Abstract
To investigate the factors involved in the sorting of cargo proteins into COPII endoplasmic reticulum (ER) to Golgi apparatus transport vesicles, we have created a strain of S. cerevisiae (p24Δ8) that lacks all eight members of the p24 family of transmembrane proteins (Emp24p, Erv25p, and Erp1p to Erp6p). The p24 proteins have been implicated in COPI and COPII vesicle formation, cargo protein sorting, and regulation of vesicular transport in eukaryotic cells. We find that p24Δ8 cells grow identically to wild type and show delays of invertase and Gas1p ER-to-Golgi transport identical to those seen in a single Δemp24 deletion strain. Thus, p24 proteins do not have an essential function in the secretory pathway. Instead, they may serve as quality control factors to restrict the entry of proteins into COPII vesicles.
In eukaryotic cells, vesicular transport connects the organelles of the secretory pathway (1). Although vesicular coat proteins for several such transport steps have been described (2, 3), regulation of vesicle budding and mechanisms of cargo protein inclusion remain poorly understood. Recently, a family of type I transmembrane proteins of 23 to 27 kDa, termed p24 proteins, has been implicated in the formation of vesicles and the selection of cargo in both directions between the endoplasmic reticulum (ER) and the Golgi apparatus. These proteins (named Emp24p, Erv25p, and Erp1p to Erp6p in Saccharomyces cerevisiae, with homologs in all eukaryotes examined) have a luminal domain of about 180 amino acids with a predicted coiled coil, and a short (10–15 amino acids) cytosolic tail (4–6). The cytosolic sequences of all p24 proteins are similar, and some contain K(X)KXX motifs for COPI-dependent Golgi-to-ER retrieval (7, 8). The luminal domains diverge between proteins, which allows a classification into α, β, γ, and δ subtypes (6). p24 family members form heterotetrameric complexes that seem to contain one protein of each class (4).
p24 proteins were identified as major components of COPI coated vesicles derived from CHO cell Golgi membranes (5) and of COPII coated vesicles budded from yeast microsomes (9). All p24 proteins seem to cycle between the ER and the Golgi apparatus (10, 11). At steady state, Emp24p and Erv25p have been found mainly in the ER in S. cerevisiae (9, 12), whereas in mammalian cells, p24 proteins are localized to the Golgi apparatus and the tubulovesicular compartment on the cis side of the Golgi stack (13, 14). The reason for the apparent difference in localization is not clear.
Three roles have been proposed for p24 proteins. First, a role in the formation of secretory vesicles in S. cerevisiae is suggested by the observation that in a sec18-1 Δemp24 double mutant, the number of vesicles accumulated at the restrictive temperature is significantly lower than in a sec18-1 strain (5). The nature of the accumulated vesicles is unknown, as sec18 mutants are blocked not only in ER-to-Golgi transport but also in later steps of the secretory pathway (15) and endocytosis (16); they also vesiculate the Golgi apparatus itself (17). Both COPI coatomer and the COPII component Sec23/24p bind to some cytoplasmic tails of p24 proteins (6, 14, 18); this interaction could create binding sites for COPI and COPII coats on Golgi and ER membranes and thus support the formation of vesicles. Indeed, binding of coatomer to liposomes was shown to depend on p24 cytosolic tail peptides coupled to lipids (18). In another study using a different lipid composition, this dependence was not observed (19).
A role for p24 proteins as transport receptors for secretory cargo was proposed because of their divergent luminal domains and their ability to oligomerize (4, 6, 10, 12). Combinatorial p24 complexes could theoretically exhibit a variety of luminal cargo binding sites (5). Accordingly, deletion of EMP24 in S. cerevisiae leads to a delay in the maturation of invertase and Gas1 proteins, whereas carboxypeptidase Y (CPY) and pro-α factor are secreted at wild-type rates. This delay has led to the suggestion that Emp24p may be a specific receptor that mediates uptake of Gas1p and invertase into COPII vesicles (9). However, specific association of any p24 protein with vesicular cargo has not yet been shown, and oligomerization of p24 proteins is not random but comprises specific complexes of α, β, γ, and δ subclasses (4).
p24 proteins also act as negative regulators of vesicle budding or cargo sorting. Deletion of EMP24/BST2 suppresses the otherwise lethal effects of a mutation or deletion of the COPII gene, SEC13 (20), but not other COPII mutations such as sec12-1. It was suggested that in Δsec13 cells, COPII trafficking is impaired, and that deletion of EMP24 removes a block that normally restricts the entry of proteins into newly formed COPII vesicles. Suppression of the sec13-1 temperature-sensitive defect also occurs in Δerv25 and Δerp1, but not in any of the Δerp2 to Δerp6 single-deletion strains (4).
