Abstract
DNA superhelical tension, an important feature of genomic organization, is known to affect the interactions of intercalating molecules with DNA. However, the effect of torsional tension on nonintercalative DNA-binding chemicals has received less attention. We demonstrate here that the enediyne calicheamicin γ1I, a strand-breaking agent specific to the minor groove, causes ≈50% more damage in negatively supercoiled plasmid DNA than in DNA with positive superhelicity. Furthermore, we show that the decrease in damage in positively supercoiled DNA is controlled at the level of thiol activation of the drug. Our results suggest that supercoiling may affect both the activity of nonintercalating genotoxins in vivo and the accessibility of glutathione and other small physiologic molecules to DNA-bound chemicals or reactions occurring in the grooves of DNA.
Superhelical tension plays a role in many aspects of DNA physiology including transcription (1), replication (1), and repair (2). The genome of Escherichia coli has a net superhelical density (σ) of ≈−0.05 (supercoils per helical turn), while there appears to be no net superhelical tension in the genomes of Drosophila melanogaster and humans (3). However, as a consequence of the loop organization of the genome in higher eukaryotes (4), transcriptionally active DNA contains high levels of localized torsional tension—negative in the 5′-ends and positive in the 3′-ends (5–11)—that is consistent with the twin-domain model of Liu and Wang (5) for transcriptionally induced DNA supercoiling. Though superhelical tension has well-defined effects on the interactions of proteins and small DNA intercalators, its effects on other molecules that interact with DNA have not been fully explored.
The effects of torsional tension on small molecule-DNA interactions are a direct consequence of changes in DNA structure and dynamics. Negative and positive superhelical tension occur when the helix is unwound or over wound, respectively (12), and it has been estimated that as much as 30% of torsional tension is expressed in alterations of twist (helical repeat) with the remaining 70% expressed as writhing of the helix (13–15). Torsional tension has several consequences for DNA secondary structure, including formation of Z-DNA, cruciforms, and single-stranded regions (12, 16–19). In the case of negatively supercoiled DNA, the under wound state of the helix favors binding of intercalating molecules because they induce local unwinding that is dissipated as global overwinding of the helix (9, 20).
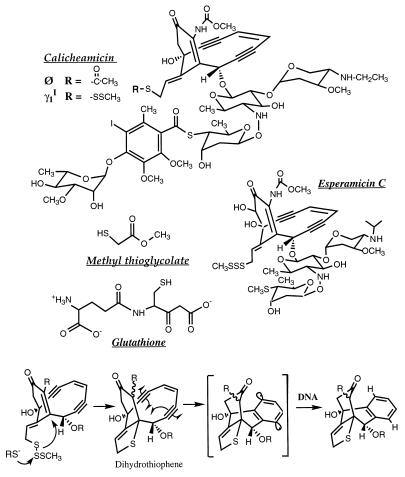
By analogy to intercalators, the DNA interactions of other molecules that are sensitive to twisting and writhing of the helix should also be affected by superhelical tension. We have tested this hypothesis with the enediyne antibiotic calicheamicin γ (Fig. 1). The enediyne family is a structurally diverse group of DNA-damaging molecules that are cytotoxic at picomolar to femtomolar concentrations (21–23). This lethality is likely due to the production of high levels (>95%) of double strand DNA damage (24) following reductive activation, presumably by glutathione in vivo, to form a diradical intermediate that binds in the minor groove and abstracts hydrogen atoms from deoxyribose (Fig. 1; reviewed in refs. 22, 25–27). In the present studies, we have employed an analog of calicheamicin γ, calicheamicin Ø, in which the methyl trisulfide trigger has been replaced by a thioacetate group that undergoes hydrolysis in the absence of thiols (Fig. 1). The resulting diradical intermediate is identical in structure to that arising from calicheamicin γ (23), as are the sequence-selectivity (23), the chemistry, and the bistranded nature of the DNA lesions produced by the two drugs (P.C.D., unpublished observations).
Figure 1.
Structures of enediynes and thiols, and the mechanism of thiol activation of calicheamicin γ. Hydrolysis of the thioacetate group of calicheamicin Ø leads to the formation of the same diradical intermediate as formed with calicheamicin γ.
By using highly positively supercoiled DNA produced by a recently developed technique (28), we have investigated the effect of superhelical tension on calicheamicin-DNA interactions. We found that calicheamicin preferentially damages negatively supercoiled DNA and that this difference is due to accessibility of glutathione to DNA-bound drug. Our results suggest that DNA supercoiling may have significant effects on small molecule-DNA interactions and on chemical reactions that occur in proximity to DNA.
MATERIALS AND METHODS
Reagents.
Calicheamicin γ1I was provided by George Ellestad (Wyeth-Ayerst Research). Calicheamicin θ1I was synthesized as described elsewhere (23).
