Abstract
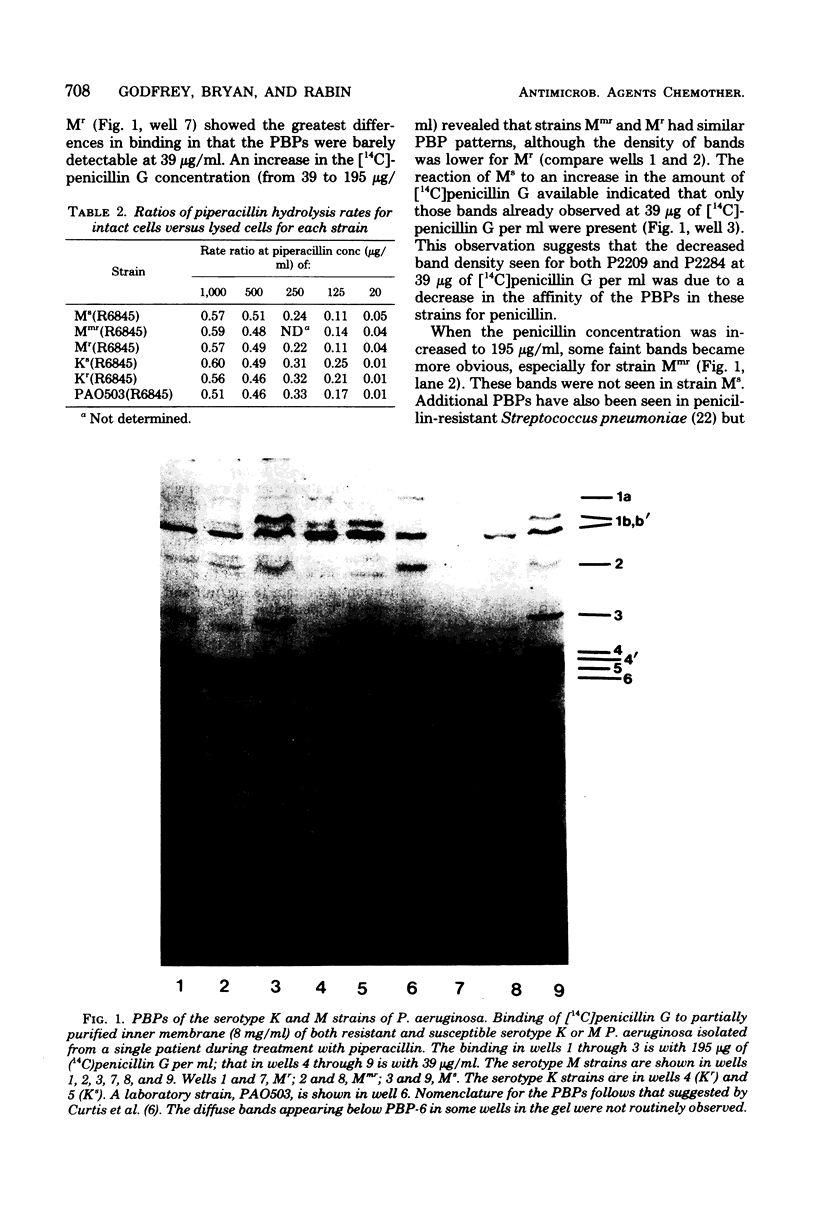
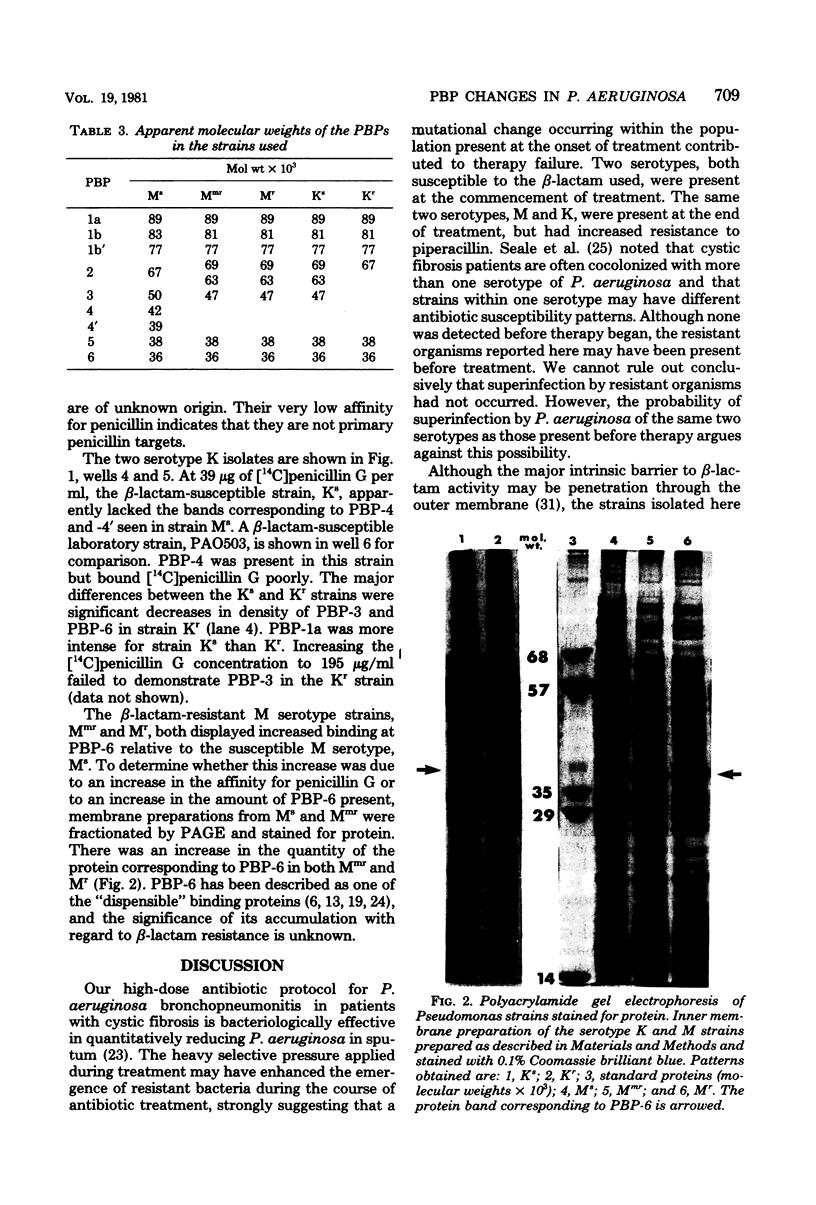
The emergence of beta-lactam-resistant strains of Pseudomonas aeruginosa in a cystic fibrosis patient treated with high-dose tobramycin and piperacillin was studied. Two serotypes, M and K, were present before treatment and persisted, with changes in their beta-lactam resistance spectra, during treatment. The resistance was correlated with changes in the penicillin-binding proteins (PBPs) in both serotypes. In the low-level-resistant serotype K organism, PBP-3 either was absent or had lost the ability to bind [14C]penicillin G. Tow serotypes M strains, one with low- and one with high-level resistance to several antipseudomonal beta-lactam antibiotics, were isolated at progressively later stages of therapy. Several differences were noted between the PBP patterns of the resistant M and the susceptible M strains. The affinity for [14C]penicillin G was reduced in both resistant strains. PBP bands, with the exception of PBP-6 in the most resistant M type, were barely or not detectable at a [14C]penicillin G concentration of 39 microgram/ml. The graduated decrease in affinity for [14c]penicillin G was correlated with increasing beta-lactam resistance and with an increase in the quantity of the protein corresponding to PBP-6. The emergence of the low-level-resistant strains midway through, and of the highly resistant strain in the final stages of, the reported treatment strongly suggested that the resistance resulted from mutation in those strains present before treatment selected for by the high-dose piperacillin treatment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauernfeind A., Petermüller C. Chemotherapy with cefotaxime: how to avoid selection of Pseudomonas aeruginosa. J Antimicrob Chemother. 1980 Sep;6(5):671–672. doi: 10.1093/jac/6.5.671. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M., Tseng J. T. Transferable drug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1972 Jan;1(1):22–29. doi: 10.1128/aac.1.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Affinities of penicillins and cephalosporins for the penicillin-binding proteins of Escherichia coli K-12 and their antibacterial activity. Antimicrob Agents Chemother. 1979 Nov;16(5):533–539. doi: 10.1128/aac.16.