Abstract
Ethylene responses in Arabidopsis are controlled by the ETR receptor family. The receptors function as negative regulators of downstream signal transduction components and fall into two distinct subfamilies based on sequence similarity. To clarify the levels of functional redundancy between receptor isoforms, combinatorial mutant lines were generated that included the newly isolated ers1-2 allele. Based on the etiolated seedling growth response, all mutant combinations tested exhibited some constitutive ethylene responsiveness but also remained responsive to exogenous ethylene, indicating that all five receptor isoforms can contribute to signaling and no one receptor subtype is essential. On the other hand, light-grown seedlings and adult ers1 etr1 double mutants exhibited severe phenotypes such as miniature rosette size, delayed flowering, and sterility, revealing a distinct role for subfamily I receptors in light-grown plants. Introduction of an ein2 loss-of-function mutation into the ers1 etr1 double mutant line resulted in plants that phenocopy ein2 single mutants, indicating that all phenotypes observed in the ers1 etr1 double mutant are EIN2 dependent.
INTRODUCTION
Studies in Arabidopsis have provided a relatively detailed model of the primary steps in the signaling pathway elicited by the gaseous plant hormone ethylene. According to this model, ethylene is perceived by a family of five membrane-bound receptors related to bacterial two-component regulators (for review, see Bleecker and Kende, 2000). These receptors are thought to transmit signal through the interaction with a Raf-related Ser/Thr kinase, CTR1 (Kieber et al., 1993; Clark et al., 1998). The receptor/CTR1 complex acts to negatively regulate ethylene-response pathways by suppressing the activity of the putative ion channel, EIN2 (Alonso et al., 1999). Ethylene binding to the receptor complex inhibits signaling, leading to an increase in EIN2 activity and an upregulation of ethylene-response pathways (Hua and Meyerowitz, 1998). The EIN2 protein is thought to stimulate ethylene responses through the activation of a transcriptional cascade mediated by the EIN3 family of transcription factors (Chao et al., 1997; Solano et al., 1998).
This model of ethylene signal transduction provides a conceptual framework that is consistent with phenotypes exhibited by plants with mutations in the genetically defined ethylene-signaling components. The placement of CTR1 between the receptors and EIN2 in the pathway model is based on epistatic relationships determined by double mutant analyses and on data demonstrating physical interactions between the transmitter domains of ETR1, ETR2, and ERS1 and the regulatory domain of CTR1 (Roman et al., 1995; Clark et al., 1998; Cancel and Larsen, 2002). The role of ethylene as an inverse agonist is supported by the discovery that Arabidopsis lines containing loss-of-function mutations in three or more ethylene receptor genes exhibit a constitutive ethylene-response phenotype, similar to the phenotype of loss-of-function mutants in CTR1 (Kieber et al., 1993; Hua and Meyerowitz, 1998).
Although the five members of the Arabidopsis ethylene receptor family share a high degree of sequence identity, each has distinguishing characteristics. All members contain an N-terminal membrane-associated sensor domain, which shows high-affinity ethylene binding in the case of ERS1 and ETR1 (Schaller and Bleecker, 1995; Schaller et al., 1995; Hall et al., 2000). Additional studies with ETR1 indicated that ethylene binding is mediated through a copper cofactor (Rodriguez et al., 1999). The C-terminal domains of the receptors show varying degrees of sequence identity to the His kinase catalytic domains of bacterial two-component regulators. In bacterial systems, these proteins transduce signal via the autophosphorylation of a His residue in the kinase transmitter domain, followed by the transfer of phosphate to an Asp residue in the receiver domain of a response regulator protein (West and Stock, 2001).
The ETR1, ETR2, and EIN4 receptors are termed “hybrid kinases” because they include a C-terminal receiver domain, whereas the ERS1 and ERS2 receptors lack this domain. The residues thought to be essential for His kinase activity are conserved in ETR1 and ERS1 (Chang et al., 1993; Hua et al., 1995) but are not conserved completely in ETR2, EIN4, and ERS2 (Hua et al., 1998; Sakai et al., 1998). Based on overall sequence similarity, the members of the ETR receptor family can be divided into two subfamilies: subfamily I, which includes ETR1 and ERS1; and subfamily II, which includes ETR2, EIN4, and ERS2.
All members of the ETR family are functionally linked to ethylene signaling by virtue of the fact that specific missense mutations in the ethylene binding domain of any one family member leads to dominant ethylene insensitivity that affects responses throughout the plant (Bleecker et al., 1988; Hua et al., 1995, 1998; Sakai et al., 1998). No ethylene-response phenotype is exhibited by etr1, etr2, ein4, or ers2 single loss-of-function mutants, indicating some level of redundancy between receptor isoforms (Hua and Meyerowitz, 1998). In addition, the constitutive ethylene-response phenotype observed in various receptor triple-mutant combinations provides evidence that each receptor isoform makes some contribution to signaling through the ethylene-response pathway. However, differences in the structural features of individual receptor isoforms raise the question of whether specific members of the ETR family perform unique functions.
The studies of receptor-deficient Arabidopsis lines also raised the question of whether the ETR family mediates signaling events distinct from the CTR1/EIN2-dependent ethylene-response pathway. A general growth deficiency in etr1 loss-of-function mutants was suggested to be an ethylene-independent phenotype (Hua and Meyerowitz, 1998). In addition, the quadruple loss-of-function etr1 etr2 ein4 ers2 mutant displays phenotypes that are not observed in ctr1 loss-of-function lines or in wild-type plants treated continuously with high levels of ethylene, including a substantial delay in flowering time and sterility (Hua and Meyerowitz, 1998). It was not established whether these severe phenotypes required EIN2 function or whether such phenotypes resulted from the ethylene receptors regulating additional pathways. Furthermore, no loss-of-function ERS1 mutant had been identified, allowing for studies of the specific contribution of ERS1 to ethylene signaling. Recently, a loss-of-function allele of ERS1, ers1-2, was isolated (Zhao et al., 2002; Wang et al., 2003). As with previous loss-of-function receptor mutants, ers1-2 single mutants exhibited no obvious defects. However, an ers1 etr1 double loss-of-function line showed a severe constitutive response phenotype, suggesting that subfamily I receptors play a particularly important role in signaling.
To extend our understanding of the distinct functions of specific ethylene receptor isoforms in Arabidopsis growth and development, we report here the detailed characterization of combinatorial loss-of-function receptor mutants, including ers1 etr1 double mutants. In addition to displaying severe constitutive ethylene-response phenotypes, the ers1 etr1 double mutant showed a number of defects in reproductive development not usually associated with ethylene signaling. However, evidence is presented here that even these severe phenotypic effects are mediated through EIN2.
