Abstract
BACKGROUND
Carcinoma of the prostate (CaP) is the most commonly diagnosed cancer in men in the United States. Signal transduction molecules such as tyrosine kinases play important roles in CaP. Src, a nonreceptor tyrosine kinase (NRTK) and the first proto-oncogene discovered is shown to participate in processes such as cell proliferation and migration in CaP. Underscoring NRTK's and, specifically, Src's importance in cancer is the recent approval by the US Food and Drug Administration of dasatinib, the first commercial Src inhibitor for clinical use in chronic myelogenous leukemia (CML). In this review we will focus on NRTKs and their roles in the biology of CaP.
MATERIALS AND METHODS
Publicly available literature from PubMed regarding the topic of members of NRTKs in CaP was searched and reviewed.
RESULTS
Src, FAK, JaK1/2, and ETK are involved in processes indispensable to the biology of CaP: cell growth, migration, invasion, angiogenesis, and apoptosis.
CONCLUSIONS
Src emerges as a common signaling and regulatory molecule in multiple biological processes in CaP. Src's relative importance in particular stages of CaP, however, required further definition. Continued investigation of NRTKs will increase our understanding of their biological function and potential role as new therapeutic targets.
Keywords: Nonreceptor tyrosine kinase, prostate cancer, Src, FAK, ETK
Introduction
Carcinoma of the prostate (CaP) is the most commonly diagnosed cancer in American men, consisting of more than 33% of all new cancer cases. Though many patients are diagnosed with CaP, it has a relatively low mortality rate when compared to other cancers. Nevertheless, it remains the third leading cause of cancer-related deaths in men in the United States, with about 27,350 estimated CaP-related deaths in 2006 in the United States [1]. Because CaP growth is facilitated by androgen exposure and because androgen withdrawal leads to apoptosis of CaP cells, the current treatment of choice for recurrent or metastatic CaP includes castration through chemical or surgical means. Nearly all patients, however, relapse with androgen-independent (AI) disease after androgen ablation therapy. Ultimately, the uncontrolled growth of AI metastatic tumors leads to patient mortality.
Tyrosine kinases (TKs) are signaling molecules well known for their roles in human diseases such as diabetes and cancer. Indeed, v-Src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (Src), a nonreceptor tyrosine kinase (NRTK), was the first proto-oncogene discovered. More than a quarter of a century has passed since the discovery of Src, and the studies on TKs are coming to fruition with the development and use of tyrosine kinase-based target-specific therapy such as Gleevec, Iressa, and Herceptin for therapy against chronic myelogenous leukemia (CML), lung cancer, and breast cancer, respectively. Dasatinib, a dual Src/v-Abl Abelson murine leukemia viral oncogene homolog (Abl) inhibitor with antimigratory activity in prostate cancer cells in culture was recently approved by the US Food and Drug Administration for use in patients with CML [2]. Further underscoring the importance of NRTKs, AZD0530 is another dual Src/Abl inhibitor that is currently in multicenter phase II clinical trials for multiple types of malignancies, including prostate cancer. In this review we will focus on each of the NRTKs and what is known about their respective roles in the biological processes of cell proliferation, migration, invasion, apoptosis, and angiogenesis in CaP.
There are several NRTK families. These are classified based on their structural similarities (Figure 1): Abl, tyrosine kinase nonreceptor (TnK), C-terminal Src kinase (CSK), focal adhesion kinase (FAK), feline sarcoma oncogene/fujinami avian sarcoma viral oncogene homolog (FeS), Janus kinase (JaK), Src, Tec protein kinase (Tec), and spleen tyrosine kinase (SYK). Though these NRTK families are extensively and individually reviewed elsewhere, this is the first time they are summarily discussed in relation to CaP.
Figure 1.
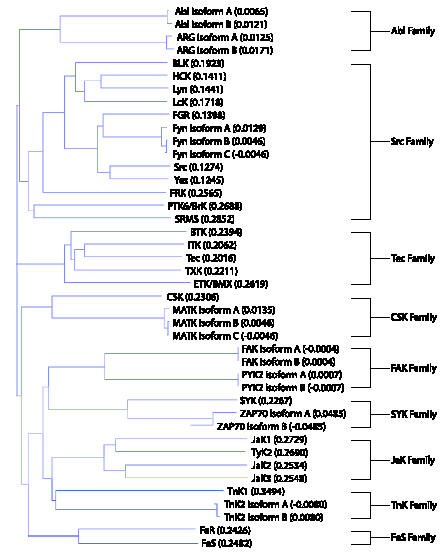
NRTK families and their members in a guide tree. Protein sequences are obtained from Entrez Gene and aligned using Vector NTI Advance software (Invitrogen, Carlsbad, CA). Vector NTI Advance uses the neighbor-joining method of phylogenetic tree construction by Saitou and Nei [127]. The numbers in parentheses after each kinase reflect the calculated distance values between pairs of analyzed sequences.
Profiles of NRTKs in CaP
In 1996, Robinson et al. [3] led the first attempt at profiling the expression of TKs in CaP. Using a modified and improved reverse transcriptase-polymerase chain reaction approach, they identified nine NRTKs expressed in CWR22, a CaP xenograft. NRTKs include lymphocyte-specific protein tyrosine kinase (LcK), v-Yes-1 Yamaguchi sarcoma viral oncogene homolog 1(Yes), Abl, Abelson-related gene (ARG), JaK1, tyrosine kinase 2 (TyK2), and endothelial/epithelial tyrosine kinase/bone marrow X kinase (ETK/BMX). Furthermore, Furthermore, ARG was found in several other CaP cell lines, which include PC-3, DU145, and LNCaP. In a similar study, Moore et al. [4] used degenerate polymerase chain reaction against conserved kinase catalytic subdomains and found that Abl, JaK1, JaK2, and TyK2 are expressed in surgically removed CaP tissues. In CWR22Rv1, DU145, LNCaP and PC3 cell lines, 18 NRTKs are expressed. This was confirmed by our internal data and also cross-referenced with several published reports (Figure 2).
Figure 2.
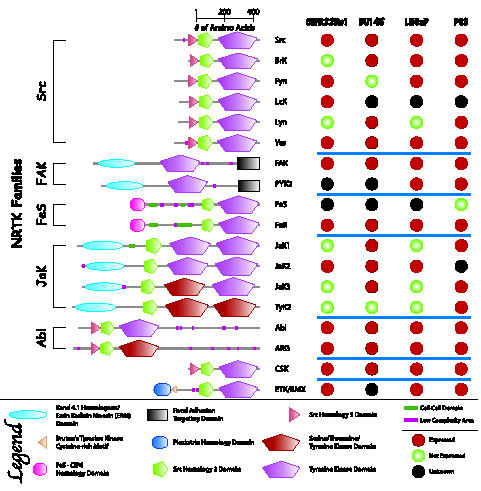
Summary of NRTK mRNA or protein expression in CWR22Rv1, DU145, LNCaP, and PC3 cell lines based on internal data and published reports. NRTK domain drawings and domain information were derived from Simple Modular Architecture Research Tool (SMART, Heidelberg, Germany).
Src Family
As the first human proto-oncogene discovered, Src's history spans nearly a century and has been extensively reviewed [5–22]. Members of the Src family include B lymphoid tyrosine kinase (BLK), breast tumor kinase/protein tyrosine kinase 6 (BrK/PTK6), Gardner-Rasheed feline sarcoma viral oncogene homolog (FGR), Fyn oncogene related to Src, FGR, Yes (Fyn), hemopoietic cell kinase (HCK), LcK, v-Yes-1 Yamaguchi sarcoma viral-related oncogene homolog (Lyn), Src, Src-related kinase lacking C-terminal regulatory tyrosine and N-terminal myristoylation sites (SRMS), Yes, and Yes-related kinase (YRK). Of these, FGR, Fyn, LcK, Lyn, Src, and Yes are expressed in either CaP tumor samples or cell lines. Src, FGR, Fyn, LcK, and Lyn in particular have been the most widely studied in CaP.
Src
The premier member of its namesake family, Src is extensively studied in cancer biology. Less is known, however, about Src biology in CaP. Though there are no published reports of Src expression or activation levels in clinical CaP specimens, Src is implicated in CaP through its association with factors that correlate positively with the presence or the progression of CaP disease, such as protein kinase C (PKC) ε, endothelial-derived gene 1 (EG-1), and a truncated form of c-kit [23–25]. As further evidence of Src's possible involvement in CaP, DRS, a negative Src regulator, is down-regulated in CaP tissues and in prostate intraepithelial neoplasia relative to normal and benign prostate hyperplasia (BPH) tissues [26]. Thus, there is circumstantial clinical evidence that Src plays a role in CaP through its interactions with other factors of significance in CaP.