Positive supercoiling of plasmid pUC19 was achieved by using a recombinant archaeal histone HMf (rHMf) synthesized in E. coli from the cloned hmfB gene from Methanothermus fervidus as described elsewhere (28). Negatively supercoiled pUC19, isolated from DH5α E. coli by standard techniques, was subjected to the same treatment as the positively supercoiled DNA except for the addition of topoisomerase-containing chicken blood extract. Sham reactions were instead performed with the dilution and storage buffer used with the chicken blood extract (28). Both plasmid substrates were quantitated by using the Hoechst dye 33258 (Sigma) fluorescence quantitation assay (29).
In both DNA substrates, care was taken to remove contaminants that could interfere with the drug-induced DNA damage. To remove trace quantities of nonspecifically bound positively charged peptides, the DNA was adjusted to 2M NaCl and incubated at room temperature for 2 hr. The DNA was then dialyzed at 4°C against Chelex-treated phosphate buffer (50 mM, pH 7.4) containing the metal chelating agent, diethylenetriaminepentaacetic acid, and finally dialyzed against phosphate buffer alone to remove the chelator.
Calicheamicin γ-Induced Changes in DNA Supercoiling.
The effect of calicheamicin γ and esperamicin C on the topology of negatively supercoiled pUC19 was determined by sucrose density gradient centrifugation as described elsewhere (30). Briefly, a mixture of [3H]-labeled, nicked plasmid, and [14C]-labeled negatively supercoiled plasmid was layered on a 5–20% sucrose density gradient (10 mM Hepes/1 mM EDTA/10% dimethylformamide, pH 7) containing various concentrations of calicheamicin γ. The gradients were centrifuged at 248,000 × g for 1.5 hr at 4°C. Fractions were collected from the top of the tube and [3H] and [14C] were quantitated by scintillation counting. The peak radioactive fraction for each isotope was used to calculate the sedimentation of the form I DNA relative to the form II DNA. Calicheamicin γ and esperamicin C were found to be soluble and chemically stable for up to 10 hr in the sucrose buffer, and there was no detectable nicking of the plasmid DNA (data not shown).
Treatment of Supercoiled pUC19 with DNA-Damaging Agents.
Damage reactions were performed in 25 μl reaction volumes (10 mM Hepes/1 mM EDTA/8% MeOH, pH 7.0) containing 30 μg/ml of either positively or negatively supercoiled pUC19 DNA. Calicheamicin γ was added to each sample and allowed to incubate at ambient temperature for 5 min. The drug was then activated by the addition of either glutathione to 10 mM or methyl thioglycolate to 1 mM and the reaction carried out for 30 min at ambient temperature; in one experiment, the reaction was allowed to proceed for up to 24 hr. Calicheamicin θ reactions were performed in a similar manner except that thiols were omitted and the damage reaction was allowed to proceed for 16 hr at 37°C. In both cases, putrescine was added to each sample to 100 mM and the samples were incubated at 37°C for 30 minutes to express abasic sites as strand breaks (24). Following treatment, DNA samples were purified by passage over G-50 Sephadex spin columns. The DNA was then relaxed with chicken blood extract (28), to remove any superhelical dependent biases in subsequent electrophoresis or probing; this treatment did not affect the number of strand breaks in the DNA substrates (data not shown). Finally, the DNA was purified by proteinase K digestion (100 μg/ml; 1% SDS; 2 hr at 37°C) followed by phenol/chloroform and chloroform/isoamyl alcohol extractions and ethanol precipitation.
DNA Damage Analysis.
DNA from each sample was resolved on a 1.5% agarose gel (Tris-borate-EDTA) containing 60 μM chloroquine (Sigma). The gels were washed extensively in water to remove the chloroquine and then stained with 0.5 μg/ml ethidium bromide for UV photography. Following exposure to 254 nm light for 5 min to ensure nicking of the plasmid DNA, the gels were dried on a conventional gel dryer for 45 min at ambient temperature and 45 min at 60°C. DNA topoisomers were then quantified by in situ hybridization in the dried agarose gel with a pUC19 random primer probe according to the protocol of Lueders and Fewell (31). The relative intensities of the plasmid forms were determined on a PhosphorImager (Molecular Dynamics) and the quantity of strand breaks was calculated from the proportion of supercoiled (form I) DNA converted to nicked (form II) DNA and linear (form III). Drug concentrations were selected so that the number of strand breaks would not exceed “single-hit” conditions according to a Poisson distribution, which corresponds to no more than ≈30% of the supercoiled DNA molecules sustaining damage (24).
In all experiments, we have compared the ratios of damage in the two supercoiled forms rather than comparing the absolute levels of damage. This was necessitated by moderate experiment-to-experiment variability in the absolute quantity of damage. However, within a single experiment, the absolute levels of DNA damage are comparable between samples.
