Abstract
To elucidate the cellular functions of phospholipase A2 in plants, an Arabidopsis cDNA encoding a secretory low molecular weight phospholipase A2 (AtsPLA2β) was isolated. Phenotype analyses of transgenic plants showed that overexpression of AtsPLA2β promotes cell elongation, resulting in prolonged leaf petioles and inflorescence stems, whereas RNA interference–mediated silencing of AtsPLA2β expression retards cell elongation, resulting in shortened leaf petioles and stems. AtsPLA2β is expressed in the cortical, vascular, and endodermal cells of the actively growing tissues of inflorescence stems and hypocotyls. AtsPLA2β then is secreted into the extracellular spaces, where signaling for cell wall acidification is thought to occur. AtsPLA2β-overexpressing or -silenced transgenic plants showed altered gravitropism in inflorescence stems and hypocotyls. AtsPLA2β expression is induced rapidly by auxin treatment and in the curving regions of inflorescence stems undergoing the gravitropic response. These results suggest that AtsPLA2β regulates the process of cell elongation and plays important roles in shoot gravitropism by mediating auxin-induced cell elongation.
INTRODUCTION
Phospholipase A2 (PLA2) hydrolyzes glycerophospholipids at the sn-2 position to yield free fatty acids and lysophospholipids. PLA2s have been implicated in diverse cellular responses in animal systems, including bioactive lipid production, host defense, and signal transduction (Li-Stiles et al., 1998; Zhang et al., 1999). PLA2 activities also have been implicated in important cellular processes in plants, such as lipid metabolism (Chapman, 1998; May et al., 1998), defense signaling (Munnik et al., 1998; Roos et al., 1999), and auxin-stimulated cell growth (Scherer and Arnold, 1997; Paul et al., 1998). Auxin induces a rapid increase of free fatty acid (FFA), lysophosphatidylcholine (LPC), and lysophosphatidylethanolamine (LPE) levels in microsomes, which suggests that PLA2 may be activated during auxin-stimulated cell growth (Scherer, 1996; Paul et al., 1998). Furthermore, the auxin-dependent elongation of hypocotyl segments is inhibited by treatment with PLA2 inhibitors (Scherer and Arnold, 1997), suggesting that PLA2 may be involved in auxin signaling leading to cell elongation.
The lysophospholipid LPE was found to retard senescence in leaves, flowers, and fruit (Farag and Palta, 1993a; Kaur and Palta, 1997) and to accelerate fruit ripening (Farag and Palta, 1993b) when applied as a spray. LPE inhibits phospholipase D, a key enzyme in promoting the membrane deterioration that leads to plant senescence (Ryu et al., 1997). PLA2 also has been suggested to be involved in plant defense responses to wound stress and pathogen elicitors (Creelman and Mullet, 1997; Lee et al., 1997; Chapman, 1998; Senda et al., 1998; Narváez-Vásquez et al., 1999). The activation of oxylipin-based chemical defenses in diatoms is initiated by PLA2 activity (Pohnert, 2002). During embryo maturation, PLA-like activities have been implicated in the selective removal of unusual fatty acids from phospholipids in the endoplasmic reticulum for their reincorporation into triacylglycerols (Ståhl et al., 1995, 1998, 1999).
Despite the potentially significant physiological roles of PLA2 in plants, little is known about the nature of plant secretory low molecular weight PLA2 (sPLA2) enzymes and genes. To date, sPLA2 activity in plants has been demonstrated only in more or less purified preparations (Ståhl et al., 1998, 1999), and putative sPLA2 genes were cloned recently from carnation and rice (Kim et al., 1999; Ståhl et al., 1999). A molecular understanding of these enzymes is necessary for the delineation of their cellular functions. To gain insight into the cellular functions of sPLA2, we cloned a sPLA2 gene from Arabidopsis (AtsPLA2β) and generated transgenic Arabidopsis plants that silence AtsPLA2 expression as a result of RNA interference (RNAi) or overexpress AtsPLA2. Here, we report that AtsPLA2β plays important roles in cell elongation and shoot gravitropism in Arabidopsis.
RESULTS
Molecular Cloning and Sequence Analysis of Arabidopsis PLA2 cDNA
Using the previously published N-terminal amino acid sequence of an elm PLA2 (Ståhl et al., 1998) as a query, we identified and cloned an Arabidopsis sPLA2 cDNA. AtsPLA2β is located on the long arm of chromosome II and is registered as a hypothetical protein (At2g19690). We found that its coding region was predicted incorrectly and have registered the correct cDNA nucleotide sequence with the National Center for Biotechnology Information. The deduced amino acid sequence of the encoded AtsPLA2β protein is shown in Figure 1. The 590-bp clone includes a 441-bp open reading frame between nucleotides 20 and 459, which encodes a protein of 147 amino acids with a predicted molecular weight of 16,288 and a pI of 8.23 (Figure 1).
Figure 1.
Amino Acid Sequence Comparison of AtsPLA2β with Other Plant sPLA2s and Snake Venom sPLA2 (Group IA).
The cleavage sites for the removal of N-terminal signal peptides as predicted by the pSORT program are indicated by closed triangles. Residues that are conserved completely among the five sequences are indicated by black boxes, and partially conserved residues are indicated by gray boxes. The Ca2+ binding domain and the active site motif of animal sPLA2s and the corresponding domain sequences in the plant sPLA2s are underlined. The Cys residues that are conserved completely between venom sPLA2, AtsPLA2β, and the three putative plant sPLA2s are indicated by open triangles.
Sequence analysis revealed that AtsPLA2β contains catalytic motif sequences identified in animal sPLA2s, such as the Ca2+ binding loop and active site (Figure 1) (White et al., 1990). The active site motif of AtsPLA2β contains a well-conserved His/Asp dyad (Figure 1), indicating that AtsPLA2β belongs to a class of PLA2 proteins that use a catalytic His according to Six and Dennis (2000). Disulfide bridges are important for PLA2 protein stability; the number of bridges is used as a criterion to classify animal PLA2s. The AtsPLA2β amino acid sequence includes 12 Cys residues that potentially can form six intramolecular disulfide bridges. Seven of the 12 Cys residues in AtsPLA2β align well with Cys in cobra venom sPLA2 (Figure 1).
Of the putative plant sPLA2s that have been reported, AtsPLA2β shares overall amino acid identities of 46, 30, and 28% with rice sPLA2 isoform I, rice sPLA2 isoform II, and carnation sPLA2, respectively. However, in a 33–amino acid sequence that contains the catalytic motifs, the identity becomes 84, 81, and 75%, respectively. This result indicates that AtsPLA2β is related most closely to the putative rice sPLA2 isoform I.
Signal Peptide and the Mature Form of AtsPLA2β Expressed in Escherichia coli
Sequence analysis using the pSORT program (http://psort.nibb.ac.jp.ggoa/) predicted that AtsPLA2β, like animal sPLA2s, has a potential N-terminal signal peptide (Figure 1). The predicted cleavage site of the signal peptide lies between Ser-28 and Glu-29, which would result in a mature protein of 119 amino acids. A fusion protein (DsbC-sPLA2) containing the mature form of AtsPLA2β was produced in E. coli and purified from the resulting cell-free extract (Figure 2A). The free mature AtsPLA2β protein was purified after enzymatic dissociation from the DsbC fusion protein and purification (Figure 2A, lane 2). The molecular mass of mature AtsPLA2β was ∼13 kD, which is in good agreement with the calculated molecular mass of 13.2 kD (pI = 7.7).
Figure 2.
Production of Recombinant AtsPLA2β and Thin Layer Chromatography Analysis of Its Hydrolytic Activity.
(A) Recombinant mature AtsPLA2β proteins were separated by 12% SDS-PAGE and stained with Coomassie blue. Lane 1, DsbC-AtsPLA2β fusion proteins purified from crude extracts of recombinant E. coli and digested with the enzyme rEK; lane 2, free mature form of AtsPLA2β isolated from the mixture shown in lane 1 with the S-Tag rEK purification kit.
(B) Thin layer chromatography analysis of the hydrolytic activity of recombinant AtsPLA2β. The substrate PC is hydrolyzed into LPC and FFA by PLA2 activity. Lane 1, snake venom sPLA2; lane 2, DsbC purified from E. coli transformed with the vector pET40; lanes 3 and 4, DsbC-AtsPLA2β fusion proteins purified from E. coli transformed with pET40 containing the full-length AtsPLA2β cDNA. The total amount of protein loaded in lane 4 was twice the amount loaded in lane 3.