Similarly, in Caenorhabditis elegans, loss or truncation of the Emp24p ortholog, SEL-9, or the Erv25p ortholog, F47G9.1, increases the function of the mutant genes lin-12(n676-n930) and glp-1(e2142), homologs of the Drosophila notch cell surface receptor gene. In the case of GLP-1, this is due to the release of a block that causes the mutant protein to remain in the ER. Thus, in glp-1(q415); sel-9(ar174) double mutants, GLP-1(q415) can leave the ER, and its plasma membrane localization is restored at least partially (21).
To define the roles of the p24 proteins in S. cerevisiae, we have tested the function of the early secretory pathway in a strain deleted for all eight genes of this family.
Materials and Methods
Media, Yeast Strains, and Genetic Methods.
Growth and maintenance of yeast strains (listed in Table 1) followed standard techniques (22). The record of ARY104 in ref. 4 is amended here. Deletion of ERP3 to ERP6 genes was performed with a kanamycin-resistance cassette and retrieval of the marker gene, by cre/lox- mediated excision (23). The oligonucleotides used to disrupt p24 genes are shown in Table 2. Clones were verified for proper insertion of the deletion construct and for marker excision by PCR using primers that hybridized ca. 300 bp upstream and downstream of the deleted gene. Disruption of IRE1 in RSY1888 was achieved with a plasmid provided by P. Walter (24) and verified by PCR.
Table 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| DHY9 (wild type) | MATα ura3-52 lys2-801 ade2-101 trp1-Δ1 his3-Δ200 leu2-Δ1 (derivative of YPH274, ATCC) | A. Rowley |
| DHY3 (Δemp24) | DHY9, Δemp24∷HIS3 | A. Rowley |
| ARY104 (p24Δ4) | DHY9, Δemp24∷HIS3 Δerv25∷HIS3 Δerp1∷TRP1 Δerp2∷HIS3 | A. Rowley |
| RSY1888 (p24Δ8) | DHY9, Δemp24∷HIS3 Δerv25∷HIS3 Δerp1∷TRP1 Δerp2∷HIS3 Δerp3∷loxP Δerp4∷loxP Δerp5∷loxP Δerp6∷loxP-Kanr-loxP | This study |
| ECY1 | RSY1888, ire1∷URA3 | This study |
| PC70 | MATα ura3 leu2 trp1 ret1-1 | F. Letourneur |
| YTX106 | MATα his3-Δ200 lys2-801am leu2-3,-112 trp1-Δ1 ura3-52 ubc7∷LEU2 | T. Sommer |
| EGY021.2 | MATa sec21∷HIS3 trp1 leu2 suc2-Δ9 (pLEU2 sec21-3) | S. Emr |
Affiliations: F. Letourneur, Institut de Biologie et Chimie des Protéines/Centre National de la Recherche Scientifique UPR 412, Lyon, France; T. Sommer, Max Delbrück Centre for Molecular Medicine, Berlin; S. Emr, University of California, San Diego.
Table 2.
Oligonucleotides used to disrupt p24 genes
| Disruption | Sequence | Name |
|---|---|---|
| ERP3, 5′ | ATAGTTACCGTACTTGAAGGGACACTGTGAACTGACTAAAAAACTCAGCTGAAGCTTCGTACGCT | oEC11 |
| ERP3, 3′ | TATATTCTCAAGTTGATAGAAAATGCAGGAACAATACACAACTATATAGGCCACTAGTGGATCTG | oEC12 |
| ERP4, 5′ | TGAAGTTTTTTCATAAATATATATTCCCAATCATCGCTAGGAATTCAGCTGAAGCTTCGTACGCT | oEC13 |
| ERP4, 3′ | CCTTAGCACAGCTGATCCAACAATTTTAAGAGCTTGAAAAGCAACATAGGCCACTAGTGGATCTG | oEC14 |
| ERP5, 5′ | TTACTTAAGGGAACACATCAAGCATTCGGTGTCTCACAGGCTACTCAGCTGAAGCTTCGTACGCT | oEC15 |
| ERP5, 3′ | TTCGTAATGAGCGATATATAAACTCTATATAACAAACATGGTATAATAGGCCACTAGTGGATCTG | oEC16 |
| ERP6, 5′ | GCTTTCTTCCTTATCGCCTCAATCTGAAAGGATCTAGATTTGCCACAGCTGAAGCTTCGTACGCT | oEC17 |
| ERP6, 3′ | GCTAAGGATTCAATTTTTTGATATGTACGGTCATAATACTTTCTGATAGGCCACTAGTGGATCTG | oEC19 |
Biochemical Characterization.
Analysis of general secretion competence (25), Kar2p secretion (4), invertase secretion caused by the constitutive pCYI-20 expression plasmid (26, 27), the time course of invertase secretion (28), pulse–chase experiments for Gas1p and CPY (4) and for the invertase-Wbp1p fusion protein (8, 27), and electron microscopy (29) were performed as described.