Determination of Relative Binding Affinities of Calicheamicin γ with Positively and Negatively Supercoiled pUC19.
The relative affinity of calicheamicin γ for positively and negatively supercoiled pUC19 was determined by a competitive DNA damage assay. Calicheamicin-induced DNA damage in a [32P]-labeled DNA fragment was measured in the presence of varying concentrations of unlabeled, supercoiled pUC19, which competed with the labeled DNA for drug binding. The relative reduction in damage to the labeled strand is directly correlated with the overall binding constant for calicheamicin with the supercoiled DNA competitor. While this assay cannot be used to determine absolute binding constants, the relative binding affinity can be defined for the two supercoiled DNA substrates. The 215-bp HindIII/PvuII fragment of pUC19 was 5′-[32P] end-labeled at the HindIII site as described elsewhere (24). The labeled fragment was mixed with calf thymus DNA (final 0.3 μg/ml) and 0.3 nM calicheamicin γ in 10 mM Hepes, 1 mM EDTA, pH 7. This mixture was then divided into 8 μl aliquots and 1 μl of supercoiled DNA was added to each aliquot. After 5 min of equilibration, 1 μl of 100 mM glutathione (pH 7) or 10 mM methyl thioglycolate was added and the damage reaction was allowed to proceed for 30 min at ambient temperature; the drug reaction was complete within this time (data not shown). Abasic sites were converted to strand breaks by treatment with putrescine (100 mM) for 30 min at 37°C (24). Following addition of glycerol-based loading buffer, the entire sample was resolved on a 10% nondenaturing polyacrylamide gel. Radioactivity in a DNA fragment produced by cleavage at an AGGA site in the labeled DNA was quantified relative to total radioactivity in the lane by PhosphorImager analysis. The ratio of binding constants for the supercoiled DNA substrates is proportional to the concentration of supercoiled DNA necessary to produce equivalent amounts of damage in the radiolabeled marker DNA.
RESULTS
In the Presence of Glutathione, Calicheamicin γ Produces More Damage in Negatively Supercoiled DNA than in Positively Supercoiled DNA.
We investigated the effect of superhelical tension on the damage produced by calicheamicin γ in highly positively and negatively supercoiled pUC19. The positively supercoiled pUC19 was prepared by using a recently developed technique that employs HMf proteins (28) and the DNA had an average σ ≈+0.04, with a maximal ΔLk of +17. The negatively supercoiled plasmid was estimated to have σ ≈ −0.05 (ΔLk ≈ −14) by two-dimensional gel electrophoresis (data not shown).
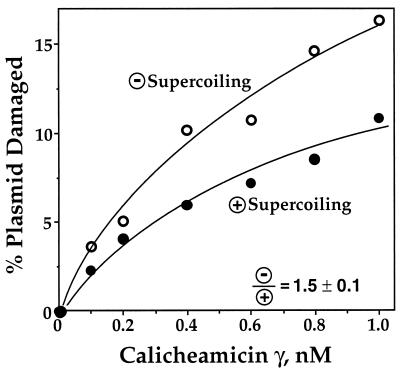
As shown in Fig. 2, calicheamicin γ activated by glutathione produced 50% more damage in the negatively supercoiled substrate than in positively supercoiled DNA. The reaction was assumed to be complete after 30 min because no further changes were noted with incubations up to 24 hr (data not shown). As observed in previous studies (24), calicheamicin caused only bistranded DNA lesions, as indicated by the fact that only linear, form III DNA was produced in the damage reaction (data not shown).
Figure 2.
Calicheamicin γ produces 50% more damage in negatively supercoiled DNA than in positively supercoiled DNA when activated by glutathione. Samples of negatively and positively supercoiled pUC19 were treated with calicheamicin γ and 10 mM glutathione as described in Materials and Methods and gel-resolved plasmid topoisomers were quantified by PhosphorImager analysis of probed gels; representative experimental results are shown in the graph. The average ratio of double-strand breaks in negatively supercoiled DNA to double-strand breaks in positively supercoiled DNA is 1.5 ± 0.1 (n = 5).
Calicheamicin γ Induces Negative Writhing of Plasmid DNA.