More is known about Src in CaP in vitro. Src is expressed in commonly used CaP cell lines CWR22Rv1, DU145, LAPC-4, LNCaP, and PC-3 (Figure 3). At first glance, Src protein expression levels in CaP cell lines do not positively correlate with the aggressiveness, AI state, or the proliferation rates of these cell lines. It is important to note, however, that wild-type cellular Src is not normally constitutively active. Its main role is to transduce signals of upstream activators. In cancer, the upstream signals may be aberrant, thus leading to improper activation of Src and its downstream pathways. One such pathway in CaP is Src activation by neuroendocrine ligands [27].
Figure 3.
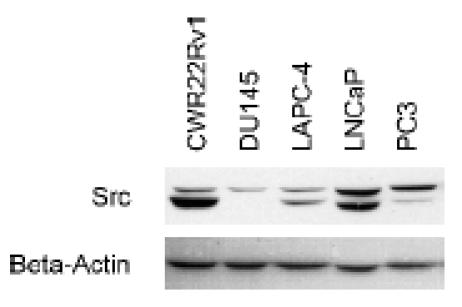
Western blot analysis of total Src protein expression levels in prostate cancer cell lines. Src is shown as a doublet upon probing in most cell lines. Internal overexpression data (not shown) indicate that both bands are Src and that the doublet is not a result of nonspecific probing of other Src family kinase members.
Neuroendocrine differentiation in CaP is theorized to be in part responsible for the development of AI CaP through the secretion of neuroendocrine ligands. There is evidence that Src takes part in AI cell proliferation. Cyclic adenosine monophosphate (cAMP) analogs are able to activate Src following neuroendocrine differentiation, perhaps secondary to secreted neuroendocrine factors such as gastrin-releasing peptide and lysophosphatidic acid (LPA) [28–31]. LPA is thought to promote cell proliferation through the v-Ha-ras Harvey rat sarcoma viral oncogene homolog (Ras)-v-raf-1 murine leukemia viral oncogene homolog 1 (Raf)-ERK1/2 pathway in Src-dependent fashion. Bombesin, a Xenopus gastrin-releasing peptide homolog, can also activate ERK1/2 through Src, possibly through epidermal growth factor (EGF) receptor transactivation [32]. Once ERK1/2 has been activated, it can then activate the androgen receptor (AR) in an AI manner, which promotes cell growth [27,33]. In addition to LPA and bombesin, non-neurotrophic factors such as interleukin-8 (IL-8) and insulin-like growth factor-1 (IGF-1) also promote AI cell growth through Src [34,35].
In addition to cell proliferation, Src also takes part in antiapoptotic pathways in CaP. Bombesin, endothelin (ET1), met proto-oncogene (Met), and dihydrotestosterone-activated AR all inhibit apoptosis through Src activation [26,36–38]. There is, however, no consensus mechanism by which Src promotes cell survival. Nuclear factor κB (NF-κB)-v-akt murine thymoma viral oncogene homolog 1 (Akt)-p21-associated kinase 1 (PAK1) pathway, MEK1/2-ERK1/2-CREB pathway, and signal and transducer of transcription 3 (STAT3)-dependent down-regulation of B-cell lymphoma leukemia (BCL-xL) and myeloid cell leukemia sequence 1 (MCL-1) are all pathways by which Src inhibits apoptosis [39].
Src is involved in other aspects of CaP biology: cell migration and adhesion. Src interacts with the extracellular signals through the IL-8 receptor, Met, β1 integrins, Kangai 1/cluster designation 82 (KAI1/CD82), and CD44 [23,34,40,41]. CD44 is a cell surface glycoprotein involved in cell-cell and cell-matrix adhesions. KAI1/CD82 functions as a metastasis suppressor, disrupting integrin-induced Src activation [42]. Intracellularly, Src modulates cell migration and adhesion through its interaction with FAK and p130 CRK-associated substrate (p130CAS) [2].
In addition to cell migration, Src also assists in tumor invasion through its regulation of matrix metalloproteinases (MMPs). MMPs aid in invasion through the degradation of the extracellular matrix. Bombesin promotes Src-dependent tumor progression and metastasis through the activation of MMP9 in conjunction with β1 integrins [43]. Src inhibition, on the other hand, decreases MMP9 activity levels [2,44].
Induction of angiogenesis by malignant cells is required for continued cell proliferation and metastasis, and vascular endothelial growth factor (VEGF) is a critical angiogenic factor. Src participates in angiogenesis in CaP through the JaK1-STAT3-VEGF pathway [45]. Src activation is also required for VEGF expression in simulated hypoxia environment through increased levels of hypoxia-inducible factor 1α (HIF-1α) and activation of STAT3; as additional evidence of Src's involvement in angiogenesis, overexpression of active Src leads to increased VEGF expression [46]. Expression of the melanoma-differentiation-associated gene-7, a Src inhibitor, on the other hand, inhibits the subsequent downstream STAT3-VEGF pathway [46,47].
Src is also of particular interest in CaP in part because of its interaction with steroid receptors. There is evidence that low amounts of AR and androgen lead to Src activation in the cytoplasm, thereby triggering downstream signaling events independent of AR's transcriptional and DNA-binding activity [38,48]. In fact, dominant negative Src can inhibit DNA synthesis following stimulation with low amounts of synthetic androgen. AR overexpression and higher concentrations of androgen, however, seem to bypass the Src pathway, leading to AR translocation to the nucleus and AR transcriptional activity-based DNA synthesis.
In addition to the aforementioned activation of Src by androgen-activated AR, Src also associates with AR and estrogen receptor (ER) upon stimulation with estradiol, ultimately resulting in increased cell proliferation [38,49,50]. It is thought that Src serves as a scaffolding protein for the AR-ER complex. Steroidal ligand, however, is not necessary for AR-Src complex formation. Upon EGF stimulation, preformed heterodimers of ERα and AR form a complex with EGF receptor and Src, resulting in the activation and phosphorylation of EGF receptor, DNA synthesis, and cytoskeletal changes [51]. On the other hand, DOC-2/DAB2, a tumor suppressor and a negative Src regulator protein, is reported to inhibit AR's mitotic effects through the disruption of the AR-Src complex [52,53]. Thus, taken together with reports of AI AR activation by Src, AR and Src seem to be able to reciprocally transactivate, depending on the concentration and type of stimulatory ligand.
There are few published reports on cellular elements that negatively regulate Src in CaP. In addition to DOC-2/DAB2, tumor growth factor (TGF) β is reported to decrease both Src expression and its corresponding activity. This is shown by the accumulation of unphosphorylated form of SH2-containing protein (SHC) and a subsequent decrease in complex formation between SHC and growth factor receptor-bound protein 2 (Grb2) [54].
BrK/PTK6
BrK is an Src family member, and little is known about it in CaP. In patient samples, BrK is detected in the nuclei of normal luminal epithelial tissues and well-differentiated tumors, but not in poorly differentiated tumors [55]. Localization of BrK in CaP cell lines LNCaP, which is poorly tumorigenic, and PC-3, which is more aggressive, is primarily nuclear and cytoplasmic, respectively. Though PC-3 expressed more BrK than LNCaP did, BrK is less active in PC-3 cells. Thus, the localization of BrK may play a role in the differentiation of CaP and its aggressiveness.
FGR/Src-2
FGR is an Src kinase family member. It is a negative regulator of phosphatase and tensin homolog (PTEN) and a positive regulator of both Ras and Raf1, thus inhibiting apoptosis and stimulating cell growth, respectively [56]. Though little is known about FGR in CaP, FGR may be overexpressed in CaP, as shown by FGR DNA amplification in patient tumor tissues transitioning from androgen-dependent to AI states [56]. Thus, FGR may play a role in CaP growth and survival.
Fyn
Fyn is an Src family kinase member. It is involved in LNCaP mitogenesis following prolactin stimulation [57]. Though it is suggested that Fyn participates in prolactin-induced cell proliferation through K+ ion channels, further studies are necessary in order to elucidate the mechanism of Fyn-modulated prolactin-induced cell proliferation in CaP.
LcK
LcK is an Src family kinase member. It is expressed in CWR22 xenograft cells [3]. Little else is known about the role of LcK in CaP.
Lyn
Lyn is an Src family kinase member expressed in normal prostate, 95% of primary CaP, and AI PC-3 and DU145 cells [58]. Lyn knockout mice have abnormal prostate gland development. Treatment with KRX-123, a Lyn-specific inhibitor, results in the inhibition of cell growth in DU145 and PC-3 cell lines. DU145 explants in mice treated with KRX-123 were found to also undergo apoptosis. Thus, Lyn seems to play a role in the proliferation and the apoptosis of CaP.