Results
Deletion of All p24 Genes.
No single p24 gene deletion affects S. cerevisiae viability, but four single deletions, namely emp24, erv25, erp1, and erp2, display secretory phenotypes. Deletion of these four genes in a single strain (p24Δ4) does not cause any additional phenotypes compared with the single Δemp24, possibly because all proteins that are missing in this quadruple deletion strain are members of a single heteromeric complex and become almost undetectable when EMP24 or ERV25 is deleted (4). This result still allows the hypothesis that the members of the p24 family have essential but overlapping functions. Indeed, some redundancy between Erp1p and Erp6p and between Erp2p and Erp4p in the suppression of Kar2p secretion from cells has been observed (4).
We used a marker retrieval method (23) to delete the remaining four p24 open reading frames from the p24Δ4 strain. The resulting strain (p24Δ8), in which all eight deletions were again confirmed by PCR, grew with kinetics identical to wild type at 30°C, 34°C, and 37°C (data not shown). Mitochondrial, peroxisomal, and vacuolar functions seemed intact, judged by the ability of all deletion strains to grow like wild type on agar plates with high (1 M NaCl) and low (0.5× YPD) osmolarity (30), 500 mM Ca2+ (31), 100 mM phosphate pH 7.5 (32), and the nonfermentable carbon sources oleate, glycerol, and lactate (30, 33).
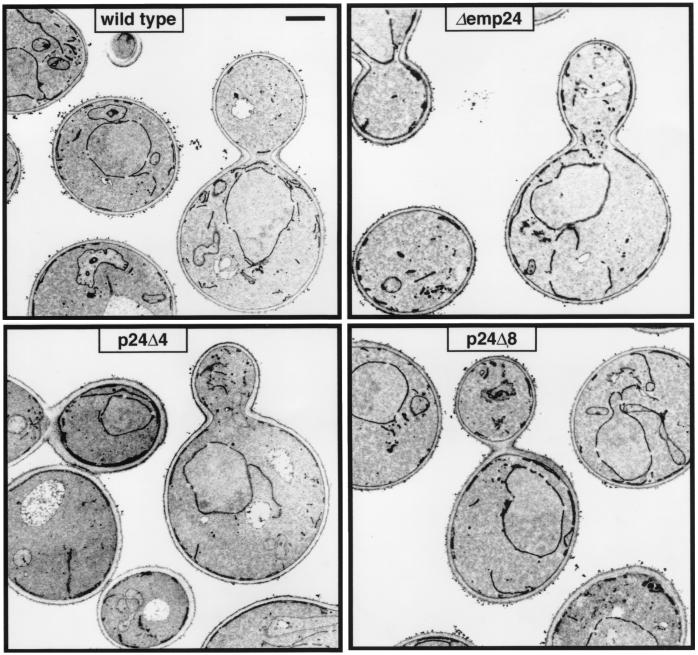
We examined the p24 deletion strains by electron microscopy to see whether any abnormality of the intracellular membrane system was apparent. Yeast cells that bear mutant alleles of COPI or COPII genes, and are thus blocked in protein transport between the ER and the Golgi apparatus, accumulate ER membranes (34–36). In our Δemp24, p24Δ4, and p24Δ8 strains, no striking differences from wild type were observed (Fig. 1), in particular no accumulation of ER or any other intracellular membranes. From examining a large number of sections, we gained the impression that the mutant strains showed a subtle but notable increase in the diameter and electron density of the nuclear envelope and the ER, but we were unable to quantify this observation. From the viability of the p24Δ8 strain, and the intact endomembrane system, we conclude that neither COPI- nor COPII-mediated transport is blocked in the absence of p24 proteins.
Figure 1.
Cells bearing p24 deletions exhibit no morphological abnormalities. Electron micrographs of logarithmic-phase cells grown in YPD (yeast extract/peptone/dextrose) at 30°C. (Bar = 1 μm.)
ER-to-Golgi Transport of Proteins in the p24Δ8 Mutant.
The delay in transport of Gas1p and invertase in strains lacking EMP24 or ERV25 (see Introduction) has been interpreted as evidence for a role of Emp24p as a transport receptor for these proteins, and it was suggested that the continuing transport of these and other proteins in Δemp24 cells is caused by partial functional redundancy of Emp24p and Erv25p with the remaining p24 family members. If this is so, the p24Δ8 strain should exhibit a severe block in the transport of at least some secretory proteins.
