Abstract
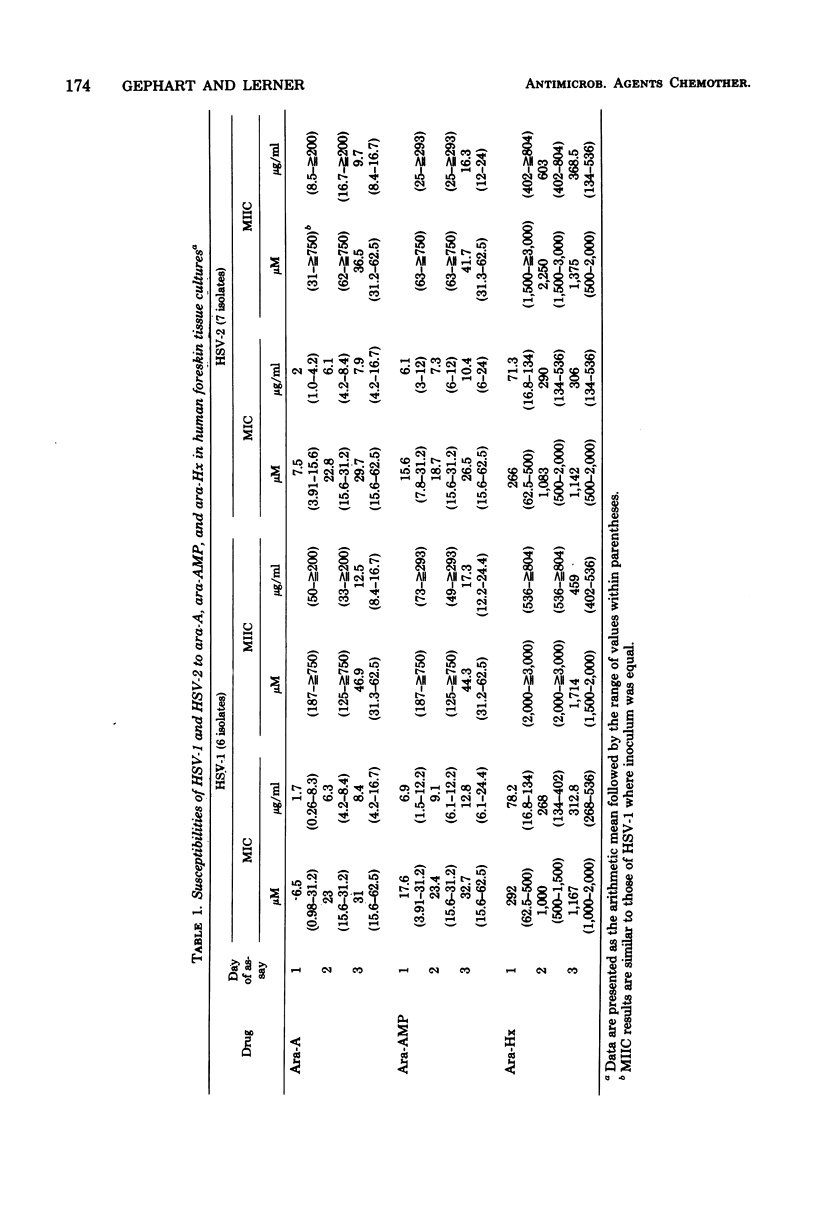
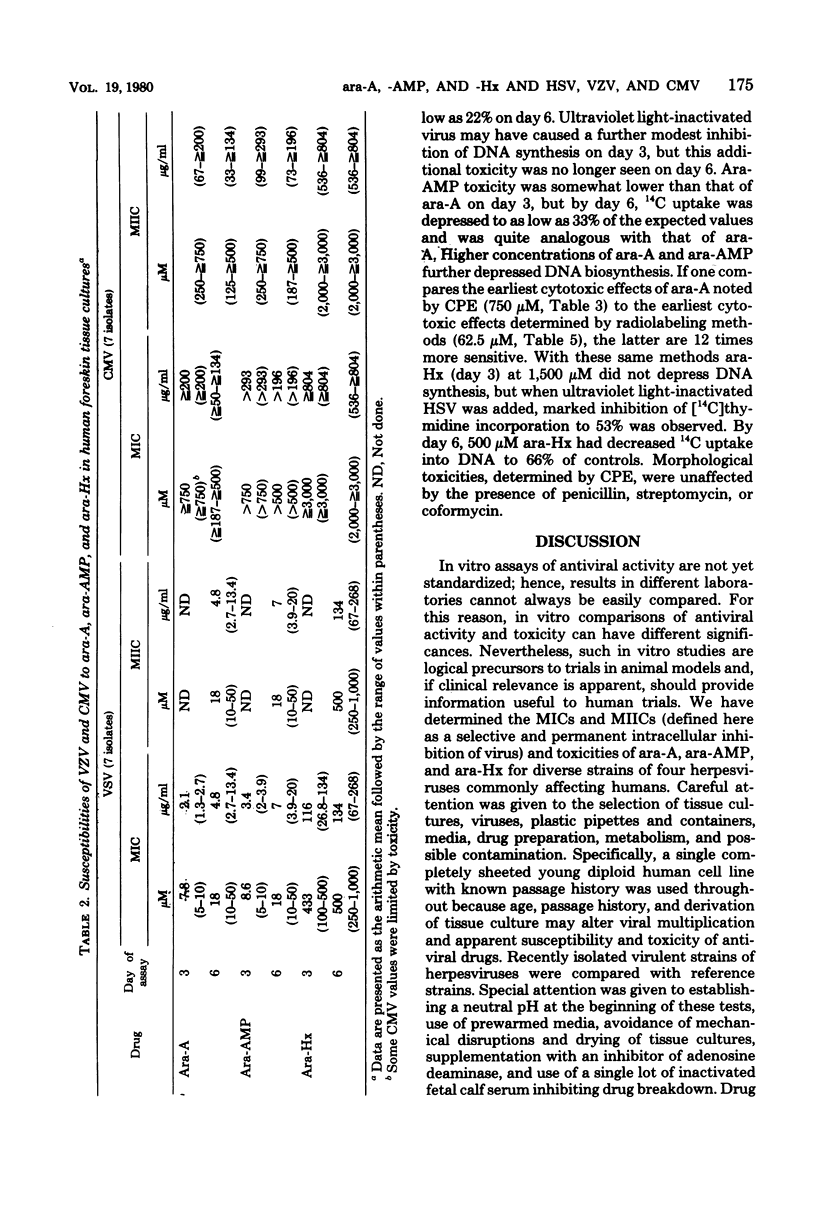
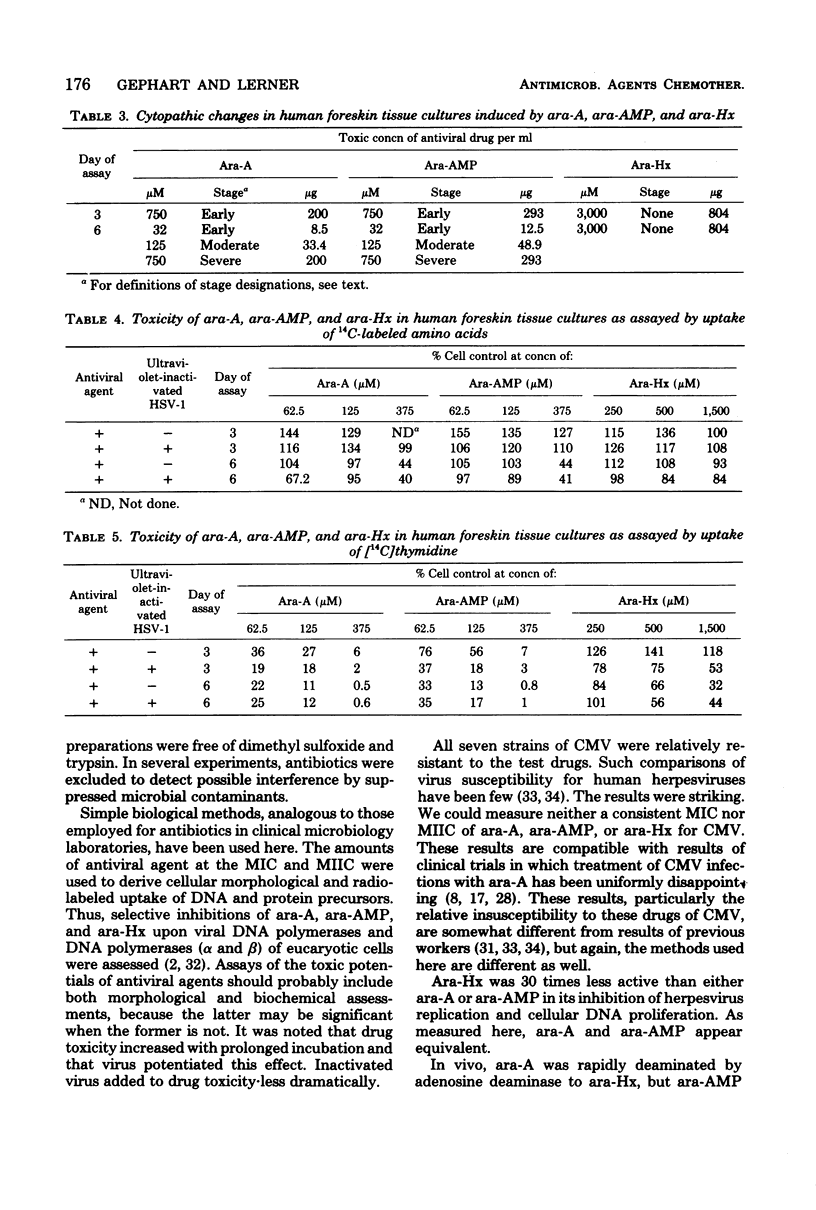
In a single line of human foreskin fibroblasts, minimum inhibitory concentrations (MICs) and the minimum intracellular virus inactivation concentrations (MIICs) of arabinosyladenine, arabinosylhypoxanthine, and arabinosyladenine 5'-monophosphate were assayed for a number of recent isolates of herpes simplex virus types 1 and 2 (HSV-1, HSV-2), varicella-zoster virus (VZV), and cytomegalovirus (CMV). (The term MIIC is used here to describe the selective qualitative intracellular inhibition of the virus inoculum in the primary tissue cultures. The inoculum is not recoverable in subcultures free of antiviral agent.) MICs and MIICs of each of the antiviral agents were readily obtained for each strain of HSV-1, HSV-2, and VZV tested, but all seven strains of CMV tested were much more resistant. At the endpoint, there was little variation in the MICs or MIICs among strans of the same virus. Final MIC results for HSV-1 and HSV-2 were complete after 3 days of incubation; CMV and VZV results required as long as 6 days. The MIC for each herpesvirus increased with incubation, but at the endpoint the MIC and MIIC were approximately equal. VZV was most susceptible to each drug, followed by HSV-1 and HSV-2. The latter two viruses were quite similar. There was no difference in antiviral susceptibilities among any of the strains of HSV-1, HSV-2, VZV, or CMV tested. The toxicities of arabinosyladenine, arabinosylhypoxanthine, and arabinosyladenine 5'-monophosphate were simultaneously compared by using both microscopic cytotoxicity and inhibition of uptakes of [14C]thymidine into cellular deoxyribonucleic acid and of 14C-labeled amino acids into protein. The selective inhibition of each antiviral agent against viral and cellular deoxyribonucleic acid polymerases was confirmed. Simultaneous assays of antiviral and anticellular activities of antiviral agents may be useful in projecting further in vivo experiments.