Functional Analysis of Recombinant AtsPLA2β
To determine if the recombinant protein is functional, both the unprocessed and mature forms of AtsPLA2β were tested for enzymatic activity. The fusion protein containing AtsPLA2β was capable of hydrolyzing the substrate phosphatidylcholine (PC) into two products that comigrate with the LPC and FFA standards (Figure 2B, lanes 3 and 4). Commercial snake venom sPLA2 completely hydrolyzed the substrate into the same products (Figure 2B, lane 1), whereas the protein DsbC alone exhibited only background levels of hydrolysis activity (Figure 2B, lane 2). The specific activity of the free mature form of AtsPLA2β was determined to be 530 nmol·min−1·mg−1 protein, which is twice as great as that of its free unprocessed form. The free mature form of AtsPLA2β still was active after 5 min of boiling treatment but lost 37% of its original activity after treatment with 5 mM DTT.
The specificity of AtsPLA2β with respect to the sn position in PC was determined by incubating the free mature form with three different substrates (Table 1). Radioactive LPC was not detected among reaction products when the protein was incubated with 1-palmitoyl-2-14C-palmitoyl-PC or 1-palmitoyl-2-14C- linoleoyl-PC, indicating that the enzyme does not act at the sn-1 position of PC (Table 1). When 1,2-14C-dipalmitoyl-PC was used as a substrate, similar levels of radioactive carbon were measured in the LPC and FFA spots. These results suggest that AtsPLA2β catalyzes hydrolysis at the sn-2 position; therefore, it is a PLA2 and not a PLA1 enzyme. At the sn-2 position, AtsPLA2β protein hydrolyzed palmitoyl acyl chains at a higher rate than linoleoyl acyl chains (Table 1).
Table 1.
sn Specificity and Acyl Preference of the E. coli–Expressed Mature Enzyme of AtsPLA2β for the Substrate PC
| Percent of Input 14C Recovered as
|
||
|---|---|---|
| Substrate | LPC | FFA |
| 1-Palmitoyl-2-14C-palmitoyl-PC | 1.90 ± 0.10 | 73.40 ± 1.75 |
| 1-Palmitoyl-2-14C-linoleoyl-PC | 1.50 ± 1.20 | 62.20 ± 2.20 |
| 1,2-14C-Dipalmitoyl-PC | 34.60 ± 0.10 | 48.70 ± 0.29 |
Values shown are means ± SE obtained from three independent in vitro assays (n = 3).
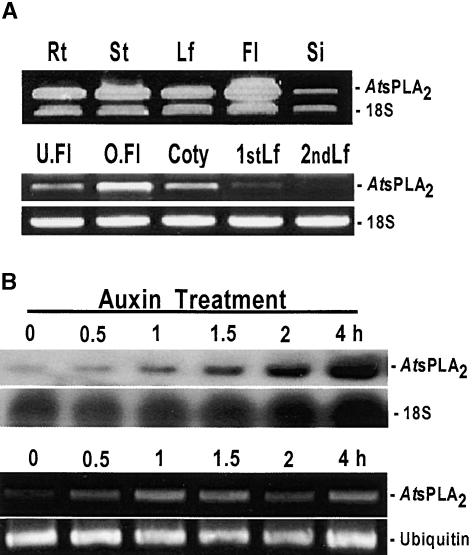
Tissue-Specific and Auxin-Induced Expression of the AtsPLA2β Transcript
To investigate the expression of the AtsPLA2β gene in different plant organs, we isolated total RNA from Arabidopsis roots, stems, leaves, flowers, and siliques. Because we were unable to detect sPLA2 transcripts in any of these tissues by RNA gel blot analysis (data not shown), we used quantitative reverse transcriptase–mediated (RT) PCR to amplify cDNA from low-abundance sPLA2 mRNA. The concomitant amplification of an 18S rRNA served as an internal standard. The ratio 1:9 (rRNA primer: competitor) allowed nearly equal amplification of sPLA2 cDNA and rRNA cDNA (Figure 3A, top gels), indicating that AtsPLA2β transcripts are extremely rare. AtsPLA2β transcripts were detected at similar levels in Arabidopsis roots, stems, leaves, and siliques but at higher levels in flowers (Figure 3A, top). The AtsPLA2β transcription levels also depended on the developmental stage (Figure 3A, bottom gels). A higher level of AtsPLA2β expression was detected in open flowers than in unopened flowers, and cotyledons expressed more transcripts than the primary and secondary leaf tissues. We also examined whether the expression of the AtsPLA2β gene is responsive to auxin. Both RNA gel blot and RT-PCR analyses showed that AtsPLA2β expression was induced rapidly after treatment with 20 μM indoleacetic acid (Figure 3B). An increase in the amount of AtsPLA2β transcripts was detected within 30 min. Using 18S and ubiquitin levels as references, the results of RNA gel blot and RT-PCR analyses indicated that the induction of AtsPLA2β transcripts peaked after 1 to 1.5 h and remained steady over the 4-h auxin treatment (Figure 3B).
Figure 3.
Tissue-Specific and Auxin-Induced Expression of AtsPLA2β Transcripts.
(A) The top gel shows tissue-specific expression. Total RNA was isolated from the root (Rt), stem (St), leaf (Lf), flower (Fl), and silique (Si) tissues of 5-week-old plants, reverse transcribed, and used as the template for PCR with primers that amplify a 436-bp AtsPLA2β fragment and a 315-bp 18S rRNA fragment. The primers used to amplify the 18S fragment were a mixture of 18S primers and 18S competitors (1:9, v/v). Thirty-five cycles of RT-PCR were used to amplify both AtsPLA2β and 18S rRNA fragments. The bottom gels show developmental stage–specific expression. Total RNA was isolated from unopened flowers (U.Fl) and opened flowers (O.Fl) of 5-week-old plants and from cotyledons (Coty), primary leaves (1stLf), and secondary leaves (2ndLf) of 2-week-old plants. Thirty-five cycles of RT-PCR were used to amplify AtsPLA2β, and 20 cycles were used to amplify 18S fragments with 18S primers without 18S competitors.
(B) The top gels show RNA gel blot results. Total RNA was extracted from 10-day-old seedlings in a Petri dish at 0, 0.5, 1, 1.5, 2, and 4 h after treatment with 20 μM indoleacetic acid. The blot membrane with the AtsPLA2β probe was exposed to x-ray film for 15 days, and the blot membrane with the 18S rRNA probe was exposed for 1 day. The bottom gels show RT-PCR results with mRNA isolated from total RNA. Twenty-nine and 20 cycles of RT-PCR were used to amplify AtsPLA2β and ubiquitin fragments, respectively.
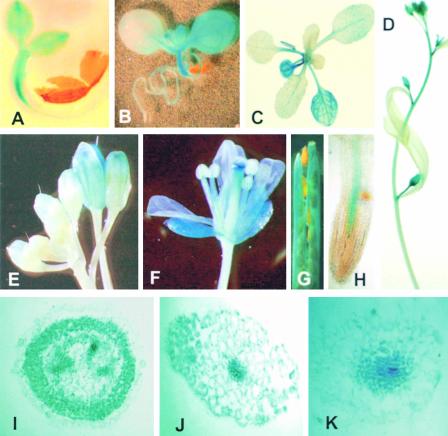
The tissue- and developmental stage–specific profile of the AtsPLA2β promoter activity was investigated using histochemical β-glucuronidase (GUS) assays. GUS activity was present in all tissues at the actively growing young stages of Arabidopsis seedlings (Figure 4A). When plants grew, however, AtsPLA2β expression was evident only in cotyledons, primary leaves, and hypocotyls (Figures 4B and 4C). The activity decreased in the secondary leaves, being detectable only in vascular tissues (Figure 4C). In the roots as well, expression was localized mostly in vascular tissues (Figures 4B and 4H). AtsPLA2β expression became highly active when floral shoots bolted, especially in the young or actively growing tissues of inflorescence stems (Figure 4D). AtsPLA2β promoter activity gradually was stimulated in opening flowers (Figures 4E and 4F), as shown by RT-PCR analysis (Figure 3A, bottom gels). In the full open floral tissues, expression was high in the filaments, styles, sepals, and pedicels but low or undetectable in petals, anthers, stigmas, and ovaries (Figure 4F). In the siliques, GUS staining was observed in the carpels, false septa, and pedicels but not in the maturing seeds (Figure 4G). Cross-sectional analyses showed strong AtsPLA2β promoter activity in the cortical, vascular, and endodermal tissues of inflorescence stems and hypocotyls (Figures 4I and 4J), but AtsPLA2β expression in the root was localized in the vascular tissues (Figure 4K).