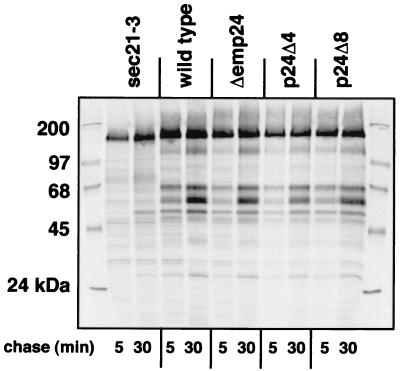
We first examined whether the general secretion competence of the cells was altered (Fig. 2). We labeled cells with 35S-labeling mix for 10 min and chased for 5 or 30 min. Proteins were then precipitated from the medium, resolved by SDS–PAGE, and detected by autoradiography. With wild-type cells, we observed the typical pattern of the major secreted proteins of S. cerevisiae (25). In sec21-3 cells, which carry a defective gene for the γ subunit of COPI coatomer, protein secretion was seriously compromised at the restrictive temperature, because a block of COPI traffic exerts an indirect inhibitory effect on ER-to-Golgi transport (25). We found that in the Δemp24 strain, as well as in our p24Δ4 and p24Δ8 mutants, the amount and composition of the secreted protein pool were indistinguishable from wild type. This result rules out severe defects in both COPII- and COPI-mediated vesicular transport in these strains. However, proteins that are bound to the membrane (such as Gas1p), retained in the periplasmic space (such as invertase), directed to intracellular compartments (such as CPY), or of low abundance, are not detected in this assay; thus, more specific defects could have escaped our attention.
Figure 2.
Cells bearing p24 deletions are secretion competent. Cells were preshifted to 37°C to induce the sec21-3 phenotype, pulse labeled for 10 min, and chased for the indicated times. Labeled proteins from the supernatant were separated by SDS–PAGE and detected by autoradiography.
We therefore examined the intracellular transport of individual proteins that show a delay in transport in the single Δemp24 strain, namely Gas1p and invertase, by pulse–chase analysis. We reasoned that if these proteins are fully dependent on members of the p24 family for ER-to-Golgi transport, no Golgi modification should occur in p24Δ8 cells.
Gas1p, a glycosyl-phosphatidylinositol (GPI) anchored protein targeted to the plasma membrane, is produced as a 105-kDa form in the ER which matures into a 125-kDa glycosylated form in the Golgi apparatus. In Δemp24 cells, its maturation to the Golgi form is delayed by 15–30 min, consistent with a slower transport of the protein out of the ER; but after 60 min of chase time, no difference is apparent between wild type and the Δemp24 mutant (9, 37). We found that in the p24Δ4 and p24Δ8 deletion strains, Gas1p matured exactly as in Δemp24 (Fig. 3). Thus, Gas1p does not depend exclusively on p24 proteins as receptors for its exit from the ER.
Figure 3.
The Gas1p maturation delay of Δemp24 cells is not exacerbated in p24Δ4 and p24Δ8 cells. Cells were pulse labeled for 5 min and chased for the indicated times. Gas1p was immunoprecipitated from cell extracts, resolved by SDS–PAGE, and detected by autoradiography. Migration positions of mature (125-kDa) and immature (105-kDa) forms are indicated.
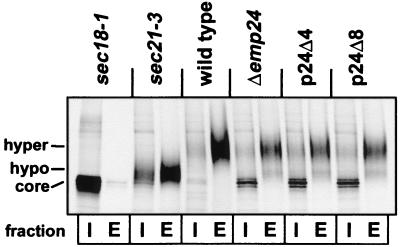
To determine whether the same was true for the soluble protein invertase (Suc2p), we performed pulse–chase experiments examining the internal and external (periplasmic) levels of this protein, expressed from a 2 μ plasmid under the control of the CPY promoter (Fig. 4). The ER form, core glycosylated to an apparent molecular mass of approximately 70 kDa, is modified in the cis-Golgi to a 90-kDa form, hyperglycosylated in the distal Golgi, and secreted into the periplasmic space, where it is retained. Initially, we performed an experiment with a single chase point (10 min; see Fig. 4). At this time, invertase from wild-type cells is present almost entirely in the periplasmic space in a hyperglycosylated form. However, in sec18 cells (where fusion of COPII vesicles with the Golgi is blocked), no extracellular invertase and no maturation of the intracellular form were detected, indicating that ER-to-Golgi transport of invertase was abolished. In sec21-3 COPI mutant cells, secretion was partially blocked after 10 min, and the secreted protein was hypoglycosylated. In contrast, in Δemp24, p24Δ4, and p24Δ8 cells, invertase was partially retained in the ER, and the secreted protein was mainly hyperglycosylated. In the p24Δ4 and p24Δ8 cells, no exacerbation of the retention phenotype observed in Δemp24 (9) was seen at the 10-min time point (Fig. 4). When we induced endogenous invertase by shifting cells into 0.1% glucose and measured amounts of intracellular and extracellular invertase at time-points of 5, 15, 30, and 60 min by pulse–chase analysis and immunoprecipitation, we could see no difference in the kinetics of invertase processing or secretion between Δemp24, p24Δ4, and p24Δ8 cells (data not shown). Thus, secretion of invertase, like that of Gas1p, does not depend entirely on members of the p24 family as ER-to-Golgi protein transport receptors.