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams H. G., Benson E. A., Alexander E. R., Vontver L. A., Remington M. A., Holmes K. K. Genital herpetic infection in men and women: clinical course and effect of topical application of adenine arabinoside. J Infect Dis. 1976 Jun;133 (Suppl):A151–A159. doi: 10.1093/infdis/133.supplement_2.a151. [DOI] [PubMed] [Google Scholar]
- Bennett L. L., Jr, Shannon W. M., Allan P. W., Arnett G. Studies on the biochemical basis for the antiviral activities of some nucleoside analogs. Ann N Y Acad Sci. 1975 Aug 8;255:342–358. doi: 10.1111/j.1749-6632.1975.tb29242.x. [DOI] [PubMed] [Google Scholar]
- Bryson Y. J., Connor J. D. In vitro susceptibility of varicella zoster virus to adenine arabinoside and hypoxanthine arabinoside. Antimicrob Agents Chemother. 1976 Mar;9(3):540–543. doi: 10.1128/aac.9.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson Y. J., Kronenberg L. H. Combined antiviral effects of interferon, adenine, arabinoside, hypoxanthine arabinoside, and adenine arabinoside-5'-monophosphate in human fibroblast cultures. Antimicrob Agents Chemother. 1977 Feb;11(2):299–306. doi: 10.1128/aac.11.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson Y., Connor J. D., Sweetman L., Carey S., Stuckey M. A., Buchanan R. Determination of plaque inhibitory activity of adenine arabinoside (9-beta-D-arabinofuranosyladenine) for herpesviruses using an adenosine deaminase inhibitor. Antimicrob Agents Chemother. 1974 Jul;6(1):98–101. doi: 10.1128/aac.6.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch'ien L. T., Cannon N. J., Charamella L. J., Dismukes W. E., Whitley R. J., Buchanan R. A., Alford C. A., Jr Effect of adenine arabinoside on severe Herpesvirus hominis infections in man. J Infect Dis. 1973 Nov;128(5):658–663. doi: 10.1093/infdis/128.5.658. [DOI] [PubMed] [Google Scholar]