Figure 4.
Histochemical Localization of GUS Activity in Arabidopsis (Col-0) Plants Transformed with the AtsPLA2β Promoter–GUS Construct.
(A) A 2-day-old seedling.
(B) A 6-day-old seedling.
(C) A 3-week-old plant.
(D) Inflorescence stems from a 4-week-old plant.
(E) and (F) Floral tissues from a 5-week-old plant.
(G) Silique tissues from a 6-week-old plant.
(H) Roots from a 4-week-old plant.
(I) Cross-section of the young inflorescence stem of a 5-week-old plant.
(J) Cross-section of the hypocotyl of a 10-day-old plant.
(K) Cross-section of the main root of a 2-week-old plant.
AtsPLA2β Is Secreted into Cell Wall/Extracellular Spaces
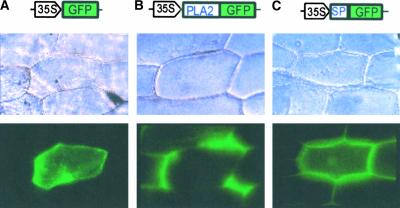
The identification of a putative signal peptide in AtsPLA2β indicates that, like animal sPLA2s, the enzyme is likely to be a secreted protein. Computer analysis with the pSORT program predicted that AtsPLA2β is located in the cell wall/extracellular space (certainty = 0.82) and in the vacuole (certainty = 0.41). To determine experimentally the subcellular localization of AtsPLA2β, we introduced vector constructs encoding AtsPLA2β–green fluorescent protein (GFP) fusion proteins into onion epidermal cells. In these vector constructs, GFP was fused either to the C terminus of AtsPLA2β or to the putative signal peptide alone, as indicated in Figure 5. Although the GFP control localized inside the cytoplasm (Figure 5A), the AtsPLA2β-GFP fusion protein accumulated densely in the interstices of the cell wall/extracellular space (Figure 5B). The signal peptide–GFP fusion protein also was observed in the intercellular space (Figure 5C), but with a more diffuse distribution compared with that of the AtsPLA2β-GFP fusion protein. This is probably the result of the high mobility of the soluble GFP protein after dissociation from the signal peptide during secretion. These observations suggest that expressed AtsPLA2β protein is secreted into the cell wall/extracellular space and that the secretion of the protein is mediated by the signal peptide.
Figure 5.
Subcellular Localization of AtsPLA2β Proteins.
GFP fusion constructs under the control of the 35S promoter of Cauliflower mosaic virus were introduced into onion epidermal cells by particle bombardment. A construct encoding only GFP was used as a control (A). GFP was fused in-frame to the C terminus of the full open reading frame of AtsPLA2β (B) or to the C terminus of the signal peptide sequence of AtsPLA2β (C). The top row depicts vector fusion constructs, the middle row depicts the onion cell structure observed by light microscopy, and the bottom row depicts the cytolocalization of GFP and GFP fusion proteins observed by fluorescence microscopy.
Upregulation or Downregulation of AtsPLA2β Expression Alters Shoot Gravitropism
To gain further insights into the cellular functions of AtsPLA2β, we generated transgenic Arabidopsis plants (ecotype Columbia [Col-0]) that overexpress AtsPLA2β or suppress AtsPLA2β expression as a result of RNAi-mediated silencing. In three independent AtsPLA2β-overexpressing and three independent AtsPLA2β-silenced T3 transgenic lines, RT-PCR and PLA2 activity assays demonstrated increased and reduced levels of gene expression and enzyme activity, respectively, compared with wild-type plants (Figures 6A and 6B). The inflorescence stems of wild-type and AtsPLA2β-overexpressing transgenic plants were analyzed for gravitropic responses (Figures 6C to 6F). After being placed horizontally, wild-type stems began to bend upward, curving >100° within 60 min, which is indicative of a strong negative gravitropic response (Figure 6C). By contrast, transgenic Arabidopsis plants constitutively expressing AtsPLA2β greatly reduced gravitropic responses (Figures 6D to 6F), whereas the plants transformed with the vector pBI121 showed gravitropic responses similar to those of wild-type plants (data not shown). Over the course of time (Figure 7A), the stems of transgenic plants constitutively expressing AtsPLA2β showed a significantly slower gravitropic response. Although wild-type stems bent by ∼50° at 30 min after gravistimulation, the stems of plants overexpressing AtsPLA2β showed little response (Figure 7A). Wild-type stems curved up to 125° from 90 to 120 min. After 2 h, they began to curve back, defining a final angle of 90° by 3 h. The stems of AtsPLA2β-overexpressing plants bent by 90° within 90 min but did not bend further (Figure 7A). Hence, from 3 h after gravistimulation, the stems of both wild-type and transgenic plants resumed vertical growth (Figure 7A).
Figure 6.
Gravitropic Responses of the Inflorescence Stems, Hypocotyls, and Roots of Transgenic Arabidopsis Plants (Col-0) That Overexpress AtsPLA2β or Silence AtsPLA2β Expression as a Result of RNAi Compared with Wild-Type Plants.
(A) and (B) Relative RT-PCR and PLA2 activity in the inflorescence stems of three 5-week-old independent T3 transgenic Arabidopsis lines that overexpress AtsPLA2β (A) and in the leaf tissues of three 2-week-old independent T3 transgenic Arabidopsis lines that suppress AtsPLA2β expression as a result of RNAi (B). Thirty-five and 20 cycles of RT-PCR were used to amplify AtsPLA2β and 18S rRNA fragments, respectively. WT, wild type.
(C) to (F) Gravitropic responses of the inflorescence stems of 5-week-old wild-type plants (C) and three independent T3 transgenic lines overexpressing AtsPLA2β ([D] to [F]) after 60 min of horizontal gravistimulation.
(G) to (L) Gravitropic responses of the hypocotyls and roots of 3-day-old dark-grown wild-type plants (G), two independent T3 transgenic lines that overexpress AtsPLA2β ([H] and [I]), and three independent T3 transgenic lines that suppress AtsPLA2β expression as a result of RNAi ([J] to [L]) after 26 h of horizontal gravistimulation.
Figure 7.
Time Course of the Gravitropic Response of the Inflorescence Stems of Wild-Type and Transgenic Arabidopsis Plants, and Gravitropic Response–Specific Activation of the AtsPLA2β Promoter in the Curving Regions of Inflorescence Stems.
(A) Data for wild-type (WT) plants (Col-0; open circles) and AtsPLA2β-overexpressing transgenic plants (T3 1-1 line; closed circles). Approximately 30 individual inflorescence stems were examined in each of four independent experiments. The vertical error bars represent se values.
(B) Histochemical staining in the inflorescence stems of transgenic Arabidopsis (Col-0) carrying the AtsPLA2β promoter–GUS construct at different stages of the gravitropic response. A control stem without gravistimulation (left) and stems with curvatures of 80° (middle) and 125° (right) are shown. The curving regions of the stems with strong GUS activity are indicated with arrows.
The hypocotyls of transgenic Arabidopsis seedlings that overexpress AtsPLA2β or suppress AtsPLA2β expression as a result of RNAi-mediated silencing also showed altered gravitropic responses compared with wild-type plants (Figures 6G to 6L). Although the hypocotyls of wild-type plants had bent almost vertically at 26 h after horizontal gravistimulation, the hypocotyls of AtsPLA2β-overexpressing or -silenced transgenic seedlings showed significantly slower gravitropic responses (Table 2). However, the roots of the transgenic seedlings showed more or less similar gravitropic responses to wild-type roots (Table 2).
Table 2.
