Abstract
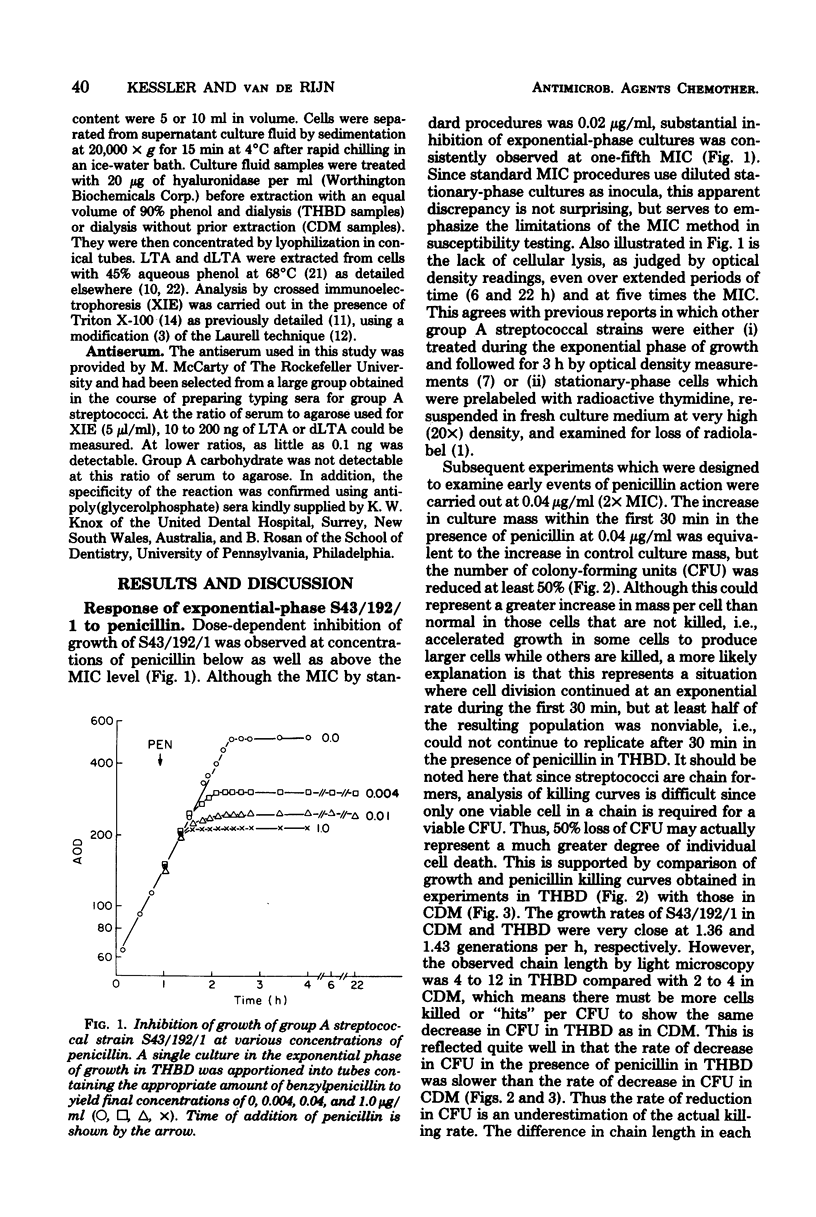
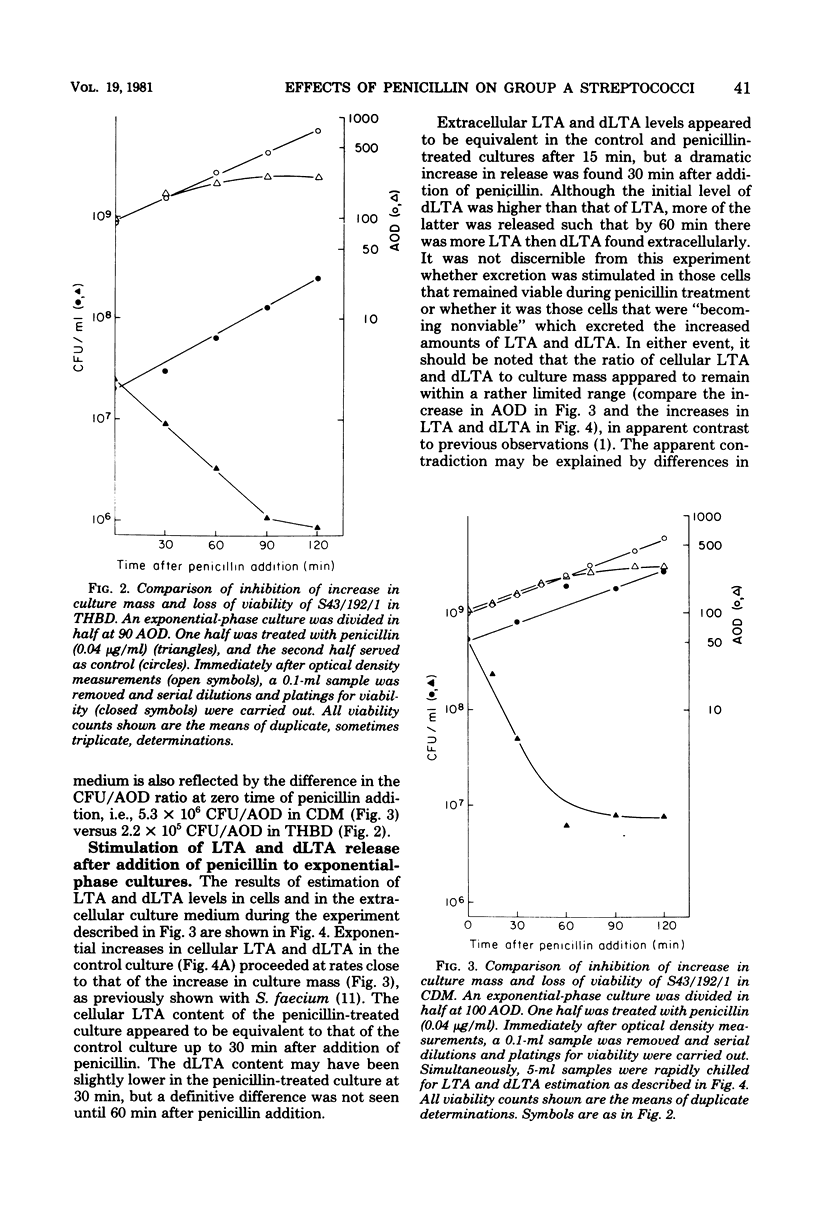
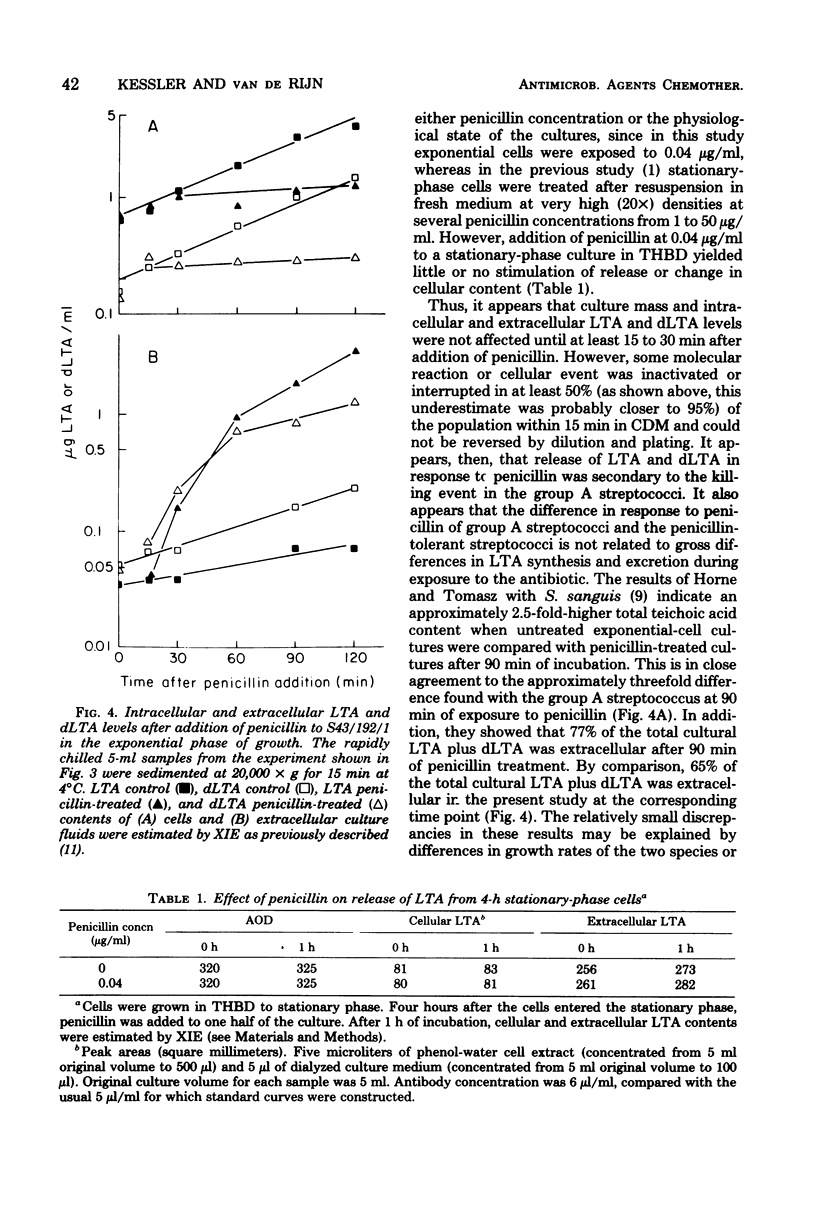
The rate of killing of a group A streptococcal species by penicillin was compared with the release of lipoteichoic acid (LTA) and its deacylated derivative, dLTA. Although there was no stimulation of release from stationary-phase cells in the presence of penicillin, there was dramatic release of LTA and dLTA from exponential-phase cells after the addition of penicillin. Although decreases in viability were observed within 15 min after addition of penicillin, culture mass and LTA content did not appear to be affected until after 30 min. Stimulation of release of LTA and dLTA appeared to take place after 15 but before 30 min after addition of penicillin. These observations are interpreted to indicate that the stimulation of release of LTA and dLTA in response to penicillin is secondary to the killing event.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M. L., Beachey E. H. Excretion of lipoteichoic acid by group A streptococci. Influence of penicillin on excretion and loss of ability to adhere to human oral mucosal cells. J Clin Invest. 1978 Mar;61(3):671–677. doi: 10.1172/JCI108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Wong W., Young F. E., Gilpin R. W. Isolation and characterization of a mutant of Staphylococcus aureus deficient in autolytic activity. J Bacteriol. 