- Ch'ien L. T., Cannon N. J., Whitley R. J., Diethelm A. G., Dismukes W. E., Scott C. W., Buchanan R. A., Alford C. A., Jr Effect of adenine arabinoside on cytomegalovirus infections. J Infect Dis. 1974 Jul;130(1):32–39. doi: 10.1093/infdis/130.1.32. [DOI] [PubMed] [Google Scholar]
- Champney K. J., Lauter C. B., Bailey E. J., Lerner A. M. Antiherpesvirus activity in human sera and urines after administration of adenine arabinoside: in vitro and in vivo synergy of adenine arabinoside and arabinosylhypoxanthine in combination. J Clin Invest. 1978 Dec;62(6):1142–1153. doi: 10.1172/JCI109233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C. T., Feng K. K., Brahmacupta N. Synergistic antiviral effects of adenine arabinoside and humoral antibodies in experimental encephalitis due to Herpesvirus hominis. J Infect Dis. 1976 Feb;133(2):157–167. doi: 10.1093/infdis/133.2.157. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Plunkett W. The utilization of nucleotides by animal cells. Ann N Y Acad Sci. 1975 Aug 8;255:269–286. doi: 10.1111/j.1749-6632.1975.tb29235.x. [DOI] [PubMed] [Google Scholar]
- Connor J. D., Sweetman L., Carey S., Stuckey M. A., Buchanan R. Effect of adenosine deaminase upon the antiviral activity in vitro of adenine arabinoside for vaccinia virus. Antimicrob Agents Chemother. 1974 Nov;6(5):630–636. doi: 10.1128/aac.6.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster D. J., McKinnon J. R., McGill J. I., Jones B. R., Fraunfelder F. T. Clinical evaluation of adenine arabinoside and trifluorothymidine in the treatment of corneal ulcers caused by herpes simplex virus. J Infect Dis. 1976 Jun;133 (Suppl):A173–A177. doi: 10.1093/infdis/133.supplement_2.a173. [DOI] [PubMed] [Google Scholar]
- Fiala M., Chow A. W., Miyasaki K., Guze L. B. Susceptibility of herpesviruses to three nucleoside analogues and their combinations and enhancement of the antiviral effect of acid pH. J Infect Dis. 1974 Jan;129(1):82–85. doi: 10.1093/infdis/129.1.82. [DOI] [PubMed] [Google Scholar]