Lyn may also be an important regulator of cell migration in CaP. DU145 cells treated with dasatinib, an Src family kinase inhibitor, have reduced migratory activity [2]. On the other hand, Lyn can bind with neutral endopeptidase (NEP) and act as a competitive inhibitor to the PI3K-FAK complex, resulting in decreased cell migration [59]. Lyn's role in CaP cell migration is therefore inconclusive.
In CaP, Lyn is down-regulated by TGFβ and up-regulated by KAI1/CD82 [54,60]. Despite its elevated expression following KAI1/CD82 stimulation, however, Lyn's overall kinase activity was unchanged.
FAK Family
FAK
As the predominate member of its namesake family of kinases, FAK is well studied in CaP. Several general reviews of FAK are available [61–71]. Though FAK may play roles in growth, apoptosis, and angiogenesis in CaP, FAK is known primarily for its role in cell motility and cytoskeletal rearrangement, as supported by in vivo and in vitro evidence. In clinical specimens, FAK expression and activation are uniformly higher in metastatic CaP than in normal and BPH tissues [72,73]. In vitro comparison between highly metastatic CaP cell lines and LNCaP, a cell line with lower metastatic potential, shows similar results, with increased expression and activation of FAK in the more aggressive cell lines [74]. FAK's association with molecular mediators of cell migration and adhesions are indicative of its function as well. Activated FAK complexes with β1 and α(v)β3 integrins, molecules involved in cell adhesion [75–78]. As further evidence of FAK's function as a cell motility factor, inhibition of FAK with anti-FAK (pY397) antibody or FAK-related nonkinase (FRNK) resulted in significantly decreased cell migration [79].
Bombesin and IL-8 are both G protein-coupled receptors (GPCR) that activate FAK and stimulate cell migration [34,79–81]. This is not surprising given FAK's reciprocal transactivation relationship with Src and both IL-8 and bombesin's abilities to activate Src. For bombesin to activate FAK, however, both PKC and an intact cytoskeleton are required [80,82]. Following its activation, FAK then phosphorylates p130CAS, leading to p130CAS-v-crk avian sarcoma virus CT10 oncogene homolog (CRKII) complex formation. Disruption of the p130CAS-CRKII complex by overexpressing KAI1/CD82 results in decreased cell motility [60].
Extracellularly, FAK is activated by integrins, ET1, bombesin, IL-8, and urokinase plasminogen activator (uPA), an invasion and metastasis factor in CaP [83,84]. Intracellularly, it is modulated by Src. It is important to note that Src and FAK activation often go hand in hand. They couple and reciprocally transactivate each other. There are, however, exceptions. FAK activation by autophosphorylation of tyrosine 397 is not Src-dependent; it is adhesion-dependent [74]. On the other hand, phosphorylation of tyrosine 861, which leads to increased FAK activity, is Src-dependent but not adhesion-dependent.
Though FAK is primarily a cell motility regulator, it is also involved in cell proliferation. Similar to cell migration, bombesin-induced FAK-mediated proliferation requires an intact cytoskeleton [80]. A signal downstream of FAK is ETK/BMX, an NRTK critical for bombesin-induced growth [27]. Following FAK activation of ETK/BMX, ETK/BMX subsequently activates AR, thereby inducing cell growth. Interestingly, not only can FAK indirectly activate AR, it can also be activated by membrane-associated AR in a PI3K-dependent manner [85].
In addition to migration and proliferation, FAK may also be involved in CaP angiogenesis and apoptosis. There is evidence that FAK induces VEGF transcription in an ERK1/2-dependent, Rap1-dependent, and Raf-dependent but Ras-independent manner [86]. Increased VEGF transcription may then lead to an increased level of its secreted protein and, thus, angiogenesis. In regard to apoptosis, treatment of cells with proapoptotic factors FTY720 and doxazosin both down-regulate FAK expression for reasons that are not currently known [87,88].
There are few known ways in which FAK is negatively regulated in CaP. Negative FAK regulators include PTEN, a tumor suppressor gene with dual phosphatase activity that is frequently deleted in aggressive CaP [89]. FAK may also be indirectly negatively regulated through the formation of the Lyn-PI3K-NEP complex instead of the PI3K-FAK complex [59].
Proline-rich tyrosine kinase 2/cell adhesion kinase β (PYK2/CAKβ)
PYK2/CAKβ is a member of the FAK family of tyrosine kinases. A general review of PYK2 is available [90]. It is expressed in normal prostate epithelia and BPH, but its expression level decreases with increasing grade in CaP [91]. The gene is located on chromosome 8p21.1, a site of frequent deletion in CaP [92].
Though in vivo evidence suggests that PYK2 plays a tumor suppressive role in CaP, the in vitro evidence of this hypothesis is inconclusive. In vitro experiments show that PYK2 is activated by LPA and tumor necrosis factor α. PYK2 plays a role in the activation of ERK1/2 following LPA stimulation and may thus stimulate cell proliferation [93]. In addition, cells expressing dominant negative PYK2 have decreased proliferation rates. On the other hand, PYK2 indirectly inhibits AR activation through the inactivation of an AR-associated protein, ARA55 [94]. Thus, PYK2's role in CaP may depend on the androgen sensitivity status of the cells in question and requires further investigation and clarification.
FeS Family
The FeS family of NRTKs consists of two members: FeS/FpS and FpS/FeS-related tyrosine kinase (FeR). Little is known about the FeS family in CaP. An examination of CaP cell lines PC-3, PC133, and PC135 failed to detect FeS transcript [95]. FeR expression, on the other hand, was found in CaP cell lines PC-3, DU145, and LNCaP and positively correlated with CaP versus normal and BPH tissue samples [96]. Consistent with patient sample data, cells transfected with antisense FeR grew at a slower rate and were unable to grow in an anchorage-independent fashion. In the dog model, a higher FeR expression was found in dividing versus resting prostate epithelial cells and in cells displaying basal cell hyperplasia and metaplasia following postcastration estrogen treatment [96]. Thus, FeR is likely a proliferation factor in CaP.
JaK Family
JaK1
The JaK family of kinases is well known for its role in signaling events in cells following cytokine stimulation and its association with the STAT family of kinases. Though JaK1 is present in some clinical CaP specimens, JaK1 is reported to be either negatively regulated or mutated in many CaP cell lines [4,97,98]. LNCaP is found to have both nonsense mutation and repressed JaK1 transcription whereas CWR22Rv1 and LAPC-4 have only nonsense mutations and no known transcriptional repression.
In DU145 cells, which have wild-type JaK1, there are reports that JaK1 associates with breast cancer susceptibility gene 1 (BRCA1) [99]. When BRCA1 is overexpressed, JaK1 and STAT3 become activated. Subsequent inhibition of STAT3 activation results in decreased cell proliferation as well as in apoptosis. Interestingly, inhibition of JaK1 in wild-type DU145 does not result in apoptosis [100]. Thus, it may be possible that although JaK1 activation by BRCA1 leads to increased JaK1 and STAT3 activation, STAT3 may in fact not be directly downstream of JaK1 in CaP, and their concurrent activation is coincidental.
JaK1 may also play a role in the inhibition of CaP migration and invasion following IL-10 stimulation [101]. Tissue inhibitor of metalloproteinases (TIMP) 1 is an anti-invasion factor. IL-10 is known to activate the JaK1-IL-10E1-TIMP-1 pathway in CaP [102].
JaK2
JaK2 is expressed in some CaP tissues [4]. Similar to JaK1, JaK2 is also activated by BRCA1 in DU145 cells [99]. It is interesting to note that although JaK1 inhibition does not result in apoptosis in wild-type DU145 cells, inhibition of JaK2 does [100]. Thus, STAT3 activation in DU145 may be dependent on JaK2 rather than on JaK1. Whether STAT3 is activated by JaK1 or JaK2 in CaP, however, seems to be cell line-dependent [103].
JaK2 may also be involved in cell proliferation in CaP. Tyrosine kinase inhibitor peptide (TKIP) directly inhibits JaK2 autophosphorylation, decreases STAT3 activation, and slows CaP proliferation [104]. Consistent with decreased cell proliferation and STAT3 activation, cyclin D1 level is decreased and cells are arrested in the G1 phase of the cell cycle following TKIP treatment. Thus, JaK2 may be important for CaP growth through the STAT3 pathway. In addition to STAT3, JaK2 may be of particular importance in CaP through its regulation of STAT5, a factor that positively correlates with the histological grade of CaP [105,106].
TyK2
TyK2 is expressed in some CaP tissues [4]. Though TyK2 may also be involved in CaP migration and invasion and similarly participates in the activation of IL-10E1 following IL-10 stimulation of CaP cells as JaK1, its temporal regulation profile is different from that of JaK1 [101,102].