RESULTS
Etiolated Seedling Ethylene Response of Double and Triple Loss-of-Function Mutants
The isolation of an ers1 loss-of-function mutant (Wang et al., 2003) provided opportunities to examine several novel combinations of ethylene receptor mutants and investigate the specific roles of receptor subtypes in ethylene signaling. Two important combinations included a mutant lacking both subfamily I receptors (ETR1 and ERS1) and a mutant lacking both receptors without response regulator domains (ERS1 and ERS2). To generate these mutant combinations, a homozygous ers1-2 mutant was crossed to the etr1-6, etr1-7, and ers2-3 single loss-of-function mutants (Hua and Meyerowitz, 1998).
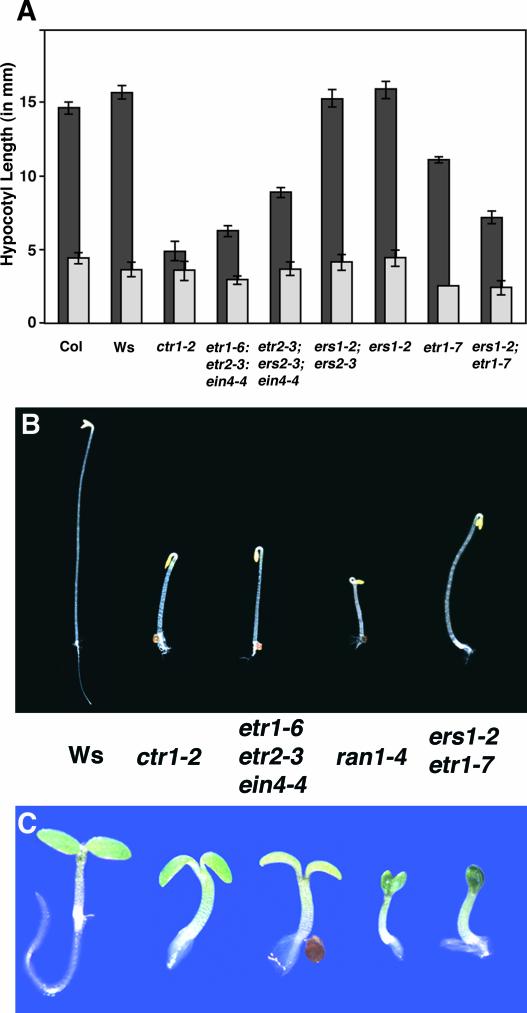
We first compared the hypocotyl ethylene responses of ers1 etr1 and ers1 ers2 double mutants with those of ctr1 mutants and the previously isolated ethylene-receptor triple mutants (Hua and Meyerowitz 1998). As shown in Figure 1A, a number of mutant lines exhibited a partial ethylene-response phenotype in the etiolated hypocotyls in the absence of exogenous ethylene. However, all mutant combinations retained the ability to respond to ethylene to some degree. This included the etr1 etr2 ein4 triple mutant, which expresses only the receptor isoforms without receiver domains (ERS1 and ERS2), and the ers1 ers2 double mutant, which only expresses receptors with receiver domains (ETR1, ETR2, and EIN4). In addition, lines expressing only subfamily I receptors (etr2 ers2 ein4) or subfamily II receptors (ers1 etr1) both exhibited some ethylene responsiveness. Assuming that the alleles used in this study are complete loss-of-function mutants, the results indicate that no particular receptor subfamily is absolutely required for ethylene signaling, at least in the context of the etiolated hypocotyl response.
Figure 1.
Light-Grown ers1 etr1 Mutants Exhibit a Severe Constitutive Ethylene-Response Phenotype, Whereas Dark-Grown Seedlings Exhibit a Partial Ethylene-Response Phenotype.
(A) ers1 etr1 mutants are capable of responding to ethylene. Hypocotyl lengths (in mm) of etiolated seedlings grown for 4 days in either air (dark gray) or 35 ppm of ethylene (light gray) are shown. At least 15 seedlings were measured for each treatment. ers1 etr1 mutants were identified phenotypically once the plates were removed from the dark and left in the light for 7 days. Col, Columbia wild type.
(B) Seedlings grown in the dark on agar plates for 4 days in air. Compared with other constitutive response mutants, ers1 etr1 seedlings exhibited only a partial inhibition of hypocotyl growth but a more complete inhibition of root growth relative to wild-type (Ws) seedlings.
(C) Seedlings grown in the light for 3 days in air. Ws seedlings appear wild type, whereas the ctr1 and etr1 etr2 ein4 mutants exhibit shorter hypocotyls, short roots with prolific root hairs, and smaller, less expanded cotyledons than wild-type seedlings. The ers1 etr1 mutants phenocopied the severe ran1 mutants, exhibiting shorter hypocotyls and roots and small, dark, unexpanded cotyledons.
Comparison of the ers1 etr1 Double Mutant with Other Constitutive Ethylene-Response Mutants
The severity of the ers1 etr1 mutant phenotypes of light-grown plants (Zhao et al., 2002; Wang et al., 2003) compared with that of other double mutant combinations prompted us to undertake a comparative study of this double mutant and other related mutants. Although the dark-grown seedling phenotype of ers1 etr1 seedlings was similar to, although less severe than, those of ctr1 and etr1-6 etr2-3 ein4-4 triple mutants (Figures 1A and 1B), the light-grown seedling phenotype of the ers1 etr1 mutant was more severe than those of ctr1 and the triple mutants (Figure 1C). Light-grown ers1 etr1 mutants exhibited some phenotypes characteristic of all three constitutive response mutants, such as thick hypocotyls and short roots with numerous root hairs. However, the cotyledons of ers1 etr1 mutants remained cupped together longer than the cotyledons of these mutants and were very small and dark green. Such phenotypes are similar to the light-grown seedling phenotype of ran1-4 (Figure 1C), a loss-of-function mutant with defects in copper transport that is thought to lack functional ethylene receptors (Hirayama et al., 1999; Himelblau and Amasino, 2000; Woeste and Kieber, 2000).