- Kern E. R., Richards J. T., Overall J. C., Jr, Glasgow L. A. Alteration of mortality and pathogenesis of three experimental Herpesvirus hominis infections of mice with adenine arabinoside 5'-monophosphate, adenine arabinoside, and phosphonoacetic acid. Antimicrob Agents Chemother. 1978 Jan;13(1):53–60. doi: 10.1128/aac.13.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. J., Friedman-Kien A. E., Yellin P. B. Orofacial herpes simplex virus infection in hairless mice: latent virus in trigeminal ganglia after topical antiviral treatment. Infect Immun. 1978 Apr;20(1):130–135. doi: 10.1128/iai.20.1.130-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. J. Isolation of herpes simplex virus clones and drug resistant mutants in microcultures. Arch Virol. 1975;49(1):73–80. doi: 10.1007/BF02175598. [DOI] [PubMed] [Google Scholar]
- Kraemer K. G., Neiman P. E., Reeves W. C., Thomas E. D. Prophylactic adenine arabinoside following marrow transplantation. Transplant Proc. 1978 Mar;10(1):237–240. [PubMed] [Google Scholar]
- LePage G. A., Naik S. R., Katakkar S. B., Khaliq A. 9-beta-D-arabinofuranosyladenine 5'-phosphate metabolism and excretion in humans. Cancer Res. 1975 Nov;35(11 Pt 1):3036–3040. [PubMed] [Google Scholar]
- Legaspi R. C., Gatmaitan B., Bailey E. J., Lerner A. M. Interferon in biopsy and autopsy specimens of brain. Its presence in herpes simplex virus encephalitis. Arch Neurol. 1980 Feb;37(2):76–79. doi: 10.1001/archneur.1980.00500510034004. [DOI] [PubMed] [Google Scholar]
- Lerner A. M., Bailey E. J. Differential sensitivity of herpes simplex virus types 1 and 2 to human interferon: antiviral effects of interferon plus 9-beta-D-arabinofuranosyladenine. J Infect Dis. 1976 Oct;134(4):400–404. doi: 10.1093/infdis/134.4.400. [DOI] [PubMed] [Google Scholar]
- Marks M. I. Variables influencing the in vitro susceptibilities of herpes simplex viruses to antiviral drugs. Antimicrob Agents Chemother. 1974 Jul;6(1):34–38. doi: 10.1128/aac.6.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. E., Maidhof A., Zahn R. K., Shannon W. M. Effect of 9-beta-D-arabinofuranosyladenine on DNA synthesis in vivo. Cancer Res. 1977 Jul;37(7 Pt 1):2282–2290. [PubMed] [Google Scholar]
