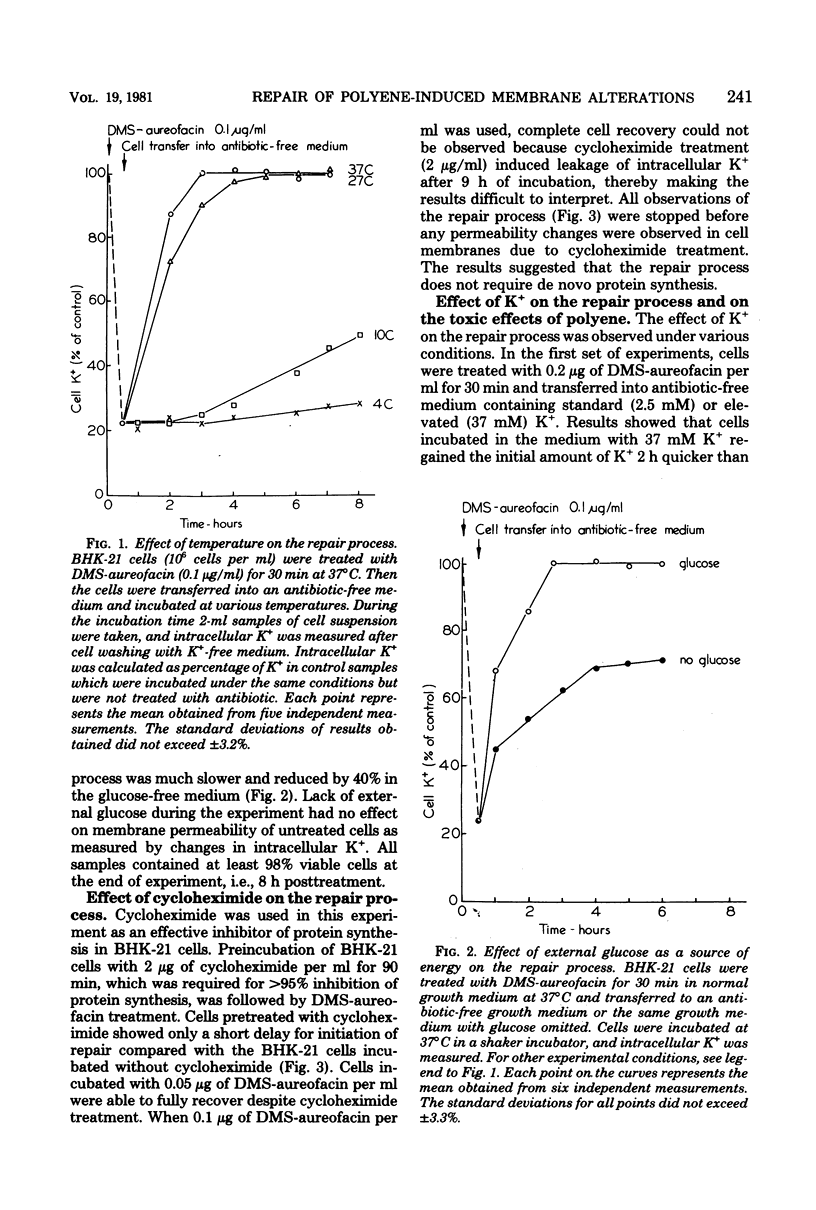
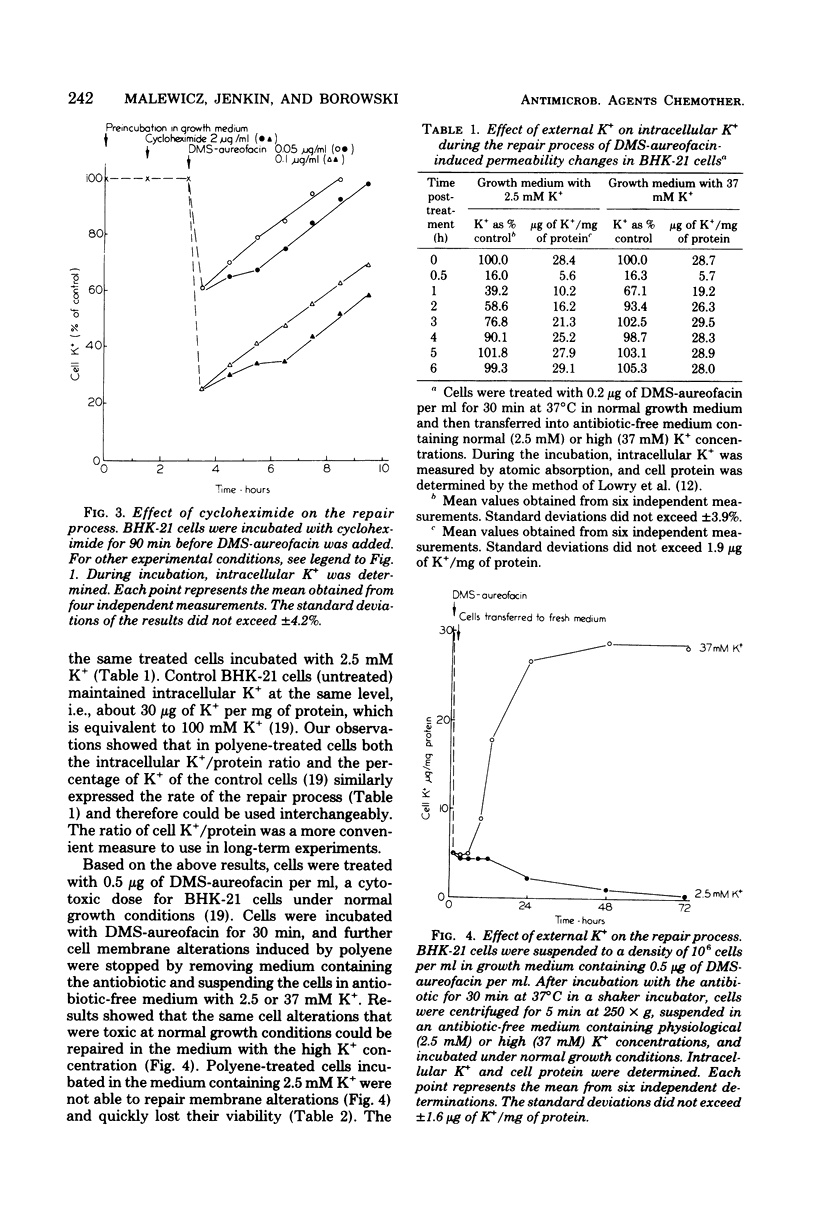
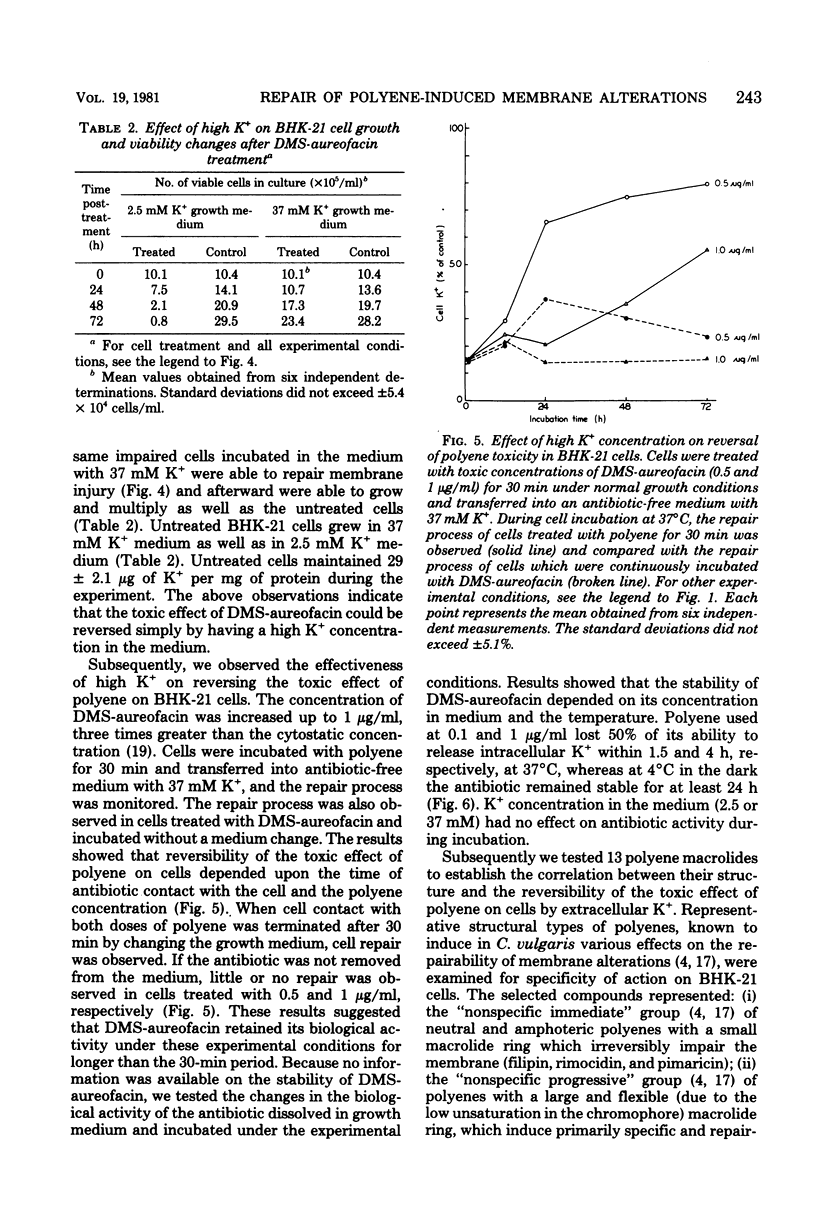
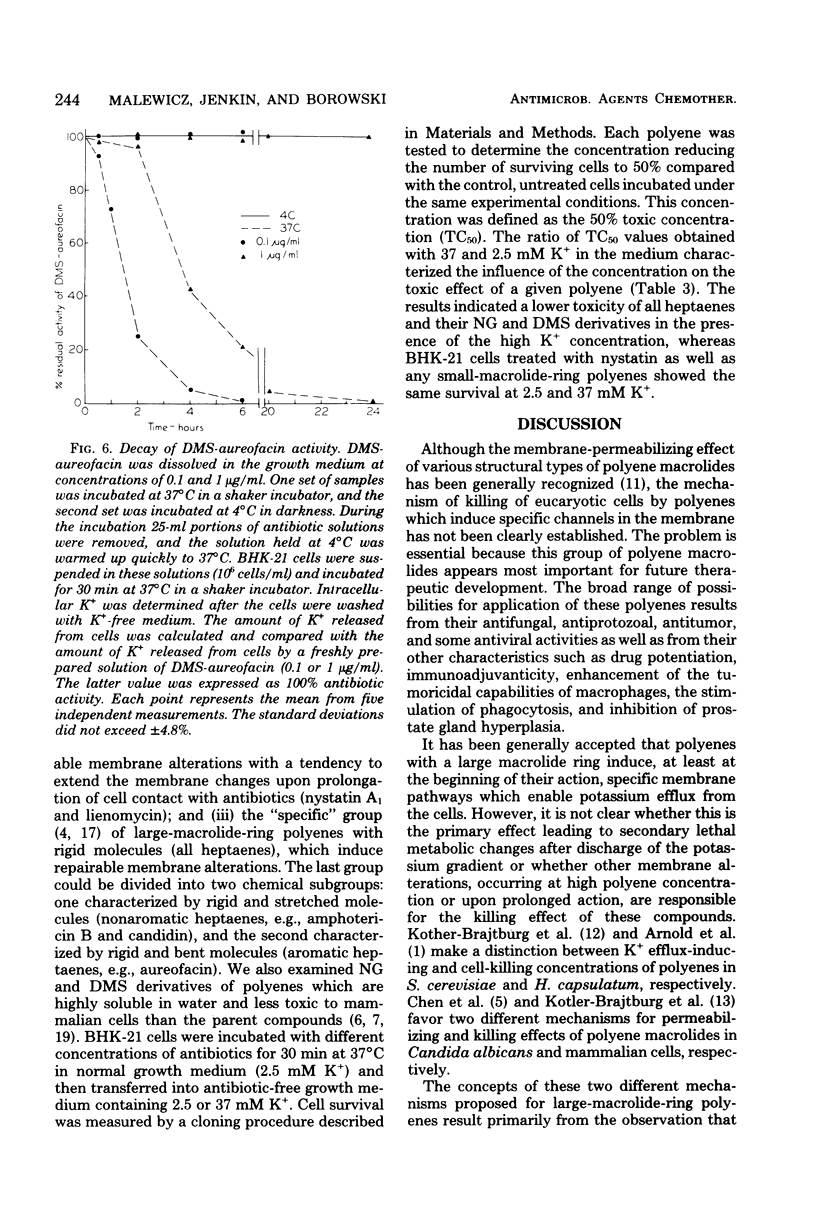
Abstract
We studied the correlation between chemical characteristics of 13 polyene macrolide antibiotics and the ability to repair the membrane permeability changes induced by polyenes in BHK-21 cells grown in shaker culture. It had been demonstrated that large-macrolide-ring polyenes with rigid molecules (heptaenes) induced specific membrane permeability pathways which were repaired by the eucaryotic cells under the proper conditions. The influence of environmental conditions on the repair process was examined. Aureofacin trimethylammonium methyl ester derivative was used as a selected representative of polyene macrolides inducing specific pathways. The factors influencing the repair process, monitored by measuring the ability of BHK-21 cells to control K+ membrane transport, were examined during and after cell contact with the antibiotic. We found that the repair process was dependent upon the temperature, the concentration of the antibiotic, time of its contact with cells, potassium concentration in the medium, and availability of