Gravitropic Responses of the Hypocotyls and Roots of Transgenic Arabidopsis Plants That Overexpress AtsPLA2β or Silence AtsPLA2β Expression Compared with Wild-Type Plants
| Overexpressing
|
RNAi Silenced
|
|||||
|---|---|---|---|---|---|---|
| Organ | Wild Type | 1-1 | 6-19 | C-5 | E-1 | R-5 |
| Hypocotyl | 88.8 ± 1.8 | 74.3 ± 1.3a | 68.0 ± 4.0b | 67.5 ± 5.3b | 68.0 ± 3.0b | 69.0 ± 1.0b |
| Root | 76.4 ± 2.2 | 69.9 ± 2.3 | 76.1 ± 1.0 | 84.3 ± 1.2 | 78.7 ± 5.8 | 74.5 ± 1.7 |
Values shown are mean curvature degrees ± SE obtained from three independent experiments (n = 60). Data were collected 26 h after horizontal gravistimulation.
Mean values are statistically different (P < 0.005) from mean values in the wild type.
Mean values are statistically different (P < 0.001) from mean values in the wild type.
We investigated whether AtsPLA2β promoter activity is induced during the gravitropic response of the florescence stems using GUS assays. Strong GUS activity was observed in the curving regions of inflorescence stems undergoing the gravitropic response, indicating gravitropic response–specific activation of the AtsPLA2β promoter (Figure 7B).
Cell Elongation Rates Are Altered in Leaf Petioles and Inflorescence Stems of Transgenic Plants
Leaf petiole lengths and leaf sizes were enlarged (∼40 to 45%) in AtsPLA2β-overexpressing transgenic plants and reduced (∼20 to 30%) in AtsPLA2β-silenced transgenic plants compared with wild-type plants (Figures 8A and 8B). To determine whether the alterations in leaf size and petiole length in the transgenic plants resulted from different levels of cell division and/or cell elongation, we measured the sizes of epidermal and cortical cells by light microscopy. The epidermal and cortical cells were significantly longer longitudinally (∼40 to 50%) in AtsPLA2β-overexpressing transgenic plants and significantly shorter (∼30 to 45%) in AtsPLA2β-silenced plants compared with wild-type plants (Figure 8C, Table 3). Because cell elongation levels reflect well the differences in petiole lengths between wild-type and AtsPLA2β-overexpressing transgenic plants, the difference likely is attributable mainly to the different levels of cell elongation. In AtsPLA2β-silenced transgenic plants, however, the degree of cell elongation decreased more than the degree of petiole length, suggesting that the shortened cell lengths might be compensated for, in part, by other factors such as cell division.
Figure 8.
Comparison of the Growth of Inflorescence Stems and Leaf Petioles in Wild-Type and Transgenic Arabidopsis Plants.
Twenty-five-day-old Arabidopsis plants (A) and leaf petioles (B), and light micrographs of the cortical cells in longitudinal sections of leaf petioles (C). Wild-type Col-0 (left), AtsPLA2β-overexpressing T3 transgenic line 1-1 (middle), and AtsPLA2β-silenced T3 transgenic line C-5 (right) are shown. Bars = 100 μm.
Table 3.
Longitudinal Lengths of Epidermal and Cortical Cells in the Petioles and Inflorescence Stems of Transgenic Plants That Overexpress AtsPLA2β or Silence AtsPLA2β Expression Compared with Wild-Type Plants
| Petiole Cell Length (μm)
|
Stem Cell Length (μm)
|
|||
|---|---|---|---|---|
| Plant Type | Epidermal | Cortical | Epidermal | Cortical |
| Wild Type | 408.7 ± 35.7 | 234.9 ± 57.72 | 261.8 ± 50.0 | 59.3 ± 8.3 |
| Overexpressing | 568.7 ± 164.1a | 354.9 ± 60.56b | 405.9 ± 70.8b | 84.3 ± 4.8b |
| RNAi silenced | 273.5 ± 43.9b | 125.7 ± 37.14b | 225.5 ± 35.7 | 38.1 ± 7.6b |
Values shown are mean lengths ± SD (n = 20).
Mean values are statistically different (P < 0.002) from mean values in the wild type.
Mean values are statistically different (P < 0.0001) from mean values in the wild type.
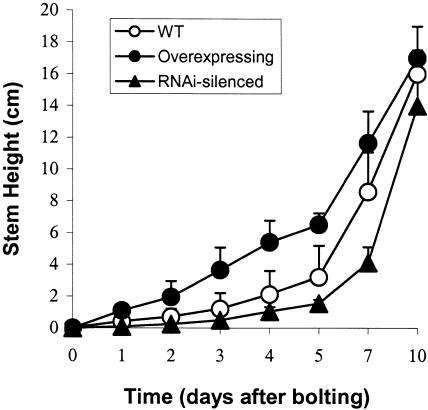
Transgenic plants overexpressing AtsPLA2β also exhibited greater inflorescence stem elongation, whereas transgenic plants with silenced AtsPLA2β expression showed retarded stem elongation compared with wild-type plants, although the developmental stages of inflorescence stems were not significantly different among wild-type and transgenic plants (Figure 8A). Microscopic analysis demonstrated that the epidermal and cortical cells in the actively growing zone of stems also were longitudinally longer in AtsPLA2β-overexpressing plants and shorter in AtsPLA2β-silenced transgenic plants compared with wild-type plants (Table 3). The difference in inflorescence stem lengths among wild-type and transgenic plants was diminished at the geometrical growth stages ∼10 days after bolting (Figure 9). These results suggest that the transient alterations in stem elongation result from differences in cell elongation rates and that AtsPLA2β plays important roles in the cellular process of cell elongation.
Figure 9.
Time Course of Longitudinal Growth in the Inflorescence Stems of Wild-Type and Transgenic Arabidopsis Plants.
Data for wild-type (WT) plants (Col-0; open circles), AtsPLA2β-overexpressing transgenic plants (T3 1-1 line; closed circles), and AtsPLA2β-silenced plants (T3 C-5 line; closed triangles). The vertical error bars represent sd values (n = 20).
DISCUSSION
We have identified an Arabidopsis cDNA encoding a sPLA2. The molecular mass of the mature form was calculated to be 13.2 kD, with a pI of 7.7. Although AtsPLA2β shares low overall amino acid identity (13 to 17%) with animal sPLA2s, catalytic motif regions of the protein—the Ca2+ binding loop and the active site—show greater identity (up to 55%). According to the criteria established for animal sPLA2s (Six and Dennis, 2000), AtsPLA2β is classified as a group-XI PLA2.
Several putative sPLA2 cDNAs have been cloned from the developing flowers of carnation and from rice (Kim et al., 1999; Ståhl et al., 1999). A Basic Local Alignment Search Tool (BLAST) search revealed that AtsPLA2β also shares sequence identity with other putative sPLA2 cDNAs from Populus, tomato, cotton, pine, soybean, and rice. It is worth noting that a full search of the Arabidopsis genome identified multiple sPLA2 genes (At2g06925, At4g29460, and At4g29470, which we named AtsPLA2α, AtsPLA2γ, and AtsPLA2δ, respectively). All of the putative enzymes encoded by these genes contain 12 conserved Cys residues, the highly conserved Ca2+ binding loop, and the active site motif. AtsPLA2β shares 31 to 57% overall identity with these Arabidopsis isoforms.
The 12 Cys residues in the mature AtsPLA2β protein may form six disulfide bonds, and the positions of these residues in AtsPLA2β are well conserved among plant sPLA2s. Most animal sPLA2s have six or seven disulfide bonds, although some have five or eight (Six and Dennis, 2000). The positioning of the Cys residues is partially conserved in spite of very low overall sequence identity. The Cys residues are known to play a critical role in the structural stability and enzymatic activity of animal sPLA2s (Six and Dennis, 2000; Valentin and Lambeau, 2000). AtsPLA2β was stable even after 5 min of boiling, but its activity decreased significantly upon treatment with a reducing agent.
Previous studies of animal sPLA2s have shown that the hydrolysis of phospholipids proceeds through the activation and coordination of a water molecule by hydrogen bonding to the active site His (Scott et al., 1990; Six and Dennis, 2000). An Asp residue provides the ligand for a crucial Ca2+ ion in forming a positively charged oxy-anion hole, stabilizing the negatively charged transition state of the PLA2 reaction. AtsPLA2β also contains the well-conserved His/Asp dyad and therefore belongs to the subgroup of PLA2s that use a catalytic His. Other conserved residues that are known to participate in hydrogen bonding networks, Tyr and Gly, also are found in the AtsPLA2β Ca2+ binding loop.