- Müller W. E., Rohde H. J., Beyer R., Maidhof A., Lachmann M., Taschner H., Kahn R. K. Mode of action of 9-beta-D-arabinofuranosyladenine on the synthesis of DNA, RNA, and protein in vivo and in vitro. Cancer Res. 1975 Aug;35(8):2160–2168. [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K., Bittlingmaier K., Falke D. Inhibition of herpesvirus DNA synthesis by 9-beta-D-arabinofuranosyladenine in cellular and cell-free systems. Ann N Y Acad Sci. 1977 Mar 4;284:34–48. doi: 10.1111/j.1749-6632.1977.tb21935.x. [DOI] [PubMed] [Google Scholar]
- Narang H. K., Codd A. A. Efficacy of adenine arabinoside (Ara-A) and cytarabine (Ara-C) in the treatment herpes encephalitis in animal model. J Antimicrob Chemother. 1978 Sep;4(5):468–469. doi: 10.1093/jac/4.5.468. [DOI] [PubMed] [Google Scholar]
- Ross A. H., Julia A., Balakrishnan C. Toxicity of adenine arabinoside in humans. J Infect Dis. 1976 Jun;133 (Suppl):A192–A198. doi: 10.1093/infdis/133.supplement_2.a192. [DOI] [PubMed] [Google Scholar]
- Rytel M. W., Kauffman H. M. Clinical efficacy of adenine arabinoside in therapy of cytomegalovirus infections in renal allograft recipients. J Infect Dis. 1976 Feb;133(2):202–205. doi: 10.1093/infdis/133.2.202. [DOI] [PubMed] [Google Scholar]
- Schabel F. M., Jr The antiviral activity of 9-beta-D-arabinofuranosyladenine (ARA-A). Chemotherapy. 1968;13(6):321–338. doi: 10.1159/000220567. [DOI] [PubMed] [Google Scholar]
- Schwartz P. M., Shipman C., Jr, Drach J. C. Antiviral activity of arabinosyladenine and arabinosylhypoxanthine in herpes simplex virus-infected KB cells: selective inhibition of viral deoxyribonucleic acid synthesis in the presence of an adenosine deaminase inhibitor. Antimicrob Agents Chemother. 1976 Jul;10(1):64–74. doi: 10.1128/aac.10.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell R. W., Allen L. B., Huffman J. H., Khwaja T. A., Tolman R. L., Robins R. K. Anti-DNA virus activity of the 5'-nucleotide and 3',5'-cyclic nucleotide of 9-beta-D-arabinofuranosyladenine. Chemotherapy. 1973;19(6):325–340. doi: 10.1159/000221473. [DOI] [PubMed] [Google Scholar]