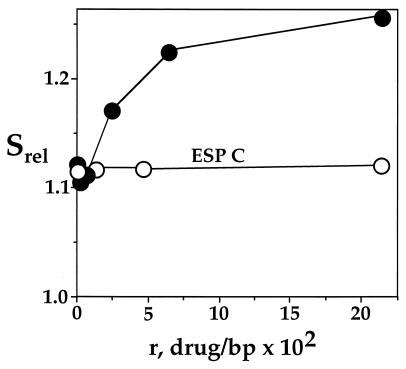
To define the basis for the supercoiling-dependent difference in calicheamicin damage, we studied the effect of calicheamicin binding on DNA topology. As shown in Fig. 3, binding of calicheamicin γ increased the sedimentation of negatively supercoiled plasmid DNA relative to nicked-open circular DNA. This is consistent with drug-induced negative writhing of the plasmid DNA. As noted previously (30), no change in supercoiling is observed for esperamicin C, an analog of calicheamicin γ missing the terminal sugar-aromatic ring (Fig. 1). This serves as a negative control for the effects of calicheamicin and demonstrates the importance of the carbohydrate side chain in calicheamicin-DNA interactions.
Figure 3.
Calicheamicin γ causes negative writhing of plasmid DNA. The effect of binding of calicheamicin γ (•) and esperamicin C (○) on the topology of negatively supercoiled pUC19 was determined by sucrose gradient centrifugation as described in Materials and Methods. The sedimentation of supercoiled pUC19 DNA was determined relative to nicked, open-circular pUC19.
The calicheamicin-dependent increase in negative writhing could be a response to changes in DNA twist, a direct response to drug-induced changes in helical writhing, or a combination of these two extremes. We have previously demonstrated that calicheamicin ɛ, the aromatized form of calicheamicin γ1I, binds to flexible DNA sequences and bends the DNA upon binding (refs. 32 and 33; A. Salzberg and P.C.D., unpublished observations). Such DNA bending can, in theory, alter the net writhing of the closed-circular plasmid DNA. On the other hand, the drug-induced increase in negative writhing is consistent with circular dichroism evidence for helical overwinding caused by binding of calicheamicin γ (34). This contrasts with the behavior of intercalating agents, such as the enediyne esperamicin A1 (30), that characteristically unwind the DNA helix upon binding (35, 36) and cause positive writhing. However, the degree to which helical winding contributes to the changes in DNA topology cannot be determined from plasmid sedimentation studies. Furthermore, a predominance of drug-induced overwinding of the helix would require that calicheamicin bind to the over wound helix of positively supercoiled DNA with higher affinity than to negatively supercoiled DNA. The following experiment demonstrates that this is not the case.
Calicheamicin γ Has Equal Binding Affinities for Negative and Positive Superhelical DNA.
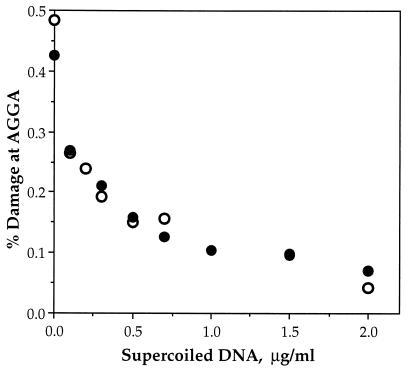
To test the hypothesis that calicheamicin γ binds preferentially to positively supercoiled DNA, we determined relative binding affinities of the drug in the two supercoiled DNA substrates. This experiment was performed by comparing the level of DNA damage in a [32P]-labeled DNA fragment in the presence of either positively or negatively supercoiled pUC19; preferential binding of calicheamicin to one of the supercoiled substrates would cause a decrease in the damage in the labeled fragment.
As shown in Fig. 4, both negatively and positively supercoiled plasmid DNA compete to the same extent in the damage assay. This is illustrated by the expected 50% reduction in damage in the labeled DNA fragment when 0.3 μg/ml of either supercoiled substrate is added to 0.3 μg/ml of the carrier DNA (average from two experiments; the labeled DNA fragment is present in trace quantities). Thus, there is no major difference in the affinity of calicheamicin γ for these two supercoiled plasmid molecules. It should be noted that similar results were obtained with both methyl thioglycolate and glutathione as drug activators.
Figure 4.
Calicheamicin γ binds with equal affinity to negatively and positively supercoiled pUC19. The relative affinity of calicheamicin γ for negatively (○) and positively (•) supercoiled pUC19 was determined by a competitive DNA damage assay, as described in Materials and Methods. Calicheamicin γ-induced DNA damage in a [32P]-labeled DNA fragment was measured in the presence of varying concentrations of unlabeled, supercoiled pUC19. The reduction of damage in the labeled DNA correlates directly with the overall binding constant for calicheamicin with the supercoiled DNA competitor. Representative experimental results are shown in the graph; the average ratio of damage in the presence of negatively supercoiled DNA to that with positively supercoiled DNA is 1.0 ± 0.1 (n = 8).
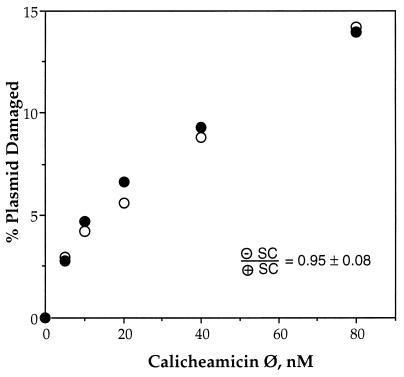
Calicheamicin Ø Produces Equivalent Amounts of Damage in Both Supercoiled Substrates.
