Abstract
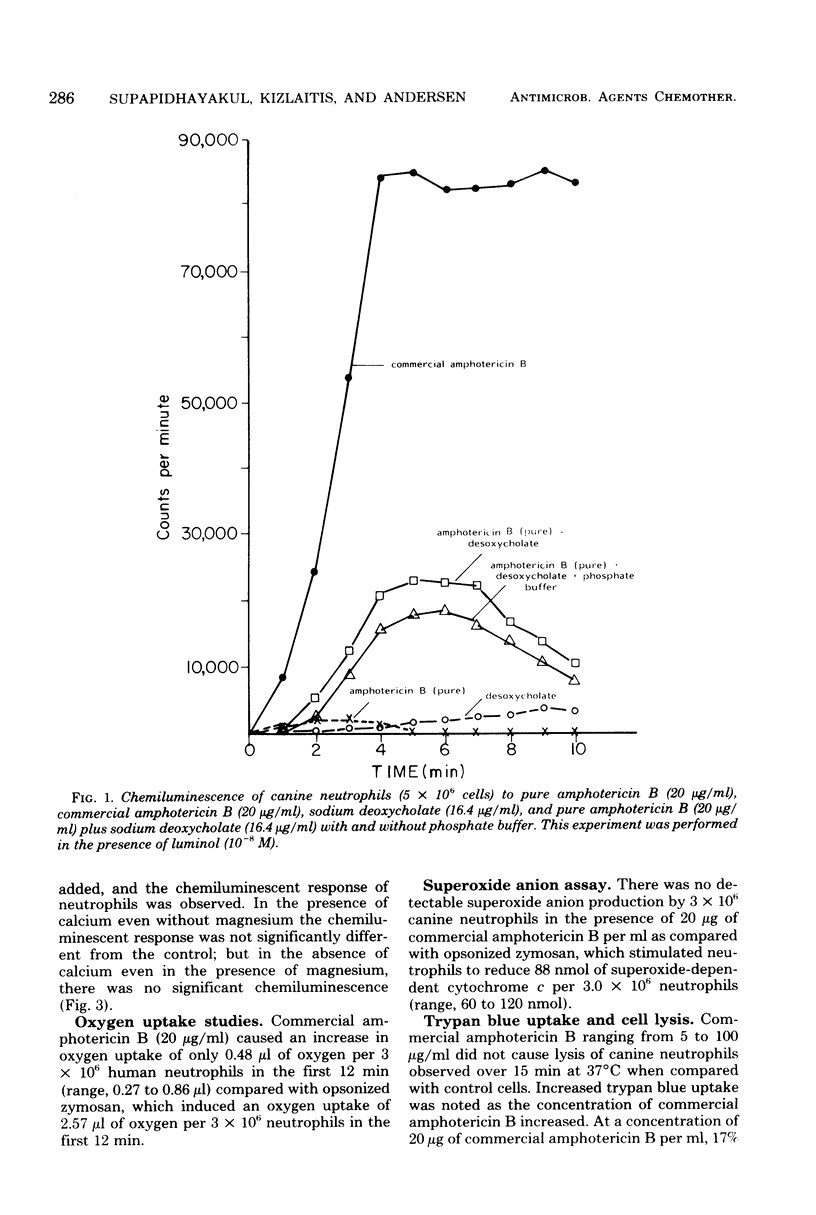
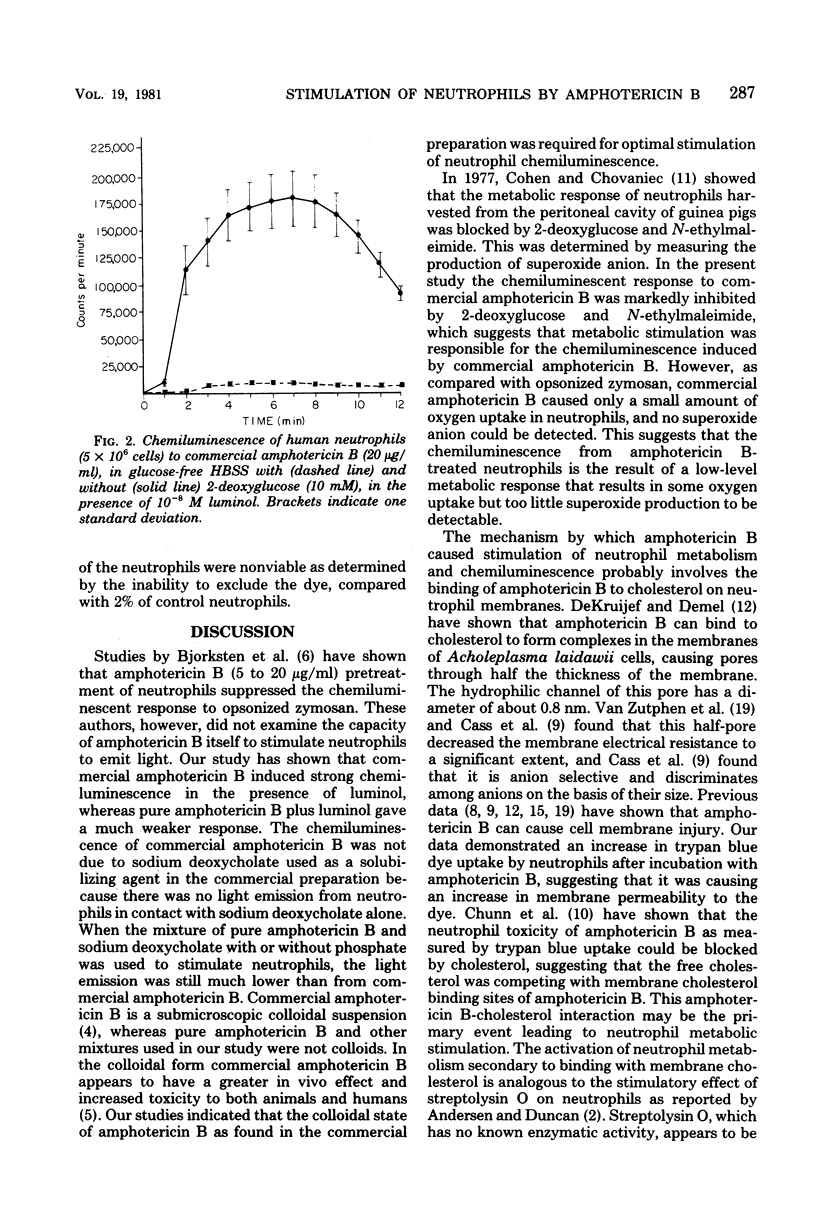
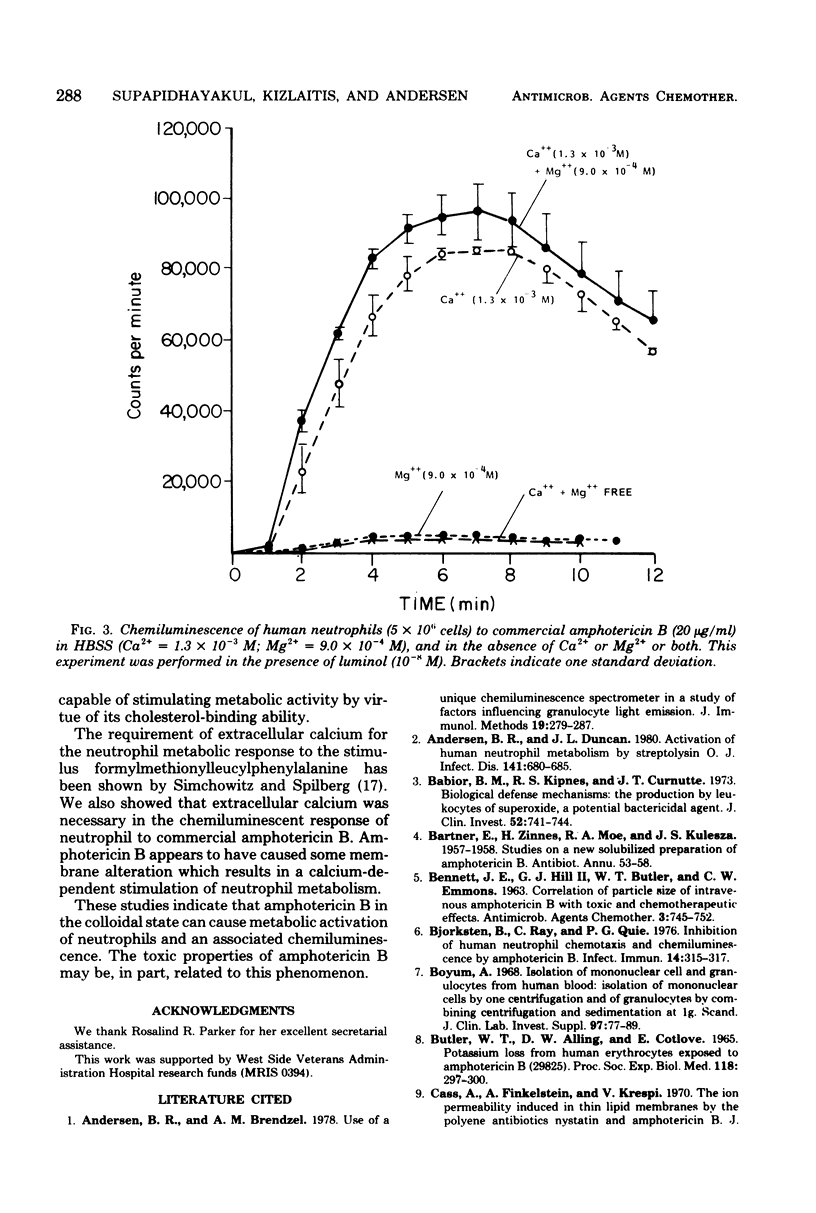
The effect of an antifungal agent, amphotericin B, on human and canine neutrophil metabolism was studied. Commercial preparations of amphotericin B in concentrations ranging from 5 to 100 micrograms/ml stimulated neutrophil chemiluminescence in the presence of 10(-8) M luminol. This response was blocked by 2-deoxyglucose, a metabolic inhibitor, and by the absence of extracellular calcium ions. Neither pure amphotericin B nor the solubilizing agent present in the commercial preparation, alone or in combination, stimulated neutrophil chemiluminescence. Commercial amphotericin B caused an increase in oxygen uptake by neutrophils but no detectable superoxide anion production. Neutrophils were injured by commercial amphotericin B, as shown by an increase in trypan blue dye uptake but not cell lysis. Binding of amphotericin B to neutrophil membrane sterol with a subsequent alteration in membrane configuration is the most likely cause of metabolic stimulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. R., Brendzel A. M. Use of a unique chemiluminescence spectrometer in a study of factors influencing granulocyte light emission. J Immunol Methods. 1978;19(2-3):279–287. doi: 10.1016/0022-1759(78)90187-4. [DOI] [PubMed] [Google Scholar]
- Andersen B. R., Duncan J. L. Activation of human neutrophil metabolism by streptolysin O. J Infect Dis. 1980 May;141(5):680–685. doi: 10.1093/infdis/141.5.680. [DOI] [PubMed] [Google Scholar]