An acyl preference was observed for AtsPLA2β. AtsPLA2β hydrolyzed palmitoyl groups at a somewhat higher rate than linoleoyl groups. This property is distinct from that of developing elm seed sPLA2, which showed a preference for caproyl and oleoyl groups (Ståhl et al., 1998). The nucleotide sequence of the elm seed sPLA2 has not been determined, but the N-terminal amino acid sequence shares low identity (42%) with that of AtsPLA2β. Because AtsPLA2β is expressed predominantly in actively growing young seedlings and floral organs, these two sPLA2s may play distinctly different roles. The elm seed sPLA2 was suggested to be involved in the removal of unusual fatty acids from membrane lipids (Ståhl et al., 1998).
Although little is known about the distribution and functions of sPLA2s in plants (Ståhl et al., 1998, 1999), accumulating biochemical evidence suggests that plant PLA2s are implicated in many important cellular activities, including auxin-stimulated cell growth (Paul et al., 1998). AtsPLA2β is tissue- and developmental stage–specifically expressed at very low levels in plant tissues. Relatively strong activities of the AtsPLA2β promoter were observed in all tissues at the stages of young seedling growth. As plants grew, however, expression gradually decreased in the primary and secondary leaves compared with that in the cotyledons. AtsPLA2β promoter activity was stimulated greatly after floral shoots had bolted, especially in young or actively growing inflorescence stem tissues and open flowers. In the open flowers, expression was detected in floral organs such as the filaments, sepals, and pedicels but was low or undetectable in petals, anthers, stigmas, ovaries, and maturing seeds. The AtsPLA2β promoter activity in different tissues and at different stages of growth was consistent with the results of RT-PCR assays. Interestingly, gene expression of AtsPLA2β was induced rapidly by auxin treatment within 30 min, suggesting that AtsPLA2β may function as a mediator of auxin signaling in response to external stimuli. Because in in vitro assays, time is required for auxin transport into the tissues, we assume that the induction of AtsPLA2β gene expression by endogenous auxin in vivo can be faster than that in vitro.
What is the role of AtsPLA2β in the growth and development of Arabidopsis? The high levels of AtsPLA2β transcript in actively growing cells suggest that AtsPLA2β functions in cell growth and proliferation. Indeed, transgenic plants overexpressing AtsPLA2β had prolonged leaf petioles and transiently more elongated inflorescence stems than did wild-type plants. By contrast, AtsPLA2β-silenced transgenic plants had shortened leaf petioles and transiently less elongated inflorescence stems. Because the elongation of stems and petioles is dependent on cell division and/or cell elongation, we measured and compared the longitudinal lengths of the epidermal and cortical cells of the petioles and stems of transgenic and wild-type plants. Cell elongation levels were significantly different among the transgenic and wild-type plants and clearly were related to the differences in petiole and stem lengths. These results suggest that the overexpression of AtsPLA2β promotes cell elongation, resulting in prolonged leaf petioles and inflorescence stems, whereas suppression of AtsPLA2β retards cell elongation, resulting in shortened leaf petioles and inflorescence stems.
These results are consistent with previously reported observations. The in vitro addition of the PLA2 enzyme products LPC and linolenic acid accelerates the elongation of maize coleoptile segments (Yi et al., 1996). Treatment of oat roots with exogenous PLA2 or with the enzyme products stimulates H+ pump activity in plasma membrane vesicles (Palmgren and Sommarin, 1989). The acidification of cell walls by the activation of H+ pumps appears to be mediated by the lysophospholipid-induced activation of protein kinase (Martiny-Baron and Scherer, 1989). In addition, LPC activates NADH oxidase, an enzyme that is involved directly in cell elongation (Brightman et al., 1991). The previously reported evidence and our results lead us to propose the role of AtsPLA2β in mediating cell elongation. AtsPLA2β is expressed in actively growing stem tissues and secreted into the extracellular space. AtsPLA2β activity yields its enzymatic products, lysophospholipids and FFAs, by hydrolyzing cell membrane phospholipids. The enzymatic products may stimulate H+ pumps so that the cell wall is acidified and also may activate other enzymes involved directly in cell elongation, including certain protein kinases.
Auxin-induced differential stem elongation has long been suggested to play a major role in the gravitropic response of plants (Kiss, 2000; Kato et al., 2002; Moore, 2002). To determine whether AtsPLA2β is involved in the gravitropic response, the inflorescence stems of wild-type and AtsPLA2β-overexpressing transgenic Arabidopsis plants were placed horizontally. The rates of gravitropic bending in all of the transgenic lines were significantly different from the rate observed in wild-type plants. The time required to bend by 90° was >30 min longer in transgenic plants than in wild-type plants. Although wild-type stems continued to bend past 90°, sPLA2-overexpressing transgenic plants, after resuming vertical growth, did not bend further during the 4-h period. The dark-grown hypocotyls of AtsPLA2β-overexpressing or -silenced transgenic Arabidopsis plants also showed altered gravitropic responses, whereas root gravitropism was not altered significantly.
The reason for the altered gravitropic response in shoots and hypocotyls, but not in roots, is not clear. One possible explanation is that in roots, AtsPLA2β expression is restricted to the vascular tissues and undetectable in the cortical and endodermal cells, where perception and signaling for gravitropic response may occur. For the altered shoot gravitropism, we believe that upregulation or downregulation of AtsPLA2 activity disrupts the asymmetric growth of hypocotyls and inflorescence stems. Because the asymmetric growth of the organs probably is mediated by the differential distribution of auxin in response to gravity stimulation (Kiss, 2000; Kato et al., 2002; Moore, 2002), we propose that AtsPLA2β may mediate auxin-induced differential cell elongation and that AtsPLA2β-mediated differential cell elongation was interfered with by the upregulation and downregulation of AtsPLA2β expression. The auxin-dependent elongation of hypocotyl segments is inhibited by treatment with PLA2 inhibitors (Scherer and Arnold, 1997). Auxin induces a rapid increase of FFA, LPC, and LPE levels, suggesting the stimulation of PLA2 activity by auxin (Paul et al., 1998). We observed that AtsPLA2β expression was activated rapidly by auxin treatment. Moreover, expression was stimulated in the curving region of inflorescence stems after horizontal gravistimulation, suggesting the gravitropic response–specific induction of AtsPLA2β expression. Based on these results, we propose that AtsPLA2β is activated by auxin and mediates auxin-induced cell elongation and that differential cell elongation induced by auxin was interfered with by the upregulation and downregulation of AtsPLA2β expression. However, the mechanisms by which AtsPLA2β mediates auxin-induced differential cell elongation during shoot gravitropism remain to be elucidated.
Recent studies of the putative phosphatidic acid–preferring phospholipase A1 (PLA1) mutant of Arabidopsis, which exhibits normal root gravitropism but impaired shoot gravitropism, suggested that the putative PLA1 regulates vacuolar membrane structure and fluidity, forms the gravity-sensing signal, or influences downstream signals in the gravitropic sensing pathway of Arabidopsis shoots (Kato et al., 2002; Morita et al., 2002). This report suggests that lysophospholipids and free fatty acids perform certain cellular roles, especially in shoot gravitropism but not in root gravitropism. It should be noted, however, that PLA2 and PLA1 act at different acyl positions in glycerophospholipids, resulting in the generation of different lysophospholipids, and they are different in their cellular localizations. Thus, the cellular role of AtsPLA2β may differ from that of the putative AtsPLA1.
Consistent with the presence of an N-terminal signal sequence, AtsPLA2β is secreted into the cell wall/extracellular space, where the signaling process for cell wall acidification may occur. The protein appears to be localized selectively to what might have been the newly formed cell plates. Lysophospholipids, the enzymatic products of PLA2, activate a protein kinase that is involved in the acidification of cell walls by activating a H+ pump (Palmgren and Sommarin, 1989). LPC is involved in transient shifts in apoplastic and intracellular pH, which are essential steps in the gravitropic response (Scott and Allen, 1999; Kiss, 2000; Boonsirichai et al., 2002; Viehweger et al., 2002). AtsPLA2β is transcribed in the cortical, vascular, and endodermal tissues of Arabidopsis stems and hypocotyls. Endodermal cells are known to be essential for the perception of gravity and the signaling pathway(s) involved in the gravitropic response (Boonsirichai et al., 2002; Kato et al., 2002; Morita et al., 2002). Cortical tissues are the sites at which asymmetric growth occurs between the upper and lower parts of responding organs. Although various enzymes, signal molecules such as Ca2+, calmodulin, and inositol 1,4,5-triphosphate, and pH change are known to be involved in the signal transduction that generates the gravitropic response (Perera et al., 1999; Scott and Allen, 1999; Fasano et al., 2001; Kato et al., 2002), the signaling mechanisms between auxin and these proteins, signal molecules, or pH change in the gravitropic response have not been elucidated. Our study suggests that AtsPLA2β regulates the process of cell elongation, possibly by activating key enzymes and signal molecules involved in cell wall acidification, and performs important roles in shoot gravitropism, possibly by mediating auxin-induced cell elongation.