The observed supercoiling-dependent bias in drug-induced damage in spite of similar binding affinities raised the possibility that supercoiling affected the drug activation step rather than the actual damage events. To test this hypothesis, we examined the effect of supercoiling on damage produced by calicheamicin Ø, an analog of calicheamicin γ with a thioacetate trigger that undergoes hydrolysis in the absence of thiols (Fig. 1).
The results of these studies are shown in Fig. 5. Calicheamicin Ø alone produced equal numbers of double-strand breaks in both plasmid substrates. This is consistent with a model in which glutathione has a reduced accessibility to drug that is bound to positively supercoiled DNA.
Figure 5.
Calicheamicin Ø is not affected by DNA supercoiling. Samples of negatively (○) and positively (•) supercoiled pUC19 were treated with calicheamicin Ø as described in Materials and Methods and gel-resolved plasmid topoisomers were quantified by PhosphorImager analysis of probed gels; representative experimental results are shown in the graph. The average ratio of double-strand breaks in negatively supercoiled DNA to double-strand breaks in positively supercoiled DNA is 0.95 ± 0.08 (n = 5).
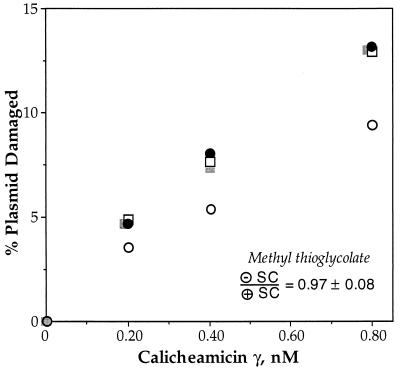
In the Presence of Methyl Thioglycolate, Calicheamicin γ Shows No Supercoiling Bias.
To test the hypothesis that the supercoiling-dependence of glutathione activation is due to the size and/or negative charge of the thiol, we studied the damage produced in supercoiled DNA when calicheamicin γ was activated by methyl thioglycolate, a small, uncharged thiol (Fig. 1). The results shown in Fig. 6 reveal that activation of calicheamicin γ by methyl thioglycolate causes the enediyne to produce equal amounts of DNA damage in both supercoiled substrates. Methyl thioglycolate does not affect the binding of the activated drug because both methyl thioglycolate and glutathione result in the same sequence-selectivity and double-strand character of calicheamicin-induced DNA damage (data not shown). It is also apparent in Fig. 6 that methyl thioglycolate and glutathione cause calicheamicin to produce equal amounts of damage in the negatively supercoiled substrate. This indicates that the supercoiling bias observed with glutathione is due to a reduction in damage in positively supercoiled DNA.
Figure 6.
Activation of calicheamicin γ by methyl thioglycolate abolishes supercoiling-dependent biases in DNA damage observed with glutathione. Samples of negatively (filled symbols) and positively (open symbols) supercoiled pUC19 were treated with calicheamicin γ and 1 mM methyl thioglycolate (squares) or 10 mM glutathione (circles) as described in Materials and Methods and gel-resolved plasmid topoisomers were quantified by PhosphorImager analysis of probed gels; representative experimental results are shown in the graph. The average ratio of double-strand breaks in negatively supercoiled DNA to double-strand breaks in positively supercoiled DNA for methyl thioglycolate is 0.97 ± 0.08 (n = 5); the average ratio for glutathione is 1.5 ± 0.1 (Fig. 2).
DISCUSSION
By using DNA substrates with high levels of positive and negative supercoiling, we have demonstrated that the damage produced by calicheamicin γ, an equilibrium-binding, minor groove-specific DNA-damaging agent, is sensitive to DNA supercoiling. The mechanistic model most consistent with our data is differential accessibility of glutathione to calicheamicin bound to DNA in different states of superhelical tension. Given the presence of high levels of both positive and negative supercoiling in transcriptionally active genes, these results have significant implications for other physiologically relevant small-molecule DNA interactions.
There are several models that account for the effect of supercoiling on the damage produced by calicheamicin γ. First, the preference for damaging negatively supercoiled DNA could reflect a higher affinity of the drug for DNA with negative torsion. Alternatively, calicheamicin may bind with higher affinity to positively supercoiled DNA and paradoxically experience less activation to produce DNA damage. This inverse relationship between binding affinity and activation has been observed by Myers et al. with dynemicin analogs (37). A third model involves calicheamicin binding to the supercoiled DNA substrates with similar affinities but in different orientations. This model requires a nonproductive binding orientation in positively supercoiled DNA. The recent studies of Epstein et al. (38) suggest a fourth model in which supercoiling affects the ability of glutathione to repair drug-induced DNA lesions. Finally, the drug may bind in similar affinity to both DNA substrates with the supercoiling effects due to differences in the ability of glutathione to activate the drug. Considering the present experimental results, only the last model is acceptable.