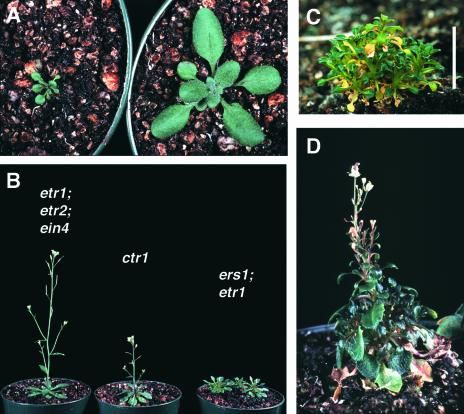
When ers1 etr1 mutants were transferred to soil, they produced miniature rosettes (Zhao et al., 2002; Wang et al., 2003) (Figures 2A and 2B). The rosette-stage phenotype of ers1 etr1 was more severe than that of ctr1 (Table 1) or the receptor triple null mutants, although it was less severe than that of ran1, which is rosette lethal (Himelblau and Amasino, 2000; Woeste and Kieber, 2000). The extremely small size of the ers1 etr1 mutants persisted throughout their life cycle; at 6 weeks, the rosette diameter of ers1 etr1 mutants averaged only 12 mm, whereas that of ctr1 mutants averaged 26 mm and that of wild-type Columbia plants averaged 104 mm (Figure 2, Table 1).
Figure 2.
ers1 etr1 Mutants Exhibit an Extremely Compact Rosette, Delayed Flowering, and Stunted Inflorescence Stems.
(A) Rosette-stage phenotype of a 3-week-old ers1-2 etr1-7 homozygous mutant is shown next to a wild-type Columbia plant (at right) for comparison.
(B) ers1-2 etr1-6 homozygous mutants (far right) photographed next to an etr1 etr2 ein4 triple mutant and a ctr1-2 mutant for comparison. All plants were 6 weeks old.
(C) Close-up of a 12-week-old ers1-2 etr1-7 mutant that has not bolted. Bar = 1 cm.
(D) Close-up of a 10-week-old ers1-2 etr1-6 mutant that has bolted and produced flowers (plant size, ∼4 cm).
Table 1.
Overview of Mutant Phenotypes
| Rosette Diameter (mm ± SD)
|
Flowering Time (DAG)
|
||||
|---|---|---|---|---|---|
| Mutant | 18 DAG | 46 DAG | Inflorescence Stem Length (cm ± SD) | Mean ± SD | NF |
| Columbia | 23.0 ± 3.3 | 104.1 ± 7.3 | 45.2 ± 4.9 | 22.4 ± 1.2 | 0 |
| Ws | 24.8 ± 2.7 | 79.2 ± 9.8 | 38.9 ± 2.3 | 18.2 ± 1.3 | 0 |
| ers1-2 | 26.7 ± 4.9 | 67.1 ± 10.8 | 47.5 ± 7.3 | 18.6 ± 0.5 | 0 |
| etr1-7 | 11.5 ± 2.4 | 53.4 ± 3.4 | 34.4 ± 2.5 | 28.4 ± 1.0 | 0 |
| ctr1-2 | 7.7 ± 1.3 | 26.3 ± 3.4 | 24.4 ± 2.4 | 26.3 ± 1.4 | 0 |
| ers1-2 etr1-7 | 4.1 ± 1.0 | 12.9 ± 1.8 | 1.8 ± 1.1 | 63.3 ± 6.3 | 5/21 |
| ein2-1 | 19.6 ± 2.0 | 90.4 ± 9.6 | 49.3 ± 4.7 | 19.1 ± 2.0 | 0 |
| ein2-1 ers1-2 etr1-7 | 24.8 ± 3.9 | 55.3 ± 11.5 | 46.1 ± 6.6 | 19.7 ± 1.3 | 0 |
n > 15 plants. For ein2-1 measurements, 11 plants were used for rosette and stem length measurements, and 6 plants were used for flowering measurements. Inflorescence stem length was measured at 8 weeks after germination, as plants were senescing and flower production terminated. Because of their delayed flowering, ers1 etr1 mutants were measured at 15 weeks after germination (n = 15 plants). Plants were grown in long days (16 h of light, 8 h of dark). DAG, days after germination; NF, number of plants that never made the transition to flowering/total number of plants analyzed.
The ers1 etr1 mutants were delayed substantially in flowering time, and some plants never appeared to make the transition from vegetative to reproductive growth before senescing (Figure 2C, Table 1). Under long-day conditions, Wassilewskija (Ws) plants flowered an average of 18 days after germination, after producing an average of 5.1 rosette leaves (Table 1). Both the triple null and ctr1 mutants were delayed slightly in flowering, bolting at 26 days after germination, after producing an average of 15 and 11 rosette leaves, respectively. On the other hand, those ers1 etr1 mutants that did flower bolted after an average of 9 weeks of vegetative growth, after producing at least 30 to 50 rosette leaves (Table 1).
The ers1 etr1 Double Mutants Show Defects in Fertility and Flower Morphology
The segregation of ers1 etr1 progeny from a self cross of an ERS1/ers1-2 etr1-7/etr1-7 parent was only 18%, rather than the expected 25% (53 etr1-7 ers1-2 double mutants, 254 wild type; χ2 = 9.4, P < 0.05). A similar segregation ratio also was obtained with self crosses of the ers1-2/ers1-2 ETR1/etr1-6 parent (data not shown), indicating a reduced transmission of the mutant ers1 allele in this background.
Fertility problems were more severe in the homozygous double mutants. The ers1 etr1 double mutants that did flower were infertile and exhibited numerous floral defects not usually associated with ethylene-mediated responses. Mutant floral buds were much smaller than wild-type buds and rarely opened normally. Instead, within most flowers, the inner floral organs remained enclosed by the sepals, and the bud eventually senesced without opening (Figure 2D). The inflorescence stems of plants that flowered were very stunted relative to those of wild-type and ctr1-2 plants at the same developmental stage. At 15 weeks, the average inflorescence stem length for the ers1-2 etr1-7 mutant was only 1.8 cm (Table 1).