1976 Mar;125(3):961–967. doi: 10.1128/jb.125.3.961-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Daneo-Moore L., Wicken A. J., Shockman G. D. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J Bacteriol. 1976 Sep;127(3):1582–1584. doi: 10.1128/jb.127.3.1582-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Horne D., Hakenbeck R., Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977 Nov;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Release of lipoteichoic acid from Streptococcus sanguis: stimulation of release during penicillin treatment. J Bacteriol. 1979 Mar;137(3):1180–1184. doi: 10.1128/jb.137.3.1180-1184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977 May;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Precursor-product relationship of intracellular and extracellular lipoteichoic acids of Streptococcus faecium. J Bacteriol. 1979 Feb;137(2):869–877. doi: 10.1128/jb.137.2.869-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., van de Rijn I., McCarty M. Characterization and localization of the enzymatic deacylation of lipoteichoic acid in group A streptococci. J Exp Med. 1979 Dec 1;150(6):1498–1509. doi: 10.1084/jem.150.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- Mychajlonka M., McDowell T. D., Shockman G. D. Inhibition of peptidoglycan, ribonucleic acid, and protein synthesis in tolerant strains of Streptococcus mutans. Antimicrob Agents Chemother. 1980 Apr;17(4):572–582. doi: 10.1128/aac.17.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Antigenic and enzymatic architecture of Micrococcus lysodeikticus membranes established by crossed immunoelectrophoresis. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3711–3715. doi: 10.1073/pnas.72.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Cornett J. B., Mychajlonka M. Does penicillin kill bacteria?. Rev Infect Dis. 1979 Sep-Oct;1(5):787–796. doi: 10.1093/clinids/1.5.787. [DOI] [PubMed] [Google Scholar]
- Tomasz A. From penicillin-binding proteins to the lysis and death of bacteria: a 1979 view. Rev Infect Dis. 1979 May-Jun;1(3):434–467. doi: 10.1093/clinids/1.3.434. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Gibbens J. W., Knox K. W. Comparative studies on the isolation of membrane lipoteichoic acid from Lactobacillus fermenti. J Bacteriol. 1973 Jan;113(1):365–372. doi: 10.1128/jb.113.1.365-372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Chemical analysis of changes in membrane composition during growth of Streptococcus pyogenes. Infect Immun. 1979 Dec;26(3):883–891. doi: 10.1128/iai.26.3.883-891.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