METHODS
Materials and Reagents
1-Palmitoyl-2-14C-palmitoyl-phosphatidylcholine (PC), 1-palmitoyl-2-14C-linoleoyl-PC, and 1,2-14C-dipalmitoyl-PC were purchased from Amersham Pharmacia Biotech. All other chemicals were purchased from Sigma unless stated otherwise. An Arabidopsis thaliana cDNA library constructed in YESTrp2 (Invitrogen, Carlsbad, CA) was obtained from Soo Young Kim (Kumho Life and Environmental Science Laboratory, Gwangju, Korea).
Cloning of an sPLA2 cDNA from Arabidopsis
Using the previously described N-terminal amino acid sequence of an elm PLA2 (Ståhl et al., 1998) as a query, we identified a related sequence in the Arabidopsis ecotype Columbia (Col-0) database (AtDB Stanford University; http://genome.www.stanford.edu/Arabidopsis/). Two PLA2-specific oligonucleotides (5′-TCGCACTTCATTGATGCG-3′ and 5′-TCATAGCTCTGTTTTCATATCATTACCT-3′) were used in combination with the T3 and T7 promoter-specific primers to isolate partial PLA2 sequences from a cDNA library. The PCR contained 6 pmol of each primer and 1 unit of ExTaq polymerase (Pan Vera, Madison, WI) and consisted of 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min. Amplified products were isolated from a 0.8% (w/v) agarose gel and cloned into pGEM-T (Promega) for sequencing using the dideoxy-Sanger method, which is based on extension of oligonucleotide primers by DNA polymerase. The complete sequence of the Arabidopsis sPLA2 gene was derived from the overlapping partial sequences.
Expression of Premature and Mature AtsPLA2β Proteins in Escherichia coli
To obtain the complete coding regions of the putative Arabidopsis PLA2 and to express the encoded proteins in E. coli, their cDNAs were amplified with the oligonucleotides 5′-GGATCCCATGATGTTTCGCACTTC- ATTGA-3′ (forward), and 5′-CTCGAGTAGCTCTGTTTTCATATCATTACCTAATTG-3′ (reverse) using the same cycling program described above. The amplified cDNAs were cloned into the expression vector pET-40b(+) (Novagen, Madison, WI). The cloned product was introduced into BL21(DE3) PLys cells (Novagen) to express the unprocessed form of AtsPLA2β with the signal peptide fused to the C terminus of DsbC in pET-40b(+). Two milliliters of Luria-Bertani medium containing 150 μg/mL ampicillin was inoculated with a single colony from the BL21(DE3) transformation, and the culture was grown at 37°C overnight. Fifty microliters of the overnight culture was added to 500 mL of Luria-Bertani medium and grown at 37°C for 3 h. Overexpression of the fusion protein then was induced by adding isopropylthio-β-galactoside (1 mM final concentration). The cells were incubated at 27°C for 2 h and harvested by centrifugation (5 min at 3000g). The cell pellet was resuspended in 1 mL of STE buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 2 mM EDTA, and 1% Triton X-100) containing 0.25 M phenylmethylsulfonyl fluoride and sonicated briefly. The solution then was centrifuged for 5 min at 20,000g, the resulting supernatant was purified with the His-Bind purification kit (Novagen), and the levels of expression were monitored with the S-Tag rapid assay kit (Novagen).
To obtain the recombinant sPLA2 protein without the signal peptide (mature form), cDNA was amplified with the oligonucleotides 5′-GGATCC- CGAGGAGTGTACAAGAACTTGCATTG-3′ (forward) and 5′-CTCGAGTAGCTCTGTTTTCATATCATTACCTAATTG-3′ (reverse) and ligated to pET40b(+). The mature form of AtsPLA2β was overexpressed in BL21(DE3) PLys cells as its C-terminal fusion with DsbC and purified as described above. The free mature form of AtsPLA2β was released from the fusion protein with the S-Tag rEK purification kit (Novagen). For protein gel blot analysis, protein extracts were mixed with loading buffer, heated at 95°C for 3 min, and loaded onto a 12% (w/v) SDS-PAGE gel. After electrophoresis, proteins were stained with Coomassie Brilliant Blue R 250.
Assays of PLA2 Activity
PLA2 activity was measured as the release of radioactive lysophosphatidylcholine (LPC) and free fatty acid (FFA) from the substrate 1,2-14C-dipalmitoyl-PC. Standard enzyme assay mixtures contained 50 mM Tris-HCl, pH 8.0, 10 mM CaCl2, 0.05% (v/v) Triton X-100, 20 μL of the substrate, and 20 μL of PLA2 extract in a total volume of 200 μL. The substrate was prepared by mixing radioactive PC (1.0 μCi, 108 mCi/mmol) with 2 μmol of unlabeled 1-palmitoyl-2-linoleoyl-PC (Avanti Polar Lipids, Alabaster, AL) in chloroform, drying the mixture under a stream of N2, and emulsifying it in 1 mL of water by sonication at room temperature. The reactions were incubated in a shaking water bath at 30°C for 30 min, 90 min, or 2.5 h and terminated by the addition of 750 μL of chloroform:methanol (1:2, v/v) followed by 200 μL of chloroform and 200 μL of KCl (2 M). After vortexing, the chloroform and aqueous phases were separated by centrifugation at 12,000g for 5 min. The chloroform phase was dried, dissolved in chloroform, and spotted onto a thin layer chromatography plate (Silica Gel G), which was developed with chloroform:methanol:NH4OH:water (65:39:4:4). Lipids on the plates were visualized by brief exposure to iodine vapor. The spots corresponding to the lipid standards FFA and LPC then were scraped into vials, and their radioactivities were determined by standard scintillation counting.
RNA Gel Blot and Reverse Transcriptase–Mediated PCR Analyses
Total RNA was isolated from Arabidopsis ecotype Col-0 roots, stems, leaves, flowers, and siliques using RNA Isolator (GIBCO) and treated with DNase RQI (Promega) to remove residual DNA. RNA gel blot analysis was performed as described (Ryu and Wang, 1995). Relative reverse transcriptase–mediated (RT) PCR was performed using an endogenous standard with the QuantumRNA 18S kit (Ambion, Austin, TX). Two micrograms of total RNA or 150 ng of mRNA isolated from total RNA was used as the template for each RT reaction. Random decamers were used as primers for the RT reactions. As an endogenous standard, an 18S rRNA or ubiquitin cDNA fragment was amplified concomitantly with PLA2. The conditions for the PCR were 20 to 35 cycles of 94°C for 30 s, 65°C for 30 s, and 72°C for 30 s. Amplified products were separated on a 1.4% (w/v) agarose gel, stained with ethidium bromide, and visualized by UV light. Candidate PLA2 DNA fragments were isolated from the gel, and their sequences were confirmed by dideoxy-Sanger sequence analysis.
Promoter Constructs, Plant Transformation, and Analysis of Transgenic Plants
Sequences beginning 699 or 469 bp upstream of the ATG start codon of AtsPLA2β were amplified by PCR from Arabidopsis genomic DNA as EcoRI-BglII fragments. 5′-oligonucleotide primers were 5′-GAATTCGAGACAACAAACAACCAGGTCG-3′ and 5′-GAATTCCACAAAGTTTAATATCTGTTCAC-3′, corresponding to −699 to −678 and −469 to −447, respectively. The 3′ end primer (5′-AGATCTATCGTCGTCGTCTCG-3′) was the reverse complement to nucleotides −13 to +5 with an added BglII site. The two 5′ fragments were used to replace the Cauliflower mosaic virus (CaMV) 35S promoter sequence of pCAMBIA1303, and the binary plasmids were transferred to Agrobacterium tumefaciens EHA105 by the freeze-thaw method. Arabidopsis plants were transformed by the in planta method (Clough and Bent, 1998). Twenty-six independent T3 transgenic lines were generated with each 699- and 469-bp promoter construct on 1× Murashige and Skoog (1962) salts (Sigma, St. Louis, MO), and 0.8% (w/v) agar medium containing 50 mg/L hygromycin. β-Glucuronidase (GUS) activity was localized according to a described procedure (Jefferson et al., 1987). The tissue-specific patterns of GUS staining were similar in all transgenic plants carrying either of the two promoter constructs. Microscopic analyses with cross-sections were performed using an Olympus BX51 microscope (Tokyo, Japan) according to Hall and Cannon (2002).