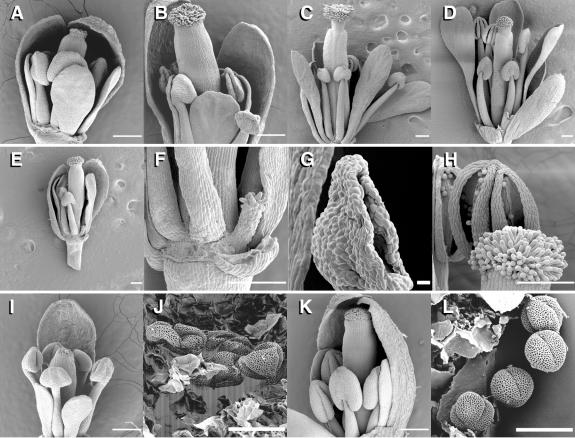
Scanning electron microscopy of floral buds indicated that with the exception of the stamens, the floral organs in the double mutants appeared fairly normal but arrested in development (Figures 3A and 3B). Often on an inflorescence, three to five green buds arrested in a similar stage of development, and the lowest buds on the stem were yellow and contained withered floral organs. Floral buds from ers1-2 etr1-7 mutants appeared as though they had progressed normally through approximately stage 11 (as defined by Bowman, 1994) (Figure 3B) but did not progress to later developmental stages that are characterized by elongating petals and stamens, bud opening, and anther dehiscence. Instead, petals remained immature, stamens never elongated above the stigma, and the mutant flowers remained almost completely closed. The ers1-2 etr1-6 mutants generally exhibited floral phenotypes that were less severe than those of the mutants just described, although the most severe mutants phenocopied the least severe ers1-2 etr1-7 mutants. The ers1-2 etr1-6 mutant flowers often matured to a point at which the pistils and petals elongated, stigmatic papillae appeared mature, and anthers shed normal-looking pollen (Figure 3C). However, in these flowers, the stamen filaments never elongated to position the anthers above the stigmatic papillae (Figure 3C), as in wild-type flowers (Figure 3D).
Figure 3.
Defects in Reproductive Development in the ers1 etr1 Mutant.
(A) First bud off the apex of the inflorescence from an ers1-2 etr1-6 mutant exhibiting a severe phenotype (approximately floral stage 10 to 11). Petals are level with the short stamens, and stigmatic papillae are beginning to appear.
(B) Older bud taken off the same inflorescence stem as the bud in (A), which also appears arrested in approximately stage 11. All buds are green, and sepals still enclose the floral organs.
(C) ers1-2 etr1-6 flower exhibiting a weaker phenotype, approximately stage 13. Petals have elongated past the sepals, and mature stigmatic papillae are present. Anthers are dehiscent but are not in the proximity of the stigma.
(D) Wild-type (Ws) flower in approximately stage 13 for comparison.
(E) Floral bud of an ers1-2 etr1-6 individual. An abnormal stamen can be seen at bottom right.
(F) Close-up of the defective stamen structure in (E).
(G) Close-up of an anther from an ers1 etr1 mutant. There is breakage along the stomium, although it is not nearly as pronounced as that in wild-type flowers.
(H) Dehiscent anther of a wild-type (Ws) individual.
(I) Floral bud from a severe ers1-2 etr1-7 mutant. Sepals were dissected off.
(J) An anther from the bud in (I) was dissected open, revealing aberrant pollen with a flattened appearance.
(K) Wild-type (Ws) bud in approximately stage 10 to 11 for comparison.
(L) An anther from the bud in (K) was dissected open, revealing normal pollen grains.
Thin bars = 240 μm; thick bars in (G), (J), and (L) = 24 μm.
The double mutant flowers often were missing or had defective stamens. Scanning electron microscopy analysis indicated that ers1-2 etr1-7 floral buds frequently possessed only four stamens. ers1-2 etr1-6 mutants often possessed four normal stamens and two defective stamens, which consisted of filamentous structures tipped with smooth, oblong cells (Figures 3E and 3F). In most flowers in which anthers had dehisced ( Figure 3G), the anthers did not open nearly as widely as in wild-type flowers (Figure 3H), and little or no pollen was shed. Dissection of anthers from ers1-2 etr1-7 mutants revealed that they did contain pollen, although it often appeared defective (Figures 3I and 3J). Mutant pollen grains exhibited normal exine patterning, but the grains appeared flattened (Figure 3J) compared with wild-type grains (Figures 3K and 3L).
To further characterize the double mutant's infertility, wild-type pollen was used to fertilize both ers1-2 etr1-6 and ers1-2 etr1-7 mutants. For the crosses to ers1-2 etr1-6 individuals, siliques containing some seeds were recovered, although they were very small and contained only 1 to 10 seeds. The seeds germinated well, and heterozygous progeny were produced (data not shown). No seeds were recovered from the ers1-2 etr1-7 mutants, even though numerous crosses were attempted. Mutant pollen also was used to fertilize wild-type pistils. However, the efficiency of the crosses was very low, and only a few successful crosses were made using pollen from ers1-2 etr1-6 individuals. No successful fertilization events were observed from crosses using ers1-2 etr1-7 as the pollen donor. Thus, both male and female fertility were affected in the double mutants.
Expression of Chitinase B in the ers1 etr1 Mutants
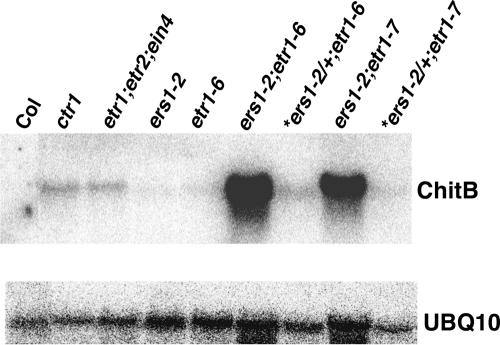
The ers1 etr1 mutants also were compared with ctr1 and the triple null mutants in their expression of an ethylene-regulated gene. As reported previously (Kieber et al., 1993; Hua and Meyerowitz, 1998), both ctr1 and the triple null mutants exhibit constitutive expression of the ethylene-response gene Chitinase B (Figure 4) (Samac et al., 1990). RNA gel blots showed greatly increased levels of Chitinase B mRNA in the ers1 etr1 mutants, even compared with levels in ctr1 and the triple null mutants. No Chitinase B expression was detected in wild-type controls, whereas a low level of expression was detected in the ers1-2 and etr1-6 lines and in siblings of the double mutants. These results confirm that the ers1 etr1 mutants exhibit a constitutive ethylene-response phenotype at the level of Chitinase B expression and that this response is even stronger than that in other constitutive ethylene-response mutants.
Figure 4.
Chitinase B mRNA Expression Is Highly Increased in ers1 etr1 Mutants.
Total RNA was isolated from rosette tissue of 3.5-week-old plants. Ten micrograms of total RNA was probed with a Chitinase B–specific probe. A ubiquitin-specific probe (UBQ10) was used as a loading control. Lanes with asterisks at top represent wild-type-looking siblings of the double mutants that were heterozygous for the ers1-2 allele and homozygous for the etr1-6 or etr1-7 allele. Col, Columbia wild type.