Members of Other NRTK Families
Abl
Abl is well known for its role in the etiology of CML following the formation of the Philadelphia chromosome (t(9:22)) and the breakpoint cluster region (Bcr)-Abl hybrid gene product. Less is known, however, about Abl in CaP. It is known that Abl is expressed in some CaP specimens and that Abl is necessary for retinoblastoma-mediated γ-radiation-induced apoptosis in DU145 cells [4,107]. There is indirect evidence that Abl may be important in CaP. Human spectrin SH domain-binding protein 1 (Hssh3bp1) is a gene that binds to Abl, possibly as a negative regulator [108]. A majority (9 of 17) of CaP tumor samples analyzed failed to express Hssh3bp1. Furthermore, Hssh3bp1 is found on chromosome 10p, a region frequently deleted in CaP. Thus, Abl may be circumstantially implicated in CaP through its association with Hssh3bp1.
Imatinib mesylate (Gleevec; Novartis, East Hanover, NJ) is a Bcr-Abl inhibitor that is clinically used for the treatment of CML. It also has activity against Kit kinase and platelet-derived growth factor (PDGF) receptor. In vitro, Gleevec inhibits CaP cell growth with IC50 in the 10-µM range [109]. In mice models, however, Gleevec's efficacy against CaP growth is inconclusive with some, but not all, studies showing growth inhibition [110–113].
Similarly, preliminary results from clinical studies also paint a mixed picture. A phase I clinical trial of Gleevec in combination with docetaxel in AI CaP showed a prostate-specific antigen (PSA) decline in 14 of 21 patients, although it is unknown whether the decline can be attributed to Gleevec or docetaxel [114]. In another AI CaP study, Gleevec in combination with zoledronic acid (Zometa, Novartis) showed no clinical effect in 15 CaP patients [115]. Lastly, as monotherapy in 16 patients with androgen-sensitive CaP, Gleevec treatment resulted in nine patients with stable PSA levels and seven patients with PSA progression [116]. Thus, clinical use of Gleevec as monotherapy in CaP may be ineffective. The efficacy of using Gleevec as an adjuvant therapy to other treatment modalities is presently unknown.
CSK
CSK is a well known negative Src regulator [117]. Little is directly known about CSK in CaP other than that it complexes with FAK in metastatic tumors and PC-3 cells [73].
ETK/BMX
Discovered in 1994, ETK/BMX belongs to the Tec family of NRTK [118]. In CaP, ETK is downstream of PI3K in the induction of the neuroendocrine differentiation of LNCaP cells following IL-6 stimulation [119]. It is also reported to function as an antiapoptotic factor. Overexpression of ETK confers resistance to apoptosis in CaP cells through its interaction with PI3K [120]. PI3K is not, however, required for ETK activation [27]. Another mechanism by which ETK may protect against apoptosis is through its interaction with p53 [121]. Interestingly, ETK also participates in the apoptotic cascade in CaP cells. Introduction of ETK's C-terminal fragment into PC-3 cells can lead to apoptosis following proteolytic cleavage of ETK by caspases [122].
ETK is also critical for cell proliferation following bombesin stimulation and AR activation in CaP [27]. ETK serves as a signal transducer between Src and FAK upstream and AR downstream. ETK alone, however, is insufficient for AR activation. ETK must be able to reciprocally transactivate with Pim1 before AR activation [123,124].
Other NRTKS
SYK and TNK1 are other NRTKs that have been studied in CaP. Virtually nothing is known about their properties and functions in prostate cancer except that the promoter region of SYK is hypermethylated and TNK1 transcript is found in prostate tissues [125,126]. SYK expression may thus be down-regulated in CaP, whereas TNK1 protein expression level remains to be investigated.
Conclusion
Much is known regarding specific NRTKs in CaP (Src, FAK, JaK1/2, and ETK), whereas less is known about the other NRTKs. Perhaps it is not a coincidence that the well-studied Src, FAK, JaK1/2, and ETK kinases are involved in processes indispensable to the pathology of CaP: cell growth, migration, invasion, angiogenesis, and apoptosis. It is therefore imperative that we learn more about these NRTKs through future studies. Although Src, FAK, JaK1/2, and ETK are important in CaP biology, we should not neglect the other NRTKs that may also play important roles in CaP and should also investigate the lesser known NRTKs.
Looking at the current literature of NRTKs in CaP, there emerges a picture of Src being an ubiquitous player in multiple biological processes interacting with numerous players in multiple signaling pathways. Src transduces signals from upstream receptors such as IL-8, EGF, IGF-1, neurotensin, ET1, and HGF/SF to downstream molecules such as FAK, ETK, JAK1/2, STAT3, Ras, ERK1/2, Akt, HIF-1α, and, of particular significance in CaP biology, AR (Figure 4). Given the preponderance of evidence in multiple biological processes linking Src to CaP, Src is likely an important point of pathway convergence in CaP. Perhaps it is not surprising then that Src is currently the only NRTK target in clinical trials for CaP, whereas no NRTK-specific therapy is available for general clinical use in CaP. What remains unclear, however, is Src's relative importance in particular stages of CaP: oncogenesis, growth, survival, AI growth, angiogenesis, and metastasis. Nevertheless, with cancer treatments moving toward targeting specific pathways, it is important that we continue investigating signaling pathways so that we can develop novel therapies through continued research.
Figure 4.
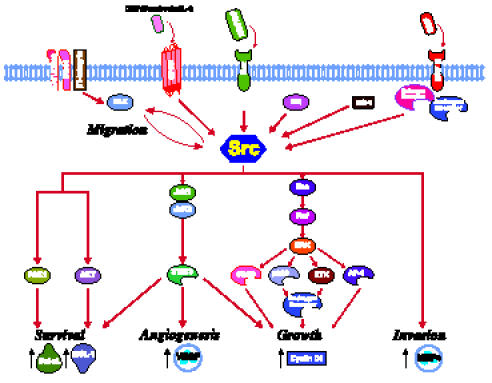
Known Src pathways in prostate cancer. The close proximity of molecules not connected with arrows denote physical association. Red arrows denote activation. Black arrows denote change in levels of molecule. Figure templates were provided by BioCarta (San Diego, CA).