Subcellular Localization Analysis of AtsPLA2β
The green fluorescent protein (GFP) was fused in frame to the C terminus of unprocessed AtsPLA2 or the signal peptide. The fusion construct was cloned into the BamHI site of the vector pBI221 (Clontech, Palo Alto, CA) and introduced into onion epidermal cells using a helium biolistic particle delivery system (Bio-Rad) as described by Shieh et al. (1993). After incubation for 24 or 48 h at 23°C, the subcellular distribution of GFP was examined by fluorescence microscopy (Olympus). A pBI221 construct containing GFP but without PLA2 was included as a control for subcellular localization.
Transgenic Plants that Overexpress AtsPLA2β or Silence AtsPLA2β Expression
To generate AtsPLA2β-overexpressing transgenic Arabidopsis plants (Col-0 ecotype), AtsPLA2β cDNA (441 bp) containing the complete open reading frame was cloned into the BamHI and NruI sites of the binary plant transformation vector pBI121 between the CaMV 35S promoter and the nopaline synthase terminator. To make the DNA construct for RNA interference–mediated silencing of the AtsPLA2β gene, partial cDNAs (99 bp) corresponding to the PA2c domain were amplified with two primer pairs: 5′BamHI (5′-CTCTTGGATCCCGATATGGGAAGTATT- GTGGG-3′) and 3′ClaI (5′-CCGTTATCGATAACACAATGGTCATGG- ATCTTAC-3′), and 5′XhoI (5′-CTCTTCTCGAGCGATATGGGAAGTATT- GTGGG-3′) and 3′KpnI (5′-CCGTTGGTACCAACACAATGGTCATGG- ATCTTAC-3′).
The BamHI-ClaI fragment was subcloned into an intron-spliced hairpin RNA vector, pKANNIBAL (Commonwealth Scientific and Industrial Research Organization Plant Industry, Canberra, Australia), in the forward orientation, and then the XhoI-KpnI fragment was integrated into the resulting vector as described above in the reverse orientation. The de novo–constructed vector was digested with NotI and subcloned into pART27 (Gleave, 1992). The construct was transformed into A. tumefaciens strain GV3101 using the freeze-thaw method described by Xie et al. (2001). A. tumefaciens–mediated in planta transformation of Arabidopsis was performed according to the method of Bechtold and Pelletier (1998). Transgenic plants were selected on 1× Murashige and Skoog (1962) salts (Sigma) and 0.8% (w/v) agar plus kanamycin (100 mg/L), transferred to soil, and grown to maturity. RT-PCR assays of transgenic plants were performed as described above. To measure PLA2 activity, total proteins were extracted in an extraction buffer (50 mM Tris-HCl, pH 7.0, 10 mM KCl, 10 mM NaF, 1 mM Na3VO4, 0.1 mM phenylmethylsulfonyl fluoride, 1 μg/mL leupeptin, and 1 μg/mL pepstatin) and assayed as described above.
Gravitropic Response Assays of Inflorescence Stems, Hypocotyls, and Roots
To examine the gravitropic responses of inflorescence stems, pots of soil-grown Arabidopsis plants with primary stems 6 to 10 cm in length were placed horizontally in the dark at 23°C. Because the stem elongation rates of wild-type and AtsPLA2β-transgenic plants were different, the heights of plant samples were synchronized by adjusting the dates that seeds were sown. As described by Kato et al. (2002), the curvature of the stems was defined as the angle formed between the direction of apical growth and the horizontal baseline. Approximately 30 individual plants from each of three independent transgenic lines were examined four times.
To determine the gravitropic responses of hypocotyls and roots, seeds were surface-sterilized, plated on Murashige and Skoog (1962) agar plates, and vernalized in the dark at 4°C for 3 days. Because the growth rates of hypocotyls and roots in the dark were not evidently different among the transgenic and wild-type plants, we did not need to synchronize them. The plates then were wrapped with aluminum foil and placed vertically in a tissue culture room at 22°C for 3 days for seedling growth in the dark. Gravistimulation was provided by turning the plates through 90°. Digital images were taken to measure the change in the orientation of the main root axis at 26 and 48 h after gravistimulation.
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact Stephen B. Ryu, sbryu@hanmail.net.
Accession Numbers
The GenBank accession number for AtsPLA2β is AF541915. The GenBank accession numbers for the sequences shown in Figure 1 are AJ238116 (putative rice sPLA2 type I), AJ238117 (putative rice sPLA2 type II), AF064732 (putative carnation sPLA2), and BAA36404 (cobra venom sPLA2). Accession numbers for the other putative sPLA2 cDNAs mentioned are as follows: AI164389 (Populus); AI487873 and BG127198 (tomato); BF275891 (cotton); AW289670 (pine); AI522932, AW318251, BE802011, and BG045671 (soybean); and C27540, D47653, D47724, and D49050 (rice).
Acknowledgments
We thank Jed Doelling and Jung Mook Kim for their critical readings of the manuscript. This work was partially supported by a grant (R01-2000-000-00128-0 to S.B.R. and J.S.S.) from the Basic Research Program of the Korea Science and Engineering Foundation, a grant (M103KD010059-03K0401-05910 to S.B.R.) from the Plant Diversity Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology of the Korean government, and a grant (CG1212 to J.S.S.) from the Crop Functional Genomics Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology. We also wish to acknowledge that our studies on the cloning and characterization of PLA2 genes began at the University of Wisconsin, Madison. Using the published N-terminal amino acid sequence of a purified Elm PLA2 as query, we identified two related sequences in Arabidopsis ecotype database. We cloned and characterized a PLA2 cDNA from Arabidopsis and named it AtsPLA2α. Using the deduced amino acid sequence of this gene as query and a full search of the Arabidopsis genome, we identified the presence of multiple sPLA2 genes, At2g06925, At4g29460, and At4g29470, which we named as AtsPLA2β, AtsPLA2γ, and AtsPLA2δ, respectively. The present study reports on the cloning and characterizing of the second gene, which resulted from the continued collaboration between J.P.P. and S.B.R. after S.B.R. moved to Kumho Life and Environmental Science Laboratory in Korea.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.014423.