Phenotype of ein2 ers1 etr1 Triple Mutants
The extreme delay in flowering, miniature size, floral defects, and sterility exhibited by the ers1 etr1 mutants are phenotypes not typically associated with ethylene responses, raising the question of whether these receptors normally regulate processes distinct from the CTR1/EIN2-dependent signal transduction pathway. To determine if any of the phenotypes exhibited by ers1 etr1 mutants result from EIN2-independent signaling activities, ers1 etr1 mutants were crossed into an ein2-1 loss-of-function mutant background.
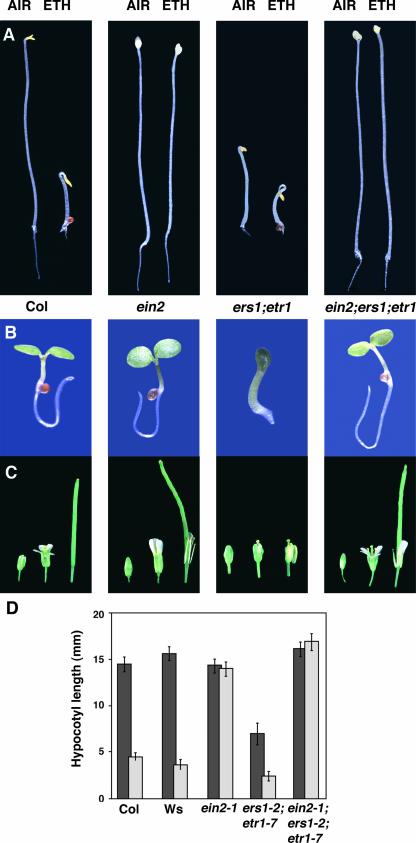
The ein2 ers1 etr1 triple mutants phenocopied ein2 mutants throughout their life cycle. Etiolated ein2 ers1 etr1 seedlings grew to the same length as the ein2-1 single mutant and were completely insensitive to ethylene (Figures 5A and 5D). In addition, light-grown ein2 ers1 etr1 seedlings no longer showed severe phenotypes such as cupped cotyledons, short roots, and thickened hypocotyls (Figure 5B). Adult ein2 ers1 etr1 mutants also produced normal-looking fertile flowers (Figure 5C) and no longer showed dwarfed stature or miniature rosettes ( Table 1). Based on these results, we conclude that the whole range of detectable phenotypes exhibited by the ers1 etr1 mutants is dependent on EIN2 function.
Figure 5.
ers1 etr1 Developmental Defects Are Rescued Completely by the ein2-1 Mutation
(A) Phenotypes of etiolated seedlings. Seedlings were grown on agar plates in the dark for 4 days in either air or 35 ppm of ethylene (Eth). ein2 ers1 etr1 triple mutants exhibit complete ethylene insensitivity.
(B) Phenotypes of light-grown seedlings. Seedlings were grown in the light for 3 days. ein2 ers1 etr1 mutants no longer exhibit cupped cotyledons, shortened roots, and prolific root hairs. Col, Columbia wild type.
(C) Floral phenotypes of adult plants. Flowers are of equivalent ages starting with developmental stage 12. ein2 ers1 etr1 mutants no longer exhibit defects in floral development.
(D) Quantification of the hypocotyl response of mutant and wild-type seedlings. Seedlings were grown on agar plates in the dark for 3 days in either air (dark bars) or 35 ppm of ethylene (light bars). At least 20 seedlings were measured for each treatment.
DISCUSSION
Receptor Redundancy
The analysis of loss-of-function mutants for four of the five Arabidopsis ethylene receptor genes led to the conclusion that there is a degree of functional redundancy between receptor isoforms (Hua and Meyerowitz, 1998). This finding now can be extended to the ERS1 gene, which shows wild-type hypocotyl elongation and vegetative shoot development. The previous study also demonstrated that combining receptor loss-of-function mutants to produce lines lacking more than one receptor isoform could result in constitutive ethylene-response phenotypes of increasing severity depending on the number of genes disrupted. The observation that different double and triple mutant combinations differ in the degree of constitutive response phenotypes raised the question of whether the individual receptor isoforms contribute quantitatively to signaling or specific subsets of receptor isoforms provide unique functional contributions.
Based on the etiolated-seedling response of plants lacking various combinations of ethylene receptors, it appears that virtually any combination of remaining receptor subtypes is capable of some level of ethylene-regulated signaling to downstream effectors (Figure 1A). Etiolated ers1 etr1 mutants show only an intermediate ethylene-response phenotype in air, and growth can be inhibited further by ethylene, indicating that in the absence of subfamily I receptors, subfamily II receptors are capable of signaling in etiolated hypocotyls and roots. Similar ethylene responsiveness in the etr2 ein4 ers2 triple mutant indicates that in the absence of subfamily II receptors, subfamily I receptors also are capable of ethylene-regulated signaling. Finally, in the etr1 etr2 ein4 triple mutant, in which only receptors lacking receiver domains should be functional, some ethylene-regulated signaling also is observed ( Hua and Meyerowitz, 1998). Together, these observations suggest some level of functional redundancy between all receptor isoforms in the etiolated-seedling growth response. This conclusion is supported by studies in tomato in which ethylene-response phenotypes obtained by antisense suppression of a tomato ETR2-like gene were compensated for by overexpression of an ERS1-like gene (Tieman et al., 2000).
However, with respect to redundancy between subfamily I and subfamily II, the intermediate constitutive response phenotype exhibited in the ers1 etr1 etiolated hypocotyl must be reconciled with the extremely severe phenotypes observed in roots and light-grown shoots. In the latter tissue, the double mutant phenotypes are more severe than either the ctr1 mutant phenotype or any of the ethylene receptor triple mutant phenotypes described previously (Hua and Meyerowitz, 1998). In fact, many of the phenotypes of the ers1-2 etr1-7 mutant are as severe as that of the loss-of-function ran1 mutants, which may entirely lack functional ethylene receptors (Woeste and Kieber, 2000). Additional recent work indicates that the deficiency in subfamily I receptors in the ers1 etr1 double mutants could not be compensated for simply by increased expression of subfamily II receptors and further supports a unique role for the subfamily I receptors. In experiments in which the ETR1 promoter was used to drive the expression of cDNAs for all five receptor isoforms in the ers1 etr1 mutant plants, subfamily I cDNAs restored normal growth, whereas subfamily II cDNAs failed to rescue the double mutant phenotype (Wang et al., 2003).