Abbreviations
- Abl
v-Abl Abelson murine leukemia viral oncogene homolog
- AI
androgen-independent
- Akt
v-akt murine thymoma viral oncogene homolog 1
- AR
androgen receptor
- ARG
Abelson-related gene
- Bcr
breakpoint cluster region
- BrK/PTK6
breast tumor kinase/protein tyrosine kinase 6
- BPH
benign prostatic hypertrophy
- BRCA1
breast cancer susceptibility gene 1
- CaP
carcinoma of the prostate
- CML
chronic myelogenous leukemia
- CRKII
v-crk avian sarcoma virus CT10 oncogene homolog
- CSK
C-terminal Src kinase
- DOC-2/DAB2
differentially expressed in ovarian cancer-2/disabled-2
- EGF
epidermal growth factor
- ER
estrogen receptor
- ERK1/2
extracellular signal-regulated kinase 1/2
- ET1
endothelin
- ETK/BMX
endothelial/epithelial tyrosine kinase/bone marrow X kinase
- FAK
focal adhesion kinase
- FeR
FpS/FeS-related tyrosine kinase
- FeS/FpS
feline sarcoma oncogene/fujinami avian sarcoma viral oncogene homolog
- FGR
Gardner-Rasheed feline sarcoma viral (v-FGR) oncogene homolog
- Fyn
Fyn oncogene related to Src, FGR, Yes
- HIF-1α
hypoxia-inducible factor 1α
- IGF-1
insulin-like growth factor 1
- IL
interleukin
- JaK
Janus kinase
- KAI1/CD82
Kangai 1/cluster designation 82
- LcK
lymphocyte-specific protein tyrosine kinase
- Lyn
v-Yes-1 Yamaguchi sarcoma viral-related oncogene homolog
- LPA
lysophosphatidic acid
- Met
met proto-oncogene (hepatocyte growth factor receptor)
- MMP
matrix metalloproteinase
- NEP
neutral endopeptidase
- NRTK
nonreceptor tyrosine kinase
- p130CAS
p130 CRKassociated substrate
- PAK1
p21-associated kinase 1
- PDGF
platelet-derived growth factor
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PSA
prostate-specific antigen
- PTEN
phosphatase and tensin homolog
- PYK2/CAKβ
proline-rich tyrosine kinase 2/cell adhesion kinase β
- Raf
v-raf-1 murine leukemia viral oncogene homolog 1
- Ras
v-Ha-ras Harvey rat sarcoma viral oncogene homolog
- SH
Src homology
- Src
v-Src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog
- STAT
signal and transducer of transcription
- SYK
spleen tyrosine kinase
- Tec
Tec protein kinase
- TGF
tumor growth factor
- TIMP
tissue inhibitor of metalloproteinase
- TKIP
tyrosine kinase inhibitor peptide
- TnK
tyrosine kinase nonreceptor
- TyK2
tyrosine kinase 2
- VEGF
vascular endothelial growth factor
- Yes
v-Yes-1 Yamaguchi sarcoma viral oncogene homolog 1
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, Mirosevich J, Lee FY, Jove R. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 3.Robinson D, He F, Pretlow T, Kung HJ. A tyrosine kinase profile of prostate carcinoma. Proc Natl Acad Sci USA. 1996;93:5958–5962. doi: 10.1073/pnas.93.12.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore TM, Garg R, Johnson C, Coptcoat MJ, Ridley AJ, Morris JD. PSK, a novel STE20-like kinase derived from prostatic carcinoma that activates the c-Jun N-terminal kinase mitogen-activated protein kinase pathway and regulates actin cytoskeletal organization. J Biol Chem. 2000;275:4311–4322. doi: 10.1074/jbc.275.6.4311. [DOI] [PubMed] [Google Scholar]
- 5.Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 6.Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- 7.Gauld SB, Cambier JC. Src-family kinases in B-cell development and signaling. Oncogene. 2004;23:8001–8006. doi: 10.1038/sj.onc.1208075. [DOI] [PubMed] [Google Scholar]
- 8.Geahlen RL, Handley MD, Harrison ML. Molecular interdiction of Src-family kinase signaling in hematopoietic cells. Oncogene. 2004;23:8024–8032. doi: 10.1038/sj.onc.1208078. [DOI] [PubMed] [Google Scholar]
- 9.Kalia LV, Gingrich JR, Salter MW. Src in synaptic transmission and plasticity. Oncogene. 2004;23:8007–8016. doi: 10.1038/sj.onc.1208158. [DOI] [PubMed] [Google Scholar]
- 10.Luttrell DK, Luttrell LM. Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene. 2004;23:7969–7978. doi: 10.1038/sj.onc.1208162. [DOI] [PubMed] [Google Scholar]
- 11.Martin GS. The road to Src. Oncogene. 2004;23:7910–7917. doi: 10.1038/sj.onc.1208077. [DOI] [PubMed] [Google Scholar]
- 12.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 13.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 14.Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds AB, Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene. 2004;23:7947–7956. doi: 10.1038/sj.onc.1208161. [DOI] [PubMed] [Google Scholar]
- 16.Shupnik MA. Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene. 2004;23:7979–7989. doi: 10.1038/sj.onc.1208076. [DOI] [PubMed] [Google Scholar]
- 17.Silva CM. Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene. 2004;23:8017–8023. doi: 10.1038/sj.onc.1208159. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez RH, Kantarjian HM, Cortes JE. The role of Src in solid and hematologic malignancies: development of new-generation Src inhibitors. Cancer. 2006;107:1918–1929. doi: 10.1002/cncr.22215. [DOI] [PubMed] [Google Scholar]
- 19.Trevino JG, Summy JM, Gallick GE. SRC inhibitors as potential therapeutic agents for human cancers. Mini Rev Med Chem. 2006;6:681–687. doi: 10.2174/138955706777435724. [DOI] [PubMed] [Google Scholar]
- 20.Summy JM, Gallick GE. Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res. 2006;12:1398–1401. doi: 10.1158/1078-0432.CCR-05-2692. [DOI] [PubMed] [Google Scholar]
- 21.Alper O, Bowden ET. Novel insights into c-Src. Curr Pharm Des. 2005;11:1119–1130. doi: 10.2174/1381612053507576. [DOI] [PubMed] [Google Scholar]
- 22.Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Wu D, Thakore CU, Wescott GG, McCubrey JA, Terrian DM. Integrin signaling links protein kinase C epsilon to the protein kinase B/Akt survival pathway in recurrent prostate cancer cells. Oncogene. 2004;23:8659–8672. doi: 10.1038/sj.onc.1207900. [DOI] [PubMed] [Google Scholar]
- 24.Paronetto MP, Farini D, Sammarco I, Maturo G, Vespasiani G, Geremia R, Rossi P, Sette C. Expression of a truncated form of the c-Kit tyrosine kinase receptor and activation of Src kinase in human prostatic cancer. Am J Pathol. 2004;164:1243–1251. doi: 10.1016/S0002-9440(10)63212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu M, Zhang L, Maul RS, Sartippour MR, Norris A, Whitelegge J, Rao JY, Brooks MN. The novel gene EG-1 stimulates cellular proliferation. Cancer Res. 2005;65:6159–6166. doi: 10.1158/0008-5472.CAN-04-4016. [DOI] [PubMed] [Google Scholar]
- 26.Kim CJ, Shimakage M, Kushima R, Mukaisho K, Shinka T, Okada Y, Inoue H. Down-regulation of drs mRNA in human prostate carcinomas. Hum Pathol. 2003;34:654–657. doi: 10.1016/s0046-8177(03)00240-5. [DOI] [PubMed] [Google Scholar]
- 27.Lee LF, Guan J, Qiu Y, Kung HJ. Neuropeptide-induced androgen independence in prostate cancer cells: roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase. Mol Cell Biol. 2001;21:8385–8397. doi: 10.1128/MCB.21.24.8385-8397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allard P, Beaulieu P, Aprikian A, Chevalier S. Bombesin modulates the association of Src with a nuclear 110-kd protein expressed in dividing prostate cells. J Androl. 2000;21:367–375. [PubMed] [Google Scholar]
- 29.Daaka Y. Mitogenic action of LPA in prostate. Biochim Biophys Acta. 2002;1582:265–269. doi: 10.1016/s1388-1981(02)00180-4. [DOI] [PubMed] [Google Scholar]
- 30.Kue PF, Daaka Y. Essential role for G proteins in prostate cancer cell growth and signaling. J Urol. 2000;164:2162–2167. [PubMed] [Google Scholar]
- 31.Bang YJ, Pirnia F, Fang WG, Kang WK, Sartor O, Whitesell L, Ha MJ, Tsokos M, Sheahan MD, Nguyen P, et al. Terminal neuroendocrine differentiation of human prostate carcinoma cells in response to increased intracellular cyclic AMP. Proc Natl Acad Sci USA. 1994;91:5330–5334. doi: 10.1073/pnas.91.12.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao D, Qu X, Weber HC. Activation of extracellular signal-regulated kinase mediates bombesin-induced mitogenic responses in prostate cancer cells. Cell Signal. 2003;15:945–953. doi: 10.1016/s0898-6568(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 33.Gong J, Zhu J, Goodman OB, Pestell RG, Schlegel PN, Nanus DM, Shen R. Activation of p300 histone acetyltransferase activity and acetylation of the androgen receptor by bombesin in prostate cancer cells. Oncogene. 2006 doi: 10.1038/sj.onc.1209231. [DOI] [PubMed] [Google Scholar]
