Abstract
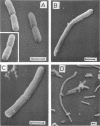
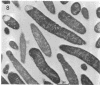
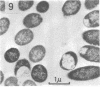
This paper presents a study of the interactions between Escherichia coli cells and nifurzide, a nitrofuran derivative which is used as an intestinal antiinfectious agent. At low concentrations of nifurzide, the growth rate of the cultures decreased, and elongated, nonseptate cells appeared. At high concentrations, complete growth inhibition occurred, accompanied by a rather strong bactericidal effect, but the appearance of the cells was normal; in particular, no bacteriolytic effect was observed. A very large number of antibiotic molecules were bound per bacterial cell. After cell disruption, similar amounts of nifurzide were found in the cytoplasm, cytoplasmic membranes, and cell wall, respectively. Most of the bound nifurzide was rapidly degraded or became protein bound. The structure of the outer membrane lipopolysaccharide appeared to have little influence on the activity of nifurzide.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ferguson C. A., Murray R. G., Lancy P., Jr Effects of some platinum IV complexes on cell division of Escherichia coli. Can J Microbiol. 1979 May;25(5):545–559. doi: 10.1139/m79-080. [DOI] [PubMed] [Google Scholar]
- Herrlich P., Schweiger M. Nitrofurans, a group of synthetic antibiotics, with a new mode of action: discrimination of specific messenger RNA classes. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3386–3390. doi: 10.1073/pnas.73.10.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliash Iu V., Lintvar'ova V. B. Vivchennia dii nitrofuraniv na NAD-zalezhni degifrogenazi zolotistogo stafilokoka. Mikrobiol Zh. 1975 Jan-Feb;37(1):106–110. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. II. Application of solutions containing lead and barium. J Biophys Biochem Cytol. 1958 Nov 25;4(6):727–730. doi: 10.1083/jcb.4.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]