Abstract
We evaluated the in vitro antimicrobial activity of Sch 24893, Sch 25298, and Sch 25393, three novel analogs of chloramphenicol and thiamphenicol. All of the analogs had minimal inhibitory concentrations of less than or equal to 10 micrograms/ml for 18 chloramphenicol-thiamphenicol-resistant strains of Shigella dysenteriae and 21 strains of resistant Salmonella typhi. The analogs were also more active than were chloramphenicol and thiamphenicol against chloramphenicol-resistant enteric bacteria, including six strains of Escherichia coli, seven strains of Klebsiella pneumoniae, and two strains of Enterobacter cloacae. Fifty-three strains of ampicillin-resistant Haemophilus influenzae were uniformly susceptible to chloramphenicol, thiamphenicol, and the three analogs. Sch 25298 was the most active compound tested (minimal inhibitory concentration, 0.5 microgram/ml for all strains). Four of seven chloramphenicol-thiamphenicol-resistant Haemophilus strains were susceptible to the fluorinated analogs. Of the three Haemophilus strains which were resistant to chloramphenicol, thiamphenicol, and the analogs, two contained less than 10% of the chloramphenicol acetyltransferase activity of the strains which were resistant to only chloramphenicol and thiamphenicol. We conclude that fluorinated analogs of chloramphenicol and thiamphenicol have considerable in vitro activity against a broad spectrum of chloramphenicol-thiamphenicol-resistant, gram-negative bacteria.
Full text
PDF
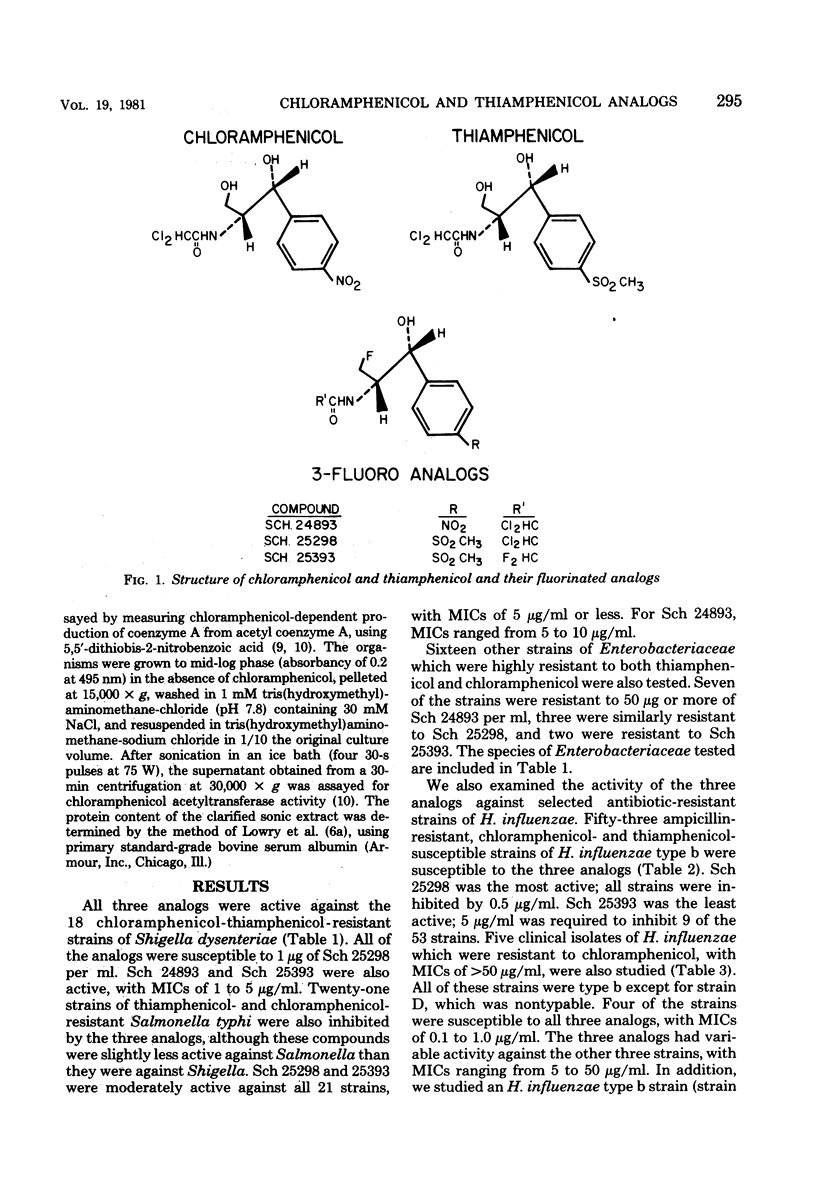
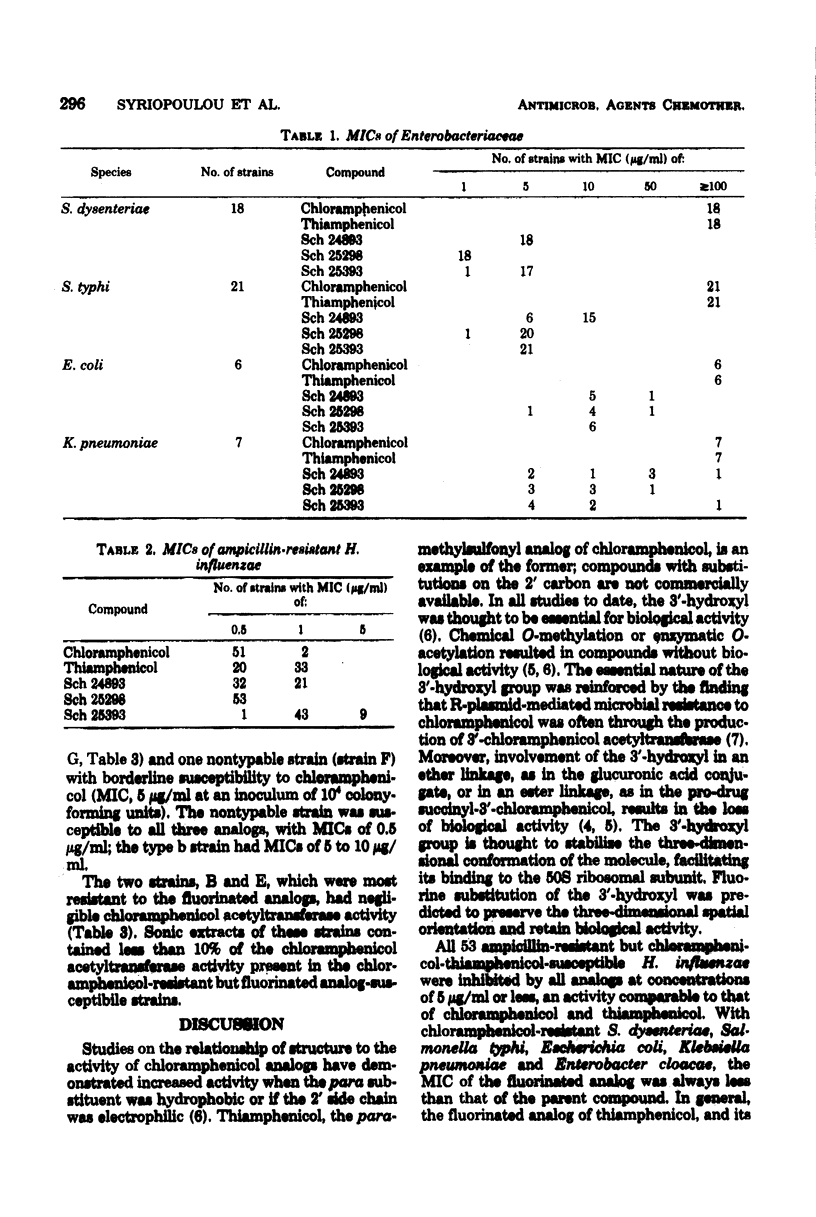
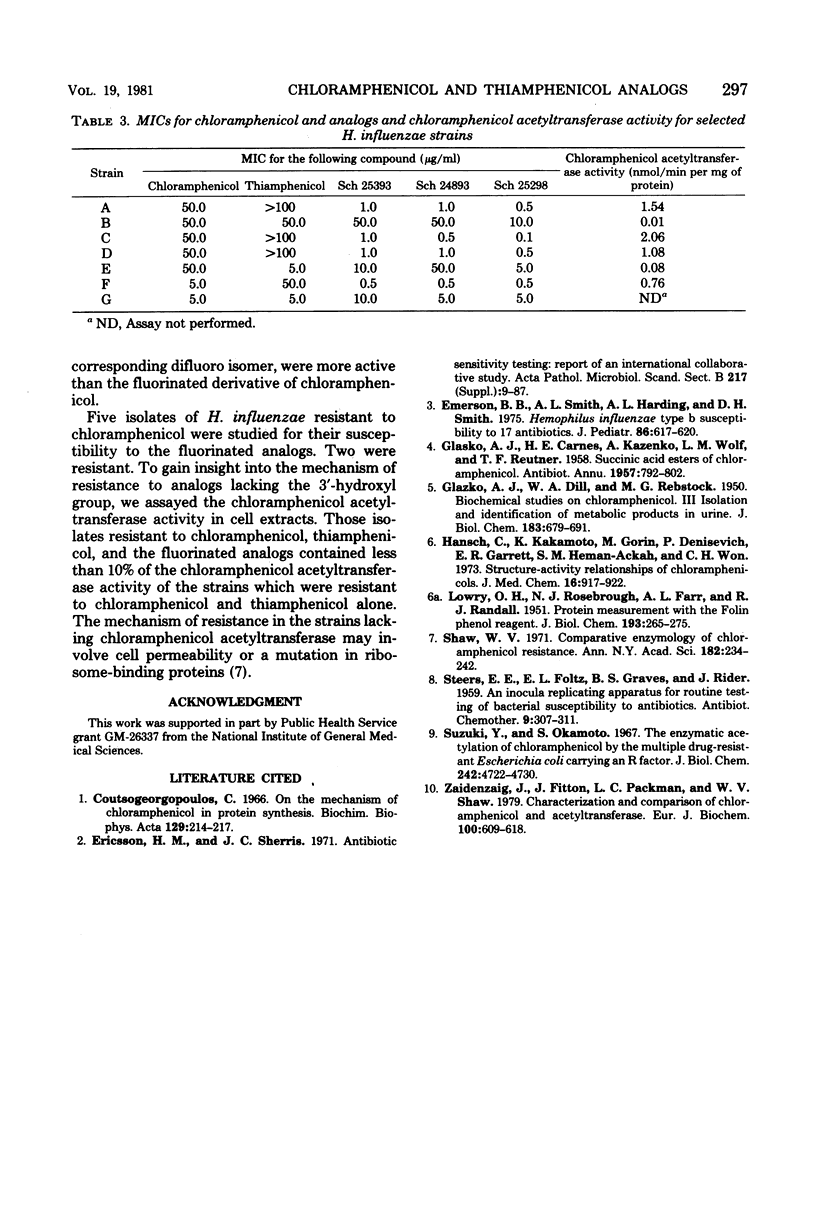
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coutsogeorgopoulos C. On the mechanism of action of chloramphenicol in protein synthesis. Biochim Biophys Acta. 1966 Oct 24;129(1):214–217. doi: 10.1016/0005-2787(66)90027-x. [DOI] [PubMed] [Google Scholar]
- Emerson B. B., Smith A. L., Harding A. L., Smith D. H. Hemophilus influenzae type B susceptibility to 17 antibiotics. J Pediatr. 1975 Apr;86(4):617–620. doi: 10.1016/s0022-3476(75)80166-1. [DOI] [PubMed] [Google Scholar]
- Hansch C., Nakamoto K., Gorin M., Denisevich P., Garret E. R., Heman-Ackah S. M., Won C. H. Structure-activity relationship of chloramphenicols. J Med Chem. 1973 Aug;16(8):917–922. doi: 10.1021/jm00266a011. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Shaw W. V. The problems of drug-resistant pathogenic bacteria. Comparative enzymology of chloramphenicol resistance. Ann N Y Acad Sci. 1971 Jun 11;182:234–242. doi: 10.1111/j.1749-6632.1971.tb30660.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Okamoto S. The enzymatic acetylation of chloramphenicol by the multiple drug-resistant Escherichia coli carrying R factor. J Biol Chem. 1967 Oct 25;242(20):4722–4730. [PubMed] [Google Scholar]
- Zaidenzaig Y., Fitton J. E., Packman L. C., Shaw W. V. Characterization and comparison of chloramphenicol acetyltransferase variants. Eur J Biochem. 1979 Oct 15;100(2):609–618. doi: 10.1111/j.1432-1033.1979.tb04208.x. [DOI] [PubMed] [Google Scholar]