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Competition of beta-lactam antibiotics for the penicillin-binding proteins of Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella aerogenes, Proteus rettgeri, and Escherichia coli: comparison with antibacterial activity and effects upon bacterial morphology. Antimicrob Agents Chemother. 1979 Sep;16(3):325–328. doi: 10.1128/aac.16.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaman W., Cates C., Snelling C. F., Lank B., Ronald A. R. Emergence of gentamicin- and carbenicillin-resistant Pseudomonas aeruginosa in a hospital environment. Antimicrob Agents Chemother. 1976 Mar;9(3):474–480. doi: 10.1128/aac.9.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980 Jul;18(1):148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. R factor variants with enhanced sex factor activity in Pseudomonas aeruginosa. Mol Gen Genet. 1976 Mar 30;144(3):243–251. doi: 10.1007/BF00341722. [DOI] [PubMed] [Google Scholar]
- Iida K., Hirata S., Nakamuta S., Koike M. Inhibition of cell division of Escherichia coli by a new synthetic penicillin, piperacillin. Antimicrob Agents Chemother. 1978 Aug;14(2):257–266. doi: 10.1128/aac.14.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya M., Strominger J. L. Simultaneous deletion of D-alanine carboxypeptidase IB-C and penicillin-binding component IV in a mutant of Escherichia coli K12. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2980–2984. doi: 10.1073/pnas.74.7.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Mikoshiba F., Nagasaki S., Matsumoto H., Tazaki T. Prevalence of Pseudomonas aeruginosa strains possessing R factor in a hospital. J Antibiot (Tokyo) 1972 Oct;25(10):607–609. doi: 10.7164/antibiotics.25.607. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labia R., Kazmierczak A., Philippon A., Le Goffic F., Faye J. C., Goldstein F. W., Acar J. F. beta-Lactamases de Pseudomonas aeruginosa et résistance a la carbénicilline. Ann Microbiol (Paris) 1975 May-Jun;126A(4):449–459. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lowbury E. J., Lilly H. A., Kidson A., Ayliffe G. A., Jones R. J. Sensitivity of Pseudomonas aeruginosa to antibiotics: emergence of strains highly resistant to carbenicillin. Lancet. 1969 Aug 30;2(7618):448–452. doi: 10.1016/s0140-6736(69)90163-9. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Takagaki Y., Maruyama I. N., Tamaki S., Nishimura Y., Suzuki H., Ogino U., Hirota Y. Mutants of Escherichia coli lacking in highly penicillin-sensitive D-alanine carboxypeptidase activity. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2976–2979. doi: 10.1073/pnas.74.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Matsuhashi M., Mitsuhashi S. Comparative studies of penicillin-binding proteins in Pseudomonas aeruginosa and Escherichia coli. Eur J Biochem. 1979 Oct;100(1):41–49. doi: 10.1111/j.1432-1033.1979.tb02031.x. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percheson P. B., Bryan L. E. Penicillin-binding components of penicillin-susceptible and -resistant strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Sep;18(3):390–396. doi: 10.1128/aac.18.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpff S. C., Greene W. H., Young V. M., Wiernik P. H. Significance of Pseudomonas aeruginosa in the patient with leukemia or lymphoma. J Infect Dis. 1974 Nov;130 (Suppl)(0):S24–S31. doi: 10.1093/infdis/130.supplement.s24. [DOI] [PubMed] [Google Scholar]
- Seale T. W., Thirkill H., Tarpay M., Flux M., Rennert O. M. Serotypes and antibiotic susceptibilities of Pseudomonas aeruginosa isolates from single sputa of cystic fibrosis patients. J Clin Microbiol. 1979 Jan;9(1):72–78. doi: 10.1128/jcm.9.1.72-78.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Richmond M. H. R factors, beta-lactamase, and carbenicillin-resistant Pseudomonas aeruginosa. Lancet. 1971 Aug 14;2(7720):342–344. doi: 10.1016/s0140-6736(71)90060-2. [DOI] [PubMed] [Google Scholar]
- Tapper M. L., Armstrong D. Bacteremia due to Pseudomonas aeruginosa complicating neoplastic disease: a progress report. J Infect Dis. 1974 Nov;130 (Suppl)(0):S14–S23. doi: 10.1093/infdis/130.supplement.s14. [DOI] [PubMed] [Google Scholar]
- Weinstein R. A., Nathan C., Gruensfelder R., Kabins S. A. Endemic aminoglycoside resistance in gram-negative bacilli: epidemiology and mechanisms. J Infect Dis. 1980 Mar;141(3):338–345. doi: 10.1093/infdis/141.3.338. [DOI] [PubMed] [Google Scholar]
- Zimmermann W. Penetration of beta-lactam antibiotics into their target enzymes in Pseudomonas aeruginosa: comparison of a highly sensitive mutant with its parent strain. Antimicrob Agents Chemother. 1980 Jul;18(1):94–100. doi: 10.1128/aac.18.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]