- BARTNER E., ZINNES H., MOE R. A., KULESZA J. S. Studies on a new solubilized preparation of amphotericin B. Antibiot Annu. 1957;5:53–58. [PubMed] [Google Scholar]
- BENNETT J. E., HILL G. J., 2nd, BUTLER W. T., EMMONS C. W. CORRELATION OF PARTICLE SIZE OF INTRAVENOUS AMPHOTERICIN B WITH TOXIC AND CHEMOTHERAPEUTIC EFFECTS. Antimicrob Agents Chemother (Bethesda) 1963;161:745–752. [PubMed] [Google Scholar]
- BUTLER W. T., ALLING D. W., COTLOVE E. POTASSIUM LOSS FROM HUMAN ERYTHROCYTES EXPOSED TO AMPHOTERICIN B. Proc Soc Exp Biol Med. 1965 Jan;118:297–300. doi: 10.3181/00379727-118-29825. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkstén B., Ray C., Quie P. G. Inhibition of human neutrophil chemotaxis and chemiluminescence by amphotericin B. Infect Immun. 1976 Jul;14(1):315–317. doi: 10.1128/iai.14.1.315-317.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cass A., Finkelstein A., Krespi V. The ion permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J Gen Physiol. 1970 Jul;56(1):100–124. doi: 10.1085/jgp.56.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunn C. J., Starr P. R., Gilbert D. N. Neutrophil toxicity of amphotericin B. Antimicrob Agents Chemother. 1977 Aug;12(2):226–230. doi: 10.1128/aac.12.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978 Apr;61(4):1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvath L., Amirault H. J., Andersen B. R. Chemiluminescence of human and canine polymorphonuclear leukocytes in the absence of phagocytosis. J Clin Invest. 