrations in wild-type and ca-1 mutant-cells of Chlamydomonas reinhardtii. Planta 196: 217-224 [Google Scholar]
- Sültemeyer DF, Fock HP, Canvin DT (1990) Mass-spectrometric measurement of intracellular carbonic-anhydrase activity in high and low Ci cells of Chlamydomonas: studies using18O exchange with13C/18O labeled bicarbonate. Plant Physiol 94: 1250-1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sültemeyer DF, Fock HP, Canvin DT (1991) Active uptake of inorganic carbon by Chlamydomonas: evidence for a simultaneous transport of HCO3- and CO2 and characterisation of active transport. Can J Bot 69: 995-1002 [Google Scholar]
- Sültemeyer DF, Miller AG, Espie GS, Fock HP, Canvin DT (1989) Active CO2 transport by the green algae Chlamydomonas reinhardtii. Plant Physiol 89: 1213-1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van K, Spalding MH (1999) Periplasmic carbonic anhydrase structural gene (Cah1) mutant in Chlamydomonas reinhardtii. Plant Physiol 120: 757-764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hunnik E, Sültemeyer D (2002) A possible role for carbonic anhydrase in the lumen of chloroplast thylakoids in green algae. Funct Plant Biol 29: 243-249 [DOI] [PubMed] [Google Scholar]
- Villarejo A, Shutova T, Moskvin O, Forssen M, Klimov VV, Samuelsson G (2002) A photosystem II-associated carbonic anhydrase regulates the efficiency of photosynthetic oxygen evolution. EMBO J 21: 1930-1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin I, Styring S, Andersson B (1988) Photosystem II disorganization and manganese release after photoinhibition of isolated spinach thylakoid membranes. FEBS Lett 233: 408-412 [Google Scholar]
- von Caemmerer S, Coleman JR, Berry JA (1983) Control of photosynthesis by RuP2 concentration: studies with high- and low-CO2 adapted cells of Chlamydomonas reinhardtii. Carnegie Inst Wash Year Book 82: 91-95 [Google Scholar]