The lack of effect of supercoiling on the results of the competitive drug binding assay appears to rule out the first two models, which depend on supercoiling-dependent differences in drug binding. This result also suggests that the main determinant of drug-induced alterations in plasmid topology is not helical over winding. If drug-induced helical over winding were solely responsible for the observed negative plasmid writhing (Fig. 3), then calicheamicin should have bound with higher affinity to positively supercoiled DNA. However, we did not detect any significant difference in the affinity of calicheamicin for either state of DNA supercoiling. Assuming that damage occurs in proportion to the level of bound drug and that calicheamicin binds to pUC19 with a micromolar binding constant (refs. 39 and 40; L.Y. and P.C.D., unpublished observations), a 50% difference in the quantity of damage would require that binding constants differ by ≈100-fold. The competitive binding assay would have detected this difference in binding affinity.
Though supercoiling may not affect the DNA binding affinity of the parent form of the drug, a parallel argument could be made for differences in the binding of the dihydrothiophene intermediate of reduced calicheamicin (Fig. 1). However, the lack of supercoiling effect observed with calicheamicin Ø and with calicheamicin γ activated by methyl thioglycolate rules out this scheme.
The third model, similar binding affinities but different binding orientations, is also inadequate to explain the results. Calicheamicin γ produces only bistranded DNA lesions and has the same sequence selectivity in both positively and negatively supercoiled DNA. Grossly different orientations of the bound drug, or reaction intermediates, would be expected to affect these features of drug-induced damage. Furthermore, the reactive intermediates for calicheamicins γ and Ø are identical, which rules out different binding orientations as the cause of the supercoiling-dependent differences.
The model that best explains the preference of calicheamicin for damaging negatively supercoiled DNA involves thiol accessibility. Two pieces of evidence support this conclusion. First, the thiol-independent calicheamicin Ø produced equal amounts of DNA damage in both supercoiled substrates. Second, activation of calicheamicin by the neutral methyl thioglycolate also produced equivalent results in both forms of supercoiled DNA. This latter result suggests that glutathione is limited in its accessibility to DNA-bound calicheamicin γ by either its negative charge or its steric bulk.
This argument is similar in nature to a model in which supercoiling differentially affects the ability of glutathione to “repair” the calicheamicin-induced damage by hydrogen transfer from the thiol to the carbon-centered radicals in deoxyribose. This phenomenon has been proposed by Epstein et al. to occur with DNA damage produced by the enediynes esperamicins A1 and C (38). According to their model, the negative charge of glutathione reduces its accessibility to drug-induced deoxyribose radicals compared with the smaller, neutrally charged methyl thioglycolate. While such repair may have occurred to some extent in the experiments, our observation of biased DNA damage cannot be explained by supercoiling-dependent differences in thiol-mediated DNA repair. If the repair phenomenon were the primary determinant of the observed effects, then we would not expect methyl thioglycolate to cause an increase—relative to glutathione—in calicheamicin-induced damage in positively supercoiled DNA (Fig. 6). Methyl thioglycolate is proposed to be more efficient at quenching drug-induced deoxyribose radicals than glutathione (38), and we would expect methyl thioglycolate to further reduce the damage in positively supercoiled DNA relative to glutathione.
All of the evidence thus points to a model in which supercoiling influences the accessibility of glutathione to DNA-bound calicheamicin. Any mechanistic proposal to explain this phenomenon must take into account two observations: (i) within 30 min, all of the drug appears to be rendered inactive toward production of DNA damage; and (ii) there appears to be no major difference in the affinity of calicheamicin for binding to supercoiled DNA of either sign. A general scheme consistent with these observations centers on a side reaction—predominantly in positively supercoiled DNA—that results in nondamaging activation of the drug by thiol or inactivation of some intermediate form of the drug. While the exact spatial relationships of drug, DNA and thiol that lead to productive activation are unknown, it is possible that positive supercoiling causes a portion of bound drug, but not “unbound” or freely solvated drug, to be inaccessible to glutathione, thus shifting the balance to nonproductive activation of calicheamicin. For example, positive supercoiling could alter the charge density around a narrowed minor groove, which could limit the accessibility of the negatively charged glutathione to drug positioned in the groove. Such changes in DNA conformation are likely given the significant portion of superhelical tension that is translated into changes in DNA twist (13–15). Another possibility lies in the handedness of the writhing of DNA helices in plectonemic supercoiling (for example, see ref. 13). Positive supercoiling causes the helices to cross one another in a left-handed superhelix with the walls of the major and minor grooves running approximately parallel to each other. In negatively supercoiled DNA, however, the walls of the grooves adopt an orthogonal orientation. Parallel placement of the grooves would create a hydrophobic “tunnel” that might protect calicheamicin from glutathione activation. Further study is necessary to identify the mechanism(s) responsible for the supercoiling-dependent differences in calicheamicin γ-induced DNA damage.