- Sidwell R. W., Arnett G., Dixon G. J., Schabel F. M., Jr Purine analogs as potential anticytomegalovirus agents. Proc Soc Exp Biol Med. 1969 Sep;131(4):1223–1230. doi: 10.3181/00379727-131-34075. [DOI] [PubMed] [Google Scholar]
- Sloan B. J., Kielty J. K., Miller F. A. Effect of a novel adenosine deaminase inhibitor (co-vidarabine, co-V) upon the antiviral activity in vitro and in vivo of vidarabine (Vira-Atm) for DNA virus replication. Ann N Y Acad Sci. 1977 Mar 4;284:60–80. doi: 10.1111/j.1749-6632.1977.tb21937.x. [DOI] [PubMed] [Google Scholar]
- Sloan B. J., Miller F. A., McLean I. W., Jr Treatment of herpes simplex virus types 1 and 2 encephalitis in mice with 9-beta-D-arabinofuranosyladenine. Antimicrob Agents Chemother. 1973 Jan;3(1):74–80. doi: 10.1128/aac.3.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley R. J., Ch'ien L. T., Dolin R., Galasso G. J., Alford C. A., Jr Adenine arabinoside therapy of herpes zoster in the immunosuppressed. NIAID collaborative antiviral study. N Engl J Med. 1976 May 27;294(22):1193–1199. doi: 10.1056/NEJM197605272942201. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Soong S. J., Dolin R., Galasso G. J., Ch'ien L. T., Alford C. A. Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis. National Institute of Allergy and Infectious Diseases collaborative antiviral study. N Engl J Med. 1977 Aug 11;297(6):289–294. doi: 10.1056/NEJM197708112970601. [DOI] [PubMed] [Google Scholar]
- Williams B. B., Bailey E. J., Lerner A. M. Inhibitory and lethal concentrations of 9-beta-D-arabinofuranosyladenine and its hypoxanthine-derivative versus herpes simplex virus, type 1. J Lab Clin Med. 1977 Apr;89(4):687–691. [PubMed] [Google Scholar]