Figure 4.
The invertase maturation delay in Δemp24 cells is not exacerbated in p24Δ4 and p24Δ8 cells. Cells expressing invertase from the CPY promoter were pulse labeled for 10 min and chased for 10 min. Spheroplasts were prepared and centrifuged to obtain the cytoplasmic (I, internal) and periplasmic (E, external) fractions. Invertase was immunoprecipitated, resolved by SDS–PAGE, and detected by autoradiography. Migration positions of core-glycosylated (ER form) and hypo- and hyperglycosylated (Golgi forms) are indicated.
We next examined the transport of CPY, a protein that is unaffected by the EMP24 deletion (9), assuming that Erp3p to Erp6p may be its specific receptors, and that its transport may be affected in the p24Δ8 strain. CPY has an apparent molecular mass of 67 kDa in the ER (p1 form), matures in the Golgi to the 69-kDa p2 form, and is finally processed in the vacuole to the mature 61-kDa form, mCPY (38). After 5 min of chase, most of the labeled CPY was in the p2 form, and maturation (indicating arrival at the vacuole) was essentially complete after 30 min in all strains. We observed no qualitative or quantitative differences between the wild type, Δemp24, p24Δ4, and p24Δ8 strains (Fig. 5). Thus, CPY transport from the ER to the vacuole is entirely independent of p24 proteins.
Figure 5.
CPY secretion is not affected in p24 deletion strains. Cells were pulse labeled for 5 min and chased for the indicated times. CPY was immunoprecipitated from cell extracts, resolved by SDS–PAGE, and detected by autoradiography. Migration positions of p1 (ER), p2 (Golgi), and mature (vacuole) forms are indicated.
The results from these pulse–chase experiments speak against an important role of p24 proteins in intracellular traffic in S. cerevisiae. The p24Δ8 strain is viable and shows no severe secretion defects. We have found no exacerbation in the p24Δ8 strain of any of the secretion defects described for Δemp24, which suggests that the function(s) of p24 proteins in ER-to-Golgi traffic is mainly borne by the complex of Emp24p, Erv25p, Erp1p, and possibly Erp2p. Accordingly, erp3 to erp6 single-deletion strains do not exhibit any secretion defects (4). We cannot rule out the possibility, however, that p24 proteins mediate the secretion of proteins that have not been examined in this context. These hypothetical proteins are then either not wholly dependent on p24 proteins for their localization or not essential for cell growth.
p24 Deletion Strains Can Retrieve Proteins from the cis-Golgi to the ER.
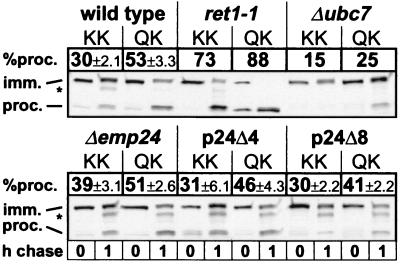
Because of their prominent presence in mammalian COPI vesicles, p24 proteins have been assumed to play a structural role in their formation (18). In this case, deletion of the p24 family should impair COPI vesicle formation and Golgi-to-ER retrieval of proteins. In mutant yeast cells where COPI-mediated trafficking is affected, COPII-dependent ER-to-Golgi traffic is usually impaired as well, and ER membranes accumulate (see refs. 35 and 36 and the sec21-3 mutant in Fig. 4). However, ER does not accumulate in our p24 deletion strains, and thus it is unlikely that any of them is totally blocked in COPI-mediated trafficking. To investigate whether a partial defect is present, we used a fusion of invertase to the C terminus of Wbp1p, which contains a transmembrane domain and a C-terminal cytosolic KKTN sequence. This fusion protein is retrieved efficiently from the cis-Golgi in wild-type cells but escapes retention in COPI mutants, where it is transported to the vacuole and proteolytically processed (8, 27). We performed quantitative pulse–chase analyses with strains expressing this protein; as the forward transport of invertase is delayed in the Δp24 strains (see Fig. 4), we used a fusion protein terminating in QKTN (which is not retrieved from the Golgi) as a forward transport control (Fig. 6). From this control, it appeared that with more p24 proteins deleted, transport of the fusion protein to the vacuole became slightly less efficient (from 53% ± 3.3% in wild-type cells to 41% ± 2.2% in p24Δ8). This effect is different from the delay of invertase trafficking (see Fig. 4), because the Suc2-Wbp1p fusion is not significantly delayed in the Δemp24 strain (51% ± 2.6% against 53% ± 3.3% in the wild type), and invertase shows no gradual increase in the severity of the delay. In the Δemp24 strain, retrieval of the Suc2-Wbp1p fusion was indeed partially defective (39% ± 3.1% reached the vacuole compared with 30% ± 3.1% in the wild type), but this deficiency was reversed in the p24Δ4 and p24Δ8 strains and is therefore not a direct consequence of the lack of p24 proteins. We do not know what the cause of the partial retrieval defect in the Δemp24 strain is, but we speculate that it may be indirect, possibly because of the presence of unstable Erv25, Erp1, and Erp2 proteins in this background (4, 12). We conclude that in S. cerevisiae, p24 proteins are not required to form Golgi-to-ER retrograde vesicles or to package the Suc2-Wbp1p cargo protein into them. p24 proteins are thus unlikely to represent important structural components of COPI vesicles.