Our observation that the preference of calicheamicin for damaging negatively supercoiled DNA is controlled at the level of thiol activation has implications for other small molecule-DNA interactions. Glutathione is believed to be involved in many oxidative damage processes (42). A supercoiling-dependent exclusion of glutathione from the major or minor grooves of DNA may affect the chemistry of oxidative DNA damage in transcriptionally active genes that are subjected to high levels of both positive and negative supercoiling (5, 9). Furthermore, the effect of supercoiling on glutathione-DNA interactions may extend to other small genotoxic molecules such as the negatively charged superoxide. We have observed that DNA damage produced by Cu(II)/H2O2 is sensitive to DNA supercoiling (43). Because glutathione appears to be capable of mediating Cu(II)-induced Fenton chemistry (44), the location, quantity, and chemistry of Cu-induced DNA damage could thus be further complicated by differential accessibility of glutathione to sites of bound Cu or DNA damage produced by Cu.
To summarize, we have shown that calicheamicin γ, a nonintercalating DNA-damaging agent, exhibits a supercoiling bias, causing more damage in negatively supercoiled plasmid DNA than in DNA with positive superhelical tension. Furthermore we have shown that this supercoiling-induced difference is controlled at the level of glutathione activation of the drug. Our findings suggest a general effect of supercoiling on the accessibility of glutathione and other small molecules to DNA.
Acknowledgments
The authors thank Dr. Kathleen Sandman and Prof. John Reeve for providing HMf proteins for the generation of positively supercoiled DNA. This work was supported by National Institutes of Health Grants CA63223 (P.C.D.), CA65375 (P.C.D.), CA46446 (K.C.N.), and the Samuel A. Goldblith Professorship (P.C.D.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Sinden R R. DNA Structure and Function. San Diego: Academic; 1994. [Google Scholar]
- 2.Koo H-S, Classen L, Grossman L, Liu L F. Proc Natl Acad Sci USA. 1991;88:1212–1216. doi: 10.1073/pnas.88.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinden R R, Carlson J O, Pettijohn D E. Cell. 1980;21:773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- 4.Nelson W G, Pienta K J, Barrack E R, Coffey D S. Annu Rev Biophys Biophys Chem. 1986;15:457–475. doi: 10.1146/annurev.bb.15.060186.002325. [DOI] [PubMed] [Google Scholar]
- 5.Liu L F, Wang J C. Proc Natl Acad Sci USA. 1987;84:7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ljungman M, Hanawalt P C. Nucleic Acids Res. 1995;23:1782–1789. doi: 10.1093/nar/23.10.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsao Y, Wu H, Liu L F. Cell. 1989;56:111–118. doi: 10.1016/0092-8674(89)90989-6. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Liu L F. J Mol Biol. 1991;219:615–622. doi: 10.1016/0022-2836(91)90658-s. [DOI] [PubMed] [Google Scholar]
- 9.Ljungman M, Hanawalt P C. Proc Natl Acad Sci USA. 1992;89:6055–6059. doi: 10.1073/pnas.89.13.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jupe E R, Sinden R R, Cartwright I L. EMBO J. 1993;12:1067–1075. doi: 10.1002/j.1460-2075.1993.tb05748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jupe E R, Sinden R R, Cartwright I L. Biochemistry. 1995;34:2628–2633. doi: 10.1021/bi00008a029. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Peck L, Becherer K. Cold Spring Harb Symp Quant Biol. 1982;47:85–91. doi: 10.1101/sqb.1983.047.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Bednar J, Furrer P, Stasiak A, Dubochet J. J Mol Biol. 1994;235:825–847. doi: 10.1006/jmbi.1994.1042. [DOI] [PubMed] [Google Scholar]