Nevertheless, arguments regarding functional redundancy between subfamily I and II receptors hinge on whether ers1-2 is a true loss-of-function mutant. Although the only detectable ERS1 message containing coding sequence also contains nine false start sites upstream of the native ATG (Wang et al., 2003), very low levels of a splice variant could result in the production of some wild-type protein. However, the relatively similar expression level of this mutant transcript in etiolated seedlings and in severely affected light-grown seedlings (data not shown) argues against this possibility, unless one assumes that the expressed splice variant is produced differentially in etiolated hypocotyls. This issue will be resolved only when additional loss-of-function alleles of ERS1 are isolated.
The Role of Subfamily I Receptors in Signal Transmission
The observation that ers1 etr1 mutants are the only ethylene receptor double mutants that exhibit a severe constitutive response phenotype reveals a critical role for subfamily I receptors in signaling. Given that only subfamily I receptors carry conserved His kinase domains, one might predict that the distinct activity provided by these two receptors in signal transmission would be through canonical His kinase–mediated phosphotransfer. However, recent experiments in which the ers1 etr1 double mutant was transformed with an etr1 genomic clone containing an inactive kinase domain demonstrated a complete restoration of wild-type growth and ethylene responsiveness to ers1 etr1 mutants. Thus, these experiments demonstrated that canonical His kinase activity is not essential for signaling by subfamily I receptors (Wang et al., 2003).
An alternative distinct role for subfamily I receptors might involve the relative strength of a direct interaction between transmitter domains of the receptors and the regulatory domain of the Raf-like CTR1 protein. Direct evidence for such an interaction has been obtained for both subfamily I receptors, with the strongest interaction observed between ETR1 and CTR1 (Clark et al., 1998). More recently, a very weak interaction between ETR2 and CTR1 was reported using two-hybrid and in vitro assays (Cancel and Larsen, 2002). If ethylene receptor output is mediated primarily through subfamily I receptors, then the contribution of subfamily II receptors could be enhanced through interactions between receptors, as was suggested recently (Chang and Stadler, 2001; Cancel and Larsen, 2002; Gamble et al., 2002). Recent studies indicate that some bacterial two-component regulators signal by the formation of higher order clusters of receptors that can crosstalk with each other (Ames et al., 2002). Such a model for the ethylene receptor family could explain the recent observation that dominant insensitivity caused by the etr1-1 mutant receptor is retained even when its transmitter domain is truncated (Gamble et al., 2002).
The Central Role of EIN2 in Ethylene Receptor Function
The possibility that the ethylene receptors may signal through alternative, ethylene-independent pathways was prompted by the pleiotropic defects observed in the ers1 etr1 mutants and the observation that etr1 loss-of-function mutants appeared to exhibit ethylene-independent growth defects (Hua and Meyerowitz, 1998). The introduction of a null allele in EIN2 can be used to test this possibility. For example, isolation of a ran1 ein2 double mutant demonstrated that adult phenotypes of the ran1 mutant were not dependent on EIN2 activity (Woeste and Kieber, 2000). By contrast, our finding that all of the ers1 etr1 mutant phenotypes are eliminated in ein2 ers1 etr1 mutants indicates that all detectable phenotypes resulting from ERS1 and ETR1 receptor deficiency are EIN2 dependent, including the putative ethylene-independent growth defects observed in the etr1 loss-of-function lines. These results support a model in which the principal function of the ethylene receptors is the negative regulation of EIN2 activity. Additionally, these data are consistent with recent findings that indicate that the apparent ethylene-independent defects in the etr1-7 mutant result from an increased sensitivity to ethylene (Cancel and Larsen, 2002).
Although the Chitinase B induction and the etiolated seedling response of ers1 etr1 mutants are well-recognized ethylene responses, the flowering and fertility defects exhibited by these mutants have not been reported as ethylene-response phenotypes. However, the severe floral phenotypes observed in the ers1 etr1 mutant may be considered extreme forms of phenotypes observed in other ethylene-signaling mutants. For example, one floral phenotype that has been documented in ctr1 mutants and EIN2 (CEND1) overexpressors is a “protruding gynoecium,” in which the pistil protrudes out of the petals and sepals before the flower opens (Kieber et al., 1993; Alonso et al., 1999). However, it is possible that in these mutants the pistil does not actually protrude early but rather the petals, sepals, and stamens are delayed in development and elongation, similar to, although less severe than, the defects in ers1 etr1 mutants. Furthermore, loss-of-function ctr1 and ran1 mutants exhibit gametophytic transmission defects, although ctr1 shows reduced transmission through the male gametophyte (Kieber et al., 1993) and ran1 is poorly transmitted through the female gametophyte (Woeste and Kieber, 2000). It is possible that the ers1 etr1 and etr1 etr2 ein4 ers2 mutants display more extreme versions of these reproductive phenotypes.
Our finding that all of the ers1 etr1 defects in fertility and development are EIN2 dependent implies that this whole continuum of phenotypes may be attributable to the unregulated activity of EIN2, a protein related to the Nramp family of membrane-associated metal transporters (Alonso et al., 1999). Given that ethylene-response pathway activation does not induce EIN2 mRNA levels (Alonso et al., 1999), it is likely that the ethylene receptors and associated transduction components negatively regulate EIN2 at the level of protein activity. Although the cytoplasmic C-terminal end of EIN2 can induce some constitutive ethylene-response phenotypes when overexpressed in Arabidopsis (Alonso et al., 1999), the mechanism by which EIN2 receives and transmits signal from receptors is not known. Elucidation of the biochemical function of EIN2 will be essential to understanding how this key component of the pathway mediates ethylene-regulated responses in plants.
METHODS
Plant Material
The ers1-2 allele of Arabidopsis thaliana carries a T-DNA insertion located in an intron within the 5′ untranslated region of the gene, 235 bp upstream of the start codon, resulting in the production of a chimeric transcript that contains at least nine AUGs upstream of the native start site (Wang et al., 2003). The etr1 loss-of-function mutant alleles were isolated as second-site suppressors of the dominant etr1-1 allele (Hua and Meyerowitz, 1998). The etr1-6 mutation results in the disruption of a splice site in the gene's second intron, whereas etr1-7 results in a stop codon at Trp-74. The ctr1-2 allele results from a deletion in nucleotides 1995 to 2011 in the fifth exon (Kieber et al., 1993). The ran1-4 allele carries a T-DNA insertion in the second intron, 9 bp downstream of the intron splice site (Himelblau and Amasino, 2001).