Figure 6.
Retrieval of Suc2-Wbp1p-KKTN (KK) and Suc2-Wbp1p-QKTN (QK) in wild-type, mutant, and p24 deletion strains. Cells were pulse labeled for 10 min and chased for 0 or 1 h. The fusion protein was immunoprecipitated from cell extracts, treated with endoglycosidase H, resolved by SDS–PAGE, and detected by autoradiography. Migration positions of immature and processed (vacuole) forms are indicated. Percentages of processed forms are averages of three experiments with standard deviations where applicable. The asterisk denotes a band that may represent either an intermediate PEP4-independent proteolytic product or nonspecific cross-reactive material, which was not included in the quantitation.
The Δp24 Strains Have No Constitutive or Early-Onset Unfolded Protein Response.
In eukaryotic cells, conditions that result in the accumulation of unfolded proteins in the ER (such as exposure to tunicamycin or a block in ER-to-Golgi transport) elicit the unfolded protein response (UPR), leading to an increase in the production of proteins that promote protein folding (39, 40). Loss of the Ire1p transmembrane kinase, which mediates the UPR, renders yeast cells hypersensitive to ER stress and accumulation of unfolded proteins (41). We reasoned that an ER-to-Golgi transport defect in the Δp24 strains may manifest itself through induction of the UPR. We introduced into these strains a plasmid (pMCZ2) that carried the β-galactosidase gene under the control of the UPR element. This procedure allows the monitoring of UPR induction by the blue color that the colonies develop on 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) plates (42). On YPD plates, neither wild-type nor Δp24 strains showed a UPR, whereas on plates containing tunicamycin, a strong rapid-onset UPR was observed in all cells. Intensity and onset of these UPR responses were identical in wild-type and Δp24 cells (data not shown), indicating that the UPR was neither inhibited nor induced by deletion of the p24 genes. Accordingly, deletion of IRE1 in p24Δ8 cells had no effects on cell growth at a range of temperatures (not shown). Thus, complete lack of p24 proteins does not lead to a significant accumulation of unfolded proteins in the ER of yeast cells.
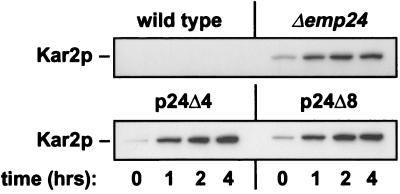
In several single p24 deletion strains, secretion of the ER chaperones Kar2p and Pdi1p into the medium has been observed (4, 12, 20). There is no further increase in Kar2p secretion when its HDEL retrieval signal is deleted, suggesting that Golgi-to-ER retrieval of Kar2p is not functional in Δemp24 cells (20). We found that this phenotype was not exacerbated in p24Δ4 and p24Δ8 cells (Fig. 7). Kar2p retention may therefore be fully deficient in Δemp24 cells already. Alternatively, the mutant cells may secrete only a small fraction of the total Kar2p produced into the medium.
Figure 7.
The Kar2p retention defect in Δemp24 cells is not exacerbated in p24Δ4 and p24Δ8 cells. Cells were transferred to fresh media, and Kar2p was immunoprecipitated from supernatants withdrawn at the indicated times, resolved by SDS–PAGE, and detected by immunoblotting.
Discussion
Deletion of the p24 Gene Family.
We report the deletion of all p24 genes from S. cerevisiae. Growth of the p24Δ8 strain (with every deletion retested) is indistinguishable from growth of wild type under all conditions examined. p24Δ8 cells show no exacerbation of the secretion phenotypes found in Δemp24, apart from a slight decrease in Suc2-Wbp1p-QKTN delivery to the vacuole. The deletions were made sequentially over a period of several months, and we are aware that bypass suppressors in other genes may have arisen during this time, masking a more severe phenotype. Still, the conclusion holds that p24 proteins are not strictly necessary for vesicular trafficking.
Role of p24 Proteins in the Generation of COPII and COPI Vesicles.
Our experiments demonstrate that in S. cerevisiae p24 proteins are required neither for the general function of the secretory pathway nor specifically to form COPI or COPII vesicles. In in vitro budding experiments using p24Δ8 microsomes, pre-pro-α factor is packaged into COPII vesicles with wild type efficiency (M. Lee, personal communication).
p24 Proteins Are Not Essential Cargo Receptors.