- 14.Adrian M, ten Heggeler-Bordier B, Wahli W, Stasiak A Z, Stasiak A, Dubochet J. EMBO J. 1990;9:4551–4554. doi: 10.1002/j.1460-2075.1990.tb07907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boles T C, White J H, Cozzarelli N R. J Mol Biol. 1990;213:931–951. doi: 10.1016/S0022-2836(05)80272-4. [DOI] [PubMed] [Google Scholar]
- 16.Lilley D M J. Cold Spring Harbor Symp Quant Biol. 1982;47:101–112. doi: 10.1101/sqb.1983.047.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Selvin P R, Cook D N, Pon N G, Bauer W R, Klein M P, Hearst J E. Science. 1992;255:82–85. doi: 10.1126/science.1553534. [DOI] [PubMed] [Google Scholar]
- 18.Shore D, Baldwin R L. J Mol Biol. 1983;170:983–1007. doi: 10.1016/s0022-2836(83)80199-5. [DOI] [PubMed] [Google Scholar]
- 19.Maxwell A, Gellert M. Adv Protein Chem. 1986;38:69–107. doi: 10.1016/s0065-3233(08)60526-4. [DOI] [PubMed] [Google Scholar]
- 20.Bauer W R, Gallo R. In: Chromosomes: Eukaryotic, Prokaryotic, and Viral. Adolph K W, editor. Boca Raton, FL: CRC; 1990. pp. 87–126. [Google Scholar]
- 21.Sullivan N, Lyne L. Mutat Res. 1990;245:171–175. doi: 10.1016/0165-7992(90)90046-m. [DOI] [PubMed] [Google Scholar]
- 22.Dedon P C, Goldberg I H. Chem Res Toxicol. 1992;5:311–332. doi: 10.1021/tx00027a001. [DOI] [PubMed] [Google Scholar]
- 23.Nicolaou K C, Li T, Nakada M, Hummel C W, Hiatt A, Wrasildo W. Angew Chem Int Ed Engl. 1994;33:183–186. [Google Scholar]
- 24.Dedon P C, Salzberg A A, Xu J. Biochemistry. 1993;32:3617–3622. doi: 10.1021/bi00065a013. [DOI] [PubMed] [Google Scholar]
- 25.Nicolaou K C, Dai W-M. Angew Chem Int Ed Engl. 1991;30:1387–1530. [Google Scholar]
- 26.Doyle T W, Borders D B. In: Enediyne Antibiotics as Antitumor Agents. Borders D B, Doyle T W, editors. New York: Marcel Dekker; 1995. pp. 1–15. [Google Scholar]
- 27.Lee M D, Ellestad G A, Borders D B. Acc Chem Res. 1991;24:235–243. [Google Scholar]
- 28.LaMarr W A, Sandman K M, Reeve J N, Dedon P C. Nucleic Acids Res. 1997;25:1660–1662. doi: 10.1093/nar/25.8.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cesarone C F, Bolognesi C, Santi L. Anal Biochem. 1979;100:188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- 30.Yu L, Golik J, Harrison R, Dedon P. J Am Chem Soc. 1994;116:9733–9738. [Google Scholar]
- 31.Lueders K K, Fewell J W. BioTechniques. 1994;16:66–67. [PubMed] [Google Scholar]
- 32.Salzberg A, Mathur P, Dedon P. In: DNA and RNA Cleavers and Chemotherapy of Cancer and Viral Diseases. Meunier B, editor. Dordrecht, The Netherlands: Kluwer Academic; 1996. pp. 23–36. [Google Scholar]
- 33.Salzberg A A, Dedon P C. J Biomol Struct Dyn. 1998;15:277–284. doi: 10.1080/07391102.1997.10508192. [DOI] [PubMed] [Google Scholar]
- 34.Krishnamurthy G, Ding W-d, O’Brien L, Ellestad G A. Tetrahedron. 1994;50:1341–1349. [Google Scholar]
- 35.Waring M. J Mol Biol. 1970;54:247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]
- 36.Lerman L S. J Mol Biol. 1961;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- 37.Myers A G, Cohen S B, Tom N J, Madar D J, Fraley M E. J Am Chem Soc. 1995;117:7574–7575. [Google Scholar]
- 38.Epstein J L, Zhang X, Doss G A, Liesch J M, Krishnan B, Stubbe J, Kozarich J W. J Am Chem Soc. 1997;119:6731–6738. [Google Scholar]
- 39.Chatterjee M, Mah S C, Tullius T D, Townsend C A. J Am Chem Soc. 1995;117:8074–8082. [Google Scholar]
- 40.Krishnamurthy G, Brenowitz M D, Ellestad G A. Biochemistry. 1995;34:1001–1010. doi: 10.1021/bi00003a035. [DOI] [PubMed] [Google Scholar]
- 41.Chatterjee M, Smith P J, Townsend C A. J Am Chem Soc. 1996;118:1938–1948. [Google Scholar]
- 42.Meister A, Anderson M E. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 43.LaMarr W A, Sandman K M, Reeve J N, Dedon P C. Chem Res Toxicol. 1997;10:1118–1122. doi: 10.1021/tx970072c. [DOI] [PubMed] [Google Scholar]
- 44.Reed C J, Douglas K T. Biochem J. 1991;275:601–608. doi: 10.1042/bj2750601. [DOI] [PMC free article] [PubMed] [Google Scholar]