- 34.Lee LF, Louie MC, Desai SJ, Yang J, Chen HW, Evans CP, Kung HJ. Interleukin-8 confers androgen-independent growth and migration of LNCaP: differential effects of tyrosine kinases Src and FAK. Oncogene. 2004;23:2197–2205. doi: 10.1038/sj.onc.1207344. [DOI] [PubMed] [Google Scholar]
- 35.Pandini G, Mineo R, Frasca F, Roberts CT, Jr, Marcelli M, Vigneri R, Belfiore A. Androgens up-regulate the insulin-like growth factor-I receptor in prostate cancer cells. Cancer Res. 2005;65:1849–1857. doi: 10.1158/0008-5472.CAN-04-1837. [DOI] [PubMed] [Google Scholar]
- 36.Fan S, Gao M, Meng Q, Laterra JJ, Symons MH, Coniglio S, Pestell RG, Goldberg ID, Rosen EM. Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene. 2005 doi: 10.1038/sj.onc.1208327. [DOI] [PubMed] [Google Scholar]
- 37.Sumitomo M, Shen R, Goldberg JS, Dai J, Navarro D, Nanus DM. Neutral endopeptidase promotes phorbol ester-induced apoptosis in prostate cancer cells by inhibiting neuropeptide-induced protein kinase C delta degradation. Cancer Res. 2000;60:6590–6596. [PubMed] [Google Scholar]
- 38.Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, Marcelli M. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64:7156–7168. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- 39.Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos AS, Carter B, Turkson J, Jove R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther. 2006;5:621–629. doi: 10.1158/1535-7163.MCT-05-0268. [DOI] [PubMed] [Google Scholar]
- 40.Zhu D, Bourguignon LY. The ankyrin-binding domain of CD44s is involved in regulating hyaluronic acid-mediated functions and prostate tumor cell transformation. Cell Motil Cytoskelet. 1998;39:209–222. doi: 10.1002/(SICI)1097-0169(1998)39:3<209::AID-CM4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Jee B, Jin K, Hahn JH, Song HG, Lee H. Metastasis-suppressor KAI1/CD82 induces homotypic aggregation of human prostate cancer cells through Src-dependent pathway. Exp Mol Med. 2003;35:30–37. doi: 10.1038/emm.2003.5. [DOI] [PubMed] [Google Scholar]
- 42.Sridhar SC, Miranti CK. Tetraspanin KAI1/CD82 suppresses invasion by inhibiting integrin-dependent crosstalk with c-Met receptor and Src kinases. Oncogene. 2006;25:2367–2378. doi: 10.1038/sj.onc.1209269. [DOI] [PubMed] [Google Scholar]
- 43.Festuccia C, Angelucci A, Gravina G, Eleuterio E, Vicentini C, Bologna M. Bombesin-dependent pro-MMP-9 activation in prostatic cancer cells requires beta1 integrin engagement. Exp Cell Res. 2002;280:1–11. doi: 10.1006/excr.2002.5609. [DOI] [PubMed] [Google Scholar]
- 44.Recchia I, Rucci N, Festuccia C, Bologna M, MacKay AR, Migliaccio S, Longo M, Susa M, Fabbro D, Teti A. Pyrrolopyrimidine c-Src inhibitors reduce growth, adhesion, motility and invasion of prostate cancer cells in vitro. Eur J Cancer. 2003;39:1927–1935. doi: 10.1016/s0959-8049(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 45.Nam S, Buettner R, Turkson J, Kim D, Cheng JQ, Muehlbeyer S, Hippe F, Vatter S, Merz KH, Eisenbrand G, et al. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc Natl Acad Sci USA. 2005;102:5998–6003. doi: 10.1073/pnas.0409467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, Gallick GE. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24:3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- 47.Inoue S, Branch CD, Gallick GE, Chada S, Ramesh R. Inhibition of Src kinase activity by Ad-mda7 suppresses vascular endothelial growth factor expression in prostate carcinoma cells. Mol Ther. 2005;12:707–715. doi: 10.1016/j.ymthe.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Castoria G, Lombardi M, Barone MV, Bilancio A, Di Domenico M, De Falco A, Varricchio L, Bottero D, Nanayakkara M, Migliaccio A, et al. Rapid signalling pathway activation by androgens in epithelial and stromal cells. Steroids. 2004;69:517–522. doi: 10.1016/j.steroids.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chieffi P, Kisslinger A, Sinisi AA, Abbondanza C, Tramontano D. 17beta-Estradiol-induced activation of ERK1/2 through endogenous androgen receptor-estradiol receptor alpha-Src complex in human prostate cells. Int J Oncol. 2003;23:797–801. [PubMed] [Google Scholar]
- 51.Migliaccio A, Di Domenico M, Castoria G, Nanayakkara M, Lombardi M, de Falco A, Bilancio A, Varricchio L, Ciociola A, Auricchio F. Steroid receptor regulation of epidermal growth factor signaling through Src in breast and prostate cancer cells: steroid antagonist action. Cancer Res. 2005;65:10585–10593. doi: 10.1158/0008-5472.CAN-05-0912. [DOI] [PubMed] [Google Scholar]
- 52.Zhoul J, Hernandez G, Tu SW, Huang CL, Tseng CP, Hsieh JT. The role of DOC-2/DAB2 in modulating androgen receptor-mediated cell growth via the nongenomic c-Src-mediated pathway in normal prostatic epithelium and cancer. Cancer Res. 2005;65:9906–9913. doi: 10.1158/0008-5472.CAN-05-1481. [DOI] [PubMed] [Google Scholar]
- 53.Zhou J, Scholes J, Hsieh JT. Characterization of a novel negative regulator (DOC-2/DAB2) of c-Src in normal prostatic epithelium and cancer. J Biol Chem. 2003;278:6936–6941. doi: 10.1074/jbc.M210628200. [DOI] [PubMed] [Google Scholar]
- 54.Atfi A, Drobetsky E, Boissonneault M, Chapdelaine A, Chevalier S. Transforming growth factor beta down-regulates Src family protein tyrosine kinase signaling pathways. J Biol Chem. 1994;269:30688–30693. [PubMed] [Google Scholar]
- 55.Derry JJ, Prins GS, Ray V, Tyner AL. Altered localization and activity of the intracellular tyrosine kinase BrK/Sik in prostate tumor cells. Oncogene. 2003;22:4212–4220. doi: 10.1038/sj.onc.1206465. [DOI] [PubMed] [Google Scholar]
- 56.Edwards J, Krishna NS, Witton CJ, Bartlett JM. Gene amplifications associated with the development of hormone-resistant prostate cancer. Clin Cancer Res. 2003;9:5271–5281. [PubMed] [Google Scholar]
- 57.Van Coppenolle F, Skryma R, Ouadid-Ahidouch H, Slomianny C, Roudbaraki M, Delcourt P, Dewailly E, Humez S, Crepin A, Gourdou I, et al. Prolactin stimulates cell proliferation through a long form of prolactin receptor and K+ channel activation. Biochem J. 2004;377:569–578. doi: 10.1042/BJ20030859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldenberg-Furmanov M, Stein I, Pikarsky E, Rubin H, Kasem S, Wygoda M, Weinstein I, Reuveni H, Ben-Sasson SA. Lyn is a target gene for prostate cancer: sequence-based inhibition induces regression of human tumor xenografts. Cancer Res. 2004;64:1058–1066. doi: 10.1158/0008-5472.can-03-2420. [DOI] [PubMed] [Google Scholar]
- 59.Sumitomo M, Shen R, Walburg M, Dai J, Geng Y, Navarro D, Boileau G, Papandreou CN, Giancotti FG, Knudsen B, et al. Neutral endopeptidase inhibits prostate cancer cell migration by blocking focal adhesion kinase signaling. J Clin Invest. 2000;106:1399–1407. doi: 10.1172/JCI10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang XA, He B, Zhou B, Liu L. Requirement of the p130CAS-Crk coupling for metastasis suppressor KAI1/CD82-mediated inhibition of cell migration. J Biol Chem. 2003;278:27319–27328. doi: 10.1074/jbc.M303039200. [DOI] [PubMed] [Google Scholar]
- 61.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Cohen LA, Guan JL. Mechanisms of focal adhesion kinase regulation. Curr Cancer Drug Targets. 2005;5:629–643. doi: 10.2174/156800905774932798. [DOI] [PubMed] [Google Scholar]
- 63.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer — a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 64.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 65.Gabarra-Niecko V, Schaller MD, Dunty JM. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003;22:359–374. doi: 10.1023/a:1023725029589. [DOI] [PubMed] [Google Scholar]