Our results lead to a restrictive conclusion. If p24 proteins are transport receptors for ER-to-Golgi trafficking, then transport of their cognate cargo either is not essential for S. cerevisiae survival or can also be achieved by different means, such as other receptors or bulk flow. In mammalian cells, ERGIC-53 is such a nonessential cargo receptor (43). In the mouse, loss of the Erv25p ortholog p23 leads to early embryonal death (44). This observation suggests that p24 proteins play a more prominent role in mammals, although a role in secretion or membrane assembly was not established in these studies.
p24 Proteins as Potential Negative Regulators of ER-to-Golgi Traffic.
p24 proteins have been suggested to restrict the entry of (especially unfolded) proteins into COPII vesicles by an unknown mechanism (see Introduction). It is not clear to us how p24 complexes suppress the bulk flow secretion of some proteins while being packaged efficiently into COPII vesicles themselves. Given their ability to interact with the COPII coat, p24 complexes may act as “placeholders” to prohibit other proteins with a lower affinity to COPII proteins from entering COPII vesicles. Cargo proteins that efficiently interact with COPII components could then displace p24 complexes from COPII budding sites, securing a space for themselves in emerging vesicles. A similar mechanism may act in the COPI-mediated retrieval pathway.
It seems surprising that the Δemp24, p24Δ4, and p24Δ8 cells do not exhibit a constitutive UPR even though they secrete Kar2p (Fig. 7). The amount of Kar2p in the cells at steady state, however, seems to be approximately the same in all four strains (S.S., unpublished results); thus, either Kar2p synthesis is up-regulated by a means other than the UPR to compensate for the loss in the mutants, or the fraction of Kar2p that is lost by secretion is relatively small.
Acknowledgments
We thank Dr. P. Walter for a plasmid and Dr. M. Lee for communicating unpublished data. S.S. is supported by a Senior Fellowship of the American Cancer Society, California Division. E.C. was supported by a Summer Research Fellowship of the Howard Hughes Medical Institute. R.D. is funded by a Wellcome Trust Senior Research Fellowship. S.M. is the recipient of a Guggenheim Foundation Fellowship. R.S. is a Howard Hughes Medical Institute Investigator.
Abbreviations
- ER
endoplasmic reticulum
- CPY
carboxypeptidase Y
- UPR
unfolded protein response
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070044097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070044097
References
- 1.Palade G. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- 2.Schekman R, Barlowe C, Bednarek S, Campbell J, Doering T, Duden R, Kuehn M, Rexach M, Yeung T, Orci L. Cold Spring Harbor Symp Quant Biol. 1995;60:11–21. doi: 10.1101/sqb.1995.060.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Rothman J E, Wieland F T. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 4.Marzioch M, Henthorn D C, Herrmann J M, Wilson R, Thomas D Y, Bergeron J J, Solari R C, Rowley A. Mol Biol Cell. 1999;10:1923–1938. doi: 10.1091/mbc.10.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stamnes M A, Craighead M W, Hoe M H, Lampen N, Geromanos S, Tempst P, Rothman J E. Proc Natl Acad Sci USA. 1995;92:8011–8015. doi: 10.1073/pnas.92.17.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominguez M, Dejgaard K, Füllekrug J, Dahan S, Fazel A, Paccaud J P, Thomas D Y, Bergeron J J, Nilsson T. J Cell Biol. 1998;140:751–765. doi: 10.1083/jcb.140.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiedler K, Veit M, Stamnes M A, Rothman J E. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- 8.Letourneur F, Gaynor E C, Hennecke S, Demolliere C, Duden R, Emr S D, Riezman H, Cosson P. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 9.Schimmöller F, Singer-Krüger B, Schröder S, Krüger U, Barlowe C, Riezman H. EMBO J. 1995;14:1329–1339. doi: 10.1002/j.1460-2075.1995.tb07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gommel D, Orci L, Emig E M, Hannah M J, Ravazzola M, Nickel W, Helms J B, Wieland F T, Sohn K. FEBS Lett. 1999;447:179–185. doi: 10.1016/s0014-5793(99)00246-x. [DOI] [PubMed] [Google Scholar]
- 11.Blum R, Pfeiffer F, Feick P, Nastainczyk W, Kohler B, Schäfer K H, Schulz I. J Cell Sci. 1999;112:537–548. doi: 10.1242/jcs.112.4.537. [DOI] [PubMed] [Google Scholar]