1978 May;61(5):1145–1154. doi: 10.1172/JCI109029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINSKY S. C. MEMBRANE STEROLS AND THE SELECTIVE TOXICITY OF POLYENE ANTIFUNGAL ANTIBIOTICS. Antimicrob Agents Chemother (Bethesda) 1963;161:387–394. [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Kobayashi G. S., Boggs S., Schlessinger D., Pandey R. C., Rinehart K. L., Jr Classification of polyene antibiotics according to chemical structure and biological effects. Antimicrob Agents Chemother. 1979 May;15(5):716–722. doi: 10.1128/aac.15.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Price H. D., Medoff G., Schlessinger D., Kobayashi G. S. Molecular basis for the selective toxicity of amphotericin B for yeast and filipin for animal cells. Antimicrob Agents Chemother. 1974 Apr;5(4):377–382. doi: 10.1128/aac.5.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Generation of superoxide radicals by human peripheral neutrophils activated by chemotactic factor. Evidence for the role of calcium. J Lab Clin Med. 1979 Apr;93(4):583–593. [PubMed] [Google Scholar]
- Woodin A. M., Wieneke A. A. Composition and properties of a cell-membrane fraction from the polymorphonuclear leucocyte. Biochem J. 1966 May;99(2):493–500. doi: 10.1042/bj0990493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijff B., Demel R. A. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. 3. Molecular structure of the polyene antibiotic-cholesterol complexes. Biochim Biophys Acta. 1974 Feb 26;339(1):57–70. doi: 10.1016/0005-2736(74)90332-0. [DOI] [PubMed] [Google Scholar]
- van Zutphen H., Demel R. A., Norman A. W., van Deenen L. L. The action of polyene antibiotics on lipid bilayer membranes in the presence of several cations and anions. Biochim Biophys Acta. 1971 Aug 13;241(2):310–330. doi: 10.1016/0005-2736(71)90031-9. [DOI] [PubMed] [Google Scholar]