References
- Bechtold, N., and Pelletier, G. (1998). In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol. 82, 259–266. [DOI] [PubMed] [Google Scholar]
- Boonsirichai, K., Guan, C., Chen, R., and Masson, P.H. (2002). Root gravitropism: An experimental tool to investigate basic cellular and molecular processes underlying mechanosensing and signal transmission in plants. Annu. Rev. Plant Biol. 53, 421–447. [DOI] [PubMed] [Google Scholar]
- Brightman, A.O., Zhu, X.Z., and Morrè, D.J. (1991). Activation of plasma membrane NADH oxidase activity by products of phospholipase A. Plant Physiol. 96, 1314–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, K.D. (1998). Phospholipase activity during plant growth and development and in response to environmental stress. Trends Plant Sci. 3, 419–426. [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Creelman, R.A., and Mullet, J.E. (1997). Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 355–381. [DOI] [PubMed] [Google Scholar]
- Farag, K.M., and Palta, J.P. (1993. a). Use of lysophosphatidylethanolamine, a natural lipid, to retard tomato leaf and fruit senescence. Physiol. Plant. 87, 515–524. [Google Scholar]
- Farag, K.M., and Palta, J.P. (1993. b). Use of natural lipids to accelerate ripening and enhance storage life of tomato fruit with and without ethephon. Horttechnology 3, 62–65. [Google Scholar]
- Fasano, J.M., Swanson, S.J., Blancaflor, E.B., Dowd, P.E., Kao, T., and Gilroy, S. (2001). Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13, 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave, A.P. (1992). A versatile binary vector system with a T-DNA organizational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol. Biol. 20, 1203–1207. [DOI] [PubMed] [Google Scholar]
- Hall, Q., and Cannon, M.C. (2002). The cell wall hydroxyproline-rich glycoprotein RSH is essential for normal embryo development in Arabidopsis. Plant Cell 14, 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, T., Morita, M.T., Fukaki, H., Yamauchi, Y., Uehara, M., Niihama, M., and Tasaka, M. (2002). SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell 14, 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur, N., and Palta, J.P. (1997). Postharvest dip in lysophosphatidylethanolamine, a natural phospholipid, may prolong vase-life of snapdragon flowers. HortScience 32, 888–890. [Google Scholar]
- Kim, J.Y., Chung, Y.S., Ok, S.H., Lee, S.G., Chung, W.I., Kim, I.Y., and Shin, J.S. (1999). Characterization of the full-length sequences of phospholipase A2 induced during flower development. Biochim. Biophys. Acta 1489, 389–392. [DOI] [PubMed] [Google Scholar]
- Kiss, J.Z. (2000). Mechanisms of the early phases of plant gravitropism. Crit. Rev. Plant Sci. 19, 551–573. [DOI] [PubMed] [Google Scholar]
- Lee, S.M., Suh, S., Kim, S., Crain, R.C., Kwak, J.M., Nam, H.G., and Lee, Y.S. (1997). Systemic elevation of phosphatidic acid and lysophospholipid levels in wounded plants. Plant J. 12, 547–556. [Google Scholar]
- Li-Stiles, B., Lo, H.-H., and Fischer, S.M. (1998). Identification and characterization of several forms of phospholipase A2 in mouse epidermal keratinocytes. J. Lipid Res. 39, 569–582. [PubMed] [Google Scholar]
- Martiny-Baron, G., and Scherer, G.F.E. (1989). Phospholipid-stimulated protein kinase in plants. J. Biol. Chem. 264, 18052–18059. [PubMed] [Google Scholar]
- May, C., Preisig-Müller, R., Höhne, M., Gnau, P., and Kindl, H. (1998). A phospholipase A2 is transiently synthesized during seed germination and localized to lipid bodies. Biochim. Biophys. Acta 1393, 267–276. [DOI] [PubMed] [Google Scholar]
- Moore, I. (2002). Gravitropism: Lateral thinking in auxin transport. Curr. Biol. 12, R452–R454. [DOI] [PubMed] [Google Scholar]
- Morita, M.T., Kato, T., Nagafusa, K., Saito, C., Ueda, T., Nakano, A., and Tasaka, M. (2002). Involvement of the vacuoles of the endodermis in the early process of shoot gravitropism in Arabidopsis. Plant Cell 14, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik, T., Irvine, R.F., and Musgrave, A. (1998). Phospholipid signaling in plants. Biochim. Biophys. Acta 1389, 222–272. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473.–497. [Google Scholar]
- Narváez-Vásquez, J., Florin-Christensen, J., and Ryan, C.A. (1999). Positional specificity of a phospholipase A activity induced by wounding, systemin, and oligosaccharide elicitors in tomato leaves. Plant Cell 11, 2249–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren, M.G., and Sommarin, M. (1989). Lysophosphatidylcholine stimulates ATP dependent proton accumulation in isolated oat root plasma membrane vesicles. Plant Physiol. 90, 1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, R.U., Holk, A., and Scherer, G.F.E. (1998). Fatty acids and lysophospholipids as potential second messengers in auxin action: Rapid activation of phospholipase A2 activity by auxin in suspension-cultured parsley and soybean cells. Plant J. 16, 601–611. [Google Scholar]
- Perera, I.Y., Heilmann, I., and Boss, W.F. (1999). Transient and sustained increases in inositol 1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc. Natl. Acad. Sci. USA 96, 5838–5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohnert, G. (2002). Phospholipase A2 activity triggers the wound-activated chemical defense in the diatom Thalassiosira rotula. Plant Physiol. 129, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, W., Dordschbal, B., Steighardt, J., Hieke, M., Weiss, D., and Saalbach, G. (1999). A redox-dependent, G-protein-coupled phospholipase A of the plasma membrane is involved in the elicitation of alkaloid biosynthesis in Eschscholtzia californica. Biochim. Biophys. Acta 1448, 390–402. [DOI] [PubMed] [Google Scholar]
- Ryu, S.B., Karlsson, B.H., Özgen, M., and Palta, J.P. (1997). Inhibition of phospholipase D by lysophosphatidylethanolamine, a lipid-derived senescence retardant. Proc. Natl. Acad. Sci. USA 94, 12717–12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, S.B., and Wang, X. (1995). Expression of phospholipase D during castor bean leaf senescence. Plant Physiol. 108, 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer, G.F.E. (1996). Phospholipid signalling and lipid-derived second messengers in plants. Plant Growth Regul. 18, 125–133. [Google Scholar]
- Scherer, G.F.E., and Arnold, B. (1997). Inhibitors of animal phospholipase A2 enzymes are selective inhibitors of auxin-dependent growth: Implications for auxin-induced signal transduction. Planta 202, 462–469. [Google Scholar]
- Scott, A.C., and Allen, N.S. (1999). Changes in cytosolic pH within Arabidopsis root columella cells play a key role in the early signaling pathway for root gravitropism. Plant Physiol. 121, 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, D.L., White, S.P., Otwinowski, Z., Yuan, W., Gelb, M.H., and Sigler, P.B. (1990). Interfacial catalysis: The mechanism of phospholipase A2. Science 250, 1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senda, K., Doke, N., and Kawakita, K. (1998). Effect of mastoparan on phospholipase A2 activity in potato tubers treated with fungal elicitor. Plant Cell Physiol. 39, 1080–1086. [Google Scholar]
- Shieh, M.W., Wessler, S.R., and Raikhel, N.V. (1993). Nuclear targeting of the maize R protein requires two nuclear localization sequences. Plant Physiol. 101, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six, D.A., and Dennis, E.A. (2000). The expanding superfamily of phospholipase A2 enzymes: Classification and characterization. Biochim. Biophys. Acta 1488, 1–19. [DOI] [PubMed] [Google Scholar]
- Ståhl, U., Banas, A., and Stymne, S. (1995). Plant microsomal acyl hydrolases have selectivities for uncommon fatty acids. Plant Physiol. 107, 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhl, U., Ek, B., and Stymne, S. (1998). Purification and characterization of a low-molecular-weight phospholipase A2 from developing seeds of elm. Plant Physiol. 117, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhl, U., Lee, M., Sjödahl, S., Archer, D., Cellini, F., Ek, B., Iannacone, R., MacKenzie, D., Semeraro, L., Tramontano, E., and Stymne, S. (1999). Plant low-molecular-weight phospholipase A2s (PLA2s) are structurally related to the animal secretory PLA2s and are present as a family of isoforms in rice (Oryza sativa). Plant Mol. Biol. 41, 481–490. [DOI] [PubMed] [Google Scholar]
- Valentin, E., and Lambeau, G. (2000). Increasing molecular diversity of secreted phospholipases A2 and their receptors and binding proteins. Biochim. Biophys. Acta 1488, 59–70. [DOI] [PubMed] [Google Scholar]
- Viehweger, K., Dordschbal, B., and Roos, W. (2002). Elicitor-activated phospholipase A2 generates lysophosphatidylcholines that mobilize the vacuolar H+ pool for pH signaling via the activation of Na+-dependent proton fluxes. Plant Cell 14, 1509–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, S.P., Scott, D.S., Otwinowski, Z., Gelb, M.H., and Sigler, P.B. (1990). Crystal structure of cobra-venom phospholipase A2 in a complex with a transition-state analogue. Science 250, 1560–1563. [DOI] [PubMed] [Google Scholar]
- Xie, M., He, Y., and Gan, S. (2001). Bidirectionalization of polar promoters in plants. Nat. Biotechnol. 19, 677–679. [DOI] [PubMed] [Google Scholar]
- Yi, H., Park, D., and Lee, Y. (1996). In vivo evidence for the involvement of phospholipase A and protein kinase in the signal transduction pathway for auxin-induced corn coleoptile elongation. Physiol. Plant. 96, 359–368. [Google Scholar]
- Zhang, Y., Lemasters, J., and Herman, B. (1999). Secretory group IIA phospholipase A2 generates anti-apoptotic survival signals in kidney fibroblasts. J. Biol. Chem. 274, 27726–27733. [DOI] [PubMed] [Google Scholar]