- 12.Belden W J, Barlowe C. J Biol Chem. 1996;271:26939–26946. doi: 10.1074/jbc.271.43.26939. [DOI] [PubMed] [Google Scholar]
- 13.Rojo M, Pepperkok R, Emery G, Kellner R, Stang E, Parton R G, Gruenberg J. J Cell Biol. 1997;139:1119–1135. doi: 10.1083/jcb.139.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms J B, Wieland F T. J Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham T R, Emr S D. J Cell Biol. 1991;114:207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prescianotto-Baschong C, Riezman H. Mol Biol Cell. 1998;9:173–189. doi: 10.1091/mbc.9.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wooding S, Pelham H R. Mol Biol Cell. 1998;9:2667–2680. doi: 10.1091/mbc.9.9.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes C A, Söllner T H, Rothman J E, Wieland F T. Cell. 1999;96:1–20. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- 19.Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L. Proc Natl Acad Sci USA. 1998;95:11199–11204. doi: 10.1073/pnas.95.19.11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elrod-Erickson M J, Kaiser C A. Mol Biol Cell. 1996;7:1043–1058. doi: 10.1091/mbc.7.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen C, Greenwald I. J Cell Biol. 1999;145:1165–1175. doi: 10.1083/jcb.145.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guthrie C, Fink G R, editors. Guide to Yeast Genetics and Molecular Biology, Methods in Enzymology. San Diego: Academic; 1991. [PubMed] [Google Scholar]
- 23.Güldener U, Heck S, Fielder T, Beinhauer J, Hegemann J H. Nucleic Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shamu C E, Walter P. EMBO J. 1996;15:3028–3039. [PMC free article] [PubMed] [Google Scholar]
- 25.Gaynor E C, Emr S D. J Cell Biol. 1997;136:789–802. doi: 10.1083/jcb.136.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson L M, Bankaitis V A, Emr S D. Cell. 1987;48:875–885. doi: 10.1016/0092-8674(87)90084-5. [DOI] [PubMed] [Google Scholar]
- 27.Gaynor E C, te Heesen S, Graham T R, Aebi M, Emr S D. J Cell Biol. 1994;127:653–665. doi: 10.1083/jcb.127.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kübler E, Schimmöller F, Riezman H. EMBO J. 1994;13:5539–5546. doi: 10.1002/j.1460-2075.1994.tb06891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiser C A, Schekman R. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- 30.Banta L M, Robinson J S, Klionsky D J, Emr S D. J Cell Biol. 1988;107:1369–1383. doi: 10.1083/jcb.107.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohya Y, Umemoto N, Tanida I, Ohta A, Iida H, Anraku Y. J Biol Chem. 1991;266:13971–13977. [PubMed] [Google Scholar]
- 32.Nelson H, Nelson N. Proc Natl Acad Sci USA. 1990;87:3503–3507. doi: 10.1073/pnas.87.9.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eide D J, Bridgham J T, Zhao Z, Mattoon J R. Mol Gen Genet. 1993;241:447–456. doi: 10.1007/BF00284699. [DOI] [PubMed] [Google Scholar]
- 34.Nakano A, Muramatsu M. J Cell Biol. 1989;109:2677–2691. doi: 10.1083/jcb.109.6.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novick P, Field C, Schekman R. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- 36.Duden R, Hosobuchi M, Hamamoto S, Winey M, Byers B, Schekman R. J Biol Chem. 1994;269:24486–24495. [PubMed] [Google Scholar]
- 37.Conzelmann A, Riezman H, Desponds C, Bron C. EMBO J. 1988;7:2233–2240. doi: 10.1002/j.1460-2075.1988.tb03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens T, Esmon B, Schekman R. Cell. 1982;30:439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- 39.Chapman R, Sidrauski C, Walter P. Annu Rev Cell Dev Biol. 1998;14:459–485. doi: 10.1146/annurev.cellbio.14.1.459. [DOI] [PubMed] [Google Scholar]
- 40.Liu E S, Ou J H, Lee A S. J Biol Chem. 1992;267:7128–7133. [PubMed] [Google Scholar]
- 41.Stroobants A K, Hettema E H, van den Berg M, Tabak H F. FEBS Lett. 1999;453:210–214. doi: 10.1016/s0014-5793(99)00721-8. [DOI] [PubMed] [Google Scholar]
- 42.Mori K, Ogawa N, Kawahara T, Yanagi H, Yura T. J Biol Chem. 1998;273:9912–9920. doi: 10.1074/jbc.273.16.9912. [DOI] [PubMed] [Google Scholar]
- 43.Appenzeller C, Andersson H, Kappeler F, Hauri H P. Nat Cell Biol. 1999;1:330–334. doi: 10.1038/14020. [DOI] [PubMed] [Google Scholar]
- 44.Denzel A, Otto F, Girod A, Pepperkok R, Watson R, Rosewell I, Bergeron J J M, Solari R C E, Owen M J. Curr Biol. 1999;10:55–58. doi: 10.1016/s0960-9822(99)00266-3. [DOI] [PubMed] [Google Scholar]