an energy source. The repair process occurred in the presence of cycloheximide, which inhibited protein synthesis in BHK-21 cells. Results showed that the repair process plays an important role in mammalian cell recovery from the toxic effects of polyenes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. N., Pringle A. T., Garrison R. G. Amphotericin B-induced changes in K+ content, viability, and ultrastructure of yeast-phase Histoplasma capsulatum. J Bacteriol. 1980 Jan;141(1):350–358. doi: 10.1128/jb.141.1.350-358.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. C., Chou D. L., Feingold D. S. Dissociation between ion permeability and the lethal action of polyene antibiotics on Candida albicans. Antimicrob Agents Chemother. 1978 Jun;13(6):914–917. doi: 10.1128/aac.13.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski L., Golik J., Kołlodziejczyk P., Pawlak J., Zielinski J. N-glycosyl derivatives of polyene macrolide antibiotics. J Antibiot (Tokyo) 1975 Mar;28(3):244–245. doi: 10.7164/antibiotics.28.244. [DOI] [PubMed] [Google Scholar]
- Falkowski L., Stefańska B., Zieliński J., Bylec E., Golik J., Kołodziejczyk P., Borowski E. Methyl esters of trimethylammonium derivatives of polyene macrolide antibiotics. J Antibiot (Tokyo) 1979 Oct;32(10):1080–1081. doi: 10.7164/antibiotics.32.1080. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Bryson V., Schaffner C. P. Polyene macrolide antibiotic cytotoxicity and membrane permeability alterations. I. Comparative effects of four classes of polyene macrolides on mammalian cells. J Cell Physiol. 1978 Dec;97(3 Pt 1):345–351. doi: 10.1002/jcp.1040970309. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Goldstein N. I., Bryson V., Schaffner C. P. Reduced toxicity of amphotericin B methyl ester (AME) vs. amphotericin B and fungizone in tissue culture. In Vitro. 1976 Feb;12(2):133–140. doi: 10.1007/BF02796361. [DOI] [PubMed] [Google Scholar]
- Guskey L. E., Jenkin H. M. The serial cultivation of suspended BHK-21/13 cells in serum-free Waymouth medium. Proc Soc Exp Biol Med. 1976 Jan;151(1):221–224. doi: 10.3181/00379727-151-39178. [DOI] [PubMed] [Google Scholar]
- Hammond S. M. Biological activity of polyene antibiotics. Prog Med Chem. 1977;14:105–179. doi: 10.1016/s0079-6468(08)70148-6. [DOI] [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Kobayashi G. S., Boggs S., Schlessinger D., Pandey R. C., Rinehart K. L., Jr Classification of polyene antibiotics according to chemical structure and biological effects. Antimicrob Agents Chemother. 1979 May;15(5):716–722. doi: 10.1128/aac.15.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Schlessinger D., Kobayashi G. S. Amphotericin B and filipin effects on L and HeLa cells: dose response. Antimicrob Agents Chemother. 1977 May;11(5):803–808. doi: 10.1128/aac.11.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B. V., Medoff G., Kobayashi G., Schlessinger D. Uptake of Escherichia coli DNA into HeLa cells enhanced by amphotericin B. Nature. 1974 Jul 26;250(464):323–325. doi: 10.1038/250323a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malewicz B., Borowski E. Energy dependence and reversibility of membrane alterations induced by polyene macrolide antibiotics in Chlorella vulgaris. Nature. 1979 Sep 6;281(5726):80–82. doi: 10.1038/281080a0. [DOI] [PubMed] [Google Scholar]
- Malewicz B., Jenkin H. M., Borowski E. Dissociation between the induction of potassium efflux and cytostatic activity of polyene macrolides in mammalian cells. Antimicrob Agents Chemother. 1980 Apr;17(4):699–706. doi: 10.1128/aac.17.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]