- 66.Hanks SK, Ryzhova L, Shin NY, Brabek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:d982–d996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- 67.Hauck CR, Hsia DA, Schlaepfer DD. The focal adhesion kinase—a regulator of cell migration and invasion. IUBMB Life. 2002;53:115–119. doi: 10.1080/15216540211470. [DOI] [PubMed] [Google Scholar]
- 68.Hecker TP, Gladson CL. Focal adhesion kinase in cancer. Front Biosci. 2003;8:s705–s714. doi: 10.2741/1115. [DOI] [PubMed] [Google Scholar]
- 69.McLean GW, Avizienyte E, Frame MC. Focal adhesion kinase as a potential target in oncology. Expert Opin Pharmacother. 2003;4:227–234. doi: 10.1517/14656566.4.2.227. [DOI] [PubMed] [Google Scholar]
- 70.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 71.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 72.Rovin JD, Frierson HF, Jr, Ledinh W, Parsons JT, Adams RB. Expression of focal adhesion kinase in normal and pathologic human prostate tissues. Prostate. 2002;53:124–132. doi: 10.1002/pros.10114. [DOI] [PubMed] [Google Scholar]
- 73.Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer. 1996;68:164–171. doi: 10.1002/(sici)1097-0215(19961009)68:2<169::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 74.Slack JK, Adams RB, Rovin JD, Bissonette EA, Stoker CE, Parsons JT. Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene. 2001;20:1152–1163. doi: 10.1038/sj.onc.1204208. [DOI] [PubMed] [Google Scholar]
- 75.Bergan R, Kyle E, Nguyen P, Trepel J, Ingui C, Neckers L. Genistein-stimulated adherence of prostate cancer cells is associated with the binding of focal adhesion kinase to beta-1-integrin. Clin Exp Metastasis. 1996;14:389–398. doi: 10.1007/BF00123398. [DOI] [PubMed] [Google Scholar]
- 76.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–1664. [PubMed] [Google Scholar]
- 77.Zheng DQ, Woodard AS, Tallini G, Languino LR. Substrate specificity of alpha(v)beta(3) integrin-mediated cell migration and phosphatidylinositol 3-kinase/AKT pathway activation. J Biol Chem. 2000;275:24565–24574. doi: 10.1074/jbc.M002646200. [DOI] [PubMed] [Google Scholar]
- 78.Bello-DeOcampo D, Kleinman HK, Deocampo ND, Webber MM. Laminin-1 and alpha6beta1 integrin regulate acinar morphogenesis of normal and malignant human prostate epithelial cells. Prostate. 2001;46:142–153. doi: 10.1002/1097-0045(20010201)46:2<142::aid-pros1018>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 79.Lacoste J, Aprikian AG, Chevalier S. Focal adhesion kinase is required for bombesin-induced prostate cancer cell motility. Mol Cell Endocrinol. 2005;235:51–61. doi: 10.1016/j.mce.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 80.Duncan MD, Harmon JW, Duncan LK. Actin disruption inhibits bombesin stimulation of focal adhesion kinase (pp125FAK) in prostate carcinoma. J Surg Res. 1996;63:359–363. doi: 10.1006/jsre.1996.0276. [DOI] [PubMed] [Google Scholar]
- 81.Aprikian AG, Tremblay L, Han K, Chevalier S. Bombesin stimulates the motility of human prostate-carcinoma cells through tyrosine phosphorylation of focal adhesion kinase and of integrin-associated proteins. Int J Cancer. 1997;72:498–504. doi: 10.1002/(sici)1097-0215(19970729)72:3<498::aid-ijc19>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Kyle E, Lieberman R, Crowell J, Kellof G, Bergan RC. Focal adhesion kinase (FAK) phosphorylation is not required for genistein-induced FAK-beta-1-integrin complex formation. Clin Exp Metastasis. 2000;18:203–212. doi: 10.1023/a:1006729106034. [DOI] [PubMed] [Google Scholar]
- 83.Margheri F, D'Alessio S, Serrati S, Pucci M, Annunziato F, Cosmi L, Liotta F, Angeli R, Angelucci A, Gravina GL, et al. Effects of blocking urokinase receptor signaling by antisense oligonucleotides in a mouse model of experimental prostate cancer bone metastases. Gene Ther. 2005 doi: 10.1038/sj.gt.3302456. [DOI] [PubMed] [Google Scholar]
- 84.Sabbisetti V, Chigurupati S, Thomas S, Shah G. Calcitonin stimulates the secretion of urokinase-type plasminogen activator from prostate cancer cells: its possible implications on tumor cell invasion. Int J Cancer. 2006;118:2694–2702. doi: 10.1002/ijc.21625. [DOI] [PubMed] [Google Scholar]
- 85.Papakonstanti EA, Kampa M, Castanas E, Stournaras C. A rapid, nongenomic, signaling pathway regulates the actin reorganization induced by activation of membrane testosterone receptors. Mol Endocrinol. 2003;17:870–881. doi: 10.1210/me.2002-0253. [DOI] [PubMed] [Google Scholar]
- 86.Sheta EA, Harding MA, Conaway MR, Theodorescu D. Focal adhesion kinase, Rap1, and transcriptional induction of vascular endothelial growth factor. J Natl Cancer Inst. 2000;92:1065–1073. doi: 10.1093/jnci/92.13.1065. [DOI] [PubMed] [Google Scholar]
- 87.Permpongkosol S, Wang JD, Takahara S, Matsumiya K, Nonomura N, Nishimura K, Tsujimura A, Kongkanand A, Okuyama A. Anticarcinogenic effect of FTY720 in human prostate carcinoma DU145 cells: modulation of mitogenic signaling, FAK, cell-cycle entry and apoptosis. Int J Cancer. 2002;98:167–172. doi: 10.1002/ijc.10178. [DOI] [PubMed] [Google Scholar]
- 88.Walden PD, Globina Y, Nieder A. Induction of anoikis by doxazosin in prostate cancer cells is associated with activation of caspase-3 and a reduction of focal adhesion kinase. Urol Res. 2004;32:261–265. doi: 10.1007/s00240-003-0365-7. [DOI] [PubMed] [Google Scholar]
- 89.Ittmann MM. Chromosome 10 alterations in prostate adeno-carcinoma (review) Oncol Rep. 1998;5:1329–1335. doi: 10.3892/or.5.6.1329. [DOI] [PubMed] [Google Scholar]
- 90.Gelman IH. Pyk 2 FAKs, any two FAKs. Cell Biol Int. 2003;27:507–510. doi: 10.1016/s1065-6995(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 91.Stanzione R, Picascia A, Chieffi P, Imbimbo C, Palmieri A, Mirone V, Staibano S, Franco R, De Rosa G, Schlessinger J, et al. Variations of proline-rich kinase Pyk2 expression correlate with prostate cancer progression. Lab Invest. 2001;81:51–59. doi: 10.1038/labinvest.3780211. [DOI] [PubMed] [Google Scholar]
- 92.Inazawa J, Sasaki H, Nagura K, Kakazu N, Abe T, Sasaki T. Precise localization of the human gene encoding cell adhesion kinase beta (CAK beta/PYK2) to chromosome 8 at p21. by fluorescence in situ hybridization. Hum Genet. 1996;98:508–510. doi: 10.1007/s004390050249. [DOI] [PubMed] [Google Scholar]
- 93.Picascia A, Stanzione R, Chieffi P, Kisslinger A, Dikic I, Tramontano D. Proline-rich tyrosine kinase 2 regulates proliferation and differentiation of prostate cells. Mol Cell Endocrinol. 2002;186:81–87. doi: 10.1016/s0303-7207(01)00667-0. [DOI] [PubMed] [Google Scholar]
- 94.Wang X, Yang Y, Guo X, Sampson ER, Hsu CL, Tsai MY, Yeh S, Wu G, Guo Y, Chang C. Suppression of androgen receptor transactivation by Pyk2 via interaction and phosphorylation of the ARA55 coregulator. J Biol Chem. 2002;277:15426–15431. doi: 10.1074/jbc.M111218200. [DOI] [PubMed] [Google Scholar]
- 95.Rijnders AW, van der Korput JA, van Steenbrugge GJ, Romijn JC, Trapman J. Expression of cellular oncogenes in human prostatic carcinoma cell lines. Biochem Biophys Res Commun. 1985;132:548–554. doi: 10.1016/0006-291x(85)91168-4. [DOI] [PubMed] [Google Scholar]
- 96.Allard P, Zoubeidi A, Nguyen LT, Tessier S, Tanguay S, Chevrette M, Aprikian A, Chevalier S. Links between Fer tyrosine kinase expression levels and prostate cell proliferation. Mol Cell Endocrinol. 2000;159:63–77. doi: 10.1016/s0303-7207(99)00205-1. [DOI] [PubMed] [Google Scholar]
- 97.Dunn GP, Sheehan KC, Old LJ, Schreiber RD. IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res. 2005;65:3447–3453. doi: 10.1158/0008-5472.CAN-04-4316. [DOI] [PubMed] [Google Scholar]
- 98.Rossi MR, Hawthorn L, Platt J, Burkhardt T, Cowell JK, Ionov Y. Identification of inactivating mutations in the JAK1, SYNJ2, and CLPTM1 genes in prostate cancer cells using inhibition of nonsense-mediated decay and microarray analysis. Cancer Genet Cytogenet. 2005;161:97–103. doi: 10.1016/j.cancergencyto.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 99.Gao B, Shen X, Kunos G, Meng Q, Goldberg ID, Rosen EM, Fan S. Constitutive activation of JAK-STAT3 signaling by BRCA1 in human prostate cancer cells. FEBS Lett. 2001;488:179–184. doi: 10.1016/s0014-5793(00)02430-3. [DOI] [PubMed] [Google Scholar]