Genetic Analysis
The etr1-6, etr1-7, etr2-3, ers2-3, ein4-4, etr1-6 etr2-3 ein4-4, and etr2-3 ers2-3 ein4-4 mutants were obtained from Elliott Meyerowitz (Hua and Meyerowitz, 1998). The ers1-2 allele was isolated in a Wassilewskija (Ws) background and was backcrossed to wild-type Ws three times before physiological analyses (Wang et al., 2003). To construct ers1 etr1 double mutants, the ers1-2 mutant was crossed to the etr1-6 and etr1-7 single mutants. F2 progeny were screened using a combination of cleaved amplified polymorphic sequence (dCAPS) markers (Michaels and Amasino, 1998) and sequencing.
For genotyping, DNA was isolated using the method of Klimyuk et al. (1993). To identify the ers1-2 allele, PCR was performed with the T-DNA primers JL202 (5′-CATTTTATAATAACGCTGCGGACATCTAC-3′) and ERSr3 (5′-TCTAATTCCATGAGTAAGCATCCTAACAT-3′). To identify a wild-type ERS1 allele, the primers ERS1F-135 (5′-ATTGGTTTCTTCTTT- ATCACACTGTTACG-3′) and ERSr3 were used. dCAPS analysis to identify the etr1-1 mutation was performed according to Hua and Meyerowitz (1998). For sequencing of etr1 mutations, ETR1 DNA was amplified using the primers eE1-1 (5′-CTGCAATTGTATTGAACCGCAATGGCC-3′) and eE1-4 (5′-GCAACATTCTGCTCCATGAGAAGGTCCC-3′). PCR products were purified using the Qiagen PCR purification kit (Valencia, CA) and sequenced. The primers ETR1F1-1 (5′-TCTCCGATTTCTTCATTGCGA- TTGCGTAT-3′) and ETR1-B (5′-TTCTTATACACTTCGTCATCAACA-3′) were used to identify the etr1-7 and etr1-6 mutations, respectively. To identify ein2 etr1 ers1 mutants, F2 progeny from a cross of an ers1-2/ERS1 etr1-7/etr1-7 plant to an ein2-1 plant were screened to confirm the ers1-2 and etr1-7 alleles, as described previously. The ein2-1 allele was confirmed by PCR using the EIN2-specific primers 5′EIN2 (5′-GGT- TTGAGATGGAATACCGTGATGG-3′) and 3′EIN2 (5′-TCAAGGATGGCAGATAAGTGTCTCC-3′) followed by sequencing with the primer ein2-1seq (5′-ATGCTCAAAATGCTTTATCTTATCCATC-3′).
Plant Growth Conditions and Measurements
Plants were grown in 16-h days in growth chambers as described by Hall et al. (1999). For rosette measurements, the diameter of the rosette was measured at its widest point. For stem measurements, plants were harvested as flower production terminated, and the primary inflorescence stem was measured from the root/inflorescence stem junction to the inflorescence tip.
Etiolated Seedling Assays
Seeds were plated on half-strength MS plates (Murashige and Skoog, 1962), treated at 4°C for 3 days, light treated for 6 h, wrapped in foil and transferred to chambers, and then gassed continuously with 100 mL/min air or ethylene (35 ppm) for 4 days. Ethylene concentrations were checked using a Perkin-Elmer 8500 gas chromatograph and compared with a 1-ppm ethylene standard. Seedling hypocotyls were measured as described by Hall et al. (1999).
Scanning Electron Microscopy Analysis of Floral Buds
For analysis by scanning electron microscopy, floral buds were fixed overnight at 4°C in 2% glutaraldehyde (in 0.05 M KPO4 buffer). Buds were dehydrated using an ethanol series from 10 to 100%. Buds then were dried using a critical point dryer, mounted on aluminum stubs (SPI Supplies, Westchester, PA) with carbon tabs, and gold coated at the Wisconsin Integrated Microscopy Facility (Madison, WI). For pollen analysis, anthers that had been critically dried and coated were broken open using a fine dissecting needle, remounted, and recoated. Samples were examined using a Hitachi S570 scanning electron microscope (Tokyo, Japan) with a digital image-capturing system (Gatan, Pleasanton, CA).
RNA Gel Blot Analysis
For Figure 4, rosette tissue was isolated from 3.5-week-old air-grown plants. RNA was isolated with the Genosys RNA isolation kit (The Woodlands, TX). Tissue was ground in liquid nitrogen and mixed with a 1-mL Genosys RNA Isolater. Tubes were frozen and then incubated for 5 min at room temperature. Chloroform extraction was performed, followed by RNA precipitation. RNA was pelleted at 4°C, washed with 80% ethanol, and resuspended in 50 μL of diethyl pyrocarbonate/water. Samples were incubated for 5 min at 60°C and stored at −80°C. RNA was quantified using a spectrophotometer (Beckman, Fullerton, CA).
For RNA gel blot analysis, total RNA was run on 1.0% agarose formaldehyde-containing gels. Gels were transferred to nitrocellulose, followed by UV light cross-linking. Probes were synthesized using the Promega Prime-a-Gene kit (Madison, WI) according to the manufacturer's instructions. Blots were hybridized at 42°C in 50% formamide, 6× SSPE (1× SSPE is 0.115 M NaCl, 10 mM sodium phosphate, and 1 mM EDTA, pH 7.4), 5× Denhardt's solution (1× Denhardt's solution is 0.02% Ficoll, 0.02% polyvinylpyrrolidone, and 0.02% BSA), and 0.5% SDS, washed with an SSPE/SDS solution, and exposed to a PhosphorImager screen (Molecular Dynamics, Sunnyvale, CA) for 1 to 3 days. To ensure equal loading, blots were stripped with a boiling solution of 0.1× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate) and 0.5% SDS and reprobed with a ubiquitin probe (UBQ10). For blots probed with Chitinase, a probe was generated with the primers 5′ChitB (5′-ATATTCATGGGGCTACTG- TTTC-3′) and 3′ChitB (5′-TTCACCGTTAATGATGTTCGT-3′).
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact Anthony Bleecker, bleecker@wisc.edu.
Acknowledgments
We thank Elliott Meyerowitz and Rick Amasino for providing seed stocks, Sara Patterson and Philip Oshel for assistance with scanning electron microscopy, Jeff Young for assistance in screening the Wisconsin T-DNA pools, Ted Anderson for plant care, and Claudia Lipke and Kandis Elliot for help with photography and illustrations. This project was funded by National Science Foundation Grant MCB #0131564.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.013060.
References
- Alonso, J., Hirayama, T., Roman, G., Nourizadeh, S., and Ecker, J. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284, 2148–2152. [DOI] [PubMed] [Google Scholar]
- Ames, P., Studdert, C.A., Reiser, R.H., and Parkinson, J.S. (2002). Collaborative signaling by mixed chemoreceptor teams in Escherichia coli. Proc. Natl. Acad. Sci. USA 99, 7060–7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker, A.B., Estelle, M.A., Somerville, C., and Kende, H. (1988). Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241, 1086–1089. [DOI] [PubMed] [Google Scholar]
- Bleecker, A.B., and Kende, H. (2000). Ethylene: A gaseous signaling molecule in plants. Annu. Rev. Cell Dev. Biol. 16, 1–18. [DOI] [PubMed] [Google Scholar]
- Bowman, J. (1994). Arabidopsis: An Atlas of Morphology and Development. (New York: Springer-Verlag).
- Cancel, J.D., and Larsen, P.B. (2002). Loss-of-function mutations in the ethylene receptor ETR1 cause enhanced sensitivity and exaggerated response to ethylene in Arabidopsis. Plant Physiol. 129, 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C., Kwok, S.F., Bleecker, A.B., and Meyerowitz, E.M. (1993). Arabidopsis ethylene-response gene ETR1: Similarity of product to two-component regulators. Science 262, 539–544. [DOI] [PubMed] [Google Scholar]
- Chang, C., and Stadler, R. (2001). Ethylene hormone receptor action in Arabidopsis. Bioessays 23, 619–627. [DOI] [PubMed] [Google Scholar]
- Chao, Q., Rothenberg, M., Solano, R., Roman, G., Terzaghi, W., and Ecker, J.R. (1997). Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89, 1133–1144. [DOI] [PubMed] [Google Scholar]
- Clark, K.L., Larsen, P.B., Wang, X., and Chang, C. (1998). Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc. Natl. Acad. Sci. USA 95, 5401–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble, R.L., Qu, X., and Schaller, G.E. (2002). Mutational analysis of the ethylene receptor ETR1: Role of the histidine kinase domain in dominant ethylene insensitivity. Plant Physiol. 128, 1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, A.E., Chen, Q.-G., Findell, J.L., Schaller, G.E., and Bleecker, A.B. (1999). The relationship between ethylene binding and dominant insensitivity conferred by mutant forms of the ETR1 ethylene receptor. Plant Physiol. 121, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, A.E., Findell, J.L., Schaller, G.E., Sisler, E., and Bleecker, A.B. (2000). Ethylene perception by the ERS1 protein in Arabidopsis. Plant Physiol. 123, 1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himelblau, E., and Amasino, R.M. (2000). Delivering copper within plant cells. Curr. Opin. Plant Biol. 3, 205–210. [PubMed] [Google Scholar]
- Himelblau, E., and Amasino, R.M. (2001). Nutrients mobilized from leaves during leaf senescence. J. Plant Physiol. 158, 1317–1323. [Google Scholar]
- Hirayama, T., Kieber, J., Hirayama, N., Kogan, M., Guzman, P., Nourizadeh, S., Alonso, J., Dailey, W., Dancis, A., and Ecker, J.R. (1999). RESPONSIVE-TO-ANTAGONIST1, a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell 97, 383–393. [DOI] [PubMed] [Google Scholar]
- Hua, J., Chang, C., Sun, Q., and Meyerowitz, E.M. (1995). Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 269, 1712–1714. [DOI] [PubMed] [Google Scholar]
- Hua, J., and Meyerowitz, E.M. (1998). Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94, 261–271. [DOI] [PubMed] [Google Scholar]
- Hua, J., Sakai, H., Nourizadeh, S., Chen, Q.G., Bleecker, A., Ecker, B., Jr., and Meyerowitz, E.M. (1998). EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 10, 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber, J.J., Rothenberg, M., Roman, G., Feldmann, K.A., and Ecker, J.R. (1993). CTR1, a negative regulator of the ethylene-response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72, 427–441. [DOI] [PubMed] [Google Scholar]
- Klimyuk, V., Carroll, B., Thomas, C., and Jones, J. (1993). Alkali treatment for rapid preparation of plant material for reliable PCR analysis. Plant J. 3, 493–494. [DOI] [PubMed] [Google Scholar]
- Michaels, S., and Amasino, R. (1998). A robust method for detecting single-nucleotide changes as polymorphic markers by PCR. Plant J. 14, 381–385. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473.–497. [Google Scholar]
- Rodriguez, F.I., Esch, J.J., Hall, A.E., Binder, B.M., Schaller, G.E., and Bleecker, A.B. (1999). A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283, 996–998. [DOI] [PubMed] [Google Scholar]
- Roman, G., Lubarsky, B., Kieber, J.J., Rothenberg, M., and Ecker, J.R. (1995). Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: Five novel mutant loci integrated into a stress response pathway. Genetics 139, 1393–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai, H., Hua, J., Chen, Q.G., Chang, C., Medrano, L.J., Bleecker, A.B., and Meyerowitz, E.M. (1998). ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 95, 5812–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samac, D., Hironaka, C., Yallaly, P., and Shah, D. (1990). Isolation and characterization of the genes encoding basic and acidic chitinase in Arabidopsis thaliana. Plant Physiol. 93, 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, G.E., and Bleecker, A.B. (1995). Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270, 1809–1811. [DOI] [PubMed] [Google Scholar]
- Schaller, G.E., Ladd, A.N., Lanahan, M.B., Spanbauer, J.M., and Bleecker, A.B. (1995). The ethylene response mediator ETR1 from Arabidopsis forms a disulfide-linked dimer. J. Biol. Chem. 270, 12526–12530. [DOI] [PubMed] [Google Scholar]
- Solano, R., Stepanova, A., Chao, Q., and Ecker, J.R. (1998). Nuclear events in ethylene signaling, a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 12, 3703–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman, D., Taylor, M., Ciardi, J., and Klee, H. (2000). The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc. Natl. Acad. Sci. USA 97, 5663–5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., Hall, A.E., O'Malley, R., and Bleecker, A.B. (2003). Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proc. Natl. Acad. Sci. USA 100, 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, A.H., and Stock, A.M. (2001). Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26, 369–376. [DOI] [PubMed] [Google Scholar]
- Woeste, K., and Kieber, J. (2000). A strong loss-of-function mutation in RAN1 results in constitutive activation of the ethylene-response pathway as well as a rosette-lethal phenotype. Plant Cell 12, 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X.-C., Xiang, Q., Mathews, D.E., and Schaller, G.E. (2002). Effect of ethylene pathway mutations upon expression of the ethylene receptor ETR1 from Arabidopsis. Plant Physiol. 130, 1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]