- 100.Barton BE, Karras JG, Murphy TF, Barton A, Huang HF. Signal transducer and activator of transcription 3 (STAT3) activation in prostate cancer: direct STAT3 inhibition induces apoptosis in prostate cancer lines. Mol Cancer Ther. 2004;3:11–20. [PubMed] [Google Scholar]
- 101.Stearns ME, Wang M, Hu Y, Garcia FU. Interleukin-10 activation of the interleukin-10E1 pathway and tissue inhibitor of metalloproteinase-1 expression is enhanced by proteasome inhibitors in primary prostate tumor lines. Mol Cancer Res. 2003;1:631–642. [PubMed] [Google Scholar]
- 102.Wang M, Hu Y, Stearns ME. A novel IL-10 signalling mechanism regulates TIMP-1 expression in human prostate tumour cells. Br J Cancer. 2003;88:1605–1614. doi: 10.1038/sj.bjc.6600855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barton BE, Murphy TF, Adem P, Watson RA, Irwin RJ, Huang HF. IL-6 signaling by STAT3 participates in the change from hyperplasia to neoplasia in NRP-152 and NRP-154 rat prostatic epithelial cells. BMC Cancer. 2001;1:19. doi: 10.1186/1471-2407-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Flowers LO, Subramaniam PS, Johnson HM. A SOCS-1 peptide mimetic inhibits both constitutive and IL-6 induced activation of STAT3 in prostate cancer cells. Oncogene. 2005;24:2114–2120. doi: 10.1038/sj.onc.1208437. [DOI] [PubMed] [Google Scholar]
- 105.Li H, Ahonen TJ, Alanen K, Xie J, LeBaron MJ, Pretlow TG, Ealley EL, Zhang Y, Nurmi M, Singh B, et al. Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res. 2004;64:4774–4782. doi: 10.1158/0008-5472.CAN-03-3499. [DOI] [PubMed] [Google Scholar]
- 106.Weiss-Messer E, Merom O, Adi A, Karry R, Bidosee M, Ber R, Kaploun A, Stein A, Barkey RJ. Growth hormone (GH) receptors in prostate cancer: gene expression in human tissues and cell lines and characterization, GH signaling and androgen receptor regulation in LNCaP cells. Mol Cell Endocrinol. 2004;220:109–123. doi: 10.1016/j.mce.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 107.Bowen C, Birrer M, Gelmann EP. Retinoblastoma protein-mediated apoptosis after gamma-irradiation. J Biol Chem. 2002;277:44969–44979. doi: 10.1074/jbc.M202000200. [DOI] [PubMed] [Google Scholar]
- 108.Macoska JA, Xu J, Ziemnicka D, Schwab TS, Rubin MA, Kotula L. Loss of expression of human spectrin src homology domain binding protein 1 is associated with 10p loss in human prostatic adenocarcinoma. Neoplasia. 2001;3:99–104. doi: 10.1038/sj.neo.7900145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kubler HR, van Randenborgh H, Treiber U, Wutzler S, Battistel C, Lehmer A, Wagenpfeil S, Hartung R, Paul R. In vitro cytotoxic effects of imatinib in combination with anticancer drugs in human prostate cancer cell lines. Prostate. 2005;63:385–394. doi: 10.1002/pros.20201. [DOI] [PubMed] [Google Scholar]
- 110.Uehara H, Kim SJ, Karashima T, Shepherd DL, Fan D, Tsan R, Killion JJ, Logothetis C, Mathew P, Fidler IJ. Effects of blocking platelet-derived growth factor-receptor signaling in a mouse model of experimental prostate cancer bone metastases. J Natl Cancer Inst. 2003;95:458–470. doi: 10.1093/jnci/95.6.458. [DOI] [PubMed] [Google Scholar]
- 111.Kim SJ, Uehara H, Yazici S, Langley RR, He J, Tsan R, Fan D, Killion JJ, Fidler IJ. Simultaneous blockade of platelet-derived growth factor-receptor and epidermal growth factor-receptor signaling and systemic administration of paclitaxel as therapy for human prostate cancer metastasis in bone of nude mice. Cancer Res. 2004;64:4201–4208. doi: 10.1158/0008-5472.CAN-03-3763. [DOI] [PubMed] [Google Scholar]
- 112.Kim SJ, Uehara H, Yazici S, He J, Langley RR, Mathew P, Fan D, Fidler IJ. Modulation of bone microenvironment with zoledronate enhances the therapeutic effects of STI571 and paclitaxel against experimental bone metastasis of human prostate cancer. Cancer Res. 2005;65:3707–3715. doi: 10.1158/0008-5472.CAN-04-3601. [DOI] [PubMed] [Google Scholar]
- 113.Corcoran NM, Costello AJ. Combined low-dose imatinib mesylate and paclitaxel lack synergy in an experimental model of extraosseous hormone-refractory prostate cancer. BJU Int. 2005;96:640–646. doi: 10.1111/j.1464-410X.2005.05699.x. [DOI] [PubMed] [Google Scholar]
- 114.Mathew P, Thall PF, Jones D, Perez C, Bucana C, Troncoso P, Kim SJ, Fidler IJ, Logothetis C. Platelet-derived growth factor receptor inhibitor imatinib mesylate and docetaxel: a modular phase I trial in androgen-independent prostate cancer. J Clin Oncol. 2004;22:3323–3329. doi: 10.1200/JCO.2004.10.116. [DOI] [PubMed] [Google Scholar]
- 115.Tiffany NM, Wersinger EM, Garzotto M, Beer TM. Imatinib mesylate and zoledronic acid in androgen-independent prostate cancer. Urology. 2004;63:934–939. doi: 10.1016/j.urology.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 116.Rao K, Goodin S, Levitt MJ, Dave N, Shih WJ, Lin Y, Capanna T, Doyle-Lindrud S, Juvidian P, DiPaola RS. A phase II trial of imatinib mesylate in patients with prostate specific antigen progression after local therapy for prostate cancer. Prostate. 2005;62:115–122. doi: 10.1002/pros.20130. [DOI] [PubMed] [Google Scholar]
- 117.Bjorge JD, O'Connor TJ, Fujita DJ. Activation of human pp60c-src. Biochem Cell Biol. 1996;74:477–484. doi: 10.1139/o96-052. [DOI] [PubMed] [Google Scholar]
- 118.Tamagnone L, Lahtinen I, Mustonen T, Virtaneva K, Francis F, Muscatelli F, Alitalo R, Smith CI, Larsson C, Alitalo K. BMX, a novel nonreceptor tyrosine kinase gene of the BTK/ITK/TEC/TXK family located in chromosome Xp22.2. Oncogene. 1994;9:3683–3688. [PubMed] [Google Scholar]
- 119.Qiu Y, Robinson D, Pretlow TG, Kung HJ. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3'-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci. 1998;95:3644–3649. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xue LY, Qiu Y, He J, Kung HJ, Oleinick NL. Etk/Bmx, a PH-domain containing tyrosine kinase, protects prostate cancer cells from apoptosis induced by photodynamic therapy or thapsigargin. Oncogene. 1999;18:3391–3398. doi: 10.1038/sj.onc.1202687. [DOI] [PubMed] [Google Scholar]
- 121.Jiang T, Guo Z, Dai B, Kang M, Ann DK, Kung HJ, Qiu Y. Bi-directional regulation between tyrosine kinase Etk/BMX and tumor suppressor p53 in response to DNA damage. J Biol Chem. 2004;279:50181–50189. doi: 10.1074/jbc.M409108200. [DOI] [PubMed] [Google Scholar]
- 122.Wu YM, Huang CL, Kung HJ, Huang CY. Proteolytic activation of ETK/Bmx tyrosine kinase by caspases. J Biol Chem. 2001;276:17672–17678. doi: 10.1074/jbc.M010964200. [DOI] [PubMed] [Google Scholar]
- 123.Kim O, Jiang T, Xie Y, Guo Z, Chen H, Qiu Y. Synergism of cytoplasmic kinases in IL6-induced ligand-independent activation of androgen receptor in prostate cancer cells. Oncogene. 2004;23:1838–1844. doi: 10.1038/sj.onc.1207304. [DOI] [PubMed] [Google Scholar]
- 124.Xie Y, Xu K, Dai B, Guo Z, Jiang T, Chen H, Qiu Y. The 44 kDa Pim-1 kinase directly interacts with tyrosine kinase Etk/BMX and protects human prostate cancer cells from apoptosis induced by chemotherapeutic drugs. Oncogene. 2006;25:70–78. doi: 10.1038/sj.onc.1209058. [DOI] [PubMed] [Google Scholar]
- 125.Wang Y, Yu Q, Cho AH, Rondeau G, Welsh J, Adamson E, Mercola D, McClelland M. Survey of differentially methylated promoters in prostate cancer cell lines. Neoplasia. 2005;7:748–760. doi: 10.1593/neo.05289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hoehn GT, Stokland T, Amin S, Ramirez M, Hawkins AL, Griffin CA, Small D, Civin CI. TnK1: a novel intracellular tyrosine kinase gene isolated from human umbilical cord blood CD34+/Lin-/CD38- stem/progenitor cells. Oncogene. 1996;12:903–913. [PubMed] [Google Scholar